Abstract
Mycosporine-like amino acids (MAAs) were studied in zooplankton from 13 Argentinian lakes covering a broad range in altitude, maximum depth and physico-chemical properties of the water. Four to nine different MAAs (predominantly porphyra-334 and shinorine) were found in the copepods Boeckella gibbosa, B. gracilipes, B. meteoris and Parabroteas sarsi, and in the ciliate Stentor amethystinus, while MAAs were undetectable in the cladoceran Daphnia middendorffiana. Among the different copepods, maximum MAA concentrations accounted for 0.25–1.31% of the dry weight, and contents were generally about three to seven times (up to 43 times) higher in the animals living in the clearest lakes compared to those occurring in low-UV systems. This variability in the content of MAAs was related to the lake altitude (r2 = 0.71), and the fraction of the water column to which 1% of the surface UV radiation at 320 nm penetrated (r2 = 0.57). Our data therefore underscore the role of MAAs as sunscreens to decrease the potential negative effects of solar radiation, but they also indicate that other environmental factors besides UV transparency play a role in determining MAA concentrations. One lake was selected to obtain additional information on the qualitative composition of MAAs in seston of <100 μm between two sampling sites and over a 2 month study period (austral summer). Six different MAAs were detected in the samples, with porphyra-334 and palythine being predominant. In the copepods collected simultaneously, there was low variation in MAA concentrations between the two sites and over time. Thus, our results suggest that under similar UV exposure conditions MAA contents of planktonic organisms show low temporal variation.
INTRODUCTION
Solar ultraviolet radiation (UVR, 290–400 nm) plays an important ecological role in many aquatic ecosystems. Both laboratory and field experiments have shown that UVR is potentially lethal to freshwater zooplankton (Siebeck, 1978; Williamson et al., 1994; Zagarese et al., 1994). UV tolerance, however, varies among species and populations (Cabrera et al., 1997; Zagarese et al., 1997a; Tartarotti et al., 1999; Leech and Williamson, 2001), and also depends on the UV transparency of the habitat (Stutzman, 1999). While in many lakes UVR is attenuated rapidly, it can penetrate deep into waters with low concentrations of dissolved organic carbon (DOC), as typically found in lakes at high elevation (Morris et al., 1995; Laurion et al., 2000).
In response to the presence of UVR, zooplankton have evolved different strategies to cope with high fluxes of solar radiation. These organisms can actively avoid UVR by vertical migration (Leech and Williamson, 2001; Rhode et al., 2001), recover from DNA damage by photoenzymatic repair (Zagarese et al., 1997a) or accumulate protective compounds such as melanin and carotenoids (Hairston, 1976; Hessen and Sørensen, 1990). Mycosporine-like amino acids (MAAs) are another family of photoprotective compounds recently described in freshwater organisms (Sommaruga and Garcia-Pichel, 1999). These compounds are colourless, show high molar extinction coefficients and have their absorption maxima (309–360 nm) within the UV-B (290–320 nm) and UV-A (320–400 nm) wavelength range. Nineteen described MAAs result from N-substitutions of different amino acid moieties to the cyclohexenone or cyclohexenimine chromophore [for review see (Karentz, 2001)]. These screening compounds are known from more than 380 species of marine organisms, ranging from bacteria, algae, corals and sea urchins to fish [for reviews see (Karentz, 2001; Shick and Dunlap, 2002)], while information on MAAs in freshwater organisms is limited to epilithic cyanobacteria, (micro)algae, and several metazooplankton species (Sommaruga and Garcia-Pichel, 1999; Xiong et al., 1999; Tartarotti et al., 2001; Gonçalves et al., 2002; Helbling et al., 2002; Laurion et al., 2002; Rocco et al., 2002).
One recent study providing information on the qualitative and quantitative composition of MAAs in different freshwater zooplankton species shows that these sunscreens are present in certain taxa but are absent in others (e.g. cladocerans), and that the variability in their concentrations is strongly related to lake transparency (Kd), lake altitude and the fraction of the water column to which 1% of the surface UVR at 320 nm (Z1%:Zmax) penetrated (Tartarotti et al., 2001). Rocco et al. (Rocco et al., 2002) compared the tolerance to UV between different populations of the copepod Boeckella poppei from the Antarctic Peninsula. They found that the population with the lowest MAA concentration also showed the lowest tolerance to solar and artificial radiation. The high concentrations of MAAs found in copepods from clear-water ecosystems (Tartarotti et al., 2001) and MAA-dependent UV tolerance (Rocco et al., 2002) suggest that these compounds constitute an important strategy to minimize UV damage in planktonic organisms.
In this investigation, our objective was to study the qualitative and quantitative composition of MAAs in different zooplankton taxa from Patagonian lakes covering a broad range of UV transparency, altitude, maximum depth and chemical properties of the lake water, thus providing a large natural gradient in UV exposure conditions. In addition, a reservoir was sampled three times during the austral summer to assess potential seasonal variation in the MAA content of zooplankton, and to follow the qualitative composition of MAAs in seston <100 μm (i.e. phytoplankton assemblages, small heterotrophic organisms, dissolved and particulate organic matter).
METHOD
Sampling sites
Thirteen lakes located in northern Patagonia (~41°S), Argentina, at elevations between 590 and 1950 m above sea level were sampled during the austral summer of 1997 (Table I). Two sites (hereinafter referred to as Upstream and Dam) of the Alicura reservoir were sampled three times during December 1997 and January 1998. The maximum depth of the study lakes ranged from ~0.3 to >400 m, vertical attenuation coefficients (Kd) at 320 nm varied from 0.14 to 166.22 m−1, and concentrations of DOC ranged from 0.38 to 521 g m−3 (Table I).
Table I.
Latitude, longitude, altitude, maximum depth (Zmax) at sampling period, diffuse attenuation coefficient at 320 nm (Kd320), dissolved organic carbon (DOC), conductivity (Cond.), and chlorophyll a (Chl a) concentrations in the studied lakes, and zooplankton species present in each lake
| Lake | Latitude (S) |
Longitude (W) |
Altitude m (a.s.l.) |
Zmax (m) |
Kd320 (m−1) |
DOC (g m−3) |
Cond. (μS cm−1) |
Chl a (mg m−3) |
Species |
|---|---|---|---|---|---|---|---|---|---|
| Lake Schmoll | 41°12′ | 71°29′ | 1950 | 5 | 0.14a | 0.38b | 6.3 | 0.14 | B. gibbosa |
| Pond | 41°12′ | 71°29′ | 1750 | ~0.3 | 1.80 | na | 23.2 | 13.39 | B. gibbosa |
| Lake Toncek | 41°12′ | 71°29′ | 1700 | 12 | 0.61a | 0.49b | 12.3 | 0.55 | B. gibbosa |
| Lake Tempanos | 41°12′ | 71°34′ | 1650 | 8 | 1.27 | na | 27.1 | 0.32 | B. gibbosa |
| Lake Jakob | 41°12′ | 71°34′ | 1550 | 25 | 0.26 | na | 13.8 | 0.26 | B. gibbosa |
| Lake Verde | 41°14′ | 71°22′ | 1545 | 5 | 2.22a | 1.23b | 29.0 | 9.72 | B. gracilipes |
| Lake Negra | 41°08′ | 71°34′ | 1100 | 10 | na | na | 5.8 | 0.12 | B. gibbosa |
| Lake Trébol | 41°03′ | 71°31′ | 764 | 12 | 3.08 | 1.70b | 67.5e | 1.34 | B. gracilipes |
| Nahuel Huapi | 41°06′ | 71°18′ | 764 | 464 | 0.42a | 0.41b | 30.9e | 0.58 | S. amethystinus |
| Alicura (Upstream) | 40°43′ | 71°05′ | 705 | 25 | 0.64c | na | 31.2–42.0d | 0.60–0.67 | B. gracilipes |
| Alicura (Dam) | 40°40′ | 71°00′ | 705 | 80 | 0.64c | na | 33.8–35.8d | 0.62–1.09 | B. gracilipes |
| Lake Puesto | 40°20′ | 70°30′ | 590 | ~1 | 131.5 | na | na | 25.07 | P. sarsi, B. meteoris |
| Lake Toro | 40°20′ | 70°30′ | 590 | ~1 | 75.6 | 521c | 3950–8800 | 1.98 | P. sarsi, D. middendorffiana |
| Lake Flamingo | 40°20′ | 70°30′ | 590 | ~1 | 166.2 | 147c | na | 19.07 | P. sarsi |
Data on UV attenuation are from Morris et al. (Morris et al., 1995) measured with a PUV 500 radiometer corresponding well with the predicted values by optical colour measurements. Data on UV attenuation for Lake Trébol and Alicura reservoir are from D. Morris and C. Osburn measured with a PUV 500 radiometer (unpublished data). na, data not available.
Data are from Morris et al. (Morris et al., 1995).
Data are from D. P. Morris (unpublished).
Data are from Temporetti (Temporetti, 1998).
Data are from Pedrozo et al. (Pedrozo et al., 1993).
Zooplankton sampling and sample processing
Depending on lake characteristics, zooplankton was collected from a boat or from the shore by either vertical (deep lakes) or horizontal (Zmax ≤2 m) net (55 μm mesh size) tows around noon. In the laboratory, the organisms were kept at 4°C and in dark conditions until further processing within 24 h. Crustacean plankton were concentrated on a net sieve of 100 μm mesh size and washed several times with tap water to remove phytoplankton. Under the stereomicroscope, CO2-narcotized copepods were separated into the different life stages present during the study period (i.e. juvenile stages copepodid CIV, copepodid CV female and male, adult female and male, egg-carrying females), while the cladoceran Daphnia middendorffiana was separated into juvenile and adult life stages. Individuals were carefully picked into Eppendorf microcentrifugation tubes (2 mL volume) kept on ice, with two to six replicates per population and/or life stage. The number of individuals per tube ranged between two (egg-carrying females of Parabroteas sarsi) and 300, but was ~60 individuals per tube in most cases. In certain circumstances, because of the low number of individuals present in the sample, different developmental stages of the copepods were pooled in one tube. The ciliate Stentor amethystinus was carefully concentrated onto a Whatman GF/F filter.
Sampling of <100 μm seston and sample processing
In addition to zooplankton samples, water samples from Alicura reservoir were taken from a boat with a Van Dorn sampler (5 L) at depths of 2, 6 and 20 m (Upstream) or 2, 6 and 30 m (Dam). Pre-filtered (100 μm mesh size; to remove large zooplankton) water samples were concentrated onto Whatman GF/F filters under low vacuum pressure. The volume filtered varied depending mainly on the amount of phytoplankton biomass present, but was generally 2–3 L.
All samples were immediately frozen (−20°C), lyophilized within 1 month and kept in dry, dark and cold (−80°C) conditions until extraction within 2 years. Karsten et al. (Karsten et al., 1998) report that macroalgae can be stored under these conditions for several years without any detectable loss of MAAs.
Extraction and HPLC analyses
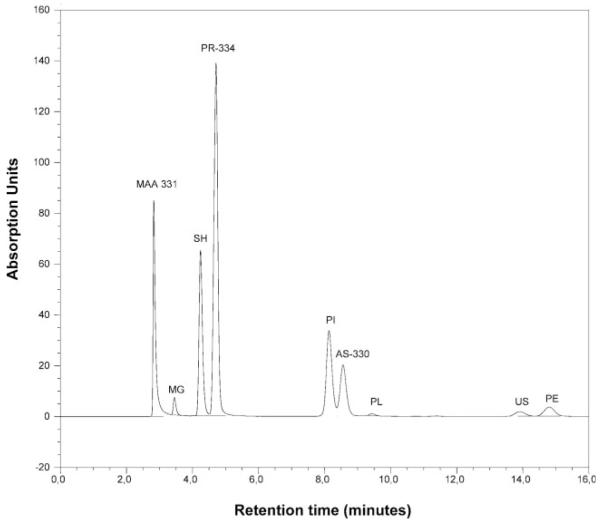
MAAs were extracted according to the most efficient protocol reported for copepods and natural phytoplankton assemblages in a study by Tartarotti and Sommaruga (Tartarotti and Sommaruga, 2002). Briefly, samples were extracted twice in 25% aqueous methanol (v:v; MeOH; HPLC grade) for 2 h in a water bath at 45°C. The extraction efficiency after two serial extractions was >98%. Samples were sonicated (1 min at 0.5 cycles and 20% amplitude; UP 200S, Dr Hielscher GmbH, Germany) on ice at the beginning of the first extraction. The pooled extracts were cleared using a 0.1 μm pore size Anodisc filter (Whatman) and evaporated to dryness under vacuum in 2 mL Eppendorf microcentrifugation tubes, using a SpeedVac concentrator (Savant). The samples were stored at −80°C for further characterization using high performance liquid chromatography (HPLC) within 1 week. For separation and quantification of MAAs, the concentrated, dried extracts were resuspended in 0.05–0.1 mL 25% MeOH (v:v), and 10–30 μL aliquots were injected in a Phenosphere 5 μm pore size RP-8 column (4.6 mm internal diameter × 25 cm, Phenomenex) protected with a RP-8 guard column (Brownlee), for isocratic reverse-phase HPLC analysis. Samples were run with a mobile phase of 0.1% acetic acid in 25% aqueous MeOH (v:v), and a flow rate of 0.79 mL min−1. The MAAs in the eluate were detected by online UV spectroscopy. Peak measurement was carried out at 310, 320, 334, and 360 nm in a Dionex system with a diode array detector (scanning from 200 to 595 nm). Individual peaks were identified by relative retention time, absorption spectra, and by co-chromatography with standards extracted from the marine alga Porphyra yezoensis and Palythoa sp. (obtained from Dr U. Karsten, University of Rostock, Rostock, Germany, and Dr M. Shick, University of Maine, Orono, USA). The total content of specific MAAs in each sample was calculated from HPLC peak areas, using published molar extinction coefficients (Tartarotti et al., 2001). The molar extinction coefficient for asterina-330 was assumed to be the same as that of palythinol (Dunlap et al., 1989). A mean molar extinction coefficient of published values (ε: 40 000) was used for one unknown compound (absorption maximum at ~331.2 nm). The molecular weights (mw) of the different MAAs are given in Figure 1. Concentrations of the different MAAs were normalized to the dry weight of the zooplankton, expressed as mg g−1 dry weight.
Fig. 1.
HPLC chromatogram of an aqueous methanolic (25%) extract of the calanoid copepod Boeckella gibbosa from Lake Negra. MAA331: unknown MAA with maximum absorption ~331 nm (assumed molecular weight, mw 332); MG, mycosporine-glycine (mw 245); SH, shinorine (mw 332); PR, porphyra-334 (mw 346); PI, palythine (mw 244); AS, asterina-330 (mw 288); PL, palythinol (mw 302); US, usujirene (mw 284); PE, palythene (mw 284).
UV attenuation measurements
Absorbance of GF/F-filtered (Whatman) samples was determined by spectrophotometry (Shimadzu) using 1 cm or 10 cm quartz cuvettes against a blank consisting of low-carbon deionized water. Absorption coefficients (a) were calculated as a = ln(10A), with A the absorbance reported for a 1 m path (m−1). The diffuse vertical attenuation coefficients (Kd) were predicted by optical colour measurements using the model equations reported in Morris et al. (Morris et al., 1995) and Laurion et al. (Laurion et al., 2000) for lakes of high altitude. In Lake Trébol and Alicura reservoir, underwater irradiance–depth profiles were obtained at ~305, 320, 340 and 380 nm, and photosynthetically active radiation (PAR; 400–700 nm) was measured with a PUV-500 radiometer (Biospherical Instruments). Diffuse attenuation coefficients (Kd) for downward irradiance were determined from the slope of the linear regression of the natural logarithm of downwelling irradiance (Ed) versus depth (z).
RESULTS
Qualitative and quantitative composition of MAAs
In total, eight different MAAs were identified in the planktonic organisms of the 14 aquatic ecosystems investigated (Figure 1): mycosporine-glycine, shinorine, porphyra-334, palythine, asterina-330, palythinol, usujirene and palythene. One unknown UV-absorbing compound with an absorption maximum at ~331.2 nm (hereinafter referred to MAA 331) was frequently found (Figure 1).
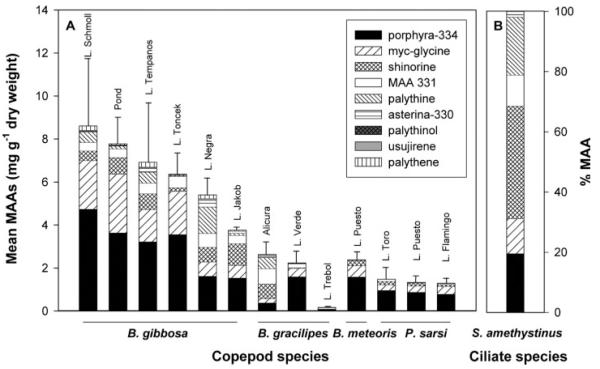
At least four distinct MAAs were detected in the calanoid copepods Boeckella gibbosa, B. gracilipes, B. meteoris and P. sarsi (Figure 2A). Boekella gibbosa living in clear-water lakes at high altitudes (Table I) contained detectable amounts of all nine screening compounds (Figures 1 and 2A). Mycosporine-glycine, shinorine, porphyra-334, palythine, usujirene, palythene and MAA 331 were present in the B. gracilipes population of Lake Verde, the same MAAs except palythene were identified in the populations of Lake Trébol and Alicura reservoir (Figure 2A). Four different MAAs (mycosporine-glycine, shinorine, porphyra-334 and MAA 331) were found in P. sarsi and B. meteoris from the shallow, highly productive, saline lakes (Figure 2A).
Fig. 2.
(A) Mean total MAA concentrations (mg g−1 dry wt) and relative contribution (%) of the different compounds to the total MAA content in the copepods Boeckella gibbosa, B. gracilipes, B. meteoris, and Parabroteas sarsi from 12 Patagonian lakes. (B) Relative contribution (%) of the different compounds to the total MAA pool in the ciliate Stentor amethystinus from Lake Nahuel Huapi.
Porphyra-334 was the predominant compound in the copepods examined, followed by mycosporine-glycine and shinorine (Figure 2A). In B. gracilipes of Alicura reservoir, however, shinorine, MAA 331, and palythine were the predominant compounds (Figure 2A). Although the MAA composition and the relative contribution of MAAs are similar among different populations, some populations show larger variability, for example, B. gibbosa from Lake Negra or B. gracilipes from Lake Verde (Figure 2A).
The highest mean concentration of a specific MAA (4.7 ± 1.8 mg porphyra-334 g−1 dry wt) was found in B. gibbosa from Lake Schmoll. This copepod population also had the highest mean total MAA concentration (8.6 ± 3.2 mg g−1 dry wt), while the lowest concentration of MAAs (0.2 ± 0.05 mg g−1 dry wt) was found in B. gracilipes from Lake Trébol (Figure 2A). In the different copepod species, maximum total concentrations of MAAs ranged from 0.25% (P. sarsi), 0.26% (B. meteoris), 0.37% (B. gracilipes) to 1.31% (B. gibbosa) of the dry weight.
Six different MAAs were identified in the ciliate S. amethystinus (Figure 2B). Shinorine, porphyra-334, and palythine were the most abundant MAAs in this ciliate species (Figure 2B). Conversely, the cladoceran Daphnia middendorffiana, occurring in the saline Lake Toro, lacked these UV-absorbing compounds.
In the <100 μm seston of Alicura reservoir, six distinct MAAs were detected (Figure 3). Porphyra-334, palythine and shinorine were the predominant compounds (Figure 3). Total MAA concentrations for water column values ranged from 0.02 to 1.51 μg L−1.
Fig. 3.
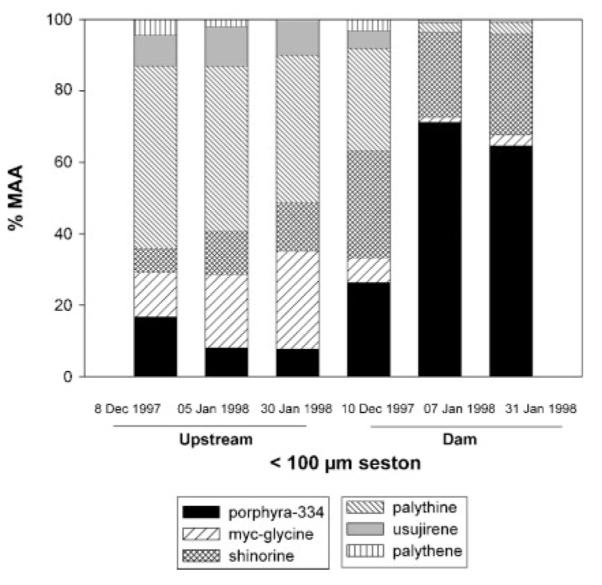
Relative contribution (%) of the different compounds to the total MAA content in the <100 μm seston from Alicura Reservoir at two sampling sites (Upstream and Dam) during the austral summer of 1997/1998.
Variation in MAA composition and concentration with time
In the <100 μm seston of Alicura reservoir, palythene made up to 4.3% of the total MAA pool (Figure 3), while this compound was not detectable in B. gracilipes, the copepod species living in Alicura reservoir (Figure 4). Conversely, the unknown MAA 331 contributed ~27% of the total MAA content in the copepod (Figure 4), a compound that was not found in the <100 μm seston (Figure 3). The relative contribution of the different compounds to the total MAA pool in the <100 μm seston varied with time and among sampling sites (Figure 3). Among different sites (Upstream and Dam) and during the 2-month study period, contents of MAAs showed low variation in B. gracilipes (Figure 4).
Fig. 4.
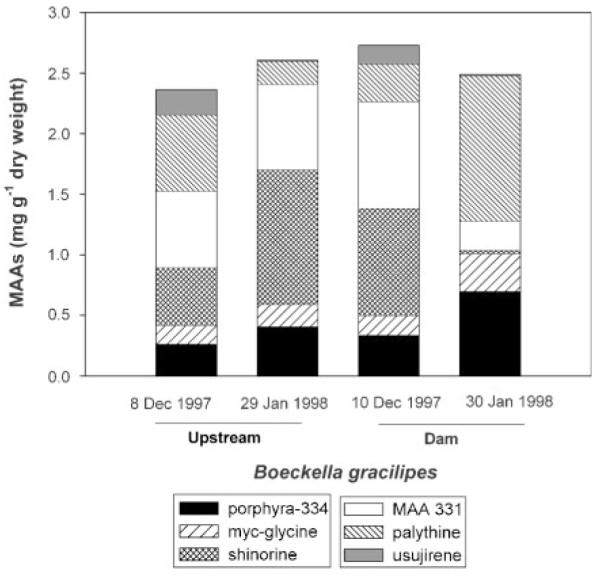
Total MAA concentrations (mg g−1 dry wt) and relative contribution (%) of the different compounds to the total MAA content in the copepod Boeckella gracilipes from Alicura reservoir at two sampling sites (Upstream and Dam) during the austral summer of 1997/1998.
Variability of MAA concentrations and MAA numbers among lakes
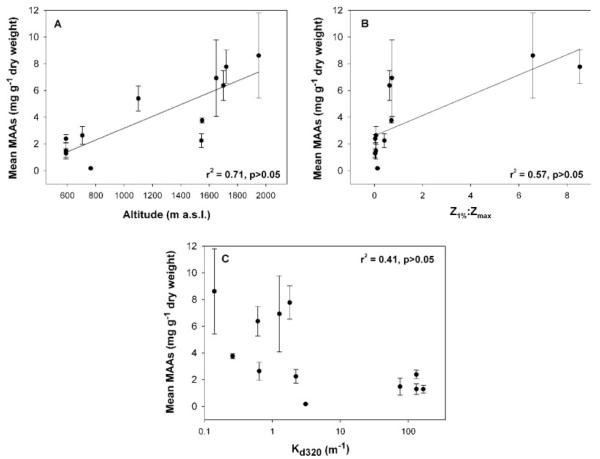
A positive relationship was found between total mean MAA concentrations of the copepods and lake elevation (r2 = 0.71; Figure 5A), and the fraction of the water column to which 1% of the surface UVR at 320 nm (Z1%:Zmax) penetrated (r2 = 0.57; Figure 5B). The highest MAA contents were generally found in the copepods from the clearest lakes (Figure 5C). However, the B. gracilipes populations from slightly turbid lakes had similar low MAA contents to those found in B. meteoris and P. sarsi living in lakes of very high UV attenuation (Table I, Figure 2A). A negative relationship was found between number of MAAs present in the different copepod species and populations and lake transparency (Kd at 320 nm) (Spearman’s rank correlation test, rS2 = −0.743).
Fig. 5.
Relationship between the mean total MAA concentrations (mg g−1 dry wt) in populations of the copepods Boeckella gibbosa, B. gracilipes, B. meteoris, and Parabroteas sarsi and (A) lake altitude, (B) the fraction of the water column to which 1% of the surface UVR at 320 nm (Z1%:Zmax) penetrated, and (C) the diffuse attenuation coefficient (Kd) at 320 nm.
DISCUSSION
Mycosporine-like amino acids are widely distributed among the zooplankton taxa of freshwater ecosystems covering a broad range in geographic location, as well as optical and chemical characteristics of the water. These screening compounds are present in all marine and fresh-water copepods examined so far (Karentz et al., 1991; Sommaruga and Garcia-Pichel, 1999; Tartarotti et al., 2001; Gonçalves et al., 2002; Helbling et al., 2002; Rocco et al., 2002; this study), however, there were no MAAs found in D. middendorffiana, confirming the lack of these compounds in cladocerans such as Daphnia, Bosmina and Chydorus (Tartarotti et al., 2001; Gonçalves et al., 2002). Interestingly, the mixotrophic ciliate S. amethystinus contained detectable amounts of six different MAAs (Figure 2B), showing the occurrence of these compounds in highly diverse planktonic groups. Apart from copepods and Stentor, MAAs are also found in rotifers (Tartarotti et al., 2001).
At present, we do not have an explanation for this taxon-specific presence or lack of MAAs, mainly because there is no information available on how these alga-derived compounds are transported from the animal’s gut to the different parts of its body. It may be speculated that such contrasts come from differences in the organism’s physiology (e.g. enzymatic activity/regulation) during the process of MAA accumulation, as both calanoid copepods and cladocerans such as Daphnia are known to feed predominantly on the same food, i.e. phytoplankton (Tartarotti et al., 2001). Although cladocerans are more UV-sensitive compared with copepods (Williamson et al., 1994; Cabrera et al., 1997; Leech and Williamson, 2000; Gonçalves et al., 2002), they have several efficient strategies for dealing with UV exposure by actively avoiding surface layers (Leech and Williamson, 2001; Rhode et al., 2001) and/or by the melanization of their carapace (Hessen and Sørensen, 1990; Rautio and Korhala, 2002).
In spite of the fact that the same types of these compounds (except for unknown MAA 331) are detected in different freshwater copepods, the number of MAAs present and their dominances vary strongly. All individuals studied contain at least two (Tartarotti et al., 2001; Gonçalves et al., 2002), three (Rocco et al., 2002) or four (Helbling et al., 2002; this study) distinct MAAs. The maximum number of MAAs in copepods is nine (B. gibbosa, this study), which is close to the maximum reported number of 10 in the coral Stylophora pistillata (Shick et al., 1999). The advantage of having several MAAs with different absorption maxima may lie in a more effective optical filter-system. Interestingly, the copepods living in highly transparent and UV-exposed lakes had nine different suncreens covering the whole MAA absorption range from 310 to 360 nm, while P. sarsi and B. meteoris, coming from aquatic systems with high UV attenuation, had only four distinct compounds (Figure 2A). In fact, the numbers of MAAs present in the different copepod species and populations correlated with the lake transparency (rS2 = −0.743).
Porphyra-334 (13 populations) and shinorine (one population) were the predominant MAAs in the calanoid copepods examined (Figure 2A). Porphyra-334 is also the main MAA in other species of the genus Boeckella (Helbling et al., 2002; Rocco et al., 2002). When comparing the composition of MAAs in <100 μm seston (mainly consisting of phytoplankton and small heterotrophic organisms) and zooplankton from Alicura reservoir (Figures 3 and 4), the lack of palythine and the presence of MAA 331 in B. gracilipes may be explained by animal metabolism or enteric bacteria which can lead to interconversions of ingested MAAs into different ones, as suggested for marine invertebrates (Dunlap and Shick, 1998). An alternative explanation may be a different (long-term) dynamic in the accumulation of MAAs in zooplankton (Tartarotti et al., 2001).
A large variability in the MAA concentrations among the different copepod species and populations examined was observed (Figure 2A). Generally, B. gibbosa had MAA concentrations between three and seven times higher than B. gracilipes, B. meteoris and P. sarsi (Figure 2A). The B. gracilipes population from Laguna Trébol presented particularly low MAA concentrations, which were 43 times lower than those in B. gibbosa from Lake Schmoll, the population with the highest concentrations (Figure 2A). Interestingly, a large variability in the content of MAAs (~13-fold) was found among populations of B. gracilipes, while there was low variance among the different P. sarsi and B. gibbosa (up to 2-fold) populations (Figure 2a). It is not clear why the population from Lake Trébol has such low MAA concentrations, particularly because Lake Verde and Trébol have similar optical properties (Table I), thus, there must also be other factors besides UV exposure which are important in regulating the MAA content in these organisms. Intra-specific variances, however, can also be shown within populations of Cyclops abyssorum tatricus (Tartarotti et al., 2001) and B. poppei (Rocco et al., 2002).
The aquatic systems studied cover a wide range of UV exposure conditions for planktonic organisms, as estimated from Kd and the ratio Z1%:Zmax. Lakes Toro, Puesto and Flamingo presented in Table I differed largely from the other study sites in these parameters. Moreover, the lakes selected in the present study cover a broad range in altitude, a factor known to affect incident UVR. Interestingly, there was a positive relationship between the variability in concentrations of MAAs and the lake altitude (r2 = 0.71), and the Z1%:Zmax ratio (r2 = 0.57). Such correlations have also been observed in C. abyssorum tatricus populations from high mountain lakes of the Central Alps where both the lake altitude and the Z1%:Zmax ratio explained 86% of the variability in MAA contents among lakes (Tartarotti et al., 2001). Thus, our results support previous findings which assume that MAAs play an important role in aquatic ecosystems where exposure to solar UVR is significant (Sommaruga and Garcia-Pichel, 1999; Tartarotti et al., 2001). Although a positive relationship was observed between concentrations of MAAs and lake transparency (Kd320 nm) (Figure 5C), a lower percentage (41%) of the variability in MAA concentration among lakes compared with that found in Alpine lakes (74%) was explained by this parameter. Therefore, our data indicate that other environmental factors (e.g. maximum depth) besides UV transparency are important in determining the MAA content of certain copepod species.
During summer there was no significant variation in the MAA concentrations of B. gracilipes from Alicura reservoir (Figure 4), suggesting that MAA concentrations are relatively stable during periods of similar solar exposure. However, long-term seasonal dynamics can be expected, as shown for marine (macro)algae (Karsten et al., 1999; Llewellyn and Harbour, 2003) and freshwater plankton (Tartarotti and Sommaruga, in preparation). Moreover, dramatic changes in UV exposure conditions (e.g. ice-melting in mountain lakes) may result in rapid (within hours) temporal changes in the MAA concentration of freshwater phytoplankton, while variances in MAA concentrations of zooplankton may have a longer time-scale (days, weeks) as they cannot immediately respond by MAA synthesis.
One might ask why MAAs can be found in copepods such as P. sarsi and B. meteoris (Figure 2A) living in saline lakes with extremely high UV attenuation (Kd320: 75.6–166.2 m−1; Table I). These lakes are very shallow (Zmax: ~1 m) and are exposed to strong winds during the summer, which may result in permanent mixing of the whole water column causing planktonic organisms to be exposed to high UV doses and fluctuating dosage rates. In these productive lakes, high zooplankton abundances can be found throughout the whole water column, including the upper few centimetres at noon (unpublished personal observations), therefore, photoprotective compounds may be essential for these animals. Parabroteas sarsi and B. meteoris not only have MAAs but they also show a strong red pigmentation which suggests the presence of high concentrations of carotenoids providing additional UV protection, as carotenoids act against photo-oxidative stress.
A further reason for the occurrence of MAAs may be that these compounds are also involved in metabolic functions other than their assumed role as sunscreens. Mycosporine-glycine, for example, is known for its antioxidant function (Dunlap and Yamamoto, 1995), interestingly, the relative proportion of this compound was high (~20% of the total MAA concentration) for both copepod species (Figure 2A). Moreover, the concentration of MAAs seems to correlate also with osmotic stress. A role for MAA in osmotic regulation has been reported for cyanobacteria (Portwich and Garcia-Pichel, 1999), thus in lakes like Lake Toro, Flamingo, or Puesto where changes in salinity may imply osmotic stress to the organisms, such an additional function cannot be excluded.
The transparent copepod populations of B. gracilipes living in Patagonian lakes lack photorecovery (Zagarese et al., 1997a), a potential strategy to repair UV-induced DNA damage. Low MAA concentrations observed in these animals (Figure 2A) may additionally be responsible for their high UV sensitivity (Zagarese et al., 1997a; Tartarotti et al., 2000). Conversely, the highest MAA concentrations were found in darkly red-pigmented B. gibbosa (Figure 2A), a species typically found in clear-water Patagonian lakes of higher elevation (Zagarese et al., 2000). Short- and long-term in situ UV exposure experiments showed that B. gibbosa was very UV-resistant (Zagarese et al., 1997a, b) and was able to recover from UV exposure (Zagarese et al., 1997a). The high UV tolerance of B. poppei from Antarctic lakes, however, is almost entirely related to the accumulation of MAAs as it shows low rates of enzymatic repair (Rocco et al., 2002). The type of habitat (e.g. shallow versus deep, clear-water versus coloured, cold versus temperate) seems to influence the presence or prominence of certain adaptation strategies or mechanisms, which again lead to the UV resistance or UV sensitivity of a certain zooplankton population.
ACKNOWLEDGEMENTS
We thank J. Chiapella, A. Diaz, M. Diaz, P. Gonzalez, B. Hargreaves, D. Morris, and C. Osburn for their help with field work; F. Pedrozo for providing logistical support, D. Morris and C. Osburn for providing their PUV data. We would like to thank R. Sommaruga and three anonymous reviewers for their useful comments on an earlier version of the manuscript. This research was supported by Fundación Antorchas (Project 14156–82), CONICET (PIP 02135), Universidad Nacional del Comahue (Program B940) and Inter-American Institute for Global Change Research (grant CRN-026). MAA analyses were carried out within the framework of a project granted by the Austrian Science Foundation (FWF P14153-BIO) to R. Sommaruga. B.T. was partially financed by the Austrian Research Association and the International Department of the University of Innsbruck.
REFERENCES
- Cabrera S, Lopéz M, Tartarotti B. Phytoplankton and zooplankton response to ultraviolet radiation in a high-altitude Andean lake: short-versus long-term effects. J. Plankton Res. 1997;19:1565–1582. [Google Scholar]
- Dunlap WC, Shick JM. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective. J. Phycol. 1998;34:418–430. [Google Scholar]
- Dunlap WC, Yamamoto Y. Small molecule antioxidants in marine organisms: antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. 1995;112B:105–114. [Google Scholar]
- Dunlap WC, Williams D. McB., Chalker BE, Banaszak AT. Biochemical photoadaptations in vision: UV-absorbing pigments in fish eye tissues. Comp. Biochem. Physiol. 1989;93B:601–607. [Google Scholar]
- Gonçalves RJ, Villafañe VE, Helbling EW. Photo-repair activity and protective compounds in two freshwater zooplankton species (Daphnia menucoensis and Metacyclops mendocinus) from Patagonia, Argentina. Photochem. Photobiol. Sci. 2002;1:996–1000. doi: 10.1039/b208145h. [DOI] [PubMed] [Google Scholar]
- Hairston NG. Photoprotection by carotenoid pigments in the copepod Diaptomus nevadensis. Proc. Natl Acad. Sci. USA. 1976;73:971–974. doi: 10.1073/pnas.73.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling EW, Zaratti F, Sala LO, Palenque ER, Menchi CF, Villafañe VE. Mycosporine-like amino acids protect the copepod Boeckella titicacae (Harding) against high levels of solar UVR. J. Plankton Res. 2002;24:225–234. [Google Scholar]
- Hessen DO, Sørensen K. Photoprotective pigmentation in alpine zooplankton populations. Aqua Fenn. 1990;20:165–170. [Google Scholar]
- Karentz D. Chemical defenses of marine organisms against solar radiation exposure: UV-absorbing mycosporine-like amino acids and scytonemin. In: McClintock JB, Baker BJ, editors. Marine Chemical Ecology. CRC Press; Boca Raton, FL: 2001. pp. 481–519. [Google Scholar]
- Karentz D, McEuen FS, Land MC, Dunlap WC. Survey of mycosporine-like amino acid compounds in Antarctic marine organisms: potential protection from ultraviolet exposure. Mar. Biol. 1991;108:157–166. [Google Scholar]
- Karsten U, Sawall T, Hanelt D, Bischof K, Figueroa FL, Flores-Moya A, Wiencke C. An inventory of UV-absorbing mycosporine-like amino acids in macroalgae from polar to warm-temperate regions. Bot. Mar. 1998;41:443–453. [Google Scholar]
- Karsten U, Bischof K, Hanelt D, Tüg H, Wiencke C. The effect of ultraviolet radiation on photosynthesis and ultraviolet-absorbing substances in the endemic Arctic macrolaga Devaleraea ramentacea (Rhodophyta) Physiol. Plant. 1999;105:58–66. [Google Scholar]
- Laurion I, Ventura M, Catalan J, Psenner R, Sommaruga R. Attenuation of ultraviolet radiation in mountain lakes: factors controlling the among- and within-lake variability. Limnol. Oceanogr. 2000;45:1274–1288. [Google Scholar]
- Laurion I, Lami A, Sommaruga R. Distribution of mycosporine-like amino acids and photoprotective carotenoids among freshwater phytoplankton assemblages. Aquat. Microb. Ecol. 2002;26:283–294. [Google Scholar]
- Leech DM, Williamson CE. Is tolerance to UV radiation in zooplankton related to body size, taxon, or lake transparency? Ecol. Appl. 2000;10:1530–1540. [Google Scholar]
- Leech DM, Williamson CE. In situ exposure to ultraviolet radiation alters the depth distribution of Daphnia. Limnol. Oceanogr. 2001;46:416–420. [Google Scholar]
- Llewellyn CA, Harbour DS. A temporal study of mycosporine-like amino acids in surface water phytoplankton from the English Channel and correlation with solar irradiation. J. Mar. Biol. Assoc. UK. 2003;83:1–9. [Google Scholar]
- Morris DP, Zagarese HE, Williamson CE, Balseiro E, Hargreaves BR, Modenutti B, Moeller R, Queimaliños C. The attenuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol. Oceanogr. 1995;40:1381–1391. [Google Scholar]
- Pedrozo F, Chillrud S, Temporetti P, Diaz M. Chemical composition and nutrient limitation in rivers and lakes of northern Patagonian Andes (39.5° – 42°S; 71°W) (Rep. Argentina) Verh. Internat. Verein. Limnol. 1993;25:207–214. [Google Scholar]
- Portwich A, Garcia-Pichel F. Ultraviolet and osmotic stresses induce and regulate the synthesis of mycosporines in the cyano-bacterium Chlorogloeopsis PCC 6912. Arch. Microbiol. 1999;172:187–192. doi: 10.1007/s002030050759. [DOI] [PubMed] [Google Scholar]
- Rautio M, Korhala A. UV-induced pigmentation in subarctic Daphnia. Limnol. Oceanogr. 2002;47:295–299. [Google Scholar]
- Rhode SC, Pawlowski M, Tollrian R. The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature. 2001;412:69–72. doi: 10.1038/35083567. [DOI] [PubMed] [Google Scholar]
- Rocco VE, Oppezzo O, Pizarro R, Sommaruga R, Ferraro M, Zagarese HE. Ultraviolet damage and counteracting mechanisms in the freshwater copepod Boeckella poppei from the Antarctic Peninsula. Limnol. Oceanogr. 2002;47:829–836. [Google Scholar]
- Shick JM, Dunlap WC. Mycosporine-like amino acids and related gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- Shick JM, Romaine-Lioud S, Ferrier-Pagès C, Gattuso J-P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999;44:1667–1682. [Google Scholar]
- Siebeck O. Ultraviolet tolerance of planktonic crustaceans. Verh. Int. Ver. Limnol. 1978;20:2469–2473. [Google Scholar]
- Sommaruga R, Garcia-Pichel F. UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake. Arch. Hydrobiol. 1999;144:255–269. [Google Scholar]
- Stutzman PL. A comparative study of ultraviolet radiation tolerance in different populations of Diaptomus minutus. J. Plankton Res. 1999;21:387–400. [Google Scholar]
- Tartarotti B, Cabrera S, Psenner R, Sommaruga R. Survivorship of Cyclops abyssorum tatricus (Cyclopoida, Copepoda) and Boeckella gracilipes (Calanoida, Copepoda) under ambient levels of solar UV radiation in two high mountain lakes. J. Plankton Res. 1999;21:549–560. [Google Scholar]
- Tartarotti B, Cravero W, Zagarese HE. Biological weighting function for the mortality of Boeckella gracilipes (Copepoda, Crustacea) derived from experiments with natural solar radiation. Photochem. Photobiol. 2000;72:314–319. doi: 10.1562/0031-8655(2000)072<0314:bwfftm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tartarotti B, Laurion I, Sommaruga R. Large variability in the concentration of mycosporine-like amino acids among zooplankton from lakes located across an altitude gradient. Limnol. Oceanogr. 2001;46:1546–1552. [Google Scholar]
- Tartarotti B, Sommaruga R. The effect of different methanol concentrations and temperatures on the extraction of mycosporine-like amino acids (MAAs) in algae and zooplankton. Arch. Hydrobiol. 2002;154:691–703. [Google Scholar]
- Temporetti P. Dinámica del fósforo en cuerpos de agua con cría intensiva de salmónidos. Centro Reginal Universitario Bariloche, Universidad Nacional del Comahue; 1998. p. 171. Doctoral thesis. [Google Scholar]
- Williamson CE, Zagarese HE, Schulze PC, Hargreaves B, Seva J. The impact of short-term exposure to UV-B radiation on zooplankton communities in north temperate lakes. J. Plankton Res. 1994;16:205–218. [Google Scholar]
- Xiong FS, Kopecky J, Nedbal L. The occurrence of UV-B absorbing mycosporine-like amino acids in freshwater and terrestrial microalgae (Chlorophyta) Aquat. Bot. 1999;63:37–49. [Google Scholar]
- Zagarese HE, Williamson CE, Mislivets M, Orr P. The vulnerability of Daphnia to UV-B radiation in the Northeastern United States. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1994;43:207–216. [Google Scholar]
- Zagarese HE, Feldman M, Williamson CE. UV-B-induced damage and photoreactivation in three species of Boeckella (Copepoda, Calanoida) J. Plankton Res. 1997a;19:357–367. [Google Scholar]
- Zagarese HE, Williamson CE, Vail TL, Olsen O, Queimaliños C. Long-term exposure of Boeckella gibbosa (Copepoda, Calanoida) to ‘in situ’ levels of solar UV-B radiation. Freshwater Biol. 1997b;37:99–106. [Google Scholar]
- Zagarese HE, Diaz M, Pedrozo F, Úbeda C. Mountain lakes in northwestern Patagonia. Verh. Internat. Verein. Limnol. 2000;27:533–538. [Google Scholar]