Abstract
We studied the quantitative and qualitative mycosporine-like amino acid (MAA) composition in phytoplankton and the copepod Cyclops abyssorum tatricus from an alpine lake over a 15-month period. Up to eight MAAs were identified in the samples, with shinorine being predominant. The MAAs occurred year round and showed a strong seasonal pattern. Compared with ice-cover periods, concentrations during the summer were on average 3.6 and 3.0 times higher in phytoplankton and C. abyssorum tatricus, respectively. During the summer, the contents of MAAs in phytoplankton decreased with depth, suggesting their photoprotective role. Chlorophyll a-specific concentrations of MAAs in phytoplankton correlated significantly with the incident solar radiation and ultraviolet (UV) water transparency (r2 ≤ 0.36), however, the strongest relationship was found with water temperature (r2 = 0.67). In zooplankton, highest contents of MAAs were found in eggs, nauplii, and young copepodids, presumably providing a high level of photoprotection for progeny. Proportions of the dominant MAAs in the copepod showed seasonal and ontogenetic variations, which were consistent with relative changes in the predominant MAA, but not other abundant MAAs, in phytoplankton. Considering a time lag of approximately 1 month between the synthesis and subsequent accumulation of these compounds, MAA concentrations in late copepodid to adult life stages were significantly correlated to those in phytoplankton. Annual patterns in MAAs with high concentrations during periods of elevated environmental stress are consistent with the idea that these compounds play an important role in protecting aquatic organisms against UV damage.
During the ice-cover season, when snow and white ice are present, lake organisms are relatively well protected from ultraviolet radiation (UVR, 290–400 nm) because irradiance values are significantly reduced and the shorter wavelengths in the UVB (290–320 nm) are excluded by the high snow albedo (Belzile et al. 2002). Prior to ice breakup and after ice out, however, lake organisms experience important qualitative and quantitative changes in UVR (Sommaruga 2001). Although damaging effects (e.g., reduced photosynthesis, survival, and reproduction) of UVR are known for planktonic organisms (Williamson et al. 1994; Cabrera et al. 1997; Villafañe et al. 1999), phytoplankton and zooplankton living in UV-exposed ecosystems have evolved several adaptive strategies to minimize potential negative impacts. For example, avoidance by swimming away from UVR is an effective way of reducing exposure: organisms migrate into protective water depths or stay deep in the water column during daytime (Leech and Williamson 2001; Alonso et al. 2004). Moreover, photoreactivation, a mechanism to cope with UV-induced DNA damage, is observed in a variety of zooplankton species (Zagarese et al. 1997; Grad et al. 2001; Gonçalves et al. 2002). Another defense line against UVR exposure is the synthesis or accumulation of photoprotective compounds. Among the colored photoprotective compounds known in planktonic organisms are melanin, which is mainly restricted to arctic and alpine cladoceran species (Hessen and Sørensen 1990), and carotenoids (Hairston 1976; Laurion et al. 2002). An additional class of photoprotective compounds are mycosporine-like amino acids (MAAs), intracellular colorless compounds with absorption maxima between 309 and 360 nm. These secondary metabolites are water soluble, have high absorptivity, and are derivatives of aminocyclohexenone or aminocycloheximine rings. Nineteen different MAAs are described so far and they are found in many marine organisms, ranging from bacteria to fish (reviewed in Karentz 2001; Shick and Dunlap 2002). Fewer studies, however, have been carried out in freshwater ecosystems, but MAAs are widespread in benthic cyanobacteria (Sommaruga and Garcia-Pichel 1999), natural phytoplankton assemblages (Laurion et al. 2002; Tartarotti et al. 2004), and zooplankton (Sommaruga and Garcia-Pichel 1999; Tartarotti et al. 2001, 2004; Rocco et al. 2002). Bacteria and phytoplankton synthesize MAAs most probably through the shikimate pathway, but the majority of heterotrophic metazoans presumably have a dietary requirement for these UV-absorbing compounds, as they lack de novo synthesis of MAAs (Carroll and Shick 1996; Newman et al. 2000; Moeller et al. 2005). MAA-dependant UV protection and tolerance in planktonic organisms is known for the cultured dinoflagellate Gymnodinium sanguineum (Neale et al. 1998), for natural populations of the copepod Boeckella poppei from the Antarctic Peninsula (Rocco et al. 2002), and for the cultured copepod Leptodiaptomus minutus (Moeller et al. 2005).
Highest MAA concentrations in lake phytoplankton assemblages are usually found in the clearest lakes (Laurion et al. 2002). However, the relationship between MAA concentration and UV transparency among natural phytoplankton assemblages is weak (Laurion et al. 2002). Conversely, in zooplankton (populations of the copepod Cyclops abyssorum tatricus) from the same lakes, 74% and 86% of the variability in MAA concentrations are explained by UV transparency and by the fraction of the water column to which 1% of the surface UVR at 320 nm penetrates, respectively (Tartarotti et al. 2001). Such correlations suggest an adaptive role of MAAs for organisms living in UV-exposed lakes, but it also implies that the synthesis of MAAs in phytoplankton and accumulation by zooplankton have different dynamics.
In freshwater organisms, data on quantitative and qualitative composition of MAAs are either for a specific sampling date (Tartarotti et al. 2001), give a comparison from summer and autumn values (Laurion et al. 2002; Tartarotti et al. 2004), or include data obtained from spring through autumn (Moeller et al. 2005), while nothing is known about potential seasonal variation in these sunscreening compounds throughout the year. Studies in marine systems, however, give a diverse picture of seasonal dynamics in MAA contents. For example, the MAA concentration in the Antarctic sea urchin Sterechinus neumayeri was not related to seasonal changes in solar exposure (Karentz et al. 1997), while the MAA contents of the coral Fungia repanda or the macroalga Devaleraea ramentacea were closely linked to seasonal variation in ambient radiation conditions (Drollet et al. 1997; Karsten et al. 1999). In a recent study by Llewellyn and Harbor (2003), MAA concentrations in surface-water phytoplankton from the English Channel were strongly related to temporal changes in solar irradiation, and a seasonal survey in the coastal waters off California showed a maximum of these UV-absorbing compounds in the spring (Whitehead and Vernet 2000).
In the present study, our objectives were (1) to determine seasonal changes in the quantitative and qualitative composition of MAAs in phytoplankton and zooplankton living in a UV-transparent alpine lake, (2) to examine ontogenetic-related and sex-specific concentrations of MAAs in C. abyssorum tatricus, the dominant zooplankton species in this lake, and (3) to relate concentrations of MAAs with factors influencing temporal changes, such as the incident levels of UVR, the UV water transparency, and the lake water temperature.
Sampling site
Gossenköllesee is located above the treeline at 2,417 m above sea level in the Austrian Central Alps (47°13′N, 11°00′E). This alpine lake has a maximum depth of 9.9 m, a surface area of 0.017 km2, a pH of ~6.9, and dissolved organic carbon concentrations of ~0.3 mg L−1. Mean values of total phosphorus (P) and total nitrogen (N) concentrations for the water column are low (~2.5 μg L−1 and ~360 μg L−1, respectively); however, N concentrations are not limiting. This lake is covered by ice and snow of up to ~2-m thickness for 7–9 months per year.
Sampling and sample processing
Water and zooplankton samples were collected between May 2001 and July 2002. Sampling was done at weekly intervals during summer (July and August) and every 2–4 weeks during the rest of the year (n = 25).
Phytoplankton
Water samples were collected from a boat at 0, 2, 4, 6, and 8 m depth with a modified 5-liter Schindler–Patalas sampler at the center of the lake around noon. From February to May 2002, the ice cover exceeded 1.3-m thickness; therefore, it was not possible to take samples from 8-m depth during this time period. Water samples (~10 liters) from each depth were pooled in large opaque carboys and processed immediately after sampling. After gentle mixing, lake water was prefiltered by a 100 μm mesh to remove zooplankton (small nauplii, like orthonauplii, could have slipped through the mesh; however, they are present at very low numbers in the water column during daytime; see Tartarotti et al. 1999) and replicated subsamples of 0.3 to 4.0 liters (depending on filter size and phytoplankton biomass) were filtered onto Whatman GF/F filters (i.e., different filters for MAAs and chlorophyll a) at low vacuum pressure (0.3–0.4 atm). Although the samples reflect the <100-μm seston, the biomass of the <100-μm seston from Gossenköllesee is dominated by phytoplankton; thus, we considered that the MAAs are mainly attributed to phytoplankton. All filters containing samples were immediately frozen and stored at −80°C until extraction within 4 months.
Zooplankton
After the water samples were taken, zooplankton was collected by vertical net (55-μm mesh size) tows. In the laboratory, the organisms were kept at 4°C and dark conditions until further processing within 24 h. The copepod C. abyssorum tatricus was concentrated on a net sieve of 100-μm mesh size and rinsed with tap water to remove phytoplankton. Under the stereomicroscope, CO2-narcotized copepods were separated into the different life stages (i.e., nauplii, juvenile-stage copepodid CI–CIV, copepodid CV female and male, adult female and male, egg-carrying females, and egg-carrying females after removal of the egg sacs). Egg sacs (removed with a needle), nauplii (separated into nauplii of different size classes referred to “small,” “medium,” and “large”), and copepodid stages CI–CIII, were transferred with a Pasteur pipette and placed on wet GF/F filters (Whatman) kept on ice, while copepodid CIV to adult life stages were picked into cooled microcentrifugation tubes. In addition, to obtain newly hatched nauplii, egg sacs were removed from females and kept under cool conditions (4–8°C) for 48–72 h. After hatching, the orthonauplii were placed onto GF/F filters as described above. The number of individuals per tube or filter, respectively, ranged from 1 (few cases of copepodid CV or adults) to 37, but generally was ~11 individuals per sample. In the case of the copepod eggs and nauplii, 24–38 egg sacs and 18–150 nauplii (50 nauplii on average), respectively, were placed on each filter. All samples consisted of two to three replicates per life stage and sampling date (except for some cases because of the very low number of a certain life stage present in the sample). Copepod samples were immediately frozen at −80°C until extraction within 4 months.
Extraction, spectrophotometric, and high-performance liquid chromatography analyses
Chlorophyll a (Chl a) was extracted in 13 mL of 90% alkaline acetone (v/v) for ~16 h in the dark at 4°C. Filters were briefly (2 min) sonicated with a tip sonicator on ice and the extracts cleared using a 0.1-μm pore-size Anodisc filter (Whatman). The extracts were scanned in a double-beam spectrophotometer (Hitachi U-2000; scans from 400 to 750 nm) against an acetone reference and using 5-cm glass cuvettes. After the measurement, 100 μL of 1 mol L−1 HCl were added, and after 5 min, the sample was scanned again to obtain phaeopigment contents. The equation of Lorenzen (1967) was used to calculate the concentration of Chl a.
MAAs were extracted according to the most efficient protocol reported for C. abyssorum tatricus and the natural phytoplankton assemblages present in the study lake (Tartarotti and Sommaruga 2002). Briefly, samples were extracted twice in 25% aqueous methanol (v/v; MeOH; high-performance liquid chromatography [HPLC] grade) at 45°C for 2 h. Samples were sonicated (1 min continuously at 40 W) on ice at the beginning of the first extraction. The pooled extracts were cleared using GF/F filters and evaporated to dryness, using a SpeedVac concentrator (Savant) at 45°C. The samples were stored at −80°C for further characterization using HPLC within 1 week. For separation and quantification of MAAs, the concentrated, dried extracts were resuspended in 0.05–0.1 mL of 25% MeOH (v/v), and 20–50-μL aliquots were injected in a Phenosphere 5-μm pore-size RP-8 column (4.6 mm inner diameter × 25 cm, Phenomenex) protected with a RP-8 guard column (Brownlee), for isocratic reverse-phase HPLC analysis. Samples were run with a mobile phase of 0.1% acetic acid in 25% aqueous MeOH (v/v), and a flow rate of 0.79 mL min−1. Peak absorbance measurements were done at 310, 320, 334, and 360 nm in a Dionex system equipped with a diode-array detector (scanning from 200 to 595 nm). Individual peaks were identified by relative retention time (order of appearance), absorption spectra, and by cochromatography with standards extracted from the marine algae Porphyra yezoensis and Palythoa sp. (courtesy of Dr. U. Karsten, University of Rostock, Rostock, Germany; and Dr. M. Shick, University of Maine, Orono, Maine). The total content of specific MAAs in each sample was calculated from HPLC peak areas, using published molar extinction coefficients (see Tartarotti et al. 2001). The molar extinction coefficient for asterina was assumed to be the same as that of palythinol (Dunlap et al. 1989). A mean molar extinction coefficient of published values (40,000 mol L−1 cm−1) was used for four unknown compounds. Concentrations of the different MAAs were normalized to the dry weight of the zooplankton (expressed as μg [μg dry weight]−1), and to the Chl a concentration of the phytoplankton (hereafter, Chl a-specific MAA concentration, expressed as μg [μg Chl a]−1), respectively. We have unpublished data (R.S.) for this lake on summer MAA concentrations in phytoplankton normalized to either protein concentration or Chl a concentration, showing that both parameters give the same trend.
Incident solar radiation and UV attenuation measurements
Global solar radiation (direct plus diffuse) was measured automatically at 15-min intervals at a nearby site (~1 km from the lake) using a star-pyranometer (Type 8102; Schenk). Due to potential variability in radiation on a daily basis, measurements were integrated over the whole wavelength band (300–3000 nm) over 2 d before sampling (“2-d-integrated solar radiation”). The downwelling irradiance was measured with a PUV-501B profiler radiometer (Biospherical Instruments Inc.) at 305, 320, 340, and 380 nm (full bandwidth at half maximum is 8–10 nm), and in the photosynthetically available radiation (PAR; 400–700 nm) band. Profiles were made at the center of the lake between ~11:00 and 14:00 h local time. The diffuse attenuation coefficient (Kd) in the water column was determined from the slope of the linear regression of the natural logarithm of downwelling irradiance (Ed) versus depth. During the ice-cover period, the sampling hole in the ice was covered with black plastic foils during the measurements. The first 3 m of the profiles, however, were not included in the calculation of the Kd.
Lake water temperature
Water temperature was measured with a thermometer (±0.1°C) placed inside the water sampler immediately after collection and checked against the temperature sensor from the PUV profiler.
Data treatment
All statistical analyses were performed using the Sigma-Stat 2.03 software package (SPSS Inc.). To investigate the effect of season (i.e., two seasons: periods of the year during which the lake was covered with ice, hereafter “ice-cover season,” and the period without ice cover, hereafter “ice-free period”) or depth on MAA concentrations, paired t-tests were performed for normally distributed and Wilcoxon signed rank tests for not normally distributed data. Data were averaged over the water column to calculate simple correlations and regressions. Analysis of variance (ANOVA) (Kruskal–Wallis one-way ANOVA on ranks) was used to test for differences in MAA concentration between the distinct life stages of C. abyssorum tatricus. Data are reported as mean ± 1 standard deviation; level of significance was set to p < 0.05.
Results
Phytoplankton
Eight different MAAs were identified in phytoplankton: mycosporine-glycine, shinorine, porphyra-334, palythine, asterina, palythinol, usujirene, and palythene. Two unknown UV-absorbing compounds with absorption maxima at ~331.6 and 332.3 nm (designated as MAA-331 and MAA-332 hereafter) were occasionally found in the samples. Shinorine was present in all samples throughout the study period; similarly, asterina was only absent in samples collected from 4-m and 6-m depth in March and April 2002. Palythine was detected in most samples, while the other compounds were less commonly found or absent during the ice-cover season (see Web Appendix 1: http://www.aslo.org/lo/toc/vol_51/issue_3/1530a1.pdf).
Shinorine constituted the largest fraction, with up to 90% of the total MAA pool (62% on average for all samples). The relative proportion of palythine (up to 63% of the total MAAs, 20% on average) and asterina (up to 42%, 9% on average) was high, while the other MAAs were present from trace levels up to 45% of the total MAA pool, but represented <4% on average (Web Appendix 1).
The composition of the predominant MAAs was very similar throughout the water column during the ice-free season. With the beginning of the ice formation and during winter, the relative proportion of shinorine was comparable with or exceeded summer and autumn percentages at the water surface (~80% of the total MAA pool), while it was lower (~50–60%) at the other sampling depths. Palythine contributed on average 13% to the total MAA pool in summer; however, during the ice-cover season, its contribution was considerably higher (average ~31%). The proportion of asterina was ~11% during the ice-free season and 4–9% during winter. All data are presented in Web Appendix 1.
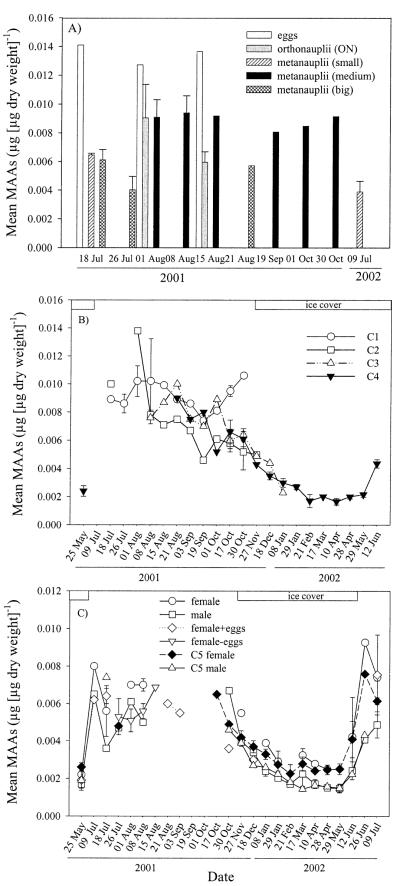
Highest Chl a-specific concentrations of MAAs were observed at 0-m depth on 15 August 2001 (1.71 ± 0.35 μg [μg Chl a]−1) and at 4-m depth on 09 July 2002 (1.74 ± 0.11 μg [μg Chl a]−1). The lowest Chl a-specific MAA content was detected just beneath the ice cover on 18 December 2001 (0.01 ± 0.001 μg [μg Chl a]−1) (Fig. 1).
Fig. 1.
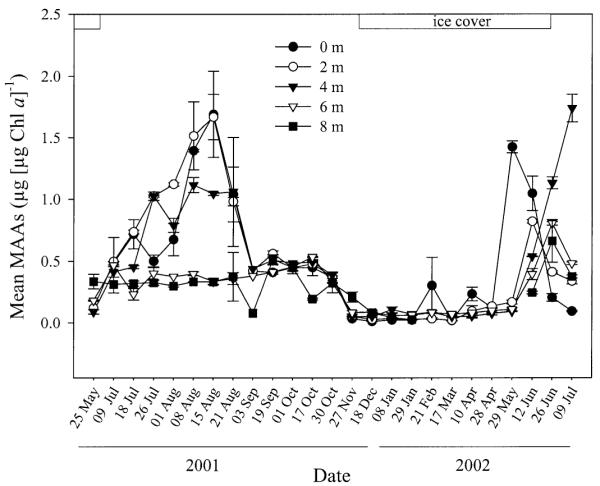
Seasonal variation of mean Chl a-specific MAA concentrations in phytoplankton at various depths from May 2001 to July 2002.
The Chl a-specific concentrations of MAAs decreased at the end of August (Fig. 1). During autumn, contents remained relatively stable but decreased at the time of the ice-cover formation (27 November 2001; Fig. 1). During winter, concentrations of MAAs were very low but started to increase with the melting of the ice cover (Fig. 1). Overall, mean Chl a-specific MAA contents averaged over the ice-free season were approximately 3.6 times higher when compared with those measured during the ice-cover period (Web Appendix 1). For the whole data set, concentrations of MAAs were significantly different between the ice-free and ice-cover season (Wilcoxon signed rank test, p < 0.001).
During summer, Chl a-specific concentrations of MAAs were typically highest at the water surface or subsurface and decreased with depth (Figs. 1, 2A). Surface MAA contents were significantly higher than those close to the lake bottom from the beginning of July to the beginning of September (paired t-test, p < 0.001, n = 15). Concentrations of MAAs showed low variance throughout the water column during the time of mixis (mid-September through the end of November) and most sampling dates in winter (Figs. 1, 2B,C). During three sampling dates under ice cover, however, a surface maximum was observed (Figs. 1, 2D). The typical summer depth profile was found prior to ice breakup (Fig. 2E). After ice out and water column mixis, highest MAA concentrations were detected at 4-m depth (Figs. 1, 2F).
Fig. 2.
Depth distribution of Chl a-specific MAA concentrations in phytoplankton during (A) summer, (B) time of water column mixis, (C, D) early and late winter, (E) prior to ice breakup, and (F) after ice-out and water column mixis.
Zooplankton
The same main MAAs were found in the copepod C. abyssorum tatricus as in phytoplankton. Shinorine, asterina, and palythine were the predominant compounds, and these MAAs were present in all life stages and throughout the study period (except for palythine in orthonauplii of one sampling date). Porphyra-334 and palythinol were detectable in most samples, while mycosporine-glycine, usujirene, and palythene were less frequently found or completely absent during the ice-cover period. In addition, two unknown compounds with absorption maxima at ~333.3 and 339.2 nm (designated as MAA-333 and MAA-339 hereafter) were occasionally observed.
Shinorine was the major MAA and made up to 97% of the total MAA concentration (63% on average for all samples). Asterina contributed up to 43% (20% on average) and palythine up to 19% (5% on average) of the total MAA pool, while the other compounds were found from trace levels up to 54%, but represented <5% on average. The maximum contribution of mycosporine-glycine, palythinol, and unknown MAA-339 was <2%, 5%, and 8%, respectively, and the latter compound was only occasionally found in the copepod samples. The contribution of mycosporine-glycine decreased with the formation of the ice cover until this compound became undetectable. It reappeared, however, in the animals collected during the last sampling date (12 June 2002) before ice out. Conversely, the contribution of palythinol was higher in the copepods sampled during the period with ice cover than during the ice-free season (1.8% and 0.4%, respectively, on average). Although the average contribution of porphyra-334 to the total MAA pool was generally low (≤3%), it made up to 10–75% of the total MAAs in some of the samples (25 May and 09 July 2001). Usujirene and palythene were less frequently found; however, in the samples where these compounds were present, they contributed on average 4% and 6% of the total MAA pool, respectively. The unknown MAA-333 was present in all samples collected on 25 May 2001 (range: 5–41%) and 01 August 2001 (range: 13–50%) and was occasionally found in the copepod samples from October 2001 to January 2002.
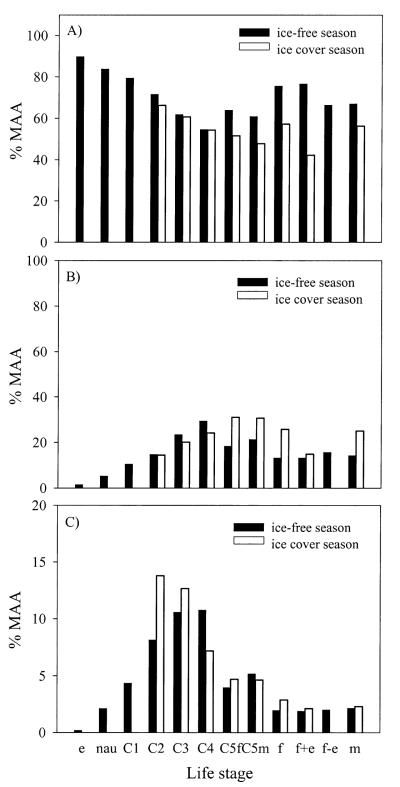
The relative proportion of the predominant MAA, shinorine, showed life–stage-specific and seasonal variations (Fig. 3). During the ice-free season, the relative contribution of shinorine decreased continually from ~90% in the eggs to 55% in copepodid C4 stages, whereas in copepodid C5 to adult life stages, it ranged from 61% to 77% (Fig. 3A). During this time, the relative proportion of shinorine in the distinct ontogenetic stages was always higher when compared with values measured under ice (Fig. 3A). Conversely, the relative contribution of asterina and palythine increased from nauplii through copepodid C3 and reached a maximum in copepodid C4 life stages during summer (Fig. 3B,C). During the ice-cover period, the relative proportion of asterina increased from 15% in copepodid C2 to 31% in copepodid C5 females, while the proportion of palythine decreased from 14% in copepodid C2 to ~3% in adult copepods (Fig. 3B,C). When comparing the ice-free season with the ice-cover one, there was no clear pattern of either higher or lower percentages for the different life stages (Fig. 3B,C), as observed for the major MAA shinorine (Fig. 3A).
Fig. 3.
(A) Life–stage-specific proportion (%) of the predominant MAAs shinorine, (B) asterina, and (C) palythine in C. abyssorum tatricus during the ice-free and ice-cover season (abbreviations: e = eggs, nau = nauplii [orthonauplii and metanauplii combined], C1–C4 = copepodid stages 1–4, C5f = copepodid C5 female, C5m = copepodid C5 male, f = female, f+e = egg-carrying female, f−e = females where eggs have been removed, m = male).
Total mean concentrations of MAAs in C. abyssorum tatricus ranged from 0.0015 ± 0.0001 (copepodid C5 male, 17 March 2002; Fig. 4C) to 0.0141 μg [μg dry weight]−1 (eggs, 18 July 2001; Fig. 4A). For all life stages, the highest total mean MAA concentration was found during the ice-free sampling period (Fig. 4). During autumn and winter, concentrations of MAAs decreased, until lowest values were found in March and April, the period with the thickest ice cover (Fig. 4). Concentrations of MAAs in C. abyssorum tatricus increased before ice out, and shortly after ice breakup, life–stage-specific MAA concentrations were similar to the ones measured the previous summer (Fig. 4B,C). Overall, life–stage-specific concentrations of MAAs averaged over the ice-free season were up to ~3 times higher than those from the ice-cover period (Fig. 5B). Concentrations of MAAs in copepodid C3 to adult copepods (except C5 males) were significantly different between the ice-free and ice-cover season (paired t-test, p < 0.05).
Fig. 4.
(A) Seasonal variation of life–stage-specific MAA concentrations in C. abyssorum tatricus eggs and nauplii, (B) copepodid C1–C4, and (C) copepodid C5 to adult from May 2001 to July 2002.
Fig. 5.
(A) Life–stage-specific MAA concentrations averaged over the study period from May 2001 to July 2002 or (B) averaged over the ice-free and ice-cover season, respectively. (A) Different letters inside the bars indicate a significant difference found with the Kruskal–Wallis ANOVA on ranks, all pairwise multiple comparison procedures (Dunn’s method, p < 0.05); eggs were not considered for the statistical analysis. Number above the bar is sample size. (B) Asterisk indicates a significant difference between ice-free and ice-cover seasons (paired t-test, p < 0.05). Abbreviations of the copepod life stages are given in Fig. 3.
When comparing within the different life stages (averaged over the total sampling period), highest total MAA concentration was found in eggs, followed by nauplii and juvenile stages copepodid CI–CIII (Fig. 5A). Contents were similarly high in females (females, females with eggs, and females where eggs have been removed) and were slightly lower in copepodid C5 females and C4, while the lowest concentrations were measured in copepodid CV males and adult males (Fig. 5A).
Relationship between concentrations of MAAs in phytoplankton and physical properties
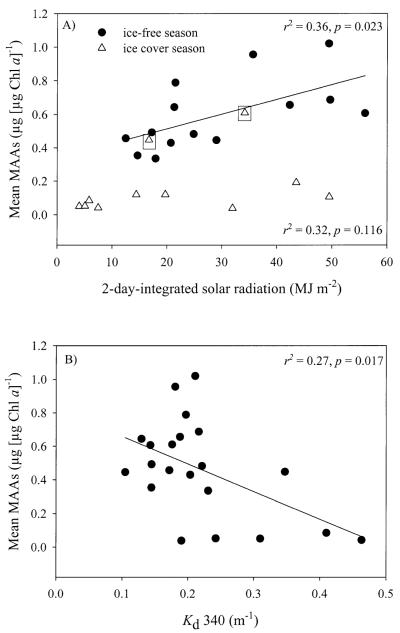
A positive linear correlation was found between mean values of Chl a-specific MAA concentrations and the 2-d-integrated solar radiation during the ice-free season (r2 = 0.36, p = 0.023, n = 14). The relationship for the data collected during the ice-cover period was similar to the one found for the ice-free period; however, it was not statistically significant (r2 = 0.32, p = 0.116, n = 9; excluding the two points with high concentration of MAAs at the last sampling dates under ice; Fig. 6A).
Fig. 6.
(A) Relationships between the Chl a-specific concentration of MAAs in phytoplankton and the 2-d-integrated solar radiation and (B) the attenuation coefficient (Kd) at 340 nm. (A) Square insets show high MAA concentrations during the last sampling dates under ice cover (29 May 2002 and 12 June 2002), which were not considered for the statistical analysis.
The mean values of Chl a-specific MAA concentrations were linearly correlated with the diffuse vertical attenuation coefficient Kd at 340 nm (r2 = 0.27, p = 0.017, n = 21; Fig. 6B). The other nominal UV wavelengths (except 305 nm) measured with the PUV radiometer led to similar conclusions. In general, the highest Chl a-normalized MAA concentrations were detected at the time of lowest UV attenuation in the water column.
Lake water temperature correlated significantly with the mean values of MAA concentrations in phytoplankton (r2 = 0.67, p < 0.0001, n = 25; Fig. 7A), whereas Chl a concentration gave a weak, not significantly different correlation (r2 = 0.16, p = 0.050, n = 25; Fig. 7B).
Fig. 7.
(A) Lake water temperature regressed against mean concentration of MAAs in phytoplankton and (B) Chl a concentration.
Relationship between concentrations of MAAs in phytoplankton and C. abyssorum tatricus
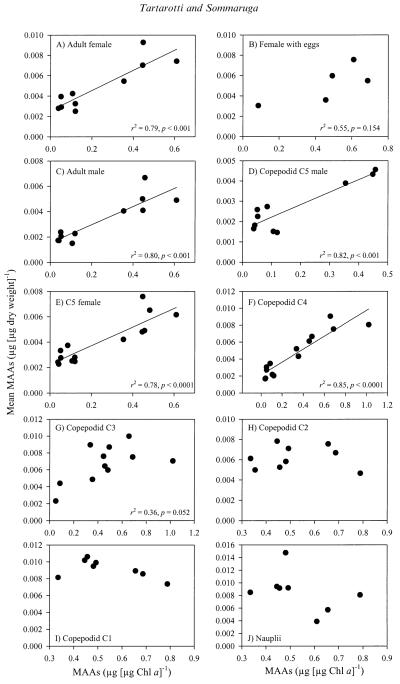
Taking into account a time period of ~3–4 weeks, to potentially cover the synthesis of MAAs and the subsequent accumulation of these compounds in the animals (i.e., phytoplankton MAA content of any given sampling date is related to copepod MAA content of ~3–4 weeks after), a positive linear relationship was found between mean values of phytoplankton MAA concentrations (chlorophyll specific) and the copepod life stages copepodid C4 to adult (Fig. 8A,C–F). In copepodid C3 stages, the highest MAA concentrations were found at the time of highest Chl a-specific MAA contents of phytoplankton; however, the relationship was not statistically significant (r2 = 0.36, p = 0.052; Fig. 8G). The MAA concentration of the life stages nauplii to C2, which occurred during the ice-free season, showed no clear relationship with the Chl a-specific MAA concentration of phytoplankton (Fig. 8H–J).
Fig. 8.
(A–J) Relationship between the Chl a-specific concentration of MAAs in phytoplankton and the life stages nauplii to adult of the copepod C. abyssorum tatricus. A time lag of ~3–4 weeks was used to potentially cover the synthesis of MAAs and the subsequent accumulation of these compounds in the animals (i.e., phytoplankton MAA content of any given sampling date is related to copepod MAA content of ~3–4 weeks after).
Discussion
Seasonal patterns and factors influencing temporal changes in MAA concentrations
Mycosporine-like amino acids were present throughout the year in phytoplankton assemblages and C. abyssorum tatricus of Gossenköllesee; however, concentrations followed strong seasonal changes (Figs. 1, 4). Contents of MAAs were low to almost undetectable during the ice-cover season, whereas they increased as the ice started to melt, and reached highest concentrations during the ice-free season (Figs. 1, 4, 5B). Annual patterns in MAAs with maximum concentrations during the ice-free period when incident levels of solar UVR are highest and minima in winter when levels are lowest are consistent with the idea that these photoprotective compounds are synthesized and accumulated in response to UV stress. Although, to our knowledge, there is no detailed information available on temporal changes of MAA concentrations for both freshwater phytoplankton and zooplankton, Laurion et al. (2002) pointed at higher MAA contents of natural phytoplankton assemblages from mountain lakes in midsummer (July) when compared with autumn (October). Furthermore, Moeller et al. (2005) reported low contents of MAAs in epilimnetic samples of the copepod Leptodiaptomus minutus during spring and a peak in mid- to late summer. Our results are also in agreement with seasonal patterns in MAA concentrations observed in phytoplankton from coastal waters (Whitehead and Vernet 2000) and surface waters of the English Channel (Llewellyn and Harbour 2003). Similar seasonal changes are also known for other photoprotective compounds. For example, a fourfold to sixfold difference between winter and summer carotenoid concentration has been observed in the freshwater copepod Diaptomus nevadensis (Hairston 1979). In subarctic Daphnia, melanin synthesis was extremely low during winter and peaked immediately after the ice break-up at the time of the maximum underwater UV intensity (Rautio and Korhala 2002).
Correlations between Chl a-specific MAA concentrations and integrated values of incident solar radiation (2-d-integrated solar radiation) and UV transparency (Kd) showed that both explained some (≤36%) of the variability found in the MAA contents throughout the year (Fig. 6). In general, MAA concentrations were greatest at the times of highest daily integral solar radiation values and lowest attenuation coefficients. The strongest relationship (r2 = 0.67) in phytoplankton, however, was found with the lake water temperature (Fig. 7A), suggesting that the synthesis of MAAs is triggered by increased sunlight intensities but also that temperature can control its rate. Rapid changes in weather conditions typically found at high altitudes during summer are a possible explanation for the weak relationship between MAA concentration and incident solar radiation. In fact, shortly before ice break-up but still under low lake water temperatures, high concentrations of MAAs in phytoplankton were found (Fig. 2E), suggesting that radiation conditions are more likely to affect MAA contents than increased water temperatures alone. The costs for the de novo synthesis of MAAs are considered high (Shick and Dunlap 2002); thus, the synthesis of increased MAA levels only at times of high UV stress would reduce the metabolic costs until an ecological benefit of the MAA production can be achieved (Michalek-Wagner 2001). Williamson et al. (2002) argued that, in zooplankton, UV tolerance via efficient photoenzymatic repair is temperature dependent, and consequently, seasonal variations in UV effects are a function of both water temperature and incident UVR, these effects being most pronounced at times when the UV : temperature ratio is highest (Williamson et al. 2002). In alpine lakes such as Gossenköllesee, where ice out is close to summer solstice, increases in surface water temperature lag incident UVR on a seasonal basis. Thus, the accumulation of high levels of MAAs in C. abyssorum tatricus at times when the UV : temperature ratio is highest (i.e., time at ice out; Fig. 4) and potential temperature-dependent DNA repair processes are limited could enhance its UV tolerance.
Distribution of MAAs in the water column
During summer, the highest Chl a-specific MAA concentrations were found at the water surface or subsurface and decreased with depth, indicating that MAAs were synthesized in response to high UV exposure (Figs. 1, 2A). The same trend was observed in several high mountain lakes sampled in midsummer (Laurion et al. 2002). Similar to the results reported in the study of Laurion et al. (2002), Chl a-specific MAA concentrations in Gossenköllesee showed higher levels at 4 m than at 1 m depth shortly after ice out (Fig. 2F). This maximum at greater depth shortly after ice out might be the result of a shift in species composition in favor of MAA-rich, UV-tolerant microalgae at that time of extreme fluctuations in physical conditions. In addition, dark-adapted, motile algae might have moved away from surface-water layers, avoiding high UV exposure. Decreasing Chl a-specific concentrations of MAAs with depth were found in several high mountain lakes even when the water column was well mixed (Laurion et al. 2002). In Gossenköllesee, however, concentrations of Chl a-specific MAAs were evenly distributed (except 17 October 2001) within the water column during the time of mixis (September through the end of November; Figs. 1, 2B). The long period of lake-water mixis goes along with decreasing levels of MAAs, which is coincident with the time of the year when lower daily incident radiation levels are found. During the ice-cover season, the concentration of MAAs was evenly distributed within the water column (Figs. 1, 2C). Some of the sampling dates in late winter and spring, however, show increased levels of Chl a-specific MAAs right beneath the ice cover (Figs. 1, 2D), suggesting that the microalgae present at that time of the year synthesize higher contents of MAAs as the light intensity is increasing with the thinning of the ice cover.
In summer (end of June to August), the MAA concentrations were similar to or exceeded the Chl a concentrations from the water surface to 4-m depth (Web Appendix 1). An increase in the ratio MAA : Chl a may result from the presence of more MAA-rich cells or from greater photoinduced MAA contents per cell. However, in natural phytoplankton assemblages, it is difficult to know whether the ability to produce significant MAA amounts is limited to certain species or taxa, as suggested by Hannach and Sigleo (1998). In Gossenköllesee, the phytoplankton species composition and dominance change abruptly after ice out (Nauwerck 1966), which may occur in favor of MAA-containing species. In fact, the summer phytoplankton assemblage in this lake consists of highly UV-resistant taxa (mainly dinoflagellates and chrysophytes) (Halac et al. 1997).
Ontogenic and sex-specific changes of MAAs in Cyclops
The total mean concentration of MAAs in copepod eggs was approximately twice the content measured in the adult females of C. abyssorum tatricus occurring during summer (Fig. 5B). We assume that high concentrations of these sunscreens are translocated from the adult female to this first life stage. The high contents in eggs are passed on to the developing embryo and the resulting orthonauplii (ON) (Fig. 4A). The ecological advantage of packing MAAs into eggs, and consequently into developing embryos and early life stages, is probably twofold: first, nauplii stages ON1 and ON2 do not feed and, thus, they cannot accumulate MAAs through their diet; and second, these early developmental stages are often exposed to higher UV doses than late copepodid and adult stages because they thrive close to the water surface during daylight hours, while the other life stages undergo diel vertical migration (Tartarotti et al. 1999). A similar pattern in MAA concentration has been found in marine organisms. For example, eggs had approximately three times the MAA content as unbleached maternal tissue of the coral Lobophytum compactum, supporting the importance of MAAs for larval survival (Michalek-Wagner and Willis 2001). In the green sea urchin Strongylocentrotus droebachiensis, MAA concentrations in eggs were more than two times higher than in ovary tissue (Adams and Shick 1996). The distribution of MAAs in the Antarctic limpet Nacella concinna showed that MAA contents were highest in ovary and eggs when compared with the gut, testis, or body tissues (Karentz et al. 1992). In addition, studies on the freshwater copepod Diaptomus nevadensis revealed that carotenoid concentrations in nauplii are between 3 and 10 times higher than in adults (Hairston 1978). Finally, a reduction in the concentration of MAAs during the ontogeny of copepods may be important if fish can see in the UV range. Browman et al. (1994) reported UV photoreception (in the long UV-A wavelength range) in zooplanktivorous fish species. Thus, it could be advantageous to not accumulate high levels of long wavelength-absorbing MAAs, such as palythene or usujirene. In fact, shorter wavelength-absorbing MAAs (e.g., shinorine, asterina, and palythine) dominated the qualitative MAA composition in C. abyssorum tatricus (Fig. 3).
High levels of MAAs in copepodid C1 life stages (Fig. 5) may result from both high sunscreen concentrations of the preceding nauplii life stage and the ability to bioaccumulate MAAs. These life stages typically occur only during summer, when MAA concentrations in phytoplankton are highest. The generally high MAA concentration in copepodid C1–C3 (Fig. 5) supports the notion that MAAs play an important photoprotective role in these early life stages, which are not able to perform strong daily vertical migrations as the late copepodid or adult stages do. Interestingly, a sex-specific variation in levels of MAAs in C. abyssorum tatricus was observed. Copepodid C5 and adult males had, on average, 32% and 40% lower MAA contents than C5 and adult females, respectively (Fig. 5A), suggesting that females need to accumulate higher MAA concentrations to translocate these compounds to the eggs. A higher concentration of MAAs in females than males has been also observed in soft corals (Michalek-Wagner 2001).
Considering a time lag of approximately 3–4 weeks between the synthesis by phytoplankton and subsequent accumulation of MAAs by C. abyssorum tatricus, we found that concentrations in copepodid C4 to adult life stages were significantly correlated to Chl a-specific levels of MAAs in phytoplankton (Fig. 8A,C–F). In contrast, nauplii and young copepodids (C1–C2) did not show a clear relationship with this parameter (Fig. 8H–J). Although C. abyssorum tatricus is considered omnivorous–carnivorous, our results suggest that these compounds are mainly derived from phytoplankton rather than from other dietary sources. The lack of a relationship between Chl a-specific MAA content and the early life stages may result from the combination of high MAA level originating from their mother together with the accumulation of new MAAs. As these life stages occur only during the ice-free season, there is less fluctuation in the generally high MAA concentrations in the phytoplankton during that time of the year. The MAA contents of C. abyssorum tatricus populations (copepodid to adult life stages) from high mountain lakes located in the Central Alps were not correlated with the concentrations of MAAs in phytoplankton when sampled simultaneously (Tartarotti et al. 2001). The findings of our present study confirm different dynamics in the accumulation of MAAs in zooplankton, as suggested by Tartarotti et al. (2001).
Our data indicate that the MAA composition of lake plankton is similar throughout periods with comparable UVR conditions. However, as water temperature and UVR decreased and the ice cover began to build up, the relative MAA composition changed and the diversity of MAAs decreased (Web Appendix 1; Fig. 3). The seasonal change in the relative proportion of the major MAA, shinorine, in phytoplankton was also observed in the different life stages of C. abyssorum tatricus (Web Appendix 1; Fig. 3A). Conversely, the relative proportion of asterina decreased in phytoplankton during the ice-cover season (Web Appendix 1), while its percentage increased in most copepod life stages present at that time of the year (Fig. 3B), responding largely to the relative decrease in shinorine. In phytoplankton, the contribution of palythine increased during the ice-cover months (except for the surface) (Web Appendix 1), whereas the fraction of this sunscreen fluctuated among the different life stages of C. abyssorum tatricus (Fig. 3C). Proportions of the dominant compounds either increased (Fig. 3A) or decreased (Fig. 3B,C) continuously from eggs to copepodid C4 life stages, while in late life stages, the relative contribution was more variable (Fig. 3). However, there was a tendency of higher/lower proportions in adult females, which was subsequently reflected in the eggs (Fig. 3), supporting the idea that the MAAs are derived from the mother. It remains unclear whether discrepancies between the relative concentrations are an indication of differences in dietary availability or if potential changes in the gut of the copepods (based on enzymatic and/or microbial processes) may play a role for seasonal changes in the relative composition of these compounds. Another possible explanation is that some MAAs (such as shinorine) are better translocated than others, as suggested by Moeller et al. (2005). The same authors report that the suite of MAAs taken up by the copepod L. minutus feeding on an MAA-rich dinoflagellate was similar to that in the microalga, although the proportions differed; the short–wavelength-absorbing MAAs (e.g., shinorine, palythine) were disproportionately accumulated over long–wavelength-absorbing MAAs (e.g., usujirene and palythene).
Conclusion
The seasonal variability in levels of MAAs shows that the ability to produce and accumulate higher levels of these sunscreens in response to periods of elevated UVR and temperature is present throughout the year in phytoplankton and zooplankton. It remains unclear whether the high MAA concentrations in C. abyssorum tatricus during summer reflect the availability of these compounds or if they are a true stress response to changes in physical conditions. In a study with cultured copepods, both factors, i.e., MAA concentration in the diet and the presence of UVR, were shown to be important for an efficient MAA accumulation (Moeller et al. 2005). A relatively long half-life (23 d in L. minutus; Moeller et al. 2005; several weeks in C. abyssorum tatricus, unpubl. data) for MAA retention, however, is potentially slow enough to sustain photoprotection through intervals of physiological stress and changes in dietary availability of MAAs, which may be particularly important in harsh environments, such as high mountain lakes.
Supplementary Material
Acknowledgments
We thank M. Orfeo, J. Hofer, A. Alfreider, H. Obwaller, C. Gritsch, S. Waldhuber, B. Sattler, and L. Baumgartner for their help during fieldwork. J. Franzoi assisted with chlorophyll analyses. T. Weintraut (TIWAG) provided the global radiation data, and U. Karsten and M. Shick donated biological materials used for MAA comparisons. Three reviewers provided helpful comments. This research was supported by the Austrian Science Fund (FWF), grant P14153 to R.S.
References
- Adams NL, Shick JM. Mycosporine-like amino acids provide protection against ultraviolet radiation in eggs of the green sea urchin Strongylocentrotus droebachiensis. Photochem. Photobiol. 1996;64:149–158. [Google Scholar]
- Alonso C, Rocco V, Barriga JP, Battini MA, Zagarese H. Surface avoidance by freshwater zooplankton: Field evidence on the role of ultraviolet radiation. Limnol. Oceanogr. 2004;49:225–232. [Google Scholar]
- Belzile C, Gibson JAE, Vincent WF. Colored dissolved organic matter and dissolved organic carbon exclusion from lake ice: Implications for irradiance transmission and carbon cycling. Limnol. Oceanogr. 2002;47:1283–1293. [Google Scholar]
- Browman HI, Novales-Flamarique I, Hawryshyn CW. Ultraviolet photoreception contributes to prey search behaviour in two species of zooplanktivorous fishes. J. Exp. Biol. 1994;186:187–198. doi: 10.1242/jeb.186.1.187. [DOI] [PubMed] [Google Scholar]
- Cabrera S, Lopéz M, Tartarotti B. Phytoplankton and zooplankton response to ultraviolet radiation in a high-altitude Andean lake: Short- versus long-term effects. J. Plankton Res. 1997;19:1565–1582. [Google Scholar]
- Carroll AK, Shick JM. Dietary accumulation of UV-absorbing mycosporine-like amino acids (MAAs) by the green sea urchin (Stongylocentrotus droebachiensis) Mar. Biol. 1996;124:561–569. [Google Scholar]
- Drollet JH, Teai T, Faucon M, Martin PMV. Field study of compensatory changes in UV-absorbing compounds in the mucus of the solitary coral Fungia repanda (Scleractinia: Fungiidae) in relation to solar UV radiation, sea-water temperature, and other coincident physico-chemical parameters. Mar. Freshw. Res. 1997;48:329–333. [Google Scholar]
- Dunlap WC, Williams D. McB., Chalker BE, Banaszak AT. Biochemical photoadaptations in vision: UV-absorbing pigments in fish eye tissues. Comp. Biochem. Physiol. 1989;93:601–607. [Google Scholar]
- Gonçalves RJ, Villafañe VE, Helbling EW. Photorepair activity and protective compounds in two freshwater zooplankton species (Daphnia menucoensis and Metacyclops mendocinus) from Patagonia, Argentina. Photochem. Photobiol. Sci. 2002;1:996–1000. doi: 10.1039/b208145h. [DOI] [PubMed] [Google Scholar]
- Grad G, Williamson CE, Karapelou DM. Zooplankton survival and reproduction responses to damaging UV radiation: A test of reciprocity and photoenzymatic repair. Limnol. Oceanogr. 2001;46:584–591. [Google Scholar]
- Hairston NG. Photoprotection by carotenoid pigments in the copepod Diaptomus nevadensis. Proc. Nat. Acad. Sci. USA. 1976;73:971–974. doi: 10.1073/pnas.73.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hairston NG. Carotenoid photoprotection in Diaptomus kenai. Verh. Internat. Verein. Limnol. 1978;20:2541–2545. [Google Scholar]
- Hairston NG. The effect of temperature on carotenoid photoprotection in the copepod Diaptomus nevadensis. Comp. Biochem. Physiol. 1979;62:445–448. [Google Scholar]
- Halac S, Felip M, Camarero L, Sommaruga-Wögrath S, Psenner R, Catalan J, Sommaruga R. An in situ enclosure experiment to test the solar UV-B impact on microplankton in a high altitude mountain lake: I) Lack of effect on phytoplankton species composition and growth. J. Plankton Res. 1997;11:1671–1687. [Google Scholar]
- Hannach G, Sigleo AC. Photoinduction of UV-absorbing compounds in six species of marine phytoplankton. Mar. Ecol. Prog. Ser. 1998;174:207–222. [Google Scholar]
- Hessen DO, Sørensen K. Photoprotective pigmentation in alpine zooplankton populations. Aqua Fennica. 1990;20:165–170. [Google Scholar]
- Karentz D. Chemical defenses of marine organisms against solar radiation exposure: UV-absorbing mycosporine-like amino acids and scytonemin. In: McClintock JB, Baker BJ, editors. Marine chemical ecology. CRC Press; 2001. pp. 481–519. [Google Scholar]
- Karentz D, Bosch I, Dunlap WC. Distribution of UV-absorbing compounds in the Antarctic limpet, Nacella concinna. Antarct. J. U.S. 1992;27:121–122. [Google Scholar]
- Karentz D, Dunlap WC, Bosch I. Temporal and spatial occurrence of UV-absorbing mycosporine-like amino acids in tissues of the Antarctic sea urchin Sterechinus neumayeri during springtime ozone-depletion. Mar. Biol. 1997;129:343–353. [Google Scholar]
- Karsten U, Bischof K, Hanelt D. The effect of ultraviolet radiation on photosynthesis and ultraviolet-absorbing substances in the endemic Arctic macroalga Devaleraea ramentacea (Rhodophyta) Physiol. Plantarum. 1999;105:58–66. [Google Scholar]
- Laurion I, Lami A, Sommaruga R. Distribution of mycosporine-like amino acids and photoprotective carotenoids among freshwater phytoplankton assemblages. Aquat. Microb. Ecol. 2002;26:283–294. [Google Scholar]
- Leech DM, Williamson CE. In situ exposure to ultraviolet radiation alters the depth distribution of Daphnia. Limnol. Oceanogr. 2001;46:416–420. [Google Scholar]
- Llewellyn CA, Harbour DS. A temporal study of mycosporine-like amino acids in surface water phytoplankton from the English Channel and correlation with solar irradiation. J. Mar. Biol. Assoc. UK. 2003;83:1–9. [Google Scholar]
- Lorenzen CJ. Determination of chlorophyll and pheopigments: Spectrophotometric equations. Limnol. Oceanogr. 1967;12:343–346. [Google Scholar]
- Michalek-Wagner K. Seasonal and sex-specific variations in levels of photo-protecting mycosporine-like amino acids (MAAs) in soft corals. Mar. Biol. 2001;139:651–660. [Google Scholar]
- Michalek-Wagner K, Willis BL. Impacts of bleaching on the soft coral Lobophytum compactum II. Biochemical changes in adults and their gametes. Coral Reefs. 2001;8:225–232. [Google Scholar]
- Moeller RE, Shawna G, Williamson CE, Dee G, Sommaruga R. Dietary acquisition of photoprotective compounds (mycosporine-like amino acids, carotenoids) and acclimation to ultraviolet radiation in a freshwater copepod. Limnol. Oceanogr. 2005;50:427–439. [Google Scholar]
- Nauwerck A. Beobachtungen über das Phytoplankton klarer Hochgebirgsseen. Schweiz Z. Hydrol. 1966;28:4–28. [Google Scholar]
- Neale PJ, Banaszak AT, Jarriel CR. Ultraviolet sunscreens in Gymnodinium sanguineum (Dinophyceae): Mycosporine-like amino acids protect against inhibition of photosynthesis. J. Phycol. 1998;34:928–938. [Google Scholar]
- Newman SJ, Dunlap WC, Nicol S, Ritz D. Antarctic krill (Euphasia superba) acquire a UV-absorbing mycosporine-like amino acid from dietary algae. J. Exp. Mar. Biol. Ecol. 2000;255:93–110. doi: 10.1016/s0022-0981(00)00293-8. [DOI] [PubMed] [Google Scholar]
- Rautio M, Korhala A. UV-induced pigmentation in subarctic Daphnia. Limnol. Oceanogr. 2002;47:295–299. [Google Scholar]
- Rocco VE, Oppezzo O, Pizarro R, Sommaruga R, Ferraro M, Zagarese HE. Ultraviolet damage and counteracting mechanisms in the freshwater copepod Boeckella poppei from the Antarctic Peninsula. Limnol. Oceanogr. 2002;47:829–836. [Google Scholar]
- Shick JM, Dunlap WC. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- Sommaruga R. The role of solar UV radiation in the ecology of alpine lakes. J. Photochem. Biol. B: Biol. 2001;62:35–42. doi: 10.1016/s1011-1344(01)00154-3. [DOI] [PubMed] [Google Scholar]
- Sommaruga R, Garcia-Pichel F. UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake. Arch. Hydrobiol. 1999;144:255–269. [Google Scholar]
- Tartarotti B, Baffico G, Temporetti P, Zagarese HE. Mycosporine-like amino acids in planktonic organisms living under different UV exposure conditions in Patagonian lakes. J. Plankton Res. 2004;26:753–762. doi: 10.1093/plankt/fbh073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartarotti B, Cabrera S, Psenner R, Sommaruga R. Survivorship of Cyclops abyssorum tatricus (Cyclopoida, Copepoda) and Boeckella gracilipes (Calanoida, Copepoda) under ambient levels of solar UV radiation in two high mountain lakes. J. Plankton Res. 1999;21:549–560. [Google Scholar]
- Tartarotti B, Laurion I, Sommaruga R. Large variability in the concentration of mycosporine-like amino acids among zooplankton from lakes located across an altitude gradient. Limnol. Oceanogr. 2001;46:1546–1552. [Google Scholar]
- Tartarotti B, Sommaruga R. The effect of different methanol concentrations and temperatures on the extraction of mycosporine-like amino acids (MAAs) in algae and zooplankton. Arch. Hydrobiol. 2002;154:691–703. [Google Scholar]
- Villafañe VE, Andrade M, Lairana V, Zaratti F, Helbling EW. Inhibition of phytoplankton photosynthesis by solar ultraviolet radiation: Studies of Lake Titicaca, Bolivia. Freshw. Biol. 1999;42:215–224. [Google Scholar]
- Whitehead K, Vernet M. Influence of mycosporine-like amino acids (MAAs) on UV absorption by particulate and dissolved organic matter in La Jolla Bay. Limnol. Oceanogr. 2000;45:1788–1796. [Google Scholar]
- Williamson CE, Zagarese HE, Schulze PC, Hargreaves B, Seva J. The impact of short-term exposure to UV-B radiation on zooplankton communities in north temperate lakes. J. Plankton Res. 1994;16:205–218. [Google Scholar]
- Williamson CE, Grad G, De Lange HJ, Gilroy S, Karapelou DM. Temperature-dependent ultraviolet responses in zooplankton: Implications of climate change. Limnol. Oceanogr. 2002;47:1844–1848. [Google Scholar]
- Zagarese HE, Feldman M, Williamson CE. UV–B-induced damage and photoreactivation in three species of Boeckella (Copepoda, Calanoida) J. Plankton Res. 1997;19:357–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.