Abstract
CD8+ cytotoxic T lymphocytes (CTL) are essential for effective immune defence against intracellular microbes and neoplasia. CTL recognize short peptide fragments presented in association with major histocompatibility complex class I (MHCI) molecules on the surface of infected or dysregulated cells. Antigen recognition involves the binding of both T cell receptor (TCR) and CD8 co-receptor to a single ligand (pMHCI). The TCR/pMHCI interaction confers antigen specificity, whereas the pMHCI/CD8 interaction mediates enhanced sensitivity to antigen. Striking biophysical differences exist between the TCR/pMHCI and pMHCI/CD8 interactions; indeed, the pMHCI/CD8 interaction can be >100-fold weaker than the cognate TCR/pMHCI interaction. Here, we show that increasing the strength of the pMHCI/CD8 interaction by ~15-fold results in non-specific, cognate antigen-independent pMHCI tetramer binding at the cell surface. Furthermore, pMHCI molecules with super-enhanced affinity for CD8 activate CTL in the absence of a specific TCR/pMHCI interaction to elicit a full range of effector functions, including cytokine/chemokine release, degranulation and proliferation. Thus, the low solution binding affinity of the pMHCI/CD8 interaction is essential for the maintenance of CTL antigen specificity.
INTRODUCTION
CD8+ cytotoxic T lymphocytes (CTLs) recognize antigenic determinants in the form of short peptides derived from endogenous proteins bound to major histocompatibility complex class I (MHCI) molecules on the surface of target cells and play a critical role in immune defence against intracellular pathogens and tumours. Antigen specificity is conferred by the T cell receptor (TCR), which interacts with the peptide-binding platform formed by the α1 and α2 domains of MHCI (1, 2). In contrast, the surface glycoprotein CD8 binds to invariant regions of MHCI and is capable of enhancing cellular sensitivity to antigen by up to six orders of magnitude (3, 4). CD8 mediates this profound enhancement of antigen sensitivity through a number of distinct mechanisms: (i) enhancement of the TCR/peptide-MHCI (pMHCI) association rate (5-7); (ii) stabilization of the TCR/pMHCI interaction (8, 9); (iii) recruitment of essential kinases to the intracellular side of the TCR/CD3/ζ complex (10, 11); and, (iv) localization of TCR/pMHCI complexes within specialized membrane micro-domains that are enriched for early intracellular signal transduction molecules and are thought to act as privileged sites for TCR-mediated cascade initiation (12, 13).
The MHCI binding site for CD8 is separate from the peptide-binding domains that are recognized by the TCR (2) and this spatial segregation allows both TCR and CD8 to bind a single MHCI molecule simultaneously (14). Thus, CTL recognition of antigen involves the binding of two receptors (TCR and CD8) to a single ligand (pMHCI), a modus operandi that is unique to αβ T cell biology. The pMHCI/CD8 interaction is characterized by very low solution affinities (KD ~150 μM) and rapid kinetics (Koff ~18 s−1) (15, 16). Indeed, the affinity of the pMHCI/CD8 interaction is even lower than the corresponding values measured for conventional molecular binding events involved in cell-cell recognition, such as the CD2/CD48 interaction (KD = 60-90 μM) (15, 17). In stark contrast, the TCR/pMHCI interaction can be more than 100-fold stronger than the pMHCI/CD8 interaction (KD range for agonists from 0.14 μM, the strongest natural TCR/pMHCI interaction measured to date) and exhibits considerably slower kinetics (Koff range for agonists from 0.01 to 1 s−1) (1, 6, 18-20). It seems extremely unlikely that the striking biophysical characteristics of the pMHCI/CD8 interaction have occurred by accident. Indeed, this conclusion is strengthened by the finding that the pMHCI/CD8 interaction is capable of exerting the vast majority of its biological function when weakened even further (21), which suggests that CD8 has specifically evolved to operate at very low solution affinities.
Here, we probe the functional significance of the low solution affinity pMHCI/CD8 interaction using pMHCI molecules with super-enhanced CD8 binding properties. Notably, we find that pMHCI molecules with affinities for CD8 that lie within the typical range for agonist TCR/pMHCI interactions (KD ~10 μM) are able to activate CTL in the absence of a specific TCR/pMHCI interaction. Thus, the biophysical characteristics of the pMHCI/CD8 interaction are essential for the maintenance of CTL antigen specificity.
MATERIALS AND METHODS
Cells
The CTL clones 003 and NT1, and the CTL line 868, are all specific for the HIV-1 p17 Gag-derived epitope SLYNTVATL (residues 77-85) restricted by HLA A*0201 (A2 from heron) (22, 23). The following A2-restricted CTL clones were also used in this study: (i) Mel13 and Melc5, specific for the Melan-A-derived epitope ELAGIGILTV (residues 26-35); and, (ii) ILA1, specific for the human telomerase reverse transcriptase (hTERT)-derived epitope ILAKFLHWL (residues 540-548) (6, 24). In addition, the following non-A2-restricted CTL clones were used: (i) the HLA A*6801-restricted CTL clone c23, specific for the HIV-1 Tat-derived epitope ITKGLGISYGR (residues 38-48) (25); (ii) the HLA B*0702-restricted CTL clone KD4, specific for the Epstein-Barr virus (EBV) EBNA3A-derived epitope RPPIFIRRL (residues 379-387); (iii) the HLA B*0801-restricted CTL clone LC13, specific for the EBV EBNA3A-derived epitope FLRGRAYGL (residues 339-347) (26, 27); and, (iv) the HLA B*3508-restricted CTL clone SB27, specific for the EBV BZLF1-derived epitope LPEPLPQGQLTAY (residues 52-64) (28, 29). All CTL were maintained in RPMI 1640 (Gibco) containing 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco), 2mM L-glutamine (Gibco) and 10% heat inactivated fetal calf serum (FCS; Gibco) (R10) supplemented with 2.5% Cellkines (Helvetica Healthcare), 200 IU/ml IL-2 (Peprotech) and 25 ng/ml IL-15 (Peprotech). Peripheral blood mononuclear cells (PBMC) were isolated by standard Ficoll-Hypaque density gradient centrifugation from healthy donor blood. 293T-CD8α cells were manufactured by introducing pBullet-human CD8α (30, 31) into 293T cells using VSV-pseudotyped MuMLV particles. 293T-CD8α cells were cultured in DMEM (Gibco) supplemented with 20% FCS (Gibco), 1mM sodium pyruvate (Gibco), 100 U/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco) and 2mM L-glutamine (Gibco). Hmy.2 C1R B (C1R) cells expressing full-length A2 and variants thereof were generated as described previously (21).
pMHCI tetramers
Tetrameric complexes of wildtype pMHCI molecules and mutants thereof were produced, stored and used as described previously (9, 21). The following A2-restricted peptide epitopes were used to refold the pMHCI molecules used in this study: SLYNTVATL (HIV-1 p17 Gag, residues 77-85), LLFGYPVYV (HTLV-1 Tax, residues 11-19), GLCTLVAML (EBV BMLF1, residues 259-267), NLVPMVATV (CMV pp65, residues 495-503), ELAGIGILTV (Melan-A, residues 26-35) and ILAKFLHWL (hTERT, residues 540-548). Tetrameric or multimeric pMHCI reagents were constructed by the addition of streptavidin conjugated to phycoerythrin (PE), quantum dot (QD) 605 or QD800 (Invitrogen) at the appropriate molar ratios.
Antibodies
The following monoclonal antibodies (mAbs) were used in this study: purified anti-human CD8 (clone DK-25; DAKO), allophycocyanin (APC)-conjugated anti-human CD8 (clone RPA-T8; BD BioSciences), fluorescein isothiocyanate (FITC)-conjugated anti-human CD8 (clone SK1; BD BioSciences), peridinin chlorophyll protein (PerCP)-conjugated anti-human CD8 (clone SK1; BD BioSciences), PE-conjugated anti-human CD8β (clone 2ST8.5H7; Beckman Coulter), PerCP-conjugated anti-human CD3 (clone SK7; BD BioSciences), FITC-conjugated anti-human αβ-TCR (clone BMA 031; Serotec), FITC-conjugated or PE-conjugated anti-human γδ-TCR (clone YB5.B8; PharMingen), APC-conjugated anti-human CD56 (clone AF12-7H3; Miltenyi), FITC-conjugated anti-human CD56 (clone MEM188; Caltag), FITC-conjugated anti-A2 (clone BB7.2; Serotec) and FITC-conjugated anti-human CD107a (clone H4A3; BD BioSciences). Unless specified, the anti-human CD8 mAbs used in this study target the α chain of the coreceptor dimer. Dead cells were excluded from flow cytometric analyses with 7-amino-actinomycin D (7-AAD; BD BioSciences).
Flow cytometry
For pMHCI tetramer staining: 2.5×106 PBMC, 5×104 CTL or 2×105 293T cells (untransfected or CD8α-transfected) were resuspended in PBS or FACS buffer (2% FCS/PBS) and stained with pMHCI tetramer at the concentrations indicated for 20-30 minutes at 37°C. Cells were subsequently stained with combinations of the mAbs described above for 30 minutes on ice. Prior to staining, 293T cells were treated with Versene (Gibco) for 10 minutes at 37°C. For anti-CD8 mAb blocking experiments: 2.5×106 PBMC were pre-treated with 10 μg/ml unconjugated anti-CD8 mAb (clone DK-25; DAKO) for 20 minutes on ice prior to staining with 10 μg/ml pMHCI tetramer for 45 minutes on ice. For A2 typing: 2.5×106 PBMC were stained with 5 μl FITC-conjugated anti-A2 mAb (clone BB7.2; Serotec) for 30 minutes on ice. Samples were then washed twice and resuspended in PBS. Data were acquired using a FACSCalibur or FACSAria™ II flow cytometer (BD) and analyzed with either CellQuest (BD BioSciences) or FlowJo (Tree Star Inc.) software.
TCR down-regulation assay
105 003 CTL per well were resuspended in a 96-well round-bottomed plate with various concentrations of the indicated PE-conjugated tetramers (A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 LLFGYPVYV or A2/Kb LLFGYPVYV) diluted in 40 μl RPMI 1640 containing 2% FCS plus penicillin, streptomycin and glutamine as above (R2) for 30 minutes at 37°C. Cells were then washed, resuspended in ice cold azide buffer (0.1% azide/2% FCS/PBS) and subsequently stained with FITC-conjugated anti-αβ-TCR (clone BMA 031; Serotec), 7-AAD (BD BioSciences) and APC-conjugated anti-CD8 (clone RPA-T8; BD BioSciences) for 30 minutes on ice. After two further washes, cells were resuspended in ice cold azide buffer. Data were acquired using a FACSCalibur flow cytometer and analyzed with CellQuest software (BD BioSciences).
Cytokine/chemokine assays: ELISA, cytometric bead array and ELISpot
CTL were incubated with C1R A2 cells, C1R A2/Kb cells or medium alone at different effector:target (E:T) ratios overnight at 37°C. Subsequent to incubation, the supernatant was harvested and assayed for MIP1β, IFNγ or RANTES by ELISA (R&D). Remaining supernatant was assayed with the human TH1/TH2 cytokine kit (BD BioSciences) according to the manufacturer’s instructions; data were acquired using a FACSCalibur flow cytometer and analyzed with CBA software (BD BioSciences). For tetramer-based ELISpot assays, 2×103 CTL +/− pMHCI tetramer at 1 μg/ml were applied to duplicate wells of PVDF-backed plates (Millipore) pre-coated with IFNγ capture antibody 1-DIK (Mabtech) in a total volume of 200 μl R2 and incubated for 4 hours at 37°C. To exclude activation by cognate peptide representation or fluorochrome-mediated aggregation, cognate A2 D227K/T228A tetramers were included as controls; these tetramers do not bind CD8 and did not activate 003 or 868 CTL, despite efficient staining in both cases (data not shown). Plates were developed according to the manufacturer’s instructions (Mabtech) and spots were counted using an automated ELISpot Reader System ELR02 (Autoimmun Diagnostika).
Degranulation assay
Surface CD107a mobilization was used to assess degranulation as described previously (32). Briefly, CTL were incubated for 4 hours at 37°C with either C1R A2 cells, C1R A2/Kb cells or medium alone at different E:T ratios; alternatively, CTL were incubated with various pMHCI tetramers. Both FITC-conjugated anti-CD107a (clone H4A3; BD BioSciences) and 0.7 μl/ml monensin (GolgiStop; BD BioSciences) were added prior to incubation. Subsequent to incubation, the cells were washed twice and resuspended in PBS. Data were acquired using a FACSCalibur flow cytometer and analyzed with FlowJo software (Tree Star Inc.).
CTL priming assay
Transfected C1R cells were pulsed with 1 μM ELAGIGILTV (Melan-A26-35) peptide for 90 minutes, irradiated and washed once in RPMI 1640 medium. Pulsed, irradiated C1R cells (2×105) were incubated with 106 fresh A2+ human PBMC in R10; 200 IU/ml interleukin (IL)-2 was added on day 3. CD8+ cells specific for Melan-A26-35 were quantified on day 10 with wildtype A2 ELAGIGILTV tetramer.
RESULTS
Generation of MHCI molecules with super-enhanced CD8 binding affinity
Tetrameric fusion molecules comprising the α1/α2 peptide binding platform of A2 and the α3 domain of H2-Kb (A2/Kb from hereon) enable the monitoring of CD8+ T cell responses in A2 transgenic mice (33). This reflects a requirement for the murine MHCI α3 domain to engage murine CD8 (11), thus enabling A2/Kb reagents to stain murine CTL with lower affinity TCR/pMHCI interactions (so-called ‘low avidity’ CTL) (22). The A2/Kb heavy chain folded with human β2m interacts strongly with human CD8 (KD ~10 μM, compared to A2 which binds to CD8 with a KD ~ 150 μM) but exhibits unaltered A2-restricted TCR binding properties (9, 22). Thus, fusing the murine α3 domain with A2 α1/α2 domains increases the strength of the pMHCI/CD8 interaction by approximately 15-fold without affecting the TCR/pMHCI interaction.
Super-enhanced CD8 binding results in non-specific pMHCI ligand interactions
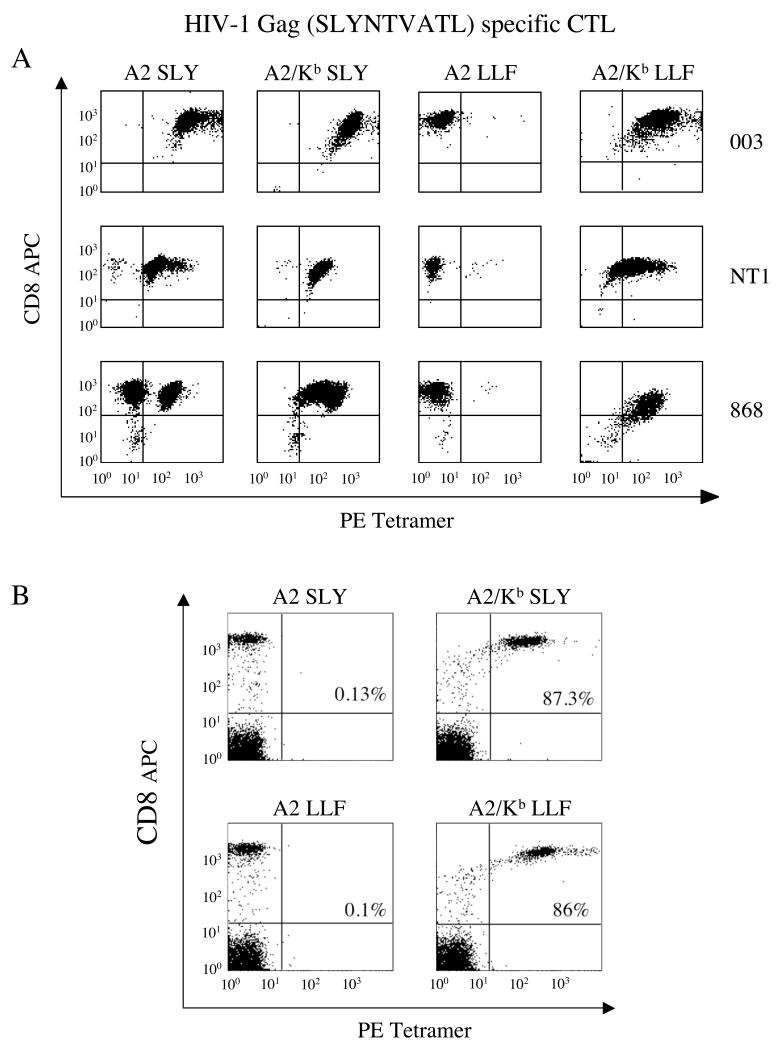
Monomeric pMHCI complexes cannot be used to examine TCR/pMHCI binding at the cell surface due to the extremely short half-life of such interactions. Increasing the valency of these molecules by avidin/biotin-based tetramerization overcomes this limitation and produces reagents that are invaluable for the identification and characterization of antigen-specific CTL (34, 35). Indeed, it is well established that wildtype tetrameric pMHCI reagents bind to cell surface TCR with exquisite specificity (34, 36). Thus, A2/Kb tetrameric reagents were generated to study the effect of super-enhanced CD8 binding on the specificity of pMHCI ligand interactions at the cell surface. Wildtype pMHCI tetrameric reagents bearing cognate peptide stained three distinct A2-restricted CTL specific for SLYNTVATL (HIV-1 p17 Gag77-85), each expressing a different TCR (19, 22, 23) (Figure 1A). Non-cognate A2 LLFGYPVYV (HILV-1 Tax11-19) tetramers failed to stain any of these in vitro expanded CTL populations to any notable extent. However, A2/Kb LLFGYPVYV tetramers stained all SLYNTVATL-specific CTL; in addition, both the A2/Kb SLYNTVATL and A2/Kb LLFGYPVYV tetramers stained the non-cognate CD8+ cell population in the 868 CTL line (Figure 1A). To examine this effect in more detail, we used A2 and A2/Kb tetramers to stain fresh human PBMC. Antigen-specific CD8+ cell populations were not identified in PBMC from healthy donors with either the A2 SLYNTVATL or A2 LLFGYPVYV tetramers (Figure 1B). In contrast, both the A2/Kb SLYNTVATL and A2/Kb LLFGYPVYV tetramers stained >85% of CD8+ cells in PBMC (Figure 1B); similar data were obtained with A2/Kb GLCTLVAML (EBV BMLF1259-267) and A2/Kb NLVPMVATV (CMV pp65495-503) tetramers (data not shown). Taken together, these data indicate that the exquisite specificity of tetrameric pMHCI reagents is lost when the strength of the pMHCI/CD8 interaction is increased by ~15-fold. Thus, the low solution affinities of the wildtype pMHCI/CD8 interaction are required to maintain pMHCI binding specificity at the cell surface.
Figure 1. The exquisite specificity of pMHCI tetramer staining is lost when the strength of the pMHCI/CD8 interaction is increased by ~15-fold.
A. The 003 or NT1 CTL clones (105 cells) or the 868 CTL line (2.5×105 cells), all specific for HIV-1 p17 Gag77-85, were stained with 1μg of the PE-conjugated tetramers A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 LLFGYPVYV or A2/Kb LLFGYPVYV in 20μl PBS for 20 minutes at 37°C. Cells were then stained with APC-conjugated anti-CD8 and 7-AAD for 30 minutes on ice, washed twice and resuspended in PBS. Data were acquired using a FACSCalibur flow cytometer and analyzed with CellQuest software. B. 2.5×105 PBMC were suspended in 250 μl FACS buffer (2% FCS/PBS), then stained with 1μg of the PE-conjugated tetramers A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 LLFGYPVYV or A2/Kb LLFGYPVYV for 20 minutes at 37°C. Each sample was subsequently stained with APC-conjugated anti-CD8, PerCP-conjugated anti-CD3 and 7-AAD for 30 minutes on ice, washed twice and resuspended in FACS buffer. Data were acquired using a FACSCalibur flow cytometer and analysed with CellQuest software by gating on the live CD3+ population. The values shown represent the percent of CD3+CD8+ cells that stain with the indicated tetramer.
A2/Kb tetramers bind the majority of CTL in peripheral blood
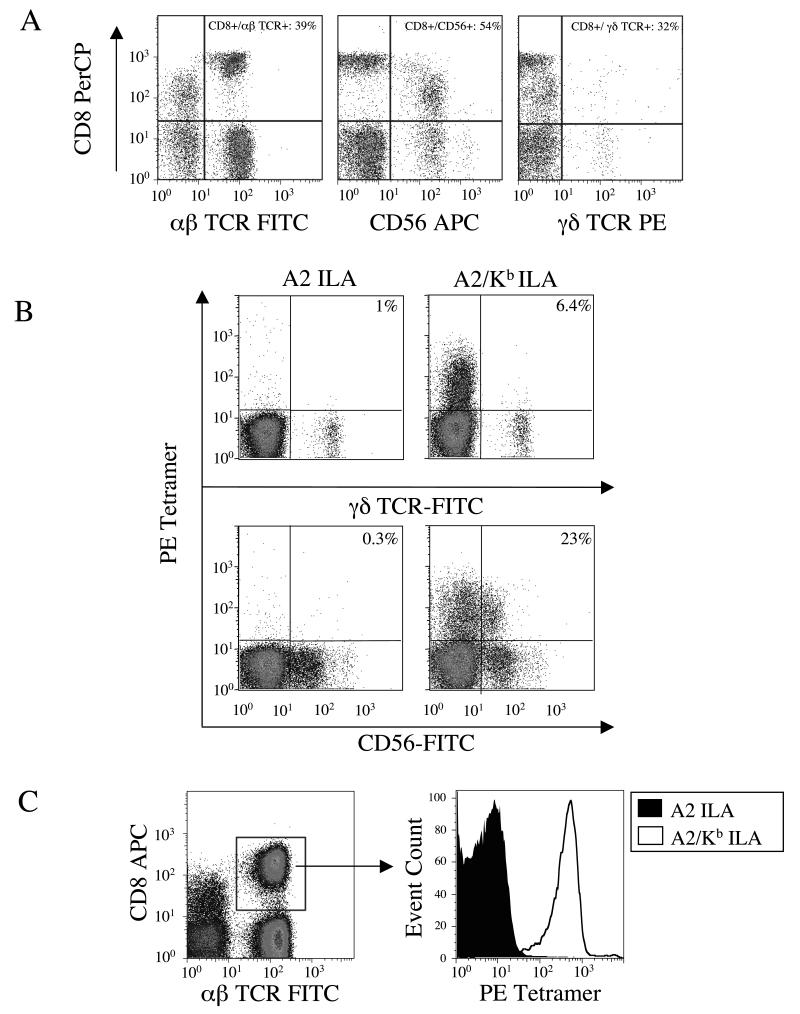
Non-cognate A2/Kb tetramers were observed to bind ~80% of the CD8α+ population in peripheral blood (Figure 1B). Although CD8α is predominantly found on the surface of αβ-TCR+ CTL, it is also found on the surface of other lymphocytes, most notably some γδ T cells and natural killer (NK) cells. We therefore sought to determine the identity of the CD8α+ cells that stain with A2/Kb tetramers. Staining of fresh ex vivo PBMC isolated from healthy A2+ donors revealed that CD8α was expressed on approximately 39%, 54% and 32 % of the αβ-TCR+, NK cell and γδ-TCR+ populations respectively, with some variation between donors (Figure 2A). The majority of γδ-TCR+ (~93.6%) and NK cells (~77%) failed to stain with the A2/Kb ILAKFLHWL (hTERT540-548) tetramer and no significant binding was observed with the corresponding A2 tetramer (Figure 2B). However, the vast majority of αβ-TCR+/CD8+ cells within the lymphocyte population stained non-specifically with the A2/Kb ILAKFLHWL tetramer (Figure 2C).
Figure 2. A2/Kb tetramers bind the majority of CTL in peripheral blood.
A. 2.5×105 PBMC from an A2+ donor were stained with PerCP-conjugated anti-CD8, 7-AAD and either FITC-conjugated anti-αβ-TCR, APC-conjugated anti-CD56 or PE-conjugated anti-γδ-TCR for 30 minutes on ice, washed twice and resuspended in PBS. B. 2.5×105 A2+PBMC were stained with 10μg/ml of the PE-conjugated tetramers A2 ILAKFLHWL or A2/Kb ILAKFLHWL for 20 minutes at 37°C. After washing, cells were subsequently stained with 7-AAD and either FITC-conjugated anti-γδ-TCR or FITC-conjugated anti-CD56 for 30 minutes on ice, washed twice and resuspended in PBS. C. 2.5×105 A2+ PBMC were stained with 10μg/ml of the PE-conjugated tetramers A2 ILAKFLHWL or A2/Kb ILAKFLHWL for 20 minutes at 37°C. After washing, cells were stained with APC-conjugated anti-CD8, FITC-conjugated anti-αβ-TCR and 7-AAD for 30 minutes on ice, washed twice and resuspended in PBS. In A, B and C, data were acquired using a FACSCalibur flow cytometer and analyzed with FlowJo software.
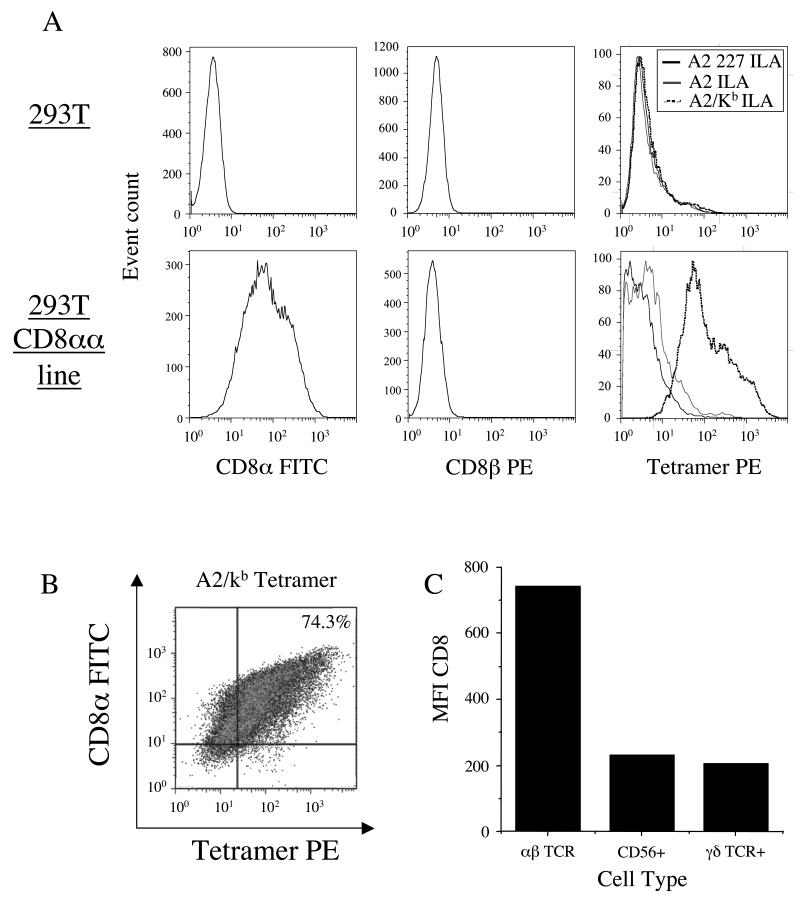
We hypothesised that most γδ-TCR+ cells and NK cells might fail to bind A2/Kb tetramers because they express the CD8αα homodimer rather than the CD8αβ heterodimer, which is expressed on the surface of CTL. Thus, we generated a 293T cell line that expressed CD8αα (Figure 3A) to examine the ability of A2/Kb tetramers to bind this homodimeric form of the CD8 coreceptor on the cell surface. In contrast to both A2 and A2 D227K/T228A tetramers, which exhibit normal and abrogated interactions with CD8 respectively, A2/Kb tetramers bound to most (74.3%) of the CD8αα+ 293T cell transfectants (Figures 3A&B); no binding was observed in the absence of CD8αα surface expression (Figure 3A). Thus, A2/Kb tetramers are capable of binding to cell surface CD8αα.
Figure 3. Non-specific A2/Kb tetramer binding is influenced by CD8 cell surface density.
A and B. 2×105 293T cells were incubated +/− 10 μg/ml of the PE-conjugated tetramers A2 D227K/T228A ILAKFLHWL, A2 ILAKFLHWL or A2/Kb ILAKFLHWL for 20 minutes at 37°C, then stained with 7-AAD and either FITC-conjugated anti-CD8 or PE-conjugated anti-CD8β for 30 minutes on ice, washed twice and resuspended in PBS. C. 2.5×105 PBMC were stained with PerCP-conjugated anti-CD8, 7-AAD and either FITC-conjugated anti-αβ-TCR, APC-conjugated anti-CD56 or PE-conjugated anti-γδ-TCR for 30 minutes on ice, washed twice and resuspended in PBS. In A, B and C, data were acquired using a FACSCalibur flow cytometer and analyzed with FlowJo software.
So, why do A2/Kb tetramers bind predominantly to the CTL population in peripheral blood and not to other cells that express CD8? Figure 3B shows that A2/Kb tetramer staining is directly proportional to the level of CD8αα expression, such that only cells with a higher level of CD8αα expression stain with this reagent. Examination of PBMC from healthy donors revealed that CD8+αβ-TCR+ cells express high levels of CD8, whereas NK and γδ-TCR+ cells express substantially lower levels (Figure 3C). Therefore, increasing the strength of the pMHCI/CD8 interaction allows pMHCI ligand binding at the cell surface that can be mediated through the engagement of either CD8αα or CD8αβ. However, our results suggest that binding is only observed when cells express CD8 at levels above a certain threshold. Importantly, these data demonstrate that TCR expression is not required for cell surface binding of A2/Kb tetramers.
A2/Kb tetramers activate CTL irrespective of TCR specificity
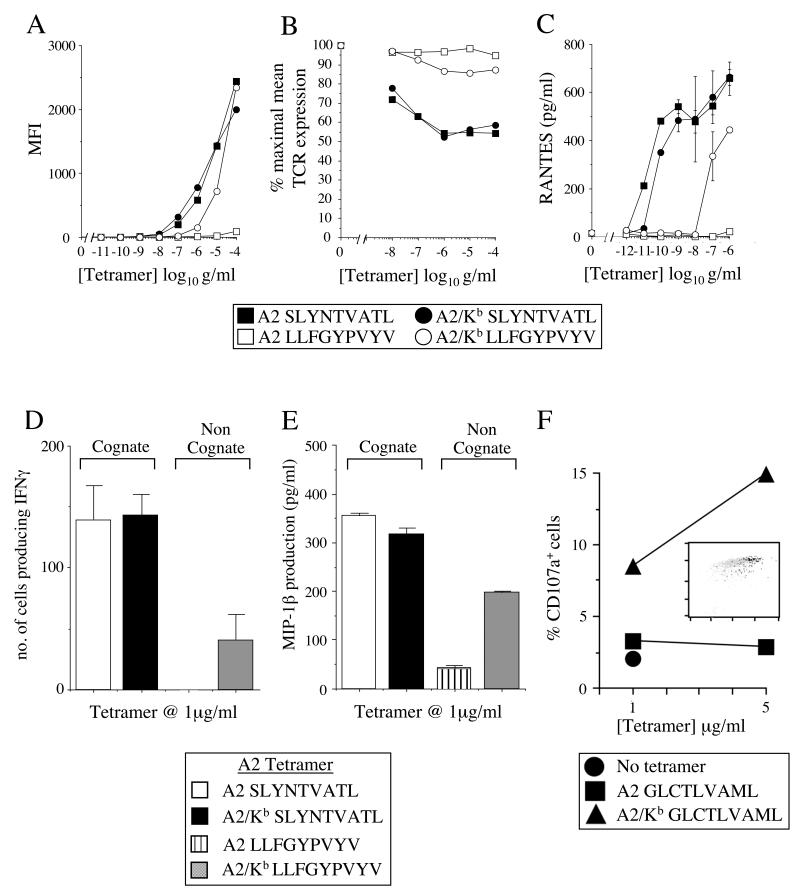
It is well established that pMHCI tetramers can activate CTL bearing cognate TCR (reviewed in (35)). However, previous studies have shown that pMHCI tetrameric binding at the cell surface does not necessarily equate with activation (11, 37). Thus, we next examined whether non-specific A2/Kb tetramer binding at the cell surface (Figures 1-3) could activate human CTL. Initially, we studied the A2-restricted SLYNTVATL-specific CTL clone 003 (23). Consistent with our findings above, both A2 SLYNTVATL and A2/Kb SLYNTVATL tetramers stained 003 CTL efficiently, as did the non-cognate A2/Kb LLFGYPVYV tetramer; no staining was observed with the A2 LLFGYPVYV tetramer (Figure 4A). On ligation, it is known that TCRs are down-regulated from the cell surface (38). The, cognate A2 tetramer was able to induce significant TCR down-regulation, even at tetramer concentrations well below the limits of detection by flow cytometry; no TCR down-regulation was observed with the non-cognate A2 LLFGYPVYV tetramer (Figure 4B). In contrast, however, both the A2/Kb SLYNTVATL and A2/Kb LLFGYPVYV tetramers induced TCR down-regulation, although this occurred to a lesser extent with the non-cognate form compared to either of the cognate tetramers (Figure 4B). This TCR down-regulation correlated with various functional readouts typical of CTL effector activity, including the production of RANTES (Figure 4C), IFNγ and MIP-1β (data not shown). Similar results were observed with SLYNTVATL-specific CTL bearing an alternative cognate TCR (Figure 4D&E). Consistent with the staining patterns (Figure 4A), the activation of CTL by non-cognate A2/Kb tetramers was less efficient than that induced by tetramers bearing the agonist peptide (Figure 4C-E).
Figure 4. A2/Kb tetramers can activate CTL in the absence of a specific TCR/pMHCI interaction.
A. 105 003 CTL were suspended in 20μl PBS and stained with the PE-conjugated tetramers A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 LLFGYPVYV or A2/Kb LLFGYPVYV at the indicated concentrations and 7-AAD for 20 minutes at 37°C. Cells were then washed twice and resuspended in PBS. Data were acquired using a FACSCalibur flow cytometer and analyzed with CellQuest software. B. 105 003 CTL were suspended in 40μl R2 with the PE-conjugated tetramers A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 LLFGYPVYV or A2/Kb LLFGYPVYV at the indicated concentrations for 30 minutes at 37°C. Cells were subsequently stained with FITC-conjugated anti-αβ-TCR, 7-AAD and APC-conjugated anti-CD8 for 30 minutes on ice in azide buffer (0.1% azide/2% FCS/PBS). After two washes, data were acquired using a FACSCalibur flow cytometer and analyzed with CellQuest software. C. 5×105 003 CTL were incubated with the PE-conjugated tetramers A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 LLFGYPVYV or A2/Kb LLFGYPVYV at the indicated concentrations. After 4 hours at 37°C, supernatants were harvested and assayed for RANTES, IFNγ and MIP-1β content by ELISA (only RANTES shown). D. 2×103 868 CTL were incubated for 4 hours at 37°C with 1μg/ml of the PE-conjugated tetramers A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 LLFGYPVYV or A2/Kb LLFGYPVYV in an IFNγ ELISpot assay. E. 1.25×105 868 CTL were incubated with 1μg/ml of the PE-conjugated tetramers A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 LLFGYPVYV or A2/Kb LLFGYPVYV for 4 hours at 37°C. The supernatant was subsequently assayed for MIP-1β content by ELISA. Figures (C-E) show the mean ± SD of two replicate assays. Results similar to (A-E) were also obtained with tetramers conjugated to fluorochromes other than PE (data not shown). F. 003 CTL were incubated with the PE-conjugated tetramers A2 SLYNTVATL, A2/Kb SLYNTVATL, A2 GLCTLVAML or A2/KbGLCTLVAML at the indicated concentrations for 4 hours at 37°C, then stained with APC-conjugated anti-CD8 for 20 minutes on ice and assayed for CD107a mobilization as described in the Materials and Methods. The inset plot shows staining for APC-conjugated anti-CD8 on the x-axis and PE-conjugated A2/Kb GLCTLVAML tetramer (5μg/ml) on the y-axis. Back-gated tetramer+CD107a+ cells are shown in black and tetramer+CD107a− cells are shown in grey. TetramerhighCD8high cells are preferentially activated by the A2/Kb tetramer.
In order to dissect this effect further at the single cell level within a clonal CTL population, we used a flow cytometric assay for degranulation based on the detection of CD107a mobilized on to the cell surface (32). The non-cognate A2/Kb tetramer, in this case folded around the GLCTLVAML peptide, induced degranulation in 15% of 003 CTL at a concentration of 5 μg/ml (Figure 4F); the cognate A2 SLYNTVATL and A2/Kb SLYNTVATL tetramers induced almost 40% degranulation (data not shown). Notably, the cells that degranulated in response to the A2/Kb GLCTLVAML tetramer were contained almost exclusively within the tetramerhighCD8high population (Figure 4F). Thus, at least to some extent, the strong interaction between A2/Kb and CD8 can bypass the requirement for a specific TCR/pMHCI interaction and non-specifically activate human CTL.
Cell surface-expressed A2/Kb activates CTL in the absence of cognate antigen
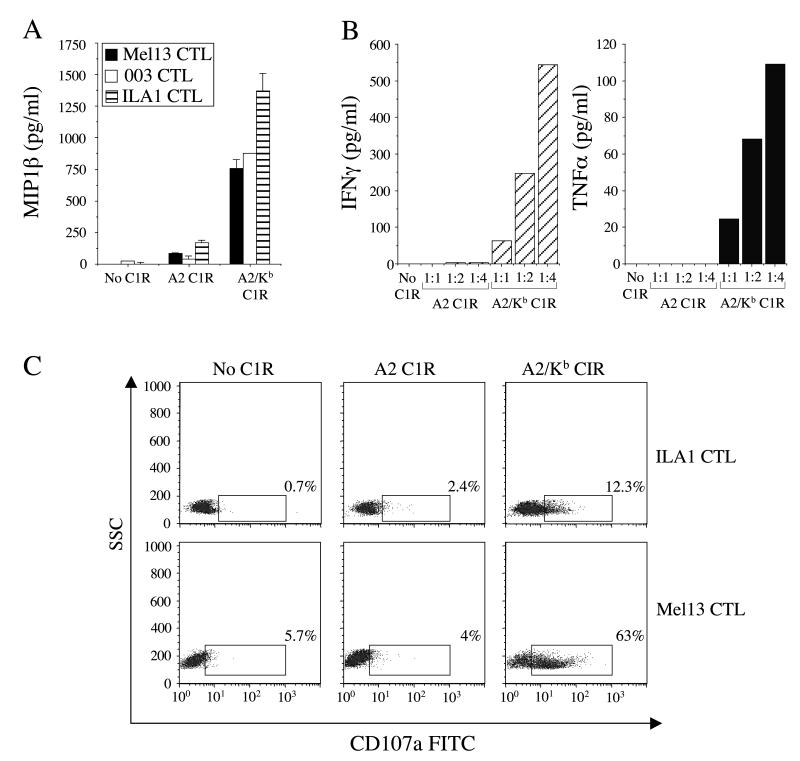
To extend our investigation to the effects of cell surface pMHCI presentation, C1R cells were transfected with either A2 or A2/Kb; stable transfectants expressing similar cell surface MHCI densities were selected as targets for further experiments. Target cells expressing either A2 or A2/Kb were incubated overnight with three A2-restricted CTL clones with different peptide specificities (Mel13, 003 and ILA1). Targets that expressed A2 failed to activate any of the CTL clones significantly above background (Figure 5A). Remarkably, however, the A2/Kb targets stimulated Mel13, 003 and ILA1 CTL to produce significant amounts of MIP-1β in the absence of specific peptide (Figure 5A). A2/Kb targets also elicited substantial levels of TNFα and IFNγ at titratable E:T ratios (Figure 5B), induced degranulation (Figure 5C) and induced significant levels of killing (data not shown) in the absence of specific TCR/pMHCI interactions.
Figure 5. Cell surface-expressed A2/Kb activates CTL in the absence of cognate antigen.
A. 2.5×104 Mel13, 003 or ILA1 CTL were incubated for 12 hours at 37°C with 105 C1R cells stably transfected to express equal levels of either A2 or A2/Kb at the cell surface. Supernatant was subsequently assayed for MIP-1β content by ELISA. The mean ± SD of two replicate assays is shown. B. 2.5×104 Mel13 CTL were incubated for 12 hours at 37°C with 105 C1R cells stably transfected to express either A2 or A2/Kb at the cell surfaceSupernatant was assayed for IFNγ and TNFα content by cytokine bead array. C. CD107a expression by ILA1 and Mel13 CTL following a 12 hour incubation at 37°C with C1R cells stably transfected to express either A2 or A2/Kb on the cell surface. For (A-C), C1R cells were not previously pulsed with peptide.
Cell surface-expressed A2/Kb primes non-cognate CTL expansions
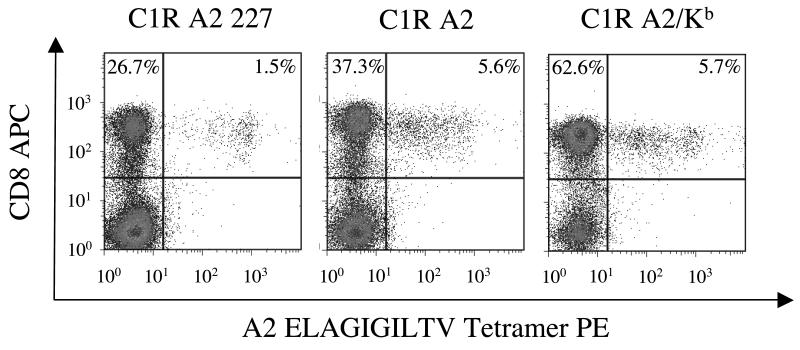
Thymic output in healthy A2+ individuals is known to generate a high frequency of naïve CD8+ T cells that can recognize the self-antigen Melan-A26-35 (39); this system can be used to examine the priming of CTL directly ex vivo (40). We exploited these observations to investigate the effect of super-enhanced pMHCI/CD8 binding on CTL priming. In priming experiments conducted with C1R target cells, the percentages of CTL specific for Melan-A26-35 that were present after 10 days in culture were related to the context of the pMHCI/CD8 interaction in which the cognate ELAGIGILTV peptide was presented. Thus, in the absence of a pMHCI/CD8 interaction (A2 D227K/T228A C1R targets), only 1.5% of the CD8+ cell population was specific for Melan-A26-35; in contrast, 5.6% and 5.7% of the CD8+ population bound the A2 ELAGIGILTV tetramer in the same experiment when priming was conducted with A2 and A2/Kb C1R targets, respectively (Figure 6). Exposure to A2/Kb C1R targets also resulted in substantial expansions of the total CD8+ population (Figure 6). Similar results were obtained with multiple donors (data not shown). Thus, target cells that express MHCI molecules with super-enhanced CD8 binding properties can induce non-specific expansions of CD8+ cells in the absence of cognate antigen.
Figure 6. Cell surface-expressed A2/Kb primes non-specific expansion of CD8+ cells.
106 A2+ PBMC were incubated with 2×105 irradiated A2 D227K/T228A, A2 or A2/Kb C1R cells that had previously been pulsed with 1μM ELAGIGILTV (Melan-A26-35) peptide in R10. From day 3, IL-2 was added in increments to reach a maximum concentration of 200 IU/ml by day 10. Lines were subsequently stained with PE-conjugated A2 ELAGIGILTV tetramer followed by APC-conjugated anti-CD8 and 7-AAD. Data were acquired using a FACSCalibur flow cytometer and analyzed with FlowJo software.
Non-specific A2/Kb-mediated CTL activation and tetramer staining are not dependent on TCR expression
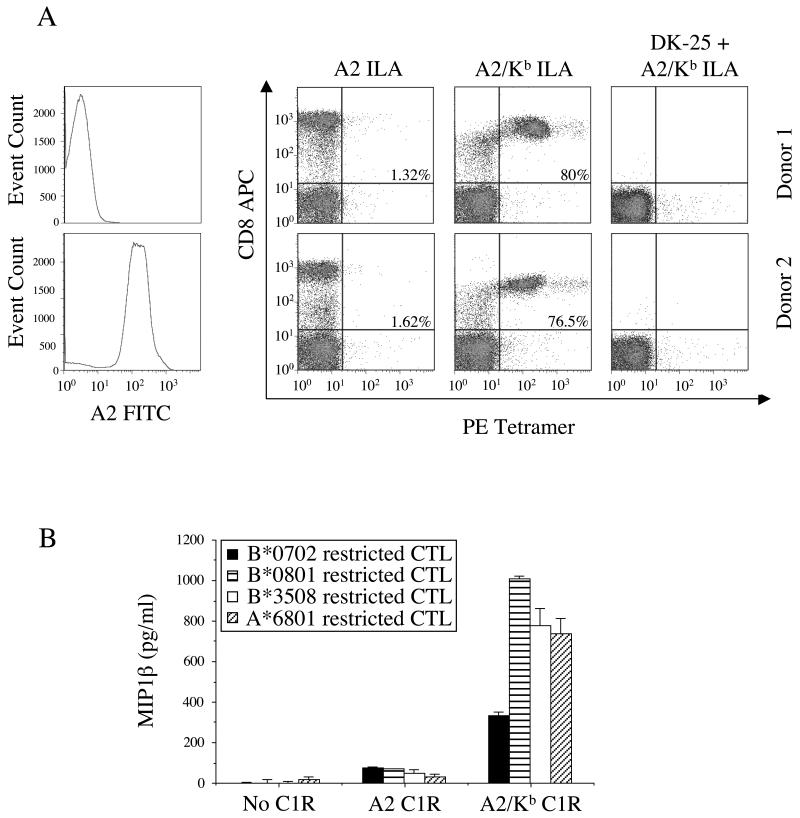
In earlier experiments, we observed that A2/Kb tetramers bound to the majority of αβ-TCR+CD8+ cells in PBMC derived from A2+ donors (Figure 2). To exclude the possibility that this phenomenon was dependent on the presence of A2-restricted TCRs, we conducted staining experiments with A2− PBMC. As previously, the A2/Kb ILAKFLHWL tetramer bound non-specifically to the majority of CD8+ cells (Figure 7A). Furthermore, A2/Kb tetramer binding favored CD8high cells and was abrogated by pre-treatment with the anti-CD8 mAb DK25 (Figure 7A). Thus, consistent with the data shown in Figure 3, non-specific A2/Kb tetramer binding is a CD8-mediated effect that is not dependent on the presence of A2-restricted TCRs. In addition, we demonstrated in earlier experiments that A2/Kb, both in soluble and cell-associated form, non-specifically activated A2-restricted CTL (Figures 4&5). To confirm that these functional correlates of non-specific binding were similarly independent of A2-restricted TCR expression, we extended our studies to CTL clones restricted by non-A2 MHCI molecules. In all cases, cell surface-expressed A2/Kb activated CTL clones regardless of restriction element (Figure 7B).
Figure 7. Non-cognate A2/Kb-mediated CTL activation and tetramer binding is not influenced by MHCI restriction.
A. 2.5×105 PBMC were suspended in 250μl FACS buffer (2% FCS/PBS) and stained with FITC-conjugated anti-A2 and 7-AAD for 30 minutes on ice, then washed twice and resuspended in PBS. For pMHCI tetramer staining experiments, 2.5×105 PBMC were suspended in 50μl FACS buffer (2% FCS/PBS) and incubated +/− 10μg/ml unconjugated anti-CD8 for 20 minutes on ice, then stained with 10μg/ml of the PE-conjugated tetramers A2 ILAKFLHWL or A2/Kb ILAKFLHWL for 45 minutes on ice. After washing, cells were subsequently stained with APC-conjugated anti-CD8 and 7-AAD, washed again and resuspended in PBS. Data were acquired using a FACSCalibur flow cytometer and analyzed with FlowJo software. B. 2.5×104 CTL were incubated for 12 hours at 37°C with 105 unpulsed C1R cells expressing either A2 or A2/Kb on the cell surface. The following CTL clones were used: (i) the HLA A*6801-restricted CTL clone c23, specific for the HIV-1 Tat-derived epitope ITKGLGISYGR (residues 38-48); (ii) the HLA B*0702-restricted CTL clone KD4, specific for the EBV EBNA3A-derived epitope RPPIFIRRL (residues 379-387); (iii) the HLA B*0801-restricted CTL clone LC13, specific for the EBV EBNA3A-derived epitope FLRGRAYGL (residues 339-347); and, (iv) the HLA B*3508-restricted CTL clone SB27, specific for the EBV BZLF1-derived epitope LPEPLPQGQLTAY (residues 52-64). Supernatant was subsequently assayed for MIP-1β content by ELISA. The mean ± SD of two replicate assays is shown.
DISCUSSION
CD8 has the potential to engage all pMHCI complexes, both self and foreign, because it binds to largely non-polymorphic regions of the MHCI molecule. Indeed, recent publications suggest that the ability of CD8 to interact with non-stimulatory pMHCI complexes lowers T cell activation thresholds and enables CTL to respond to low copy numbers of specific pMHCI (41, 42). It therefore remains unclear how the specificity of TCR recognition is maintained despite the potential for multiple pMHCI/CD8 interactions at the cell surface. One possibility resides in the fact that the binding of CD8 to MHCI is characterized by very low affinities and extremely rapid kinetics. Here, we have generated chimeric A2/Kb MHCI molecules that increase the strength of the pMHCI/CD8 interaction by ~15-fold to probe the biophysical and functional significance of the low solution binding affinities observed for the pMHCI/CD8 interaction.
Initially, we examined the effect of super-enhanced CD8 binding on pMHCI tetramer binding at the cell surface. Increasing the strength of the pMHCI/CD8 interaction by ~15-fold resulted in the total loss of pMHCI tetramer binding specificity. Thus, irrespective of restriction element and the presented peptide, A2/Kb tetramers bound to the surface of all CTL clones examined in this study and to the majority of CTL present within PBMC (Figures 1,2&7A). In addition, A2/Kb tetramers bound to the cell surface in the absence of TCR expression (Figure 3) and non-specific binding was abrogated by pre-treatment with an anti-CD8 antibody (Figure 7A), thereby demonstrating that the observed loss of pMHCI tetramer binding specificity was CD8-mediated and TCR-independent. These findings indicate that the low solution binding affinities observed for the pMHCI/CD8 interaction are essential for the preservation of pMHCI ligand binding specificity at the cell surface.
It has previously been documented that pMHCI tetramers are efficient activators of cognate CTL (reviewed in (35)). However, pMHCI tetramer staining does not necessarily equate with cellular activation. Therefore, we proceeded to examine the ability of A2/Kb tetramers to activate CTL clones. Notably, we found that A2/Kb tetramers activated CTL clones in a non-specific manner (Figure 4). Activation resulted in a full range of effector functions, including cytokine/chemokine release, degranulation and killing. Flow cytometric assessment of degranulation by analysis of CD107a mobilization revealed that CTL with higher surface expression of CD8 were the cells most likely to activate in response to A2/Kb molecules. This finding led us to examine the effects of cell-surface presented antigen. Strikingly, exposure of PBMC to C1R target cells bearing A2/Kb molecules caused a general non-specific expansion of CD8+ cells during the course of the experiment (Figure 6). Furthermore, A2/Kb C1R cells, unlike their wildtype A2 counterparts, were capable of stimulating effector function in all CTL clones tested regardless of specificity and MHCI restriction (Figures 5&7B). Although we cannot exclude the possibility that inclusion of the murine α3 domain induces conformational changes at the T cell surface on binding to CD8 that favour non-cognate activation, this seems unlikely given that: (i) the TCR binding site remains unaltered (9, 22); (ii) a degree of non-cognate activation can be observed in long-term assays with non-chimeric human MHCI molecules that exhibit incrementally enhanced CD8 binding (data not shown); and, (iii) murine and human pMHCI/CD8αα co-crystals exhibit similar binding orientations (14, 43). Furthermore, these results are consistent with the observation that thymus leukaemia antigen (TL), which interacts strongly (KD ~ 12μM) with cell surface CD8αα expressed by intraepithelial lymphocytes (IELs), can modulate T cell responses independently of the TCR (44-46).
How does a super-enhanced pMHCI/CD8 interaction result in non-specific CTL activation? We have previously demonstrated that an incremental increase in the pMHCI/CD8 interaction (A2 Q115E) results in enhanced immunogenicity of cognate antigens and that this effect is mediated by enhanced early intracellular signal transduction (9, 47). In contrast, the stimulatory properties of A2/Kb molecules exhibited no peptide specificity requirements whatsoever; indeed, cell surface-expressed A2/Kb was shown to activate even non-A2 restricted CTL clones (Figure 7B), thereby confirming that cognate TCR/pMHCI interactions are not required. Combined with the ability of A2/Kb to engage multiple CD8 molecules at the cell surface, these results suggest that A2/Kb cross-links CD8 and induces activation in an ‘antibody-like’ manner. Indeed, this is consistent with previous studies demonstrating that antibody-induced CD8 cross-linking can induce T cell signalling (48, 49) and elicit downstream effector functions such as chemokine release (50); such effects are predictable given that the CD8α tail is coupled to p56lck, an essential component of the early intracellular signalling pathway (10). It is interesting to note that the murine pMHCI/CD8 interaction is significantly stronger (KD ~ 30μM) than the equivalent human interaction (KD ~ 150μM) (11), but does not result in non-cognate CTL activation. It is therefore likely that a pMHCI/CD8 interaction affinity threshold exists for the maintenance of CTL activation specificity. The strength of the murine pMHCI/CD8 interaction is 3-fold weaker than the strength of the interaction measured between A2/Kb and human CD8, thereby still operating at a level below this threshold.
In summary, we utilized chimeric MHCI molecules that exhibit a super-enhanced interaction with CD8 to probe the physical and functional significance of the low solution binding affinities previously described for the pMHCI/CD8 interaction. We found that increasing the strength of the pMHCI/CD8 interaction by ~15-fold resulted in: (i) total loss of pMHCI binding specificity at the cell surface; (ii) non-cognate pMHCI tetramer-mediated activation; and, (iii) non-specific activation and proliferation triggered by cell surface-expressed pMHCI molecules. Thus, the low solution binding affinity of the pMHCI/CD8 interaction is essential for the preservation of pMHCI ligand binding specificity at the cell surface and its attendant functional repercussions.
ACKNOWLEDGEMENTS
We would like to thank Eddie Wang for provision of the APC-conjugated monoclonal antibody specific for human CD56.
REFERENCES
- 1.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 2.Rudolph MG, Wilson IA. The specificity of TCR/pMHC interaction. Curr Opin Immunol. 2002;14:52–65. doi: 10.1016/s0952-7915(01)00298-9. [DOI] [PubMed] [Google Scholar]
- 3.Zamoyska R. CD4 and CD8: modulators of T-cell receptor recognition of antigen and of immune responses? Curr Opin Immunol. 1998;10:82–87. doi: 10.1016/s0952-7915(98)80036-8. [DOI] [PubMed] [Google Scholar]
- 4.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 5.Gakamsky DM, Luescher IF, Pramanik A, Kopito RB, Lemonnier F, Vogel H, Rigler R, Pecht I. CD8 kinetically promotes ligand binding to the T-cell antigen receptor. Biophys J. 2005;89:2121–2133. doi: 10.1529/biophysj.105.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laugel B, van den Berg HA, Gostick E, Cole DK, Wooldridge L, Boulter J, Milicic A, Price DA, Sewell AK. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem. 2007;282:23799–23810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- 7.van den Berg HA, Wooldridge L, Laugel B, Sewell AK. Coreceptor CD8-driven modulation of T cell antigen receptor specificity. J heor Biol. 2007;249:395–408. doi: 10.1016/j.jtbi.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luescher IF, Vivier E, Layer A, Mahiou J, Godeau F, Malissen B, Romero P. CD8 modulation of T-cell antigen receptor-ligand interactions on living cytotoxic T lymphocytes. Nature. 1995;373:353–356. doi: 10.1038/373353a0. [DOI] [PubMed] [Google Scholar]
- 9.Wooldridge L, van den Berg HA, Glick M, Gostick E, Laugel B, Hutchinson SL, Milicic A, Brenchley JM, Douek DC, Price DA, Sewell AK. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J Biol Chem. 2005;280:27491–27501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 11.Purbhoo MA, Boulter JM, Price DA, Vuidepot AL, Hourigan CS, Dunbar PR, Olson K, Dawson SJ, Phillips RE, Jakobsen BK, Bell JI, Sewell AK. The human CD8 coreceptor effects cytotoxic T cell activation and antigen sensitivity primarily by mediating complete phosphorylation of the T cell receptor zeta chain. J Biol Chem. 2001;276:32786–32792. doi: 10.1074/jbc.M102498200. [DOI] [PubMed] [Google Scholar]
- 12.Arcaro A, Gregoire C, Bakker TR, Baldi L, Jordan M, Goffin L, Boucheron N, Wurm F, van der Merwe PA, Malissen B, Luescher IF. CD8beta endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes. J Exp Med. 2001;194:1485–1495. doi: 10.1084/jem.194.10.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arcaro A, Gregoire C, Boucheron N, Stotz S, Palmer E, Malissen B, Luescher IF. Essential role of CD8 palmitoylation in CD8 coreceptor function. J Immunol. 2000;165:2068–2076. doi: 10.4049/jimmunol.165.4.2068. [DOI] [PubMed] [Google Scholar]
- 14.Gao GF, Tormo J, Gerth UC, Wyer JR, McMichael AJ, Stuart DI, Bell JI, Jones EY, Jakobsen BK. Crystal structure of the complex between human CD8alpha(alpha) and HLA-A2. Nature. 1997;387:630–634. doi: 10.1038/42523. [DOI] [PubMed] [Google Scholar]
- 15.Wyer JR, Willcox BE, Gao GF, Gerth UC, Davis SJ, Bell JI, van der Merwe PA, Jakobsen BK. T cell receptor and coreceptor CD8 alphaalpha bind peptide-MHC independently and with distinct kinetics. Immunity. 1999;10:219–225. doi: 10.1016/s1074-7613(00)80022-9. [DOI] [PubMed] [Google Scholar]
- 16.Gao GF, Willcox BE, Wyer JR, Boulter JM, O’Callaghan CA, Maenaka K, Stuart DI, Jones EY, Van Der Merwe PA, Bell JI, Jakobsen BK. Classical and nonclassical class I major histocompatibility complex molecules exhibit subtle conformational differences that affect binding to CD8alphaalpha. J Biol Chem. 2000;275:15232–15238. doi: 10.1074/jbc.275.20.15232. [DOI] [PubMed] [Google Scholar]
- 17.Dustin ML, Golan DE, Zhu DM, Miller JM, Meier W, Davies EA, van der Merwe PA. Low affinity interaction of human or rat T cell adhesion molecule CD2 with its ligand aligns adhering membranes to achieve high physiological affinity. J Biol Chem. 1997;272:30889–30898. doi: 10.1074/jbc.272.49.30889. [DOI] [PubMed] [Google Scholar]
- 18.Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, Price DA, Gao GF, Sewell AK, Jakobsen BK. Human TCR-binding affinity is governed by MHC class restriction. J Immunol. 2007;178:5727–5734. doi: 10.4049/jimmunol.178.9.5727. [DOI] [PubMed] [Google Scholar]
- 19.Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, Moysey R, Cameron BJ, Vuidepot A, Purbhoo MA, Cole DK, Phillips RE, June CH, Jakobsen BK, Sewell AK, Riley JL. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchinson SL, Wooldridge L, Tafuro S, Laugel B, Glick M, Boulter JM, Jakobsen BK, Price DA, Sewell AK. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J Biol Chem. 2003;278:24285–24293. doi: 10.1074/jbc.M300633200. [DOI] [PubMed] [Google Scholar]
- 22.Choi EM, Chen JL, Wooldridge L, Salio M, Lissina A, Lissin N, Hermans IF, Silk JD, Mirza F, Palmowski MJ, Dunbar PR, Jakobsen BK, Sewell AK, Cerundolo V. High avidity antigen-specific CTL identified by CD8-independent tetramer staining. J Immunol. 2003;171:5116–5123. doi: 10.4049/jimmunol.171.10.5116. [DOI] [PubMed] [Google Scholar]
- 23.Sewell AK, Harcourt GC, Goulder PJ, Price DA, Phillips RE. Antagonism of cytotoxic T lymphocyte-mediated lysis by natural HIV-1 altered peptide ligands requires simultaneous presentation of agonist and antagonist peptides. Eur J Immunol. 1997;27:2323–2329. doi: 10.1002/eji.1830270929. [DOI] [PubMed] [Google Scholar]
- 24.Purbhoo MA, Li Y, Sutton DH, Brewer JE, Gostick E, Bossi G, Laugel B, Moysey R, Baston E, Liddy N, Cameron B, Bennett AD, Ashfield R, Milicic A, Price DA, Classon BJ, Sewell AK, Jakobsen BK. The HLA A*0201-restricted hTERT(540-548) peptide is not detected on tumor cells by a CTL clone or a high-affinity T-cell receptor. Mol Cancer Ther. 2007;6:2081–2091. doi: 10.1158/1535-7163.MCT-07-0092. [DOI] [PubMed] [Google Scholar]
- 25.Gostick E, Cole DK, Hutchinson SL, Wooldridge L, Tafuro S, Laugel B, Lissina A, Oxenius A, Boulter JM, Price DA, Sewell AK. Functional and biophysical characterization of an HLA-A*6801-restricted HIV-specific T cell receptor. Eur J Immunol. 2007;37:479–486. doi: 10.1002/eji.200636243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrows SR, Silins SL, Moss DJ, Khanna R, Misko IS, Argaet VP. T cell receptor repertoire for a viral epitope in humans is diversified by tolerance to a background major histocompatibility complex antigen. J Exp Med. 1995;182:1703–1715. doi: 10.1084/jem.182.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kjer-Nielsen L, Clements CS, Purcell AW, Brooks AG, Whisstock JC, Burrows SR, McCluskey J, Rossjohn J. A structural basis for the selection of dominant alphabeta T cell receptors in antiviral immunity. Immunity. 2003;18:53–64. doi: 10.1016/s1074-7613(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 28.Tynan FE, Burrows SR, Buckle AM, Clements CS, Borg NA, Miles JJ, Beddoe T, Whisstock JC, Wilce MC, Silins SL, Burrows JM, Kjer-Nielsen L, Kostenko L, Purcell AW, McCluskey J, Rossjohn J. T cell receptor recognition of a ‘super-bulged’ major histocompatibility complex class I-bound peptide. Nat Immunol. 2005;6:1114–1122. doi: 10.1038/ni1257. [DOI] [PubMed] [Google Scholar]
- 29.Tynan FE, Borg NA, Miles JJ, Beddoe T, El-Hassen D, Silins SL, van Zuylen WJ, Purcell AW, Kjer-Nielsen L, McCluskey J, Burrows SR, Rossjohn J. High resolution structures of highly bulged viral epitopes bound to major histocompatibility complex class I. Implications for T-cell receptor engagement and T-cell immunodominance. J Biol Chem. 2005;280:23900–23909. doi: 10.1074/jbc.M503060200. [DOI] [PubMed] [Google Scholar]
- 30.Willemsen R, Ronteltap C, Heuveling M, Debets R, Bolhuis R. Redirecting human CD4+ T lymphocytes to the MHC class I-restricted melanoma antigen MAGE-A1 by TCR alphabeta gene transfer requires CD8alpha. Gene therapy. 2005;12:140–146. doi: 10.1038/sj.gt.3302388. [DOI] [PubMed] [Google Scholar]
- 31.Willemsen RA, Sebestyen Z, Ronteltap C, Berrevoets C, Drexhage J, Debets R. CD8 alpha coreceptor to improve TCR gene transfer to treat melanoma: down-regulation of tumor-specific production of IL-4, IL-5, and IL-10. J Immunol. 2006;177:991–998. doi: 10.4049/jimmunol.177.2.991. [DOI] [PubMed] [Google Scholar]
- 32.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 33.Choi EM, Palmowski M, Chen J, Cerundolo V. The use of chimeric A2K(b) tetramers to monitor HLA A2 immune responses in HLA A2 transgenic mice. J Immunol Methods. 2002;268:35–41. doi: 10.1016/s0022-1759(02)00198-9. [DOI] [PubMed] [Google Scholar]
- 34.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 35.Wooldridge L, Lissina A, Cole DK, van den Berg HA, Price DA, Sewell AK. Tricks with tetramers: how to get the most from multimeric peptide-MHC. Immunology. 2009;126:147–164. doi: 10.1111/j.1365-2567.2008.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burrows SR, Kienzle N, Winterhalter A, Bharadwaj M, Altman JD, Brooks A. Peptide-MHC class I tetrameric complexes display exquisite ligand specificity. J Immunol. 2000;165:6229–6234. doi: 10.4049/jimmunol.165.11.6229. [DOI] [PubMed] [Google Scholar]
- 37.Schott E, Ploegh HL. Mouse MHC class I tetramers that are unable to bind to CD8 reveal the need for CD8 engagement in order to activate naive CD8 T cells. Eur J Immunol. 2002;32:3425–3434. doi: 10.1002/1521-4141(200212)32:12<3425::AID-IMMU3425>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 39.Zippelius A, Pittet MJ, Batard P, Rufer N, de Smedt M, Guillaume P, Ellefsen K, Valmori D, Lienard D, Plum J, MacDonald HR, Speiser DE, Cerottini JC, Romero P. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J Exp Med. 2002;195:485–494. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured Murine Plasmacytoid Dendritic Cells Are Capable of In Vivo Priming of Functional CD8 T Cell Responses to Endogenous but Not Exogenous Antigens. J Exp Med. 2004;199:567–579. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yachi PP, Ampudia J, Gascoigne NR, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anikeeva N, Lebedeva T, Clapp AR, Goldman ER, Dustin ML, Mattoussi H, Sykulev Y. Quantum dot/peptide-MHC biosensors reveal strong CD8-dependent cooperation between self and viral antigens that augment the T cell response. Proc Natl Acad Sci. 2006;103:16846–16851. doi: 10.1073/pnas.0607771103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kern PS, Teng MK, Smolyar A, Liu JH, Liu J, Hussey RE, Spoerl R, Chang HC, Reinherz EL, Wang JH. Structural basis of CD8 coreceptor function revealed by crystallographic analysis of a murine CD8alphaalpha ectodomain fragment in complex with H-2Kb. Immunity. 1998;9:519–530. doi: 10.1016/s1074-7613(00)80635-4. [DOI] [PubMed] [Google Scholar]
- 44.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang HC, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 45.Tsujimura K, Obata Y, Kondo E, Nishida K, Matsudaira Y, Akatsuka Y, Kuzushima K, Takahashi T. Thymus leukemia antigen (TL)-specific cytotoxic T lymphocytes recognize the alpha1/alpha2 domain of TL free from antigenic peptides. Int Immunol. 2003;15:1319–1326. doi: 10.1093/intimm/dxg131. [DOI] [PubMed] [Google Scholar]
- 46.Cole DK, Gao GF. CD8: adhesion molecule, co-receptor and immuno-modulator. Cell Mol Immunol. 2004;1:81–88. [PubMed] [Google Scholar]
- 47.Wooldridge L, Lissina A, Vernazza J, Gostick E, Laugel B, Hutchinson SL, Mirza F, Dunbar PR, Boulter JM, Glick M, Cerundolo V, van den Berg HA, Price DA, Sewell AK. Enhanced immunogenicity of CTL antigens through mutation of the CD8 binding MHC class I invariant region. Eur J Immunol. 2007;37:1323–1333. doi: 10.1002/eji.200636765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grebe KM, Clarke RL, Potter TA. Ligation of CD8 leads to apoptosis of thymocytes that have not undergone positive selection. Proc Natl Acad Sci U S A. 2004;101:10410–10415. doi: 10.1073/pnas.0402079101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke RL, Thiemann S, Refaeli Y, Werlen G, Potter TA. A new function for LAT and CD8 during CD8-mediated apoptosis that is independent of TCR signal transduction. Eur J Immunol. 2009;39:1619–1631. doi: 10.1002/eji.200839062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wooldridge L, Hutchinson SL, Choi EM, Lissina A, Jones E, Mirza F, Dunbar PR, Price DA, Cerundolo V, Sewell AK. Anti-CD8 antibodies can inhibit or enhance peptide-MHC class I (pMHCI) multimer binding: this is paralleled by their effects on CTL activation and occurs in the absence of an interaction between pMHCI and CD8 on the cell surface. J Immunol. 2003;171:6650–6660. doi: 10.4049/jimmunol.171.12.6650. [DOI] [PubMed] [Google Scholar]