Mammalian oocytes and zygotes are overtly and deceptively simple cells poised to elaborate a foundation to support the development of highly complex multi-cellular embryos. Yet these cells have turned out to be extraordinary resilient receptacles which provide a conduit for designs etched in laboratory notebooks to living animals and beyond.
The generation of the first transgenic mammals was achieved by injection of naked DNA into pro-nuclei of mouse zygotes three decades ago. This success provided the first indication that these cells were endowed with the machinery to support experimental genetic manipulation of their genomes. This remarkable feature was soon recognized to be a general property of other mammalian zygotes with the generation of transgenic rats, rabbits and farm animal species.
The technical simplicity of transgenic technology has led to its widespread application in pursuit of scientific questions as well as industrial applications such as protein production. However, the technology has, until now, only supported the insertion of exogenous DNA at random sites in the genome. It has proven to be extremely difficult to manipulate the genome in a site-specific manner by this route. Cui and colleagues, writing on page xxx of this issue, have finally overcome this barrier, making use of zinc finger nucleases (ZFNs) to stimulate targeted integration of transgenes by homologous recombination1. This technology will dramatically alter the speed and repertoire of genetic alterations that can be generated in a variety of mammalian species.
Evidence that mouse pro-nuclei are endowed with the machinery to support homologous recombination emerged more than 20 years ago, but the efficiencies were too low to be practically useful2. Instead, the technology to manipulate the mouse genome has relied on embryonic stem (ES) cells, which have been so extraordinarily receptive to homologous recombination. Up to now, mouse ES cells with targeted mutations are available for more than 12,000 genes 3, thus they have become the genetic repository for the mouse. Unfortunately, the link between cultured ES cells and production of mutant animals could not be readily established in other species. ES cell lines isolated from species other than the mouse, rarely exhibit germ line colonization, although recent success has been reported in the rat4, 5.
The lack of authentic ES cells held back attempts to manipulate endogenous genes in most mammalian species for many years. This barrier was eventually overcome by utilizing a remarkable feature of the mammalian oocyte to re-programme a somatic cell nucleus, effectively converting it to a zygotic genome6. By performing gene targeting in cultured somatic cells and then using these for nuclear transfer, it became possible to manipulate endogenous genes in several mammalian species7. Despite these successes, the technical difficulties have been substantial because of the low efficiencies of both gene targeting in somatic cells and the subsequent re-programming of their nuclei.
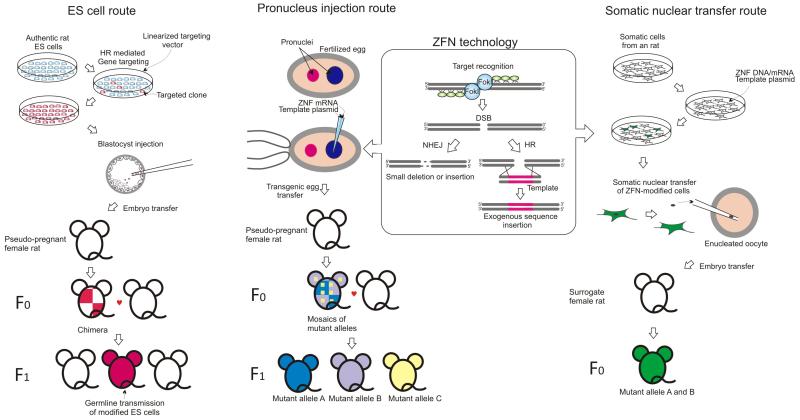
The research reported by Cui et al. provides for the first time a route to directly manipulate the rat genome, an approach which bypasses the requirement for germ-line competent ES cells or somatic nuclear transfer. This approach also offers an avenue towards targeted modification of other mammalian genomes. The major difficulty in achieving gene targeting with naked DNA injected into pro-nuclei is the very low efficiency of targeted rather than random integration2. Gene targeting is stimulated by several orders of magnitude in somatic cells by provision of a double strand break in the host genome8. Cleaving the mammalian genome at any defined site was not possible until ZFNs were developed. ZFNs are modular proteins which couple sequence-specific binding of zinc finger DNA binding domains to the nuclease domain of the restriction endonuclease FokI9. ZFNs function as homo- or hetero-dimers, cleaving DNA between two sequences recognized by the zinc-finger arrays (Figure, ZFN technology). The large number of different zinc fingers, their diversity of recognition sequences and their modularity will in principle enable them to be developed to target any DNA sequence10.
Figure.
Following the initial demonstration of sequence-specific cleavage of the Drosophila genome11, ZFNs have been shown to stimulate targeted integration of a template sequence via homologous recombination in fruit flies, plants and human cells10. Cui et al. in this issue1 and Meyer et al. in a recent report12, both demonstrate that co-injection of a pair of ZFN mRNAs with a targeting vector into pro-nuclei stimulates the frequency of gene targeting by workable levels, 2-20% (Figure, pronucleus injection route). Interestingly, live-born founder animals obtained from these experiments are mosaics which carry several different mutant alleles with deletions at the target locus, as well as correctly targeted alleles and unmodified wild-type alleles. Deletions are expected products following non-homologous end-joining (NHEJ) of cleaved DNA in the absence of targeting and have been described previously following expression of ZFNs in zygotes13. The transmission of multiple different mutant alleles from the same founder reflects germline mosacism caused by expression and cleavage activity of ZFNs after DNA replication in maternal and/or paternal pro-nuclei. The ability to simultaneously generate a spectrum of mutations can be advantageous for genetic purposes.
Despite these advances, several questions remain to be addressed about ZFN stimulated pronuclear targeting. Molecular biologists are familiar with unwanted off-target activity of restrictions enzymes. To what extent do ZFNs cleave other sites in the genome? The comparatively small size of deletions generated at illegitimate sites suggests that off-target cleavages will be hard to trace. Does the physical damage of the host genome observed in many transgenic animals generated by pronuclear injection occur in this setting too? The importance of the answers to these questions will undoubtedly depend on the frequency and type of unwanted events, their linkages to the desired genomic alterations and the context in which the technology is applied.
The mutant alleles generated using conventional gene targeting technology in mouse ES cells have steadily embodied greater degrees of sophistication over the last two decades. The repertoire of genetic alterations that can be achieved by ZFN-stimulated pronuclear targeting is fertile ground for exploration. Although mutant rats have recently been established using rat ES cell technology14 (Figure, ES cell route), ZFN targeting applied directly to the zygote (Figure, pronucleus injection route) presents considerable advantages. Pro-nuclear injection of nucleic acids is well established, widely practiced and applicable to any strain. Moreover transmission of the engineered allele from founder rats is readily achieved. Provided ZFNs with the appropriate specificity can be generated, the community of rat researchers can look forward to rats with a myriad of defined genome modifications.
This technology will also find applications in a multitude of other species, which hitherto have required somatic cell re-programming to achieve directed modifications of their genomes. While vector-chromosome gene targeting has yet to be demonstrated in the pro-nuclei of farm animal zygotes, this is likely to be possible. ZFNs have also be shown to stimulate gene targeting in a variety of species, thus this technology can also be used in combination with somatic nuclear transfer, removing a bottle neck in achieving directed modification by this route (Figure, somatic nuclear transfer route). While the promise of this technology will stimulate numerous applications, the terms, conditions and costs associated with commercially provided ZFNs can be prohibitive and may limit their potential.
References
- 1.Cui X, et al. Targeted Integration in Rat and Mouse Embryos with Zinc-finger Nucleases. Nature Biotechnology. 2010 doi: 10.1038/nbt.1731. [DOI] [PubMed] [Google Scholar]
- 2.Brinster RL, et al. Targeted correction of a major histocompatibility class II E alpha gene by DNA microinjected into mouse eggs. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:7087–7091. doi: 10.1073/pnas.86.18.7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. http://www.knockoutmouse.org/
- 4.Buehr M, et al. Capture of Authentic Embryonic Stem Cells from Rat Blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Li P, et al. Germline Competent Embryonic Stem Cells Derived from Rat Blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KHS. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 7.McCreath KJ, et al. Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature. 2000;405:1066–1069. doi: 10.1038/35016604. [DOI] [PubMed] [Google Scholar]
- 8.Jasin M. Genetic manipulation of genomes with rare-cutting endonucleases. Trends in Genetics. 1996;12:224–228. doi: 10.1016/0168-9525(96)10019-6. [DOI] [PubMed] [Google Scholar]
- 9.Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:1156–1160. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 11.Bibikova M, Golic M, Golic KG, Carroll D. Targeted Chromosomal Cleavage and Mutagenesis in Drosophila Using Zinc-Finger Nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer M, de Angelis MH, Wurst W, Kuhn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proceedings of the National Academy of Sciences. 2010;107:15022–15026. doi: 10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geurts AM, et al. Knockout Rats via Embryo Microinjection of Zinc-Finger Nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong C, Li P, Wu NL, Yan Y, Ying Q-L. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]