Abstract
Aquatic organisms respond to environmental challenges such as thermal stress with the rapid induction of highly conserved polypeptides known as stress proteins or heat shock proteins (Hsps). Solar ultraviolet radiation (UVR, 280–400 nm) is an important environmental stressor in marine ecosystems. Here, we present results of experiments conducted with the marine copepod Acartia tonsa to follow the de novo protein synthesis and measure the level of constitutive and inducible isoforms of the Hsp70 gene family of stress proteins after UV exposure. Animals were collected from Tampa Bay, Florida (USA), and exposed to solar radiation (full spectrum), UV-A (320–400 nm) and PAR (400–700 nm), or PAR only, for periods of 0.5–4 h. Controls were kept in the dark. Protein synthesis was robust under all treatments when the copepods were exposed to low solar radiation intensities. Conversely, high solar radiation intensities (both UV-B and UV-A) caused an overall suppression in the protein synthesis of the copepods with no detectable induction of stress-inducible isoforms of Hsps. Immunochemical assays (western blotting) showed that UVR increased levels (3.5–4-fold increase compared to the dark control) of the constitutively expressed 70 kDa heat-shock (Hsc70) protein in A. tonsa, without indication of inducible isoform upregulation.
Keywords: Hsc70, Hsp70, Metabolic labeling, Stress proteins, Western blot
1. Introduction
Solar ultraviolet radiation (UVR, 280–400 nm) is an important environmental stressor in both marine and freshwater ecosystems. Direct effects, such as UV-induced mortality and unsuccessful reproduction, have been reported for several zooplankton species (Karanas et al., 1979; Naganuma et al., 1997; Tartarotti et al., 2000; Williamson et al., 2001). However, much less is known about sublethal physiological and molecular responses of these organisms to UVR.
Organisms generally respond to perturbations of cellular homeostasis, including exposure to thermal or oxidative stress, hypoxia, chemicals, and toxins, with the rapid induction of highly conserved polypeptides known as stress proteins or heat shock proteins (Hsps) (see Sanders, 1993; Feder and Hofmann, 1999, for reviews). This cellular response, termed the heat shock response (HSR), has been described in numerous organisms ranging from bacteria to mammals (Schlesinger et al., 1982). With the exception of two species (a freshwater hydra, Hydra oligactis, and an Antarctic notothenioid fish, Trematomus bernacchii), the HSR has been found in every organism studied to date. Stress proteins function as molecular chaperones, which assist in protein folding and stabilizing other proteins that would otherwise be lost in response to sub-lethal denaturing stresses (Feder and Hofmann, 1999). Moreover, Hsps play an important role in maintaining protein homeostasis (Hofmann et al., 2002), and the induction of these proteins after a sub-lethal event can induce stress tolerance and protection against a subsequent stress. Several forms of Hsps are known. They are grouped into gene families based on their molecular weight, including the Hsp60, Hsp70, and Hsp90 protein families. Members of the Hsp70 gene family are the most highly conserved, abundant, and widely studied group in eukaryotic cells. Constitutive (Hsc70) or cognate members of the Hsp family can be distinguished from their inducibly expressed isoforms (Hsp70), which are upregulated in response to protein-denaturing stress.
Stress responses have been studied mainly in mammalian cell lines and in prokaryotes, or in organisms kept under constant laboratory conditions. Less information is available on the induction of Hsps in aquatic animals that have to tolerate large fluctuations in environmental and internal conditions. Stress proteins have been found in diverse marine organisms such as fish, echinoderms, mussels, snails, sponges and crustaceans (Bradley et al., 1988; Sanders et al., 1991; Dietz and Somero, 1992; Hofmann and Somero, 1995; Clegg et al., 1998; Tomanek and Somero, 2002; Choresh et al., 2004; Bonaventura et al., 2006).
In natural populations, studies focused mainly on the ecological advantage of Hsp synthesis conferring thermotolerance to cells and organisms (Hofmann et al., 2002). Much effort has been made to investigate the response of animals to varying environmental temperature, specifically organisms living in the rocky intertidal zone or estuarine shoreline which have to cope with strongly fluctuating environments (Roberts et al., 1997; Buckley et al., 2001; Helmuth and Hofmann, 2001; Botton et al., 2006). However, stresses are not limited to extreme temperature and may include several other biochemical and environmental perturbations (Feder and Hofmann, 1999). Roberts et al. (1997) for instance, suggested that the stress response in the intertidal mussel Mytilus californianus is modulated by environmental factors such as wave exposure stress or high levels of UVR in addition to body temperature.
In general, information on the role of UVR in the synthesis of Hsps is limited. In mammalian cells, the induction of Hsps has been recognized as part of an adaptive cellular UV-protective mechanism (Trautinger et al., 1996). Increases in Hsp70 mRNA levels were correlated to UV-B exposure in both mouse epidermis (Brunet and Giacomoni, 1989) and human keratinocytes (Garmyn et al., 1991). Conversely, a study with cancer cells (HeLa cells) showed that UVR down-regulated Hsp expression (Qiu et al., 1997). In aquatic organisms, heat stress enhanced protein synthesis, while UVR seriously impaired translational activity in the freshwater cyanobacterium Nostoc sp.; however, an 18 kDa protein was induced by UV-B radiation (Araoz et al., 1998). On the other hand, an induction of Hsps by UVR was demonstrated in UV-sensitive (but not in UV-tolerant) marine diatoms (Dohler et al., 1995), in sea urchin embryos/larvae (Bonaventura et al., 2005, 2006), and in short-term cultures of sea urchin coelomocytes (Matranga et al., 2006). Responses of different fish species to UVR are contradictory. In vendace (Coregonus albula), no effect on Hsp70 levels by UV-B radiation was observed, while in whitefish (C. lavaretus) UV-B upregulated Hsp70 (Vehniäinen et al., 2003). In UV-sensitive pike larvae (Esox lucius), levels of Hsp70 decreased in larvae exposed either to UV-B radiation or to retene (a polycyclic aromatic hydrocarbon). However, simultaneous UV and retene exposure tended to induce higher Hsp70 levels in these organisms (Häkkinen et al., 2004).
Here, we investigate the effect of solar UVR on the synthesis of stress proteins in individuals of the calanoid copepod Acartia tonsa (Dana), which is a prominent neritic zooplankton species in the warm temperate coastlines of Florida.
2. Materials and methods
2.1. Animal collection and experimental conditions
The estuarine copepod A. tonsa (mostly copepodid CIV and CV life stages; no egg-carrying females) was collected from Tampa Bay (University of South Florida dock), St. Petersburg, Florida, USA (27°50′ N, 82°30′ W) between June 2003 and June 2004. Horizontal net (150 μm mesh size) tows were made at the water surface in the morning, and immediately after sampling, individuals were sorted into glass containers (1 L) containing filtered (0.22 μm, Millipore), aerated seawater. During handling and maintenance, animals were kept at ambient seawater temperature. Copepods were kept in the laboratory for 24 h prior to the UV exposure experiments to minimize the effects of capture and handling stress. In addition, Hakimzadeh and Bradley (1990) observed that the uptake of radiolabeled amino acids was enhanced in the marine copepod Eurytemora affinis after a 24 h period without food. To measure the effect of UV radiation on protein synthesis, experiments consisted of the following treatments: i) full solar radiation (i.e., UV-B, UV-A and photosynthetically active radiation (PAR, 400–700 nm)), ii) UV-A and PAR (quartz tubes wrapped with Mylar® D foil to exclude most of the UV-B radiation), iii) PAR only (quartz tubes shielded with Acrylite OP-3 to remove UVR), and iv) a dark control (tubes wrapped with aluminum foil). Copepods were radiolabeled (in vivo metabolic labeling — see below) or frozen (western blots) immediately after sampling (“sampling group”, SG), and after the 24 h adjustment period (“time zero group”, t0), respectively. After the adjustment period, the quartz glass tubes were filled with seawater (0.22 μm-filtered, aerated) along with the test species, and incubated horizontally at ~15 cm water depth in a UV-transparent acrylic tank (dark bottom) filled with unfiltered seawater. A flow-through system was used to maintain constant water temperature during the experiments. The copepods were exposed to natural levels of solar radiation on the rooftop of a building next to the dock during UV peak hours on sunny days. Time course experiments (UV exposure range: 0.5–4 h; see below for determination of exposure times) were conducted to obtain detailed information on the protein synthesis of the copepods. After the end of each exposure experiment, mortality of the copepods was recorded.
2.2. Simultaneous heat and UV stress experiments
During three UV exposure experiments, the water temperature in the tank was raised ~6 °C from 25 °C (i.e., the temperature of the 24 h adjustment period in the lab) up to 30.5 ° C (i.e., the temperature during the 4 h in situ exposure), which is close to the upper thermal tolerance limit in this copepod species (Gonzalez, 1974).
2.3. Addition of antibiotics
To test for potential protein synthesis caused by bacteria (e.g., bacteria on the copepods’ exoskeleton), treatments were kept in filtered seawater without antibiotics, while a second set of treatments was pretreated with the antibiotic gentamicin (Sigma-Aldrich, 50 mg ml−1; applied at a concentration of 1 ml l−1) during the 24 h adjustment period (see above) and during the exposure period in one experimental run (17 October 2003). As gentamicin did not negatively affect the protein synthesis in A. tonsa (see Fig. 5B), it was routinely used in the exposure experiments conducted during spring and early summer of 2004.
Fig. 5.
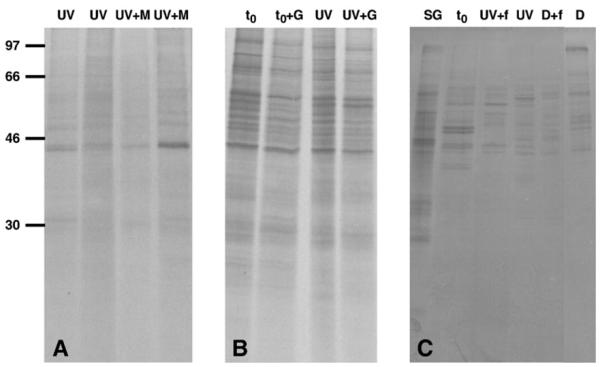
De novo protein synthesis pattern of A. tonsa individuals. (A) UV: copepods exposed to solar radiation for 4 h; UV+M: metabolic labeling with the addition of unlabeled methionine after exposure to solar radiation for 4 h (09 July 2003). (B) t0: time zero group; t0+G: time zero group with the addition of the antibiotic gentamicin during the 24 h adjustment period; UV: copepods exposed to solar radiation for 4 h; UV+G: copepods treated with gentamicin during the 24 h adjustment period followed by a 4 h solar UVR exposure (17 October 2003). (C) SG: sampling group; t0: time zero group; UV+f: copepods exposed to solar radiation for 3 h with the addition of Isochrysis sp. during the 24 h adjustment period; UV: copepods exposed to solar radiation for 3 h; D+f: copepods kept in the dark for 3 h with the addition of Isochrysis sp. as food during the 24 h adjustment period, D: copepods kept in the dark for 3 h (03 June 2004). A) ~43,000 c.p.m., B) ~77,000 c.p.m., and C) ~43,000 c.p.m. per individual lane were loaded. There was no effect of unlabeled methionine, gentamicin or food on the protein synthesis pattern. See Fig. 3 legend for detailed explanation.
2.4. Addition of unlabeled methionine
During one series of experiments (09 July 2003), treatments were radiolabeled with a 35S-labelled methionine/cysteine amino acid mixture after the end of the exposure (i.e., general procedure; see below), whereas unlabeled methionine (Sigma-Aldrich) was added to the radiolabeled methionine/cysteine mix in a second set of treatments. Unlabeled methionine (applied at a concentration of 1 ppm) was supplemented to facilitate amino acid uptake by the copepods.
2.5. Addition of food
To test for potential food effects, treatments were kept in filtered seawater without food (i.e., general procedure; see above), while a second set of treatments was fed ad libitum on the alga Isochrysis sp. during the 24 h adjustment period in one of the UV exposure experiments (03 June 2004).
2.6. Determination of exposure times
A preliminary experiment was conducted to test the UV sensitivity of A. tonsa based on its mortality, which helped to determine the duration of the in situ exposure experiments. For that purpose, copepods were exposed under full solar radiation (exposure time: 2 h and 4 h) and dark conditions, respectively (see above). The survivorship of the exposed animals was further monitored under laboratory conditions (i.e., copepods placed in microcentrifuge tubes filled with filtered seawater and kept under artificial light conditions for 5 h), which were part of the radiolabeling procedures (see below).
2.7. Heat-shock protein induction experiments: in vivo metabolic labeling
The de novo synthesis of proteins was detected by metabolic labeling with radiolabelled amino acids. Immediately after the end of the UV exposure experiments, the copepods (all treatments) were brought back to the laboratory and individually placed in 1.5 ml microcentrifuge tubes containing 0.5 ml of 0.22 μm-filtered seawater (aerated and pre-equilibrated to ambient exposure temperatures). After a pre-incubation period of 1 h, 1.85 × 106 Bq of 35S-labelled methionine/cysteine amino acid mixture (Trans-35, MP Biomedicals) were added to the seawater. After the 5 h radiolabeling incubation period (i.e., under artificial light conditions, without UVR), copepods were rinsed twice with 1 ml 0.22 μm-filtered seawater, frozen immediately in liquid nitrogen, and stored for a maximum of 3 week s at −80 °C until analysis. Samples were homogenized in 100 μl of homogenization buffer and processed using the protocol described in the sample preparation section below. Samples from the Hsp induction experiments were not assayed for protein concentration; instead, a small fraction of each sample (10 μl) was analyzed in a liquid scintillation counter (TM Analytic Delta 300 Liquid Scintillation System) to determine the amount of radioactivity (counts per minute, c.p.m.) per milliliter for each copepod extract.
2.8. Preparation of samples
Copepods were homogenized in homogenization buffer consisting of 50 mM Tris–HCl (pH 6.8), 4% sodium dodecyl sulfate (SDS), 1 mM EDTA (pH 8.0), and 1 mM phenylmethylsulfonylfluoride (PMSF). At the beginning of the homogenization, samples were sonicated for 20 s at 26 W (Ultrasonic Processor, Cole Parmer). Homogenates were heated at 100 °C for 5 min and centrifuged for 15 min at 12,000 g. The resulting supernatant was analyzed for total protein content using a Bicinchoninic Acid Assay (BCA protein assay, Pierce) prior to electrophoresis.
2.9. Electrophoresis and fluorography
Proteins were separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE; 12% acrylamide gels), and individual lanes were loaded with equivalent amounts of radioactivity (~27,000–87,000 c.p.m., depending on the radioactivity of the samples). Gels were run at 20 mA for approximately 4 h. A 14C-methylated molecular weight marker (Amersham) was included on each gel as an internal standard. Following electrophoresis, gels were fixed (30% methanol and 10% acetic acid (v/v)) for at least 30 min, washed in Milli-Q water for 30 min, and treated with an autoradiographic enhancer (EN3HANCE; Perkin Elmer) according to the manufacturer’s protocol. Gels were then dried at 55 °C for 1 h 50 min, exposed to X-ray film (BioMax MR, Kodak) at −80 °C for an empirically determined period (i.e., 3 days to 3 weeks, depending on radioactivity), developed, and autoradiographs were scanned.
2.10. Western blotting
Solid-phase immunoblotting (western blots) and scanning densitometry were used to determine the levels of both the constitutive and inducible isoforms of Hsp70 in A. tonsa. The immunochemical assay was conducted according to a modified version of the protocol described by Hofmann and Somero (1996). Immediately after the end of the UV exposure experiments, individuals (three copepods per sample) were placed in microcentrifuge tubes kept on ice, frozen in liquid nitrogen, and stored at −80 °C until further processing within 1 month. Equal amounts of protein (6 μg total protein) from each sample were separated on 10% polyacrylamide gels. A sample of purified hsc70 (0.2 μg of bovine hsc70; Stressgen, SPP-751) was included on each gel as a positive control, and as an internal standard to allow comparison of multiple western blots. Each gel also contained prestained molecular weight markers (Kaleidoscope Prestained Standard, broad range; Biorad). Following electrophoresis, proteins were transferred to a nitrocellulose membrane (Osmonics, NitroBind, 0.45 μm) via semi-dry electrophoretic transfer. Transfers were conducted at 115 mA for 1.5 h with a transfer buffer consisting of 25 mM Tris, 192 mM glycine, and 20% methanol. Following transfer, blots were blocked at 4 °C overnight in phosphate-buffered saline (PBS; pH 7.4) containing 5% (w/v) non-fat dry milk. The immunodetection was performed using an anti-Hsp70 rat monoclonal antibody (Affinity Bioreagents; MA3-001, diluted 1:2500; 1.5 h with constant shaking) which crossreacts with the constitutive and inducible isoforms of Hsc/Hsp70. Blots were sequentially incubated with a bridging antibody (rabbit anti-rat IgG, A-4000, Vector Laboratories; diluted 1:2000) for 30 min, followed by a horseradish-peroxidase-conjugated secondary antibody (donkey anti-rabbit; Amersham; diluted 1:2000) for 1 h with constant shaking. An enhanced chemiluminescence (ECL) detection method was used to visualize proteins that cross-reacted with the antibodies according to the manufacturer’s instructions (Amersham). The chemiluminescent signal was detected using ECL-Hyperfilm (Amersham). The blots were scanned and band intensity from each western blot was quantified using Quantity One software (Biorad).
2.11. Incident solar radiation
An IL 1700 radiometer (International Light) or a portable LI-1800 (LI-COR) spectroradiometer were used to measure incident solar radiation next to the experimental set-up during the exposure experiments. The former instrument measured at 15 min intervals, while the latter measured global solar radiation (direct plus diffuse; 300–850 nm) at 5 min intervals.
2.12. Chlorophyll α, CDOM, salinity and temperature
Surface water samples were collected with a bucket from the University of South Florida dock on the day of the UV exposure experiments (May–June 2004) around noon. For colored dissolved organic matter (CDOM), an important optical component of aquatic systems, the GF/F-filtered seawater (replicates) was stored in amber, precombusted (2 h at 450 °C) glass bottles at −20 °C until subsequent analysis within 3 months. Samples were filtered (0.2 μm, Millipore) and measured with a Perkin-Elmer Lambda 18 UV/VIS spectrophotometer (wavelength range: 200–800 nm) using a 10-cm quartz cuvette, referenced against 0.2 μm membrane-filtered Milli-Q water. For chlorophyll a (Chl a) measurements, 0.8–1.0 l (replicates) of seawater were filtered onto glass fiber filters (Whatman GF/F) immediately after sampling. Pigments were extracted with 100% methanol in the dark at 0 °C for 24 h. The extracts were measured in a fluorometer (10-AU Turner) and the equations of Holm-Hansen et al. (1965) were used to calculate the concentration of pigments. Salinity was measured with a portable refractometer, and water temperature was recorded at the collection site and throughout the exposure experiments with a thermometer (±0.1 °C) to monitor temperature consistency in the tank.
3. Results
3.1. Environmental conditions
Throughout the experimental period, CDOM absorption coefficients at 320 nm (aCDOM(320) range: 4.40±0.78–5.79±0.45 m−1), Chl a concentrations (range: 5.9±0.45–7.7±0.05 μg l−1), and salinity (range: 24–27) showed low variation. During the UV exposure experiments, the water temperature in the tank ranged from 23.0 °C (October) to 27.5 °C (June). Solar irradiance data are shown for a clear day with low solar radiation intensities (October) (Fig. 1A), and for a typical spring day (May) with high solar radiation intensities (Fig. 1B).
Fig. 1.
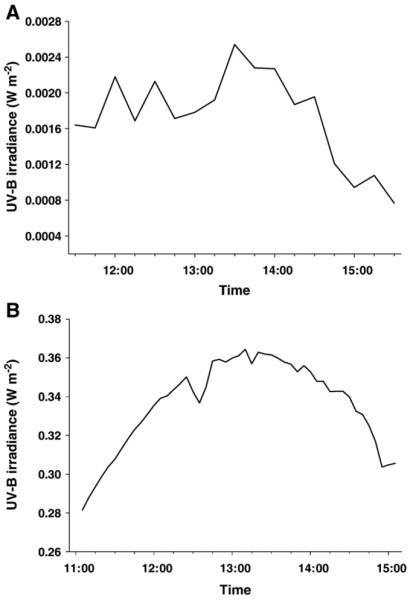
A) Solar irradiance data (measured with an IL 1700 radiometer) for a clear day with low solar radiation intensities (17 October 2003), and B) for a typical spring day (06 May 2004) with high solar radiation intensities (measured with a LI-1800 spectroradiometer; data are given for a wavelength of 320 nm).
3.2. Mortality
The results of the experiment to test the UV sensitivity of A. tonsa showed that the mortality of the copepods was 27% and 40% in the treatments receiving 2 h and 4 h full solar radiation, respectively, and 11% in the dark treatment (Fig. 2). The post exposure mortality (surviving animals kept under PAR in the laboratory for 5 h) was 25% for both UV exposure groups and 19% for the dark treatment (Fig. 2, inset). During the UV exposure experiments, mortality of the copepods was ≤35% (data not shown), except for the experiment to test the effect of food on protein synthesis where the mortality varied from 33% to 100%.
Fig. 2.
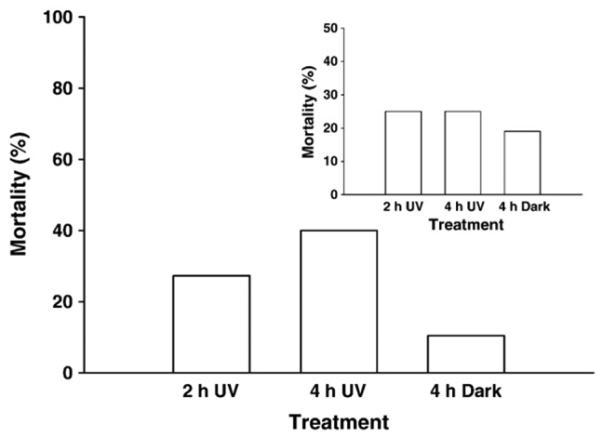
Mortality of A. tonsa (n = 20 animals) after exposure to solar radiation (UV) for 2 h and 4 h, respectively, or when kept in the dark (D) for 4 h (17 June 2003). The data of the inset show the post exposure mortality (animals were kept under PAR in the laboratory for 5 h).
3.3. De novo protein synthesis pattern
In vivo metabolic labeling was used to characterize the effects of exposure to solar UV radiation in A. tonsa. Protein synthesis was robust under all treatments when the copepods were exposed to low (October) solar radiation intensities (Fig. 3). In contrast, overall protein synthesis was strongly suppressed in the treatments receiving UVR during experiments conducted under high (May) solar radiation conditions (Fig. 4). Both UV-B and UV-A radiation affected the de novo synthesis of proteins (Fig. 4). The copepods exposed to PAR only or those kept in the dark showed the same robust protein synthesis pattern as the sampling and the time zero groups (Fig. 4). In none of the time-course experiments, however, did UV exposure result in the detectable induction of any molecular mass size class of Hsps (i.e., no noticeable expression of heat-shock proteins in the 60–90 kDa molecular mass range or in the low molecular mass range) (Figs. 3 and 4).
Fig. 3.
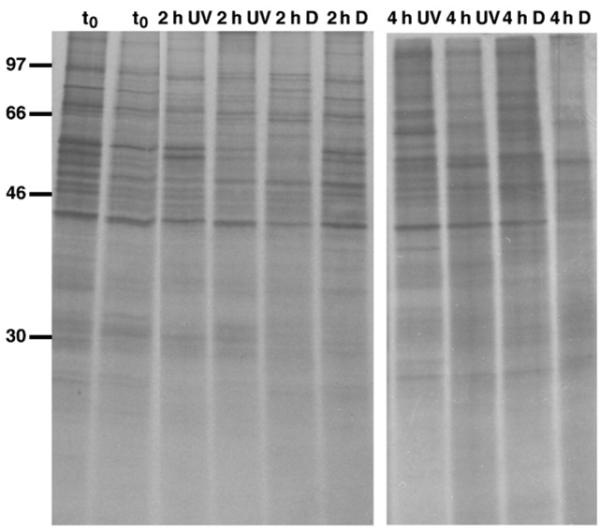
De novo protein synthesis pattern of A. tonsa after exposure to low solar radiation intensities (UV) or when kept in the dark (D) for 2 h and 4 h, respectively; t0: time zero group (17 October 2003). There was no effect of UVR on protein synthesis pattern at low solar radiation levels. Following exposure copepods were radiolabelled (35S-labelled methionine/cysteine) for 5 h at ambient seawater temperature, and proteins were separated on 12% SDS-polyacrylamide gels, which were dried and exposed to X-ray films; ~77,000 c.p.m. (left panel) and ~52,000 c.p.m. (right panel) per individual lane were loaded; each lane represents a single copepod, and individual samples were run in duplicate. The positions of 14C-labelled protein molecular mass markers are indicated in kDa on the left.
Fig. 4.
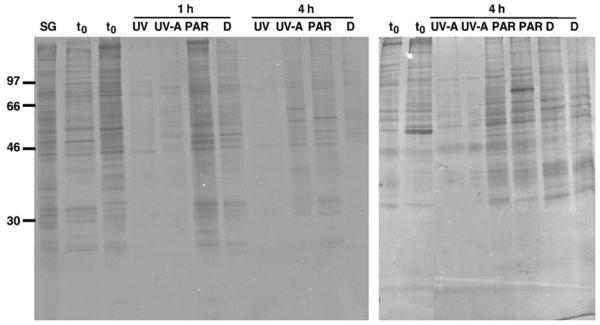
De novo protein synthesis pattern of A. tonsa after exposure to high solar radiation intensities (UV) or when kept in the dark (D) for 1 h and 4 h, respectively (06 May 2004). SG: sampling group, t0: time zero group, UV-A: UV-A and PAR, PAR: PAR only (UV-shielded treatment); ~79,000 c.p.m. (left panel) and ~87,000 c.p.m. (right panel) per individual lane were loaded. See Fig. 3 legend for detailed explanation.
Neither the addition of unlabeled methionine (Fig. 5A), antibiotics (Fig. 5B), nor food (Fig. 5C) during the 24 h adjustment period affected the patterns of the de novo protein synthesis in A. tonsa during the experiments.
For the simultaneous heat and UV stress experiments (~6 °C increase of water temperature), an overall suppression in protein patterns (i.e., faint bands) in both the UV-exposed and dark treatments was observed (Fig. 6).
Fig. 6.
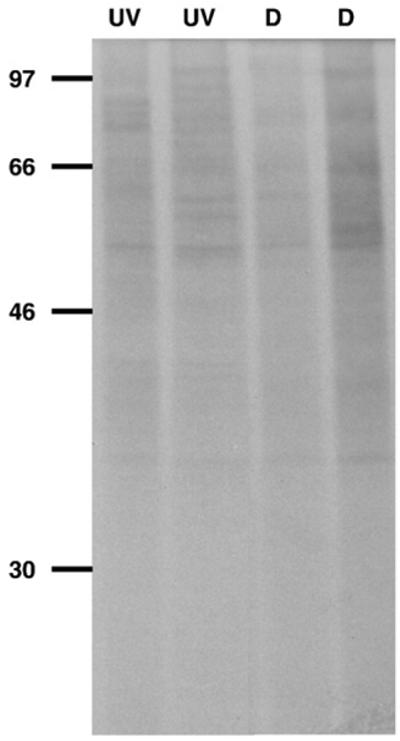
De novo protein synthesis pattern of A. tonsa after simultaneous UV and heat (water temperature increase of ~6 °C) stress. Copepods were exposed to solar radiation (UV) or were kept in the dark (D) for 4 h, respectively (25 June 2003). ~80,000 c.p.m. per individual lane were loaded. See Fig. 3 legend for detailed explanation.
3.4. Hsp70 expression
Western blotting was used to detect both the constitutive and stress-induced forms of Hsc/Hsp70 in A. tonsa. There was one predominant band with an apparent molecular mass of ~75 kDa detected in the copepod samples (Fig. 7A), which we assume to be the constitutively expressed isoform of the 70 kDa heat-shock protein gene family. Exposure to UVR led to an increase in the levels of constitutively expressed forms of the 70 kDa Hsp family. After 4 h of exposure, Hsp70 levels were four-fold and 3.5-fold higher in the copepods receiving full solar radiation and UV-A and PAR, respectively, compared to the levels in the dark control. The Hsp70 levels of the UV-shielded and dark treatments were similar to those found in the sampling and time zero groups and in the different treatments after exposure for 1 h (Fig. 7). However, there was no induction of UV-induced Hsp70 isoforms, which is in accordance with the results of the metabolic labeling experiments.
Fig. 7.
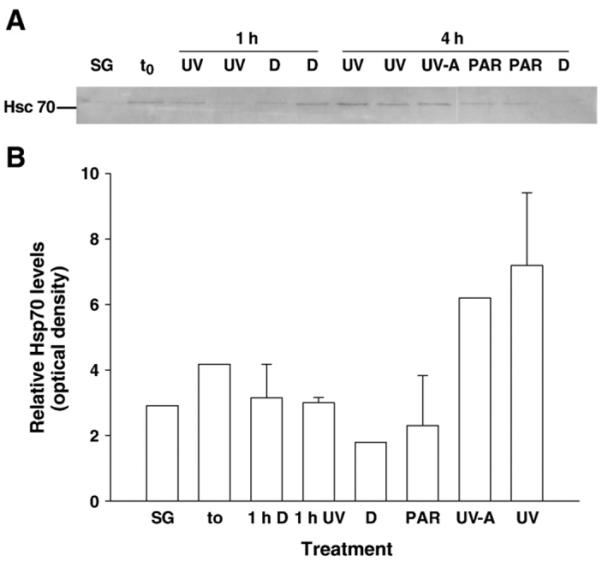
A) Western blot detection of constitutively expressed 70 kDa Hsp isoforms in A. tonsa from UV exposure (1 h or 4 h, respectively) experiments (13 May 2004). Copepod samples were screened for 70 kDa Hsp isoforms using an anti-Hsp70 rat monoclonal antibody. Each lane contains 6 μg of total protein; the standard shown on the left is bovine brain heat shock cognate 70 (Hsc 70). SG: sampling group, t0: time zero group, UV: copepods exposed to solar radiation, UV-A: copepods exposed to UV-A and PAR, PAR: copepods exposed to PAR only (UV-shielded treatment), D: copepods kept in the dark. B) Relative levels of constitutively expressed 70 kDa hsp isoforms in A. tonsa after exposure to solar radiation for 1 h and 4 h, respectively (13 May 2004). Error bars indicate + 1 s.d.
4. Discussion
Our results obtained with solid-phase antibody (western) analysis show that UVR increased levels of constitutively expressed 70 kDa heat-shock proteins in the marine copepod A. tonsa when exposed to high solar radiation intensities (Fig. 7B); however, without indication of stress-inducible isoform upregulation (Fig. 7A). Despite the elevated concentrations of endogenous Hsps in these animals, decreased survival rates (Fig. 2) and suppression in the de novo protein synthesis (Fig. 4) indicate that irreversible protein denaturation occurred at high solar radiation conditions. These findings suggest that the presence of elevated concentrations of constitutively expressed Hsp 70 may not be sufficient to rescue UV-damaged proteins. Similar results have been observed in intertidal mussels (Mytilus trossulus) exposed to high temperatures during summer (Hofmann and Somero, 1995). Although Hsp70 levels were higher in summer–compared to winter-collected animals–elevated concentrations of irreversibly denatured, i.e. ubiquitinated, proteins were still found, indicating that the heat shock response was most likely not able to prevent irreversible protein denaturation in the face of heat stress.
There is little information on the heat shock response in marine copepods. Bradley et al. (1988) found that the overall de novo synthesis of proteins decreased after heat shock in the estuarine copepod E. affinis, while a new set of proteins, including 70 kDa proteins, was upregulated. In the same species, heat-induced expression of proteins in the 60 kDa and 70 kDa ranges was observed regardless of salinity concentrations (Gonzalez and Bradley, 1994). The HSR in E. affinis is considered a transient response: a time-course experiment revealed that the synthesis of normal (not heat-shock) proteins is strongly inhibited up to 8 h after a heat shock stimulus. A gradual return of normal protein synthesis is observed after 10 h, but most Hsps are still being synthesized even 24 h post-heat shock (Hakimzadeh and Bradley, 1990). Kimmel and Bradley (2001) assume that changes in protein expression, measured as presence or absence of protein spots compared to control, are adaptations to differences in temperature and salinity in E. affinis. A genomic approach (mRNA expression) was used to detect thermal stress in the calanoid copepod Calanus finmarchicus. A four-fold induction of hsp70 expression was found in copepods exposed to both short-term (30 min) and longterm (48 h) temperature stress (Voznesensky et al., 2004).
Stress proteins are known to be upregulated in response to a wide variety of physical and chemical stressors, and they play a significant role in conferring tolerance to harsh conditions on these organisms. Although metabolic labeling yielded evidence for induction of Hsps in the marine copepod E. affinis (Bradley et al., 1988), none of the A. tonsa individuals examined in the metabolic labeling experiments showed any indication of induction of stress-inducible proteins (Figs. 3 and 4). Neither 30 min of UV exposure (data not shown), which might not have been enough time to activate the HSR, nor 1 to 4 h of UV exposure and 6 h of recovery/labeling period led to an Hsp induction in A. tonsa when labeled in vivo (Figs. 3 and 4). Generally, Hsps build up in cells upon stress as soon as 5 h following the event (Hofmann and Somero, 1996; Tomanek and Somero, 2002). In human keratinocytes, for example, UVR increased hsp72 mRNA with peak levels observed 1–3 h post-UV exposure, and constitutively expressed Hsp72 protein showed maximum levels 6 h following stress (Zhou et al., 1998). In aquatic organisms, transcription of hsp69 mRNA occurred within 3 h of stress recovery after heat exposure in oyster (Ostrea edulis) (Piano et al., 2004). Induction of hsp70 mRNA and protein synthesis in response to stress has also been documented in copepods. In Hemidiaptomus roubaui, a tissue-specific expression of Hsp70 was induced by short-term (3 h) heat stress (Liberge and Barthélémy, 2007). In this freshwater copepod, heat exposure resulted in the expression of the stress-inducible Hsp70 isoform, specifically expressed in the shell glands and genital double somite of adult copepods, while the constitutive form (Hsc70) was expressed in the brain and muscle cells (Liberge and Barthélémy, 2007). In the marine C. finmarchicus, expression of hsp70 was rapid (within 4 h) in response to a short heat shock (30 min) (Voznesensky et al., 2004). We conclude that the UV exposure time (up to 4 h) and the subsequent recovery/labeling period (6 h) were within the range of induction of stress protein synthesis as shown for different cell types in even closely related organisms.
Why the UV-induced increase in constitutively expressed 70 kDa heat-shock protein (Fig. 7) is not evident in the overall UV-suppressed de novo protein synthesis pattern (Fig. 4) is unknown. Different degrees of inducibility (constitutively expressed, constitutively expressed but increasing during or after stress, exclusively inducible) are known for entire families of Hsps (Feder and Hofmann, 1999). Among the possible explanations is that a time difference in inducibility may have caused the upregulation of Hsc70 to be completed by the time the radiolabel was introduced. Another possibility is the existence of this constitutive protein within the cell in an inactive or conjugated state and its activation by perturbations such as UVR. Since the protein exists already in an inactive state, no new protein would need to be synthesized. Thus, constitutively expressed 70 kDa heat-shock proteins would not take up the radiolabel and would not appear on the fluorograms, but would show as an Hsp increase in the western blots. During the simultaneous heat and UV stress experiments (maximum water temperature: 30.5 °C), an overall decrease in protein expression regardless of treatment was observed (Fig. 6). Although exposure to temperatures 5–10 °C above body temperature have been found to result in Hsp induction (Feder and Hofmann, 1999), the absolute increase in the water temperature (~6 °C) might have been too low to induce specific stress protein upregulation in A. tonsa. However, 32 °C is considered to be the upper thermal tolerance limit for this species (Gonzalez, 1974), thus A. tonsa was exposed to stringent heat conditions. The overall suppression in constitutive protein production even in the solar radiation-shielded treatment (Fig. 6) makes clear that the copepods were at their physiological limit. General protein synthesis is inhibited after extreme (heat) challenges as a result of phosphorylation of initiation factors such as eIF2 α, which disrupts ribosomal assembly and inactivates capbinding proteins (Scorsone et al., 1987; Zapata et al., 1991). On the other hand, when exposing the animals to lower UV doses and constantly lower (27.0 °C) water temperatures, protein synthesis was robust in all treatments and did not differ from the time zero group (Fig. 3). Unfortunately, to our knowledge, there exists no information on the potential synthesis of Hsps following heat stress in A. tonsa. Heat stress, however, also did not provoke the expression of inducible Hsp isoforms in the mussel Mytilus galloprovincialis and in the clams Tapes philippinarum and Scapharca inaequivalvis (Piano et al., 2004). Moreover, in oyster (O. edulis), heat caused overexpression of constitutive Hsp70 isoforms in the digestive gland, without appearance of an inducible isoform. In contrast, thermal stress caused the expression of a 69 kDa inducible isoform in gills of the same species (Piano et al., 2004). Although extremely unusual, complete absence of a HSR has been observed in the freshwater cnidarian H. oligactis (Bosch et al., 1988) and the Antarctic fish T. bernacchii (Hofmann et al., 2000), both living exclusively in cold, thermally stable environments. All these findings suggest a strong species- and tissue-specificity of the heat shock response. Another aspect might be that both the transcriptional and post-transcriptional regulatory steps are required for the production of Hsps. For example, Wu et al. (1986) showed that human Hsp promoter activity and protein accumulation following oxidative stress may be uncoupled. Moreover, increased heat shock promoter activation through HSF binding but no Hsp70 message or protein production was found in cells after hydrogen peroxide exposure (Bruce et al., 1993). Whether differences in Hsp70 accumulation following a perturbation such as UV stress are related to events distal to promoter activation and may require post-transcriptional regulatory steps remains unclear.
Species-dependent accumulation of Hsps may play an important role in the physiological stress response of aquatic organisms. For example, in fish, UV-B radiation, retene alone, and simultaneous exposure had no effect on Hsp70 levels in vendace (C. albula), while in whitefish (C. lavaretus) both UV-B radiation and retene alone upregulated Hsp synthesis (Vehniäinen et al., 2003). In spike larvae (E. lucius), however, Hsp70 levels decreased in the animals exposed either to UV-B or to retene, while simultaneous exposure induced Hsp70 synthesis (Häkkinen et al., 2004). Differences in the upregulation of Hsps have even been shown on a genotype level. Interclonal variation in the induction of stress proteins was found in Daphnia magna when facing predator stress. Both the constitutive levels of Hsp60 and the increase in Hsp60 concentrations upon exposure to fish kairomones varied between different clones, showing a dynamic induction of stress proteins upon predation risk (Pauwels et al., 2005).
Planktonic organisms have several strategies to protect themselves from harmful UVR (see Karentz, 2001, for review). While there exists no information on the presence of photoprotective UV-absorbing mycosporine-like amino acids in this calanoid copepod, carotenoids (mostly astaxanthin), which act against photo-oxidative stress, have been found in pooled samples of several Acartia species including A. tonsa from the Baltic Sea (Lotocka et al., 2004). A. tonsa from Tampa Bay is slightly colored, thus, we assume the presence of carotenoids in the study organism. Although A. tonsa is known to vertically migrate (Cuker and Watson, 2002), it is frequently found at shallow depths (i.e., top meters of the water column) during the day and is therefore exposed to harmful intensities in UV-transparent systems such as Tampa Bay (aCDOM(320): <6 m−1). UV-induced DNA damage may have occurred during the in situ experiments; however, as A. tonsa was exposed to artificial light (PAR, no UVR) following UV exposure, the presence of longer-wavelength photoreactivating radiation might have allowed potential photoenzymatic repair of DNA damage. Relatively high levels of DNA repair have been observed in Euphausia superba (krill) under laboratory conditions (Malloy et al., 1997), while ambient levels of DNA damage and subsequent repair are not known.
5. Conclusion
Molecular approaches serve as effective tools to measure stress responses in aquatic organisms. Protein synthesis and induction of constitutively expressed chaperones belonging to the 70 kDa Hsp family appear to be useful indicators of UV-induced sublethal stress in marine copepods. In general, organisms from warmer regions such as Tampa Bay may be well adapted to high temperatures and high solar radiation intensities, both known to potentially stimulate stress protein induction in aquatic organisms.
Acknowledgements
We are grateful to J. Donnelly, D. English, R. Russell, S. Samson, and C. Simoniello for technical assistance. We thank J. Cannizzaro and C. Du for chlorophyll and CDOM measurements, and N. Elsaessar for providing Isochrysis. We also thank R. Sommaruga for helpful comments on the manuscript. This research was funded by Austrian Science Fund (FWF) grant J2266-B06 (B.T.), and FWCC contract 99086 and NSF OPP 0523244 (J.J.T.). [SS]
References
- Araoz R, Lebert M, Häder DP. Translation activity under ultraviolet radiation and temperature stress in the cyanobacterium Nostoc sp. J. Photochem. Photobiol. 1998;47:115–120. [Google Scholar]
- Bonaventura R, Poma V, Costa C, Matranga V. UV-B radiation prevents skeleton growth and stimulates the expression of stress markers in sea urchin embryos. Biochem. Biophys. Res. Commun. 2005;328:150–157. doi: 10.1016/j.bbrc.2004.12.161. [DOI] [PubMed] [Google Scholar]
- Bonaventura R, Poma V, Russo R, Zito F, Matranga V. Effects of UV-B radiation on development and hsp70 expression in sea urchin cleavage embryos. Mar. Biol. 2006;149:79–86. [Google Scholar]
- Bosch TCG, Krylow SM, Bode HR, Steele RE. Thermotolerance and synthesis of heat shock proteins: these responses are present in Hydra attenuata but absent in Hydra oligactis. Proc. Natl. Acad. Sci. U. S. A. 1988;85:7927–7931. doi: 10.1073/pnas.85.21.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton ML, Pogorzelska M, Smoral L, Shehata A, Hamilton MG. Thermal biology of horseshoe crab embryos and larvae: a role for heat shock proteins. J. Exp. Mar. Biol. Ecol. 2006;336:65–73. [Google Scholar]
- Bradley BP, Hakimzadeh R, Vincent JS. Rapid responses to stress in Eurytemora affinis. Hydrobiologia. 1988;167/168:197–200. [Google Scholar]
- Bruce JL, Price BD, Coleman CN, Calderwood SK. Oxidative injury rapidly activates the heat shock transcription factor but fails to increase levels of heat shock proteins. Cancer Res. 1993;53:12–15. [PubMed] [Google Scholar]
- Brunet S, Giacomoni PU. Heat shock mRNA in mouse epidermis after UV irradiation. Mutat. Res. 1989;219:217–224. doi: 10.1016/0921-8734(89)90003-9. [DOI] [PubMed] [Google Scholar]
- Buckley BA, Owen ME, Hofmann GE. Adjusting the thermostat: the threshold induction temperature for the heatshock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J. Exp. Biol. 2001;204:3571–3579. doi: 10.1242/jeb.204.20.3571. [DOI] [PubMed] [Google Scholar]
- Choresh O, Loya Y, Müller WEG, Wiedenmann J, Azem A. The mitochondrial 60-kDa heat shock protein in marine invertebrates: biochemical purification and molecular characterization. Cell Stress Chaperones. 2004;9:38–47. doi: 10.1379/469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg JS, Uhlinger KR, Jackson SA, Cherr GN, Rifkin E, Friedman CS. Induced thermotolerance and the heat shock protein-70 family in the Pacific oyster Crassostrea gigas. Mol. Mar. Biol. Biotech. 1998;7:21–30. [Google Scholar]
- Cuker BE, Watson MA. Diel vertical migration of zooplankton in contrasting habitats of the Chesapeake Bay. Estuaries. 2002;25:296–307. [Google Scholar]
- Dietz TJ, Somero GN. The threshold induction temperature of the 90 kDa heat shock protein is subject to acclimatization in eurythermal goby fishes (genus Gillichthys) Proc. Natl. Acad. Sci. U. S. A. 1992;89:3389–3393. doi: 10.1073/pnas.89.8.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohler G, Hoffmann M, Stappel U. Pattern of proteins after heat-shock and UV-B radiation of some temperate marine diatoms and the Antarctic Odontella weissflogii. Botanica Acta. 1995;108:93–98. [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Garmyn M, Yaar M, Holbrook N, Gilchrest BA. Immediate and delayed molecular response of human keratinocytes to solar-simulated irradiation. Lab. Invest. 1991;65:471–478. [PubMed] [Google Scholar]
- Gonzalez JG. Critical thermal maxima and upper lethal temperatures for the calanoid copepods Acartia tonsa and A. clausi. Mar. Biol. 1974;27:219–223. [Google Scholar]
- Gonzalez CRM, Bradley BP. Salinity stress proteins in Eurytemora affinis. Hydrobiologia. 1994;293:461–468. [Google Scholar]
- Hakimzadeh R, Bradley BP. The heat-shock response in the copepod Eurytemora affinis (POPPE) J. Therm. Biol. 1990;15:67–77. [Google Scholar]
- Häkkinen J, Vehniäninen E, Oikari A. High sensitivity of northern pike larvae to UV-B but no UV-photoinduced toxicity of retene. Aquat. Toxicol. 2004;66:393–404. doi: 10.1016/j.aquatox.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Helmuth BST, Hofmann GE. Microhabitats, thermal heterogeneity, and patterns of physiological stress in the rocky intertidal zone. Biol. Bull. 2001;201:374–384. doi: 10.2307/1543615. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Somero GN. Evidence for protein damage at environmental temperatures: seasonal changes in levels of ubiquitin conjugates and hsp70 in the intertidal mussel Mytilus trossulus. J. Exp. Biol. 1995;198:1509–1518. doi: 10.1242/jeb.198.7.1509. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Somero GN. Interspecific variation in thermal denaturation of proteins in the congeneric mussels Mytilus trossulus and M. galloprovincialis: Evidence from the heat shock response and protein ubiquitination. Mar. Biol. 1996;126:65–75. [Google Scholar]
- Hofmann GE, Buckley BA, Airaksinen S, Keen JE, Somero GN. Heat-shock protein expression is absent in the Antarctic fish Trematomus bernacchii (Family Nototheniidae) J. Exp. Biol. 2000;203:2331–2339. doi: 10.1242/jeb.203.15.2331. [DOI] [PubMed] [Google Scholar]
- Hofmann GE, Buckley BA, Place SP, Zippay ML. Molecular chaperones in ectothermic marine animals: biochemical function and gene expression. Integr. Comp. Biol. 2002;42:808–814. doi: 10.1093/icb/42.4.808. [DOI] [PubMed] [Google Scholar]
- Holm-Hansen O, Lorenzen CJ, Holmes RW, Strickland JD. Fluorometric determination of chlorophyll. J. Cons. Perm. Int. Explor. Mer. 1965;30:3–15. [Google Scholar]
- Karanas JJ, Van Dyke H, Worrest RC. Mid-ultraviolet (UV-B) sensitivity of Acartia clausii Giesbrecht (Copepoda) Limnol. Oceanogr. 1979;24:1104–1116. [Google Scholar]
- Karentz D. Chemical defenses of marine organisms against solar radiation exposure: UV-absorbing mycosporine-like amino acids and scytonemin. In: McClintock JB, Baker BJ, editors. Marine chemical ecology. InCRC Press; 2001. pp. 481–519. [Google Scholar]
- Kimmel DG, Bradley BP. Specific protein responses in the calanoid copepod Eurytemora affinis (Poppe, 1880) to salinity and temperature variation. J. Exp. Mar. Biol. Ecol. 2001;266:135–149. [Google Scholar]
- Liberge M, Barthélémy RM. Localisation of metallothionein, heat shock protein (Hsp70), and superoxide dismutase expression in Hemidiaptomus roubaui (Copepoda, Crustacea) exposed to cadmium and heat stress. Can. J. Zool. 2007;85:362–371. [Google Scholar]
- Lotocka M, Styczynska-Jurewicz E, Bledzki LA. Changes in carotenoid composition in different developmental stages of copepods: Pseudocalanus acuspes Giesbrecht and Acartia spp. J. Plankton Res. 2004;26:159–166. [Google Scholar]
- Malloy KD, Holman MA, Mitchell D, Detrich HW. Solar UVB-induced DNA damage and photoenzymatic DNA repair in Antarctic zooplankton. Proc. Natl. Acad. Sci. 1997;94:1258–1263. doi: 10.1073/pnas.94.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga V, Pinsino A, Celi M, Di Bella G, Natoli A. Impacts of UV-B radiation on short-term cultures of sea urchin coelomocytes. Mar. Biol. 2006;149:25–34. [Google Scholar]
- Naganuma T, Inoue T, Uye S. Photoreactivation of UV-induced damage to embryos of a planktonic copepod. J. Plankton Res. 1997;19:783–787. [Google Scholar]
- Pauwels K, Stoks R, De Meester L. Coping with predator stress: interclonal differences in induction of heat-shock proteins in the water flea Daphnia magna. J. Evol. Biol. 2005;18:867–872. doi: 10.1111/j.1420-9101.2005.00890.x. [DOI] [PubMed] [Google Scholar]
- Piano A, Valbonesi P, Fabbri E. Expression of cytoprotective proteins, heat shock protein 70 and metallothioneins, in tissues of Ostrea edulis exposed to heat and heavy metals. Cell Stress Chaperones. 2004;9:134–142. doi: 10.1379/483.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Welk JF, Jurivich DA. Ultraviolet light attenuates heat-inducible gene expression. J. Cell. Physiol. 1997;172:314–322. doi: 10.1002/(SICI)1097-4652(199709)172:3<314::AID-JCP5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Roberts DA, Hofmann GE, Somero GN. Heat-shock protein expression in Mytilus californianus: acclimatization (seasonal and tidal-height comparisons) and acclimation effects. Biol. Bull. 1997;192:309–320. doi: 10.2307/1542724. [DOI] [PubMed] [Google Scholar]
- Sanders BM. Stress proteins in aquatic organisms: an environmental perspective. Crit. Rev. Toxicol. 1993;23:49–75. doi: 10.3109/10408449309104074. [DOI] [PubMed] [Google Scholar]
- Sanders BM, Hope C, Pascoe VM, Martin LS. Characterization of the stress protein response in 2 species of collisella limpets with different temperature tolerances. Physiol. Zool. 1991;64:1471–1489. [Google Scholar]
- Schlesinger MJ, Tissières A, Ashburner M. Heat Shock Proteins: From Bacteria to Man. Cold Spring Harbor Laboratory Press; New York: 1982. [Google Scholar]
- Scorsone KA, Panniers R, Rowlands AG, Henshaw EC. Phosphorylation of eukaryotic initiation factor 2 during physiological stresses which affect protein synthesis. J. Biol. Chem. 1987;262:14538–14543. [PubMed] [Google Scholar]
- Tartarotti B, Cravero W, Zagarese HE. Biological weighting function for the mortality of Boeckella gracilipes (Copepoda, Crustacea) derived from experiments with natural solar radiation. J. Photochem. Photobiol. B. 2000;72:314–319. doi: 10.1562/0031-8655(2000)072<0314:bwfftm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tomanek L, Somero GN. Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. J. Exp. Biol. 2002;205:677–685. doi: 10.1242/jeb.205.5.677. [DOI] [PubMed] [Google Scholar]
- Trautinger F, Kindås-Mügge I, Knobler RM, Hönigsmann H. Stress proteins in the cellular response to ultraviolet radiation. J. Photochem. Photobiol. B. 1996;35:141–148. doi: 10.1016/s1011-1344(96)07344-7. [DOI] [PubMed] [Google Scholar]
- Vehniäinen E-R, Häkkinen J, Oikari A. Photoinduced lethal and sublethal toxicity of retene, a polycyclic aromatic hydrocarbon derived from resin acid, to coregonid larvae. Environ. Toxicol. Chem. 2003;22:2295–3000. doi: 10.1897/02-569. [DOI] [PubMed] [Google Scholar]
- Voznesensky M, Lenz PH, Spanings-Pierrot C, Towle DW. Genomic approaches to detecting thermal stress in Calanus finmarchicus (Copepoda: Calanoida) J. Exp. Mar. Biol. Ecol. 2004;311:37–46. [Google Scholar]
- Williamson CE, Neale PJ, Grad G, DeLange HJ, Hargreaves BR. Beneficial and detrimental effects of UV radiation: implications of variation in the spectral composition of environmental radiation for aquatic organisms. Ecol. Appl. 2001;11:1843–1857. [Google Scholar]
- Wu BJ, Kingston RE, Morimoto RI. Human HSP70 promoter contains at least two distinct regulatory domains. Proc. Natl. Acad. Sci. U. S. A. 1986;83:629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata JM, Maroto FG, Sierra JM. Inactivation of mRNA cap-binding protein complex in Drosophila melanogaster embryos under heat shock. J. Biol. Chem. 1991;266:16007–16014. [PubMed] [Google Scholar]
- Zhou XW, Tron VA, Li G, Trotter MJ. Heat shock transcription factor-1 regulates heat shock protein-72 expression in human keratinocytes exposed to ultraviolet B light. J. Invest. Dermatol. 1998;111:194–198. doi: 10.1046/j.1523-1747.1998.00266.x. [DOI] [PubMed] [Google Scholar]


