Abstract
The cellular reaction to the DNA-damaging agents may modulate individual’s cancer susceptibility. This reaction is mainly determined by the efficacy of DNA repair, which in turn, may be influenced by the variability of DNA repair genes, expressed by their polymorphism. The hOGG1 gene encodes a glycosylase of base excision repair and RAD51 specifies a key protein in homologues recombination repair. Both proteins can be involved in the repair of DNA lesions, which are known to contribute to endometrial cancer. In the present work we determined the extent of basal DNA damage and the efficacy of removal of DNA damage induced by hydrogen peroxide and N-methyl-N′-nitro N-nitrosoguanidyne (MNNG) in peripheral blood lymphocytes of 30 endometrial cancer patients and 30 individuals without cancer. The results from DNA damage and repair study were correlated with the genotypes of two common polymorphisms of the hOGG1 and RAD51 genes: a G>C transversion at 1245 position of the hOGG1 gene producing a Ser → Cys substitution at the codon 326 (the Ser326Cys polymorphism) and a G>C substitution at 135 position of the RAD51 gene (the 135G>C polymorphism). DNA damage and repair were evaluated by alkaline single cell gel electrophoresis and genotypes were determined by restriction fragment length polymorphism PCR. We observed a strong association between endometrial cancer and the C/C genotype of the 135G>C polymorphism of the RAD51 gene. Moreover, there was a strong correlation between that genotype and endometrial cancer occurrence in subjects with a high level of basal DNA damage. We did not observe any correlation between the Ser326Cys polymorphism of the hOGG1 gene and endometrial cancer. Our result suggest that the 135G>C polymorphism of the RAD51 gene may be linked to endometrial cancer and can be considered as an additional marker of this disease.
Keywords: DNA damage, DNA repair, Endometrial cancer, RAD51, hOGG1, Genetic polymorphism
Introduction
Endometrial cells are constantly under the oxidative stress during menstrual cycles [1]. The stress is generated in the metabolic reactions of estrogens, producing reactive oxygen species (ROS), which can cause damages to biomolecules, including DNA [2]. ROS may induce mutations in proto-oncogenes and tumor suppressor genes, as well as in other genes important for induction, promotion and progression of cancer, accelerating up malignant transformation [3, 4]. Oxidative damages to the DNA bases are mainly removed by base excision repair (BER) pathway. hOGG1 is a key BER enzyme to recognize and excise of 8-hydroxy-deoxyguanine (8-oxo-dG), which cause G>A transversion [5–8].
hOGG1, the gene encoding hOGG1, is highly polymorphic [9]. A C>G transversion at the 1245 position in the exon 7 of the gene results in a serine to cysteine substitution in the codon 326 (the Ser326Cys polymorphism; rs 1052133). The role of this polymorphism in the catalytic activity of the hOGG1 protein is still controversial. Several studies have suggested that Cys326 allele may be associated with the increased risk cancers [10–21]. For prostate cancer it was shown that Cys326Cys variant of hOGG1 reduced cancer risk of more aggressive disease [22].
Hydroxyl radicals can attack sugar-phosphate backbone of DNA, producing single strand breaks, which could be converted into double strand breaks (DSBs) in replication or generation of another single strand break on the opposite DNA strand. RAD51-depended homologous DNA recombination repair (HRR) is one of major pathways for the repair of DSBs in human cells. HRR has been implicated in the resuming of stalled replication fork and RAD51 protein plays essential role in this process. HRR is also involved in the repair of DNA-protein cross-links in cooperation with nucleotide excision repair (NER). Human RAD51 is required for meiotic and mitotic recombination and plays a central role in homology-dependent recombinational repair of DSBs [23, 24]. A single nucleotide polymorphism in the 5′-untranslated region (5′-UTR) of the human RAD51 gene, resulting in a G>C substitution at the 135 position (the 135G>C polymorphism; c. −98 G>C; rs 1801320), has been identified [25]. We showed previously that this polymorphism was not an independent marker in breast cancer, but it could be associated with an increased gastric cancer risk and an increased breast cancer risk in BRCA2 mutation carriers [26, 27]. Similar results came from other laboratories [25, 28].
In the present work we tried to correlate the genetic constitution expressed by genotypes of the Ser326Cys and 135G>C polymorphisms of the hOGG1 and RAD51 genes, respectively, with susceptibility to DNA damage and efficacy of DNA repair in human lymphocytes of endometrial cancer patients. To evaluate the extent of DNA damage, the effectiveness of DNA repair and the sensitivity to exogenous mutagens we determined: (1) the level of basal DNA damage and (2) the capacity to remove DNA damage induced by hydrogen peroxide and N-methyl-N′-nitro N-nitrosoguanidyne (MNNG) in peripheral blood lymphocytes of endometrial cancer patients and healthy individuals. DNA damage and repair were evaluated by alkaline single cell gel electrophoresis (comet assay).
Materials and methods
Patients
Blood samples were obtained from 30 patients with histologically confirmed endometrial cancer (median age 55 years) treated at the Department of Oncological Surgery, N. Copernicus Hospital (Lodz, Poland) and Polish Mother’s Memorial Hospital (Lodz, Poland) in 2006 and 2007 and 30 cancer-free age-matched women. Blood from patients was collected before surgical treatment and radiotherapy. The study was approved by the Local Ethic Committee and each patient gave a written consent.
Cell preparation
Blood samples were immediately transported to the laboratory on ice. Peripheral blood lymphocytes (PBL) were isolated by centrifugation in a density gradient of Histopaque-1077 (15 min, 280 × g). The pellet containing PBL was resuspended in RPMI 1640 medium to give about (1–3) × 105 cells per ml and further processed.
Comet assay
The comet assay was performed under alkaline conditions essentially according to the procedure of Singh et al. [29] with some modifications as described previously [30].
Peripheral blood lymphocytes were incubated with hydrogen peroxide (10 mM for 10 min at 0°C) and MNNG (200 nM for 1 h at 37°C) to evaluate the efficacy of DNA repair. After incubation, cells were washed and incubated in a fresh, drug-free medium for 2 h at 37°C. The comets were observed at ×200 magnification in an Eclipse fluorescence microscope (Nikon, Tokyo, Japan) attached to a COHU 4910 video camera (Cohu, San Diego, CA, USA) equipped with UV-1 filter block (an excitation filter of 359 nm and a barrier filter of 461 nm) and connected to a personal computer-based Lucia-Comet v. 4.51 image analysis system (Laboratory Imaging, Prague, Czech Republic). The mean of percentage of DNA in the tail of comet for a total of 100 cells was calculated. All the values in this study were expressed as mean ± SE.
Genotype determination
Genomic DNA was prepared by the standard phenol procedure as we described previously [31]. RAD51 genotyping was analyzed by a PCR amplification of a 157-bp region around the 135th nucleotide. This region contains a single MvaI site that was abolished in the 135C variant. Wild type alleles were digested by MvaI (Fermentas, Vilnius, Lithuania) producing 86 and 71 bp length products. The 135C variant of RAD51 was not digested by the enzyme, giving a single 157 bp PCR product. PCR was performed in a MT Research, INC thermal cycler with the following primers: sense: 5′-TGGGAACTGCAACTCATCTGG-3′ and 5′-GCGCTCCTCTCTCCAGCAG-3′ at a final Mg2+ concentration of 1.5 mM and annealing temperature of 53°C. After overnight digestion with the enzyme, the samples were separated onto a 8% polyacrylamide gel. The genotypes of the other polymorphism were determined with the following primers: sense 5′-GTTTTCACTAATGAGCTTGC-3′, antisense 5′-AGTGGTATAATCATGTGGGT-3′ at a final Mg2+ concentration of 1.5 mM and annealing temperature of 57°C. The 200 bp PCR product was digested overnight with 5 U of the restriction enzyme SatI (Fermentas, Vilnius, Lithuania). The Cys allele was digested into 100 bp fragments whereas the Ser variant remained intact. The PCR products were run on a 8% of polyacrylamide gel [12, 26, 27].
Data analysis
Distribution of genotypes and alleles between groups were analyzed using the χ2-test. Relationship between genotype and cancer was assessed by the unconditional logistic regression (quasi-Newton method). For each SNP, odds ratio (OR) was estimated. Wild type alleles or additional homozygous variants were used as reference groups. Efficacy of DNA repair was analyzed using Wilcoxon matched pair test. Analyses were performed using STATISTICA 8.0 package (Statsoft, Tulusa, OK, USA) and P < 0.05 was considered statistically significant.
Results
DNA damage
The mean extent of endogenous DNA damage measured as the percentage of DNA in comet tail of the lymphocytes of endometrial cancer patients and controls is displayed in Fig. 1. There were no differences between the extents of DNA damage for both groups (P > 0.05).
Fig. 1.
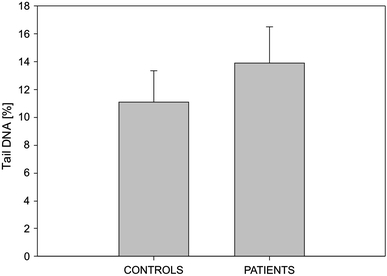
Endogenous DNA damage (DNA strand breaks and alkali labile sites), measured as the mean percentage of comet tail DNA in the alkaline comet assay in human peripheral blood lymphocytes of healthy controls and endometrial cancer patients. Thirty individuals were analyzed in either group. The results displayed are the mean ± SE
Sensitivity to DNA-damaging agents and efficacy of DNA repair
The mean percentage tail DNA of PBL from endometrial cancer patients and controls exposed to 10 mM hydrogen peroxide or 200 nM MNNG immediately after the exposure as well as 120 min thereafter are presented in Tables 1 and 2. We do not observed any differences in the mean sensitivity to hydrogen peroxide and MNNG (the mean extent of DNA damage immediately after exposure) for patients and controls. We observed a great variation in the individual efficacy of DNA repair. Both patients and controls had comparable number of subjects with inefficient DNA repair, in other words, with not any statistically significant difference between the level of DNA damage at 120 and 0 min (bolded).
Table 1.
Repair of hydrogen peroxide-induced DNA damage in 30 endometrial cancer patients and 30 healthy controls
| Endometrial cancer DNA damage ± SE | Control DNA damage ± SE | ||||||
|---|---|---|---|---|---|---|---|
| Number | 0 min | 120 min | P | Number | 0 min | 120 min | P |
| 1 | 16.5 ± 5.55 | 5.33 ± 0.79 | <0.05 | 1 | 17.3 ± 0.66 | 7.52 ± 0.91 | <0.05 |
| 2 | 9.44 ± 3.05 | 3.23 ± 0.44 | <0.05 | 2 | 15.7 ± 3.20 | 2.61 ± 0.81 | <0.05 |
| 3 | 8.43 ± 2.46 | 0.87 ± 1.12 | <0.05 | 3 | 9.59 ± 1.88 | 1.57 ± 0.87 | <0.05 |
| 4 | 12.5 ± 3.32 | 6.04 ± 1.61 | <0.05 | 4 | 19.8 ± 0.58 | 9.76 ± 2.03 | <0.05 |
| 5 | 7.99 ± 2.70 | 4.95 ± 1.17 | <0.05 | 5 | 14.8 ± 0.11 | 10.3 ± 0.52 | <0.05 |
| 6 | 7.68 ± 1.61 | 4.48 ± 2.12 | <0.05 | 6 | 58.1 ± 3.39 | 12.3 ± 3.02 | <0.05 |
| 7 | 22.6 ± 1.44 | 23.9 ± 1.72 | 0.2567 | 7 | 46.7 ± 3.36 | 6.49 ± 0.63 | <0.05 |
| 8 | 59.0 ± 12.74 | 48.3 ± 5.46 | 0.2972 | 8 | 29.9 ± 1.48 | 26.6 ± 2.12 | 0.2972 |
| 9 | 14.5 ± 1.17 | 7.06 ± 1.98 | <0.05 | 9 | 20.5 ± 2.00 | 10.8 ± 0.62 | <0.05 |
| 10 | 18.6 ± 3.15 | 11.3 ± 1.48 | <0.05 | 10 | 76.5 ± 3.61 | 8.77 ± 2.40 | <0.05 |
| 11 | 16.4 ± 2.58 | 8.43 ± 0.84 | <0.05 | 11 | 22.9 ± 0.59 | 24.0 ± 2.03 | 0.4314 |
| 12 | 25.3 ± 2.26 | 8.13 ± 1.75 | <0.05 | 12 | 22.6 ± 2.24 | 3.92 ± 0.38 | <0.05 |
| 13 | 25.7 ± 1.08 | 6.06 ± 2.46 | <0.05 | 13 | 18.2 ± 1.48 | 1.61 ± 0.45 | <0.05 |
| 14 | 67.5 ± 2.87 | 59.6 ± 2.55 | 0.2894 | 14 | 8.33 ± 0.98 | 3.77 ± 1.72 | <0.05 |
| 15 | 39.0 ± 2.45 | 25.0 ± 2.81 | <0.05 | 15 | 29.0 ± 2.32 | 1.17 ± 0.63 | <0.05 |
| 16 | 28.9 ± 2.39 | 19.0 ± 1.20 | <0.05 | 16 | 10.4 ± 1.52 | 9.19 ± 1.73 | 0.2540 |
| 17 | 47.3 ± 5.33 | 35.1 ± 2.78 | <0.05 | 17 | 9.8 ± 5.09 | 6.84 ± 1.72 | <0.05 |
| 18 | 26.6 ± 3.01 | 7.41 ± 0.84 | <0.05 | 18 | 28.3 ± 3.61 | 22.7 ± 3.96 | 0.4486 |
| 19 | 14.5 ± 3.27 | 11.8 ± 4.03 | 0.0814 | 19 | 22.2 ± 1.94 | 14.5 ± 1.52 | <0.05 |
| 20 | 12.0 ± 2.03 | 13.8 ± 2.01 | 0.0978 | 20 | 27.8 ± 1.29 | 13.7 ± 1.85 | <0.05 |
| 21 | 19.1 ± 3.64 | 6.23 ± 1.36 | <0.05 | 21 | 25.0 ± 3.14 | 12.6 ± 2.16 | <0.05 |
| 22 | 21.7 ± 3.64 | 3.66 ± 2.11 | <0.05 | 22 | 28.5 ± 3.98 | 2.87 ± 2.85 | <0.05 |
| 23 | 13.5 ± 3.26 | 7.94 ± 2.28 | <0.05 | 23 | 18.0 ± 1.11 | 3.35 ± 0.86 | <0.05 |
| 24 | 17.3 ± 1.14 | 7.52 ± 1.19 | <0.05 | 24 | 18.8 ± 2.22 | 11.3 ± 3.09 | <0.05 |
| 25 | 11.4 ± 1.06 | 11.4 ± 1.48 | 0.8281 | 25 | 7.19 ± 1.61 | 7.69 ± 1.80 | 0.1080 |
| 26 | 15.7 ± 1.12 | 3.77 ± 1.35 | <0.05 | 26 | 58.9 ± 3.07 | 8.78 ± 1.03 | <0.05 |
| 27 | 24.5 ± 2.67 | 11.9 ± 2.38 | <0.05 | 27 | 10.7 ± 1.94 | 10.5 ± 1.14 | 0.2994 |
| 28 | 23.8 ± 1.88 | 8.22 ± 1.51 | <0.05 | 28 | 24.5 ± 2.07 | 27.3 ± 1.48 | 0.2103 |
| 29 | 10.7 ± 1.44 | 10.5 ± 1.01 | 0.0767 | 29 | 11.2 ± 1.17 | 8.05 ± 0.77 | <0.05 |
| 30 | 22.1 ± 1.69 | 6.75 ± 1.64 | <0.05 | 30 | 11.9 ± 1.22 | 5.89 ± 0.88 | <0.05 |
DNA damage for each treatment was analyzed as the percentage of DNA in the tail of 100 comets; mean ± SE. The efficacy of DNA repair was calculated by comparing the extent of DNA damage immediately and 120 min after hydrogen peroxide treatment using Wilcoxon matched pairs test. The subjects with no significant repair (P > 0.05) are presented bold
Table 2.
Repair of MNNG-induced DNA damage in 30 endometrial cancer patients and 30 healthy controls
| Endometrial cancer DNA damage ± SE | Control DNA damage ± SE | ||||||
|---|---|---|---|---|---|---|---|
| Number | 0 min | 120 min | P | Number | 0 min | 120 min | P |
| 1 | 24.2 ± 3.09 | 13.3 ± 0.56 | <0.05 | 1 | 15.2 ± 2.82 | 5.35 ± 0.30 | <0.05 |
| 2 | 10.2 ± 0.68 | 3.93 ± 1.16 | <0.05 | 2 | 15.4 ± 0.67 | 3.99 ± 0.62 | <0.05 |
| 3 | 5.94 ± 1.83 | 2.41 ± 0.25 | <0.05 | 3 | 17.0 ± 1.94 | 6.16 ± 0.50 | <0.05 |
| 4 | 9.94 ± 0.75 | 5.55 ± 2.32 | 0.069 | 4 | 12.7 ± 1.54 | 7.36 ± 1.80 | <0.05 |
| 5 | 16.8 ± 1.42 | 2.61 ± 0.34 | <0.05 | 5 | 14.9 ± 0.73 | 5.22 ± 2.12 | <0.05 |
| 6 | 5.46 ± 0.71 | 3.02 ± 1.20 | <0.05 | 6 | 46.3 ± 2.02 | 21.7 ± 1.08 | <0.05 |
| 7 | 36.8 ± 3.7 | 11.4 ± 0.86 | <0.05 | 7 | 31.3 ± 0.57 | 13.5 ± 1.27 | <0.05 |
| 8 | 37.4 ± 2.25 | 37.0 ± 0.58 | 0.760 | 8 | 36.5 ± 2.15 | 36.0 ± 1.54 | 0.942 |
| 9 | 15.3 ± 2.00 | 7.56 ± 0.46 | <0.05 | 9 | 21.3 ± 1.09 | 28.1 ± 3.95 | – |
| 10 | 9.85 ± 0.73 | 5.25 ± 0.55 | <0.05 | 10 | 95.9 ± 1.74 | 14.5 ± 0.82 | <0.05 |
| 11 | 9.44 ± 1.40 | 7.57 ± 2.43 | 0.083 | 11 | 26.3 ± 1.72 | 25.6 ± 2.03 | 0.853 |
| 12 | 21.3 ± 2.25 | 8.56 ± 0.63 | <0.05 | 12 | 36.8 ± 3.81 | 11.4 ± 0.89 | <0.05 |
| 13 | 27.5 ± 0.31 | 8.81 ± 0.39 | <0.05 | 13 | 10.6 ± 0.67 | 6.51 ± 1.22 | <0.05 |
| 14 | 70.0 ± 0.74 | 54.5 ± 2.90 | 0.058 | 14 | 10.5 ± 0.79 | 3.69 ± 0.98 | <0.05 |
| 15 | 17.0 ± 0.47 | 9.54 ± 0.30 | <0.05 | 15 | 13.7 ± 1.19 | 1.41 ± 0.14 | <0.05 |
| 16 | 20.7 ± 0.39 | 25.8 ± 2.26 | 0.067 | 16 | 5.68 ± 3.95 | 5.53 ± 1.75 | 0.546 |
| 17 | 45.2 ± 0.29 | 21.5 ± 0.28 | <0.05 | 17 | 6.52 ± 0.84 | 2.23 ± 3.74 | <0.05 |
| 18 | 39.0 ± 0.51 | 7.27 ± 0.21 | <0.05 | 18 | 12.5 ± 2.03 | 14.4 ± 1.31 | 0.070 |
| 19 | 10.5 ± 1.65 | 9.44 ± 1.35 | 0.453 | 19 | 33.2 ± 0.78 | 14.6 ± 0.60 | <0.05 |
| 20 | 10.5 ± 2.89 | 14.1 ± 2.04 | 0.098 | 20 | 24.3 ± 1.75 | 15.9 ± 1.53 | <0.05 |
| 21 | 9.14 ± 2.01 | 4.23 ± 0.84 | <0.05 | 21 | 32.5 ± 1.33 | 16.1 ± 0.99 | <0.05 |
| 22 | 13.3 ± 1.27 | 4.77 ± 0.83 | <0.05 | 22 | 16.9 ± 1.10 | 6.69 ± 1.34 | <0.05 |
| 23 | 8.30 ± 3.23 | 2.77 ± 0.59 | <0.05 | 23 | 14.9 ± 2.19 | 12.8 ± 0.86 | 0.084 |
| 24 | 15.2 ± 2.42 | 5.35 ± 0.45 | <0.05 | 24 | 19.9 ± 0.40 | 2.64 ± 1.97 | <0.05 |
| 25 | 9.09 ± 1.36 | 4.26 ± 0.84 | <0.05 | 25 | 7.64 ± 0.66 | 3.2 ± 2.07 | <0.05 |
| 26 | 15.7 ± 0.66 | 3.99 ± 0.66 | <0.05 | 26 | 52.7 ± 1.95 | 11.4 ± 2.23 | <0.05 |
| 27 | 15.5 ± 2.28 | 5.79 ± 1.09 | <0.05 | 27 | 7.64 ± 0.46 | 3.20 ± 0.07 | <0.05 |
| 28 | 29.4 ± 1.22 | 19.9 ± 2.58 | <0.05 | 28 | 15.5 ± 3.81 | 14.0 ± 2.35 | 0.750 |
| 29 | 17.2 ± 0.73 | 11.7 ± 1.74 | <0.05 | 29 | 12.3 ± 0.64 | 6.06 ± 0.52 | <0.05 |
| 30 | 11.0 ± 0.35 | 6.56 ± 0.45 | <0.05 | 30 | 11.9 ± 0.92 | 4.48 ± 0.53 | <0.05 |
“–” Not estimated
DNA damage for each treatment was analyzed as the percentage of DNA in the tail of 100 comets; mean ± SE. The efficacy of DNA repair was calculated by comparing the extent of DNA damage immediately and 120 min after MNNG treatment using Wilcoxon matched pairs test. The subjects with no significant repair (P > 0.05) are presented bold
Genotype analysis
Tables 3 and 4 show the distribution of genotypes of the Ser326Cys and 135G>C polymorphism for cancer patients and controls. A strong association of the C/C genotype (OR 25.3, 95% CI 4.48–143) and the C allele (OR 7.23, 95% CI 3.20–16.35) and endometrial cancer occurrence was observed. Table 5 shows the distribution of genotypes of the 135G>C polymorphism in patients and controls with high, i.e. higher than median, level of endogenous DNA damage. There was a strong association between endometrial cancer occurrence and the C/C genotype (OR 13.2, 95% CI 1.47–123) and the C allele (OR 4.75, 95% CI 1.58–14.3) in patients with elevated level of basal DNA lesions. There were no significant differences between the distributions of the other polymorphism in cancer patients and controls with high level of endogenous DNA damage (Table 6). No correlation was found between both polymorphism and endometrial cancer occurrence in subjects with impaired DNA repair after hydrogen peroxide and MNNG treatment (data not shown).
Table 3.
The allele and genotype frequencies and odds ratio (OR) of the Ser326Cys polymorphism of the OGG1 gene in endometrial cancer patients and non-cancer patients
| Genotype or allele | Patients (n = 30) | Controls (n = 30) | OR (95% CI) |
|---|---|---|---|
| Number | Number | ||
| Ser/Ser | 23 | 22 | 1.00 ref. |
| Ser/Cys | 6 | 7 | 0.82 (0.24–2.83) |
| Cys/Cys | 1 | 1 | 0.96 (0.06–16.3) |
| Ser | 52 | 51 | 1.00 ref. |
| Cys | 8 | 9 | 0.87 (0.31–2.44) |
χ2 = 0.319031, P < 0.9886
Table 4.
The allele and genotype frequencies and odds ratio (OR) of the 135G>C polymorphism of the RAD51 gene in endometrial cancer patients and non-cancer patients
| Genotype or allele | Patients (n = 30) | Controls (n = 30) | OR (95% CI) |
|---|---|---|---|
| Number | Number | ||
| GG | 6 | 19 | 1.00 ref. |
| GC | 8 | 9 | 2.81 (0.75–10.6) |
| CC | 16 | 2 | 25.3 (4.48–143) |
| G | 20 | 47 | 1.00 ref. |
| C | 40 | 13 | 7.23 (3.20–16.4) |
χ2 = 178.5934, P < 0.0001
Table 5.
The allele and genotype frequencies and odds ratio (OR) of the Ser326Cys polymorphism of the OGG1 gene in endometrial cancer patients and non-cancer patients with high level of endogenous DNA damage
| Genotype or allele | Patients (n = 15) | Controls (n = 15) | OR (95% CI) |
|---|---|---|---|
| Number | Number | ||
| Ser/Ser | 10 | 10 | 1.00 ref. |
| Ser/Cys | 4 | 5 | 0.80 (0.16–3.88) |
| Cys/Cys | 1 | 0 | – |
| Ser | 24 | 25 | 1.00 ref. |
| Cys | 6 | 5 | 1.25 (0.34–4.64) |
“–” Not estimated; χ2 = 0.4400, P < 0.9791
Table 6.
The allele and genotype frequencies and odds ratio (OR) of the 135G>C polymorphism of the RAD51 gene in endometrial cancer patients and non-cancer patients with high level of endogenous DNA damage
| Genotype or allele | Patients (n = 15) | Controls (n = 15) | OR (95% CI) |
|---|---|---|---|
| Number | Number | ||
| GG | 2 | 6 | 1.00 ref. |
| GC | 4 | 7 | 1.71 (0.23–12.9) |
| CC | 9 | 2 | 13.2 (1.47–123) |
| G | 8 | 19 | 1.00 ref. |
| C | 22 | 11 | 4.75 (1.58–14.3) |
χ2 = 45.8208, P < 0.0001
Discussion
In our study we did not observe any difference between basal endogenous level of DNA damage in lymphocytes of endometrial cancer patients and non-cancer controls. The same concerns the susceptibility of lymphocytes to DNA-damaging agent in these two groups. On the other hand, it was shown that a long-term estrogens exposure was associated with endometrial and ovarian cancer risk [32]. Estrogen metabolites can cause DNA damage in many tissues [33, 34]. Primarily they form unstable N-3 adenine and N-7 guanine adducts resulting in mutagenic apurinic sites, witch are easily detected by comet assay. The alkaline single cell-gel electrophoresis was used to examine oestrogens (beta-estradiol, estrone and estriol) activities on the estrogen-responsive MCF-7 human breast cancer cells for chromosomal damage and DNA single-strand breaks (SSBs). Both beta-estradiol and estrone induced dose-related increases in SSBs (up to sevenfold over control) at concentrations as low as 10−9 and 10−8 M, while estriol was less genotoxic [35]. In the present study we showed that genotoxic effect of estrogens cannot be observed in non estrogen-responsive target cells, such as lymphocytes. We showed that there are no significant differences in basal endogenous DNA damage in lymphocytes from patients with endometrial cancer and healthy controls. It is in agreement with other studies performed on human lymphocytes [36–38].
Our results obtained for the 135G>C polymorphism of the RAD51 gene indicated that both the C/C genotype and the C allele are strongly associated with endometrial cancer. The 135G>C polymorphism of the RAD51 gene was reported to change breast cancer risk in BRCA2 mutations carriers [25, 26, 28]. It is located in the 5′-untranslated region of the RAD51 gene, so it could affect the gene expression and, as a consequence, alter the concentration of the final product—the RAD51 protein. RAD51 takes a part in the repair of DNA double strand breaks (DSBs), the most dangerous damage to DNA. The formation of RAD51 foci represents an important step in the repair of DNA double-strand breaks. RAD51 foci also appear during S phase and are thought to be required for the restart of stalled or broken replication fork. The RAD51 recombinase interacts directly with the breast cancer-associated tumor suppressor BRCA2 and this interaction is required for normal recombination proficiency, radiation resistance and genome stability [39–46]. There are no epidemiological studies linked the 135G>C polymorphism with endometrial cancer. Studies in mice suggested a role of polymorphic variants of RAD51 in radiation induced acute myeloid leukemia [47].
Antoniou et al. suggests an effect of the RAD51 135C homozygotes on the RAD51 alternative splicing within the 5′ UTR. Isoform for 135C genotype has lower translation efficiency and may cause an overall lower abundance of RAD51 protein [48].
Our present data confirm our previous suggestion that the 135G>C polymorphism of the RAD51 gene have a phenotypic effect, manifested in the changes in the extent of DNA damage [12].
Human tumors often display loss of heterozygosity at the hOGG1 locus. Bacterial and yeast cells deficient in the repair of 8-oxoG repair display a hypermutator phenotype [49, 50]. Mice deficient for ogg1 accumulate abnormal levels of 8-oxoG and have an elevated spontaneous mutation rate [51]. It was suggested that hOGG1–Cys326 enzyme had a lover ability to suppress mutation than wild type Ser326 protein in human cells in vitro [52]. Even though no differences in catalytic activity was shown among Ser326 and Cys326 variants in one study [36], the other shows that Ser326Cys and Cys326Cys genotypes displayed slower DNA repair than the Ser326Ser genotype [53] and the hOGG1 protein encoded by the Ser326 allele had significantly higher activity than the Cys326 variants in an in vitro complementation activities assay [6]. Interestingly, this effect is not related to difference in the expression rate of polymorphic variants. The hOGG1 Cys326Cys genotype had significantly higher expression level of the hOGG1 mRNA than Ser326Ser genotype [54, 55]. Replacement of wild type serine at residue 326 (allele frequencies of 0.55–0.67 in Asians and 0.73–0.78 in the Caucasians populations) with redox sensitive cysteine resulted in attenuated activity under oxidative stress. It is also possible that loss of phosphorylation site on Ser326 provide to aberrant nuclear localization of the Cys variant of the enzyme [56, 57].
In summary, our results suggest that the variants of the 135G>C polymorphism of the RAD51 gene can be associated with the occurrence of endometrial cancer in individuals with a high level of DNA damage. Therefore, this polymorphism can be considered as an additional marker in endometrial cancer.
Acknowledgments
This work was supported by the University of Lodz, grant number 505/376
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Sugino N. The role of oxygen radical-mediated signaling pathways in endometrial function. Placenta. 2007;28(Suppl A):S133–S136. doi: 10.1016/j.placenta.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 3.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 4.Roy D, Cai Q, Felty Q, Narayan S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. J Toxicol Environ Health B Crit Rev. 2007;10:235–257. doi: 10.1080/15287390600974924. [DOI] [PubMed] [Google Scholar]
- 5.Martinez GR, Loureiro AP, Marques SA, Miyamoto S, Yamaguchi LF, Onuki J, Almeida EA, Garcia CC, Barbosa LF, Medeiros MH, Di MP. Oxidative and alkylating damage in DNA. Mutat Res. 2003;544:115–127. doi: 10.1016/j.mrrev.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Dherin C, Radicella JP, Dizdaroglu M, Boiteux S. Excision of oxidatively damaged DNA bases by the human alpha-hOgg1 protein and the polymorphic alpha-hOgg1(Ser326Cys) protein which is frequently found in human populations. Nucleic Acids Res. 1999;27:4001–4007. doi: 10.1093/nar/27.20.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boiteux S, Dherin C, Reille F, Apiou F, Dutrillaux B, Radicella JP. Excision repair of 8-hydroxyguanine in mammalian cells: the mouse Ogg1 protein as a model. Free Radic Res. 1998;29:487–497. doi: 10.1080/10715769800300541. [DOI] [PubMed] [Google Scholar]
- 8.Radicella JP, Dherin C, Desmaze C, Fox MS, Boiteux S. Cloning and characterization of hOGG1, a human homolog of the OGG1 gene of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:8010–8015. doi: 10.1073/pnas.94.15.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishioka K, Ohtsubo T, Oda H, Fujiwara T, Kang D, Sugimachi K, Nakabeppu Y. Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Mol Biol Cell. 1999;10:1637–1652. doi: 10.1091/mbc.10.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao X, Huang J, Wu S, Lv M, Hu Y, Jianfu Su X, Luo C, Ce B. hOGG1 Ser326Cys polymorphism and susceptibility to gallbladder cancer in a Chinese population. Int J Cancer. 2007;121:501–505. doi: 10.1002/ijc.22748. [DOI] [PubMed] [Google Scholar]
- 11.Coppede F, Mancuso M, Lo GA, Carlesi C, Piazza S, Rocchi A, Petrozzi L, Nesti C, Micheli D, Bacci A, Migliore L, Murri L, Siciliano G. Association of the hOGG1 Ser326Cys polymorphism with sporadic amyotrophic lateral sclerosis. Neurosci Lett. 2007;420:163–168. doi: 10.1016/j.neulet.2007.04.067. [DOI] [PubMed] [Google Scholar]
- 12.Poplawski T, Arabski M, Kozirowska D, Blasinska-Morawiec M, Morawiec Z, Morawiec-Bajda A, Klupinska G, Jeziorski A, Chojnacki J, Blasiak J. DNA damage and repair in gastric cancer–a correlation with the hOGG1 and RAD51 genes polymorphisms. Mutat Res. 2006;601:83–91. doi: 10.1016/j.mrfmmm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Tsukino H, Hanaoka T, Otani T, Iwasaki M, Kobayashi M, Hara M, Natsukawa S, Shaura K, Koizumi Y, Kasuga Y, Tsugane S. hOGG1 Ser326Cys polymorphism, interaction with environmental exposures, and gastric cancer risk in Japanese populations. Cancer Sci. 2004;95:977–983. doi: 10.1111/j.1349-7006.2004.tb03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Chen L, Tockman MS, Elahi A, Lazarus P. The human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) DNA repair enzyme and its association with lung cancer risk. Pharmacogenetics. 2004;14:103–109. doi: 10.1097/00008571-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kau HC, Tsai CC, Hsu WM, Liu JH, Wei YH. Genetic polymorphism of hOGG1 and risk of pterygium in Chinese. Eye. 2004;18:635–639. doi: 10.1038/sj.eye.6700738. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Elahi A, Pow-Sang J, Lazarus P, Park J. Association between polymorphism of human oxoguanine glycosylase 1 and risk of prostate cancer. J Urol. 2003;170:2471–2474. doi: 10.1097/01.ju.0000087498.23008.bb. [DOI] [PubMed] [Google Scholar]
- 17.Elahi A, Zheng Z, Park J, Eyring K, McCaffrey T, Lazarus P. The human OGG1 DNA repair enzyme and its association with orolaryngeal cancer risk. Carcinogenesis. 2002;23:1229–1234. doi: 10.1093/carcin/23.7.1229. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Zheng SL, Turner A, Isaacs SD, Wiley KE, Hawkins GA, Chang BL, Bleecker ER, Walsh PC, Meyers DA, Isaacs WB. Associations between hOGG1 sequence variants and prostate cancer susceptibility. Cancer Res. 2002;62:2253–2257. [PubMed] [Google Scholar]
- 19.Le ML, Donlon T, Lum-Jones A, Seifried A, Wilkens LR. Association of the hOGG1 Ser326Cys polymorphism with lung cancer risk. Cancer Epidemiol Biomark Prev. 2002;11:409–412. [PubMed] [Google Scholar]
- 20.Xing DY, Tan W, Song N, Lin DX. Ser326Cys polymorphism in hOGG1 gene and risk of esophageal cancer in a Chinese population. Int J Cancer. 2001;95:140–143. doi: 10.1002/1097-0215(20010520)95:3<140::AID-IJC1024>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Stanczyk M, Sliwinski T, Cuchra M, Zubowska M, Bielecka-Kowalska A, Kowalski M, Szemraj J, Mlynarski W, Majsterek I (2010) The association of polymorphisms in DNA base excision repair genes XRCC1, OGG1 and MUTYH with the risk of childhood acute lymphoblastic leukemia. Mol Biol Rep. doi:10.1007/s110330100127x [DOI] [PubMed]
- 22.Nock NL, Cicek MS, Li L, Liu X, Rybicki BA, Moreira A, Plummer SJ, Casey G, Witte JS. Polymorphisms in estrogen bioactivation, detoxification and oxidative DNA base excision repair genes and prostate cancer risk. Carcinogenesis. 2006;27:1842–1848. doi: 10.1093/carcin/bgl022. [DOI] [PubMed] [Google Scholar]
- 23.Helleday T. Pathways for mitotic homologous recombination in mammalian cells. Mutat Res. 2003;532:103–115. doi: 10.1016/j.mrfmmm.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair. 2007;6:923–935. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Levy-Lahad E, Lahad A, Eisenberg S, Dagan E, Paperna T, Kasinetz L, Catane R, Kaufman B, Beller U, Renbaum P, Gershoni-Baruch R. A single nucleotide polymorphism in the RAD51 gene modifies cancer risk in BRCA2 but not BRCA1 carriers. Proc Natl Acad Sci USA. 2001;98:3232–3236. doi: 10.1073/pnas.051624098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sliwinski T, Krupa R, Majsterek I, Rykala J, Kolacinska A, Morawiec Z, Drzewoski J, Zadrozny M, Blasiak J. Polymorphisms of the BRCA2 and RAD51 genes in breast cancer. Breast Cancer Res Treat. 2005;94:105–109. doi: 10.1007/s10549-005-0672-5. [DOI] [PubMed] [Google Scholar]
- 27.Blasiak J, Przybylowska K, Czechowska A, Zadrozny M, Pertynski T, Rykala J, Kolacinska A, Morawiec Z, Drzewoski J. Analysis of the G/C polymorphism in the 5′-untranslated region of the RAD51 gene in breast cancer. Acta Biochim Pol. 2003;50:249–253. [PubMed] [Google Scholar]
- 28.Wang WW, Spurdle AB, Kolachana P, Bove B, Modan B, Ebbers SM, Suthers G, Tucker MA, Kaufman DJ, Doody MM, Tarone RE, Daly M, Levavi H, Pierce H, Chetrit A, Yechezkel GH, Chenevix-Trench G, Offit K, Godwin AK, Struewing JP. A single nucleotide polymorphism in the 5′ untranslated region of RAD51 and risk of cancer among BRCA1/2 mutation carriers. Cancer Epidemiol Biomark Prev. 2001;10:955–960. [PubMed] [Google Scholar]
- 29.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 30.Czechowska A, Poplawski T, Drzewoski J, Blasiak J. Imatinib (STI571) induces DNA damage in BCR/ABL-expressing leukemic cells but not in normal lymphocytes. Chem Biol Interact. 2005;152:139–150. doi: 10.1016/j.cbi.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Krupa R, Blasiak J. An association of polymorphism of DNA repair genes XRCC1 and XRCC3 with colorectal cancer. J Exp Clin Cancer Res. 2004;23:285–294. [PubMed] [Google Scholar]
- 32.Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000;27:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- 33.Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;27:75–93. doi: 10.1093/oxfordjournals.jncimonographs.a024247. [DOI] [PubMed] [Google Scholar]
- 34.Liehr JG. Genotoxic effects of estrogens. Mutat Res. 1990;238:269–276. doi: 10.1016/0165-1110(90)90018-7. [DOI] [PubMed] [Google Scholar]
- 35.Yared E, McMillan TJ, Martin FL. Genotoxic effects of oestrogens in breast cells detected by the micronucleus assay and the Comet assay. Mutagenesis. 2002;17:345–352. doi: 10.1093/mutage/17.4.345. [DOI] [PubMed] [Google Scholar]
- 36.Janssen K, Schlink K, Gotte W, Hippler B, Kaina B, Oesch F. DNA repair activity of 8-oxoguanine DNA glycosylase 1 (OGG1) in human lymphocytes is not dependent on genetic polymorphism Ser326/Cys326. Mutat Res. 2001;486:207–216. doi: 10.1016/s0921-8777(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 37.Tarng DC, Tsai TJ, Chen WT, Liu TY, Wei YH. Effect of human OCC1 1245C>G gene polymorphism on 8-hydroxy-2-deoxyguanosine levels of leukocyte DNA among patients undergoing chronic hemodialysis. J Am Soc Nephrol. 2001;12:2338–2347. doi: 10.1681/ASN.V12112338. [DOI] [PubMed] [Google Scholar]
- 38.Bianco NR, Chaplin LJ, Montano MM. Differential induction of quinone reductase by phytoestrogens and protection against oestrogen-induced DNA damage. Biochem J. 2005;385:279–287. doi: 10.1042/BJ20040959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Min J, Park PG, Ko E, Choi E, Lee H. Identification of Rad51 regulation by BRCA2 using Caenorhabditis elegans BRCA2 and bimolecular fluorescence complementation analysis. Biochem Biophys Res Commun. 2007;362:958–964. doi: 10.1016/j.bbrc.2007.08.083. [DOI] [PubMed] [Google Scholar]
- 40.Esashi F, Galkin VE, Yu X, Egelman EH, West SC. Stabilization of RAD51 nucleoprotein filaments by the C-terminal region of BRCA2. Nat Struct Mol Biol. 2007;14:468–474. doi: 10.1038/nsmb1245. [DOI] [PubMed] [Google Scholar]
- 41.Davies OR, Pellegrini L. Interaction with the BRCA2 C terminus protects RAD51-DNA filaments from disassembly by BRC repeats. Nat Struct Mol Biol. 2007;14:475–483. doi: 10.1038/nsmb1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petalcorin MI, Galkin VE, Yu X, Egelman EH, Boulton SJ. Stabilization of RAD-51-DNA filaments via an interaction domain in Caenorhabditis elegans BRCA2. Proc Natl Acad Sci USA. 2007;104:8299–8304. doi: 10.1073/pnas.0702805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galkin VE, Esashi F, Yu X, Yang S, West SC, Egelman EH. BRCA2 BRC motifs bind RAD51-DNA filaments. Proc Natl Acad Sci USA. 2005;102:8537–8542. doi: 10.1073/pnas.0407266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo T, Pellegrini L, Venkitaraman AR, Blundell TL. Sequence fingerprints in BRCA2 and RAD51: implications for DNA repair and cancer. DNA Repair. 2003;2:1015–1028. doi: 10.1016/S1568-7864(03)00097-1. [DOI] [PubMed] [Google Scholar]
- 45.Tarsounas M, Davies D, West SC. BRCA2-dependent and independent formation of RAD51 nuclear foci. Oncogene. 2003;22:1115–1123. doi: 10.1038/sj.onc.1206263. [DOI] [PubMed] [Google Scholar]
- 46.Pellegrini L, Yu DS, Lo T, Anand S, Lee M, Blundell TL, Venkitaraman AR. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature. 2002;420:287–293. doi: 10.1038/nature01230. [DOI] [PubMed] [Google Scholar]
- 47.Jawad M, Seedhouse CH, Russell N, Plumb M. Polymorphisms in human homeobox HLX1 and DNA repair RAD51 genes increase the risk of therapy-related acute myeloid leukemia. Blood. 2006;108:3916–3918. doi: 10.1182/blood-2006-05-022921. [DOI] [PubMed] [Google Scholar]
- 48.Antoniou AC, Sinilnikova OM, Simard J, et al. RAD51 135G>C modifies breast cancer risk among BRCA2 mutation carriers: results from a combined analysis of 19 studies. Am J Hum Genet. 2007;81(6):1186–1200. doi: 10.1086/522611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cabrera M, Nghiem Y, Miller JH. mutM, a second mutator locus in Escherichia coli that generates G.C → T.A transversions. J Bacteriol. 1988;170:5405–5407. doi: 10.1128/jb.170.11.5405-5407.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas D, Scot AD, Barbey R, Padula M, Boiteux S. Inactivation of OGG1 increases the incidence of G.C → T.A transversions in Saccharomyces cerevisiae: evidence for endogenous oxidative damage to DNA in eukaryotic cells. Mol Gen Genet. 1997;254:171–178. doi: 10.1007/s004380050405. [DOI] [PubMed] [Google Scholar]
- 51.Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci USA. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamane A, Kohno T, Ito K, Sunaga N, Aoki K, Yoshimura K, Murakami H, Nojima Y, Yokota J. Differential ability of polymorphic OGG1 proteins to suppress mutagenesis induced by 8-hydroxyguanine in human cell in vivo. Carcinogenesis. 2004;25:1689–1694. doi: 10.1093/carcin/bgh166. [DOI] [PubMed] [Google Scholar]
- 53.Aka P, Mateuca R, Buchet JP, Thierens H, Kirsch-Volders M. Are genetic polymorphisms in OGG1, XRCC1 and XRCC3 genes predictive for the DNA strand break repair phenotype and genotoxicity in workers exposed to low dose ionising radiations? Mutat Res. 2004;556:169–181. doi: 10.1016/j.mrfmmm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Kohno T, Shinmura K, Tosaka M, Tani M, Kim SR, Sugimura H, Nohmi T, Kasai H, Yokota J. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16:3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- 55.Hatt L, Loft S, Risom L, Moller P, Sorensen M, Raaschou-Nielsen O, Overvad K, Tjonneland A, Vogel U. OGG1 expression and OGG1 Ser326Cys polymorphism and risk of lung cancer in a prospective study. Mutat Res. 2008;639:45–54. doi: 10.1016/j.mrfmmm.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Smart DJ, Chipman JK, Hodges NJ. Activity of OGG1 variants in the repair of pro-oxidant-induced 8-oxo-2′-deoxyguanosine. DNA Repair. 2006;5:1337–1345. doi: 10.1016/j.dnarep.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Bravard A, Vacher M, Moritz E, Vaslin L, Hall J, Epe B, Radicella JP. Oxidation status of human OGG1-S326C polymorphic variant determines cellular DNA repair capacity. Cancer Res. 2009;69(8):3642–3649. doi: 10.1158/0008-5472.CAN-08-3943. [DOI] [PubMed] [Google Scholar]


