Abstract
Objective
To investigate antiretroviral treatment (ART) interruption in a long-term treatment cohort in South Africa.
Methods
All adults accessing ART between 2004 and 2009 were included in this analysis. Defaulting was defined as having stopped all ART drugs for more than 30 days. Treatment interrupters were patients who defaulted and returned to care during the study, whereas loss to follow-up was defined as defaulting and not returning to care. Kaplan-Meier estimates and Possion regression models were used to analyze rates and determinants of defaulting therapy and of treatment resumption.
Results
Overall rate of defaulting treatment was 12.8/100 person years (95% CI 11.4-14.4). Risk factors for defaulting were male gender, high baseline CD4 count, recency of ART initiation and time on ART. The probability of resuming therapy within 3 years of defaulting therapy was 42% (event rate=21.4/100 person-years). Factors associated with restarting treatment were female gender, older age, and time since defaulting.
Conclusion
Defaulting treatment need not be an irreversible event. Interventions to increase retention in care should target men, less immunocompromised patients and patients during the first 6 months of treatment. Resumption of treatment is most likely within the first year of interrupting therapy.
Keywords: HIV, antiretroviral, unstructured treatment interruption, loss to follow-up, Africa
Introduction
Access to antiretroviral therapy (ART) has improved substantially in resource-limited settings in Africa, Asia and South America where 90% of people with HIV/AIDS reside. According to World Health Organization (WHO) estimates, more than four million people with HIV/AIDS in low and middle income countries had initiated treatment by the end of 2008.1 Despite this success, ensuring that patients remain in care over time remains one of the major challenges in resource-limited settings. Much attention has been paid to patient adherence2-5, loss to follow-up, and mortality in ART programs in resource-limited settings.6-9 A systematic review of 33 patient cohorts from 13 African countries reported that only between 46 and 85% of patients remained in care at two years.8
The realization that a substantial proportion of patients reported as lost to follow-up may have died has led to concern that there may be significant biases in program outcome reports of survival.10 Another potential source of bias is the fact that a proportion of patients may only transiently default, returning to care at a later stage. Such unstructured treatment interruption has been reported to occur in around 20% of patients in industrialized settings.11-14 The proportion of patients who transiently interrupt treatment in resource-limited settings is largely unreported.
Treatment interruptions, planned or otherwise, have been found to increase the risk of opportunistic infection and death15-17, with viral load increase and associated CD4 decline most pronounced in the first two months.16, 18-20 Interruptions raise similar concerns with respect to drug resistance and increased mortality as sub-optimal adherence.11, 15, 21-23 However, few studies have addressed the issue of unstructured treatment interruptions in resource-limited settings. The aim of this study was to investigate the frequency and risk factors of defaulting treatment and identify factors associated with subsequent return to care in a long-term treatment cohort in South Africa.
Methods
Study site and data collection
The study was based in a peri-urban township in the greater area of Cape Town, with a population of approximately 15,000 people and an estimated adult HIV prevalence of 23% in 2005.24 The community is served by a single public-sector primary care clinic which provides ART free of charge.
ART provision began in 2004. From 2005 to 2009 ART services were partly provided according to the Antiretroviral Treatment Protocol of the Western Cape and partly through a study funded by the National Institutes of Health (NIH). Patients enrolled in the NIH-funded study could access ART with a CD4 count below 350 cells/μl or WHO stage 3 disease as compared to 200 cells/μl or WHO stage 4 disease in the provincial program. The NIH-funded study completed enrollment in 2007 after which all patients were treated in the provincial ART program.
Initial evaluation for ART eligibility included medical history, physical examination and CD4 cell count. A follow-up appointment was scheduled one to two weeks later when the laboratory results were reviewed and ART eligibility was determined. Patients eligible for ART underwent three adherence counseling sessions before starting treatment.
The initial follow-up schedule for those starting ART included one visit two weeks after ART initiation, followed by monthly visits until month three. Patients who were stable on ART and did not experience any adherence problems were thereafter seen every three months. Three attempts were made to contact patients who had missed appointments.
All patients aged ≥15 years accessing ART in the primary health care clinic between 01 March 2004 and 31 December 2009 were included in the analysis.
Sociodemographic and clinical data at baseline and laboratory data were collected prospectively using a standardized data form. All laboratory tests were performed by the National Health Laboratory Services in Cape Town.
Definitions
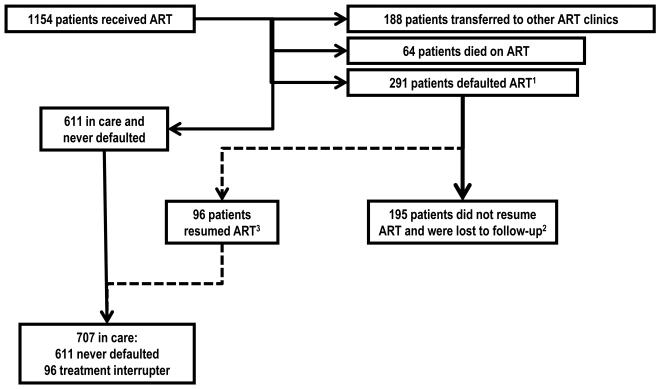
Patients defaulting treatment were defined as those who had not presented at the pharmacy for ART refills for more than 30 days. This category included patients who subsequently returned to care and restarted ART (treatment interrupters) and patients who had not returned to care at the time of censoring (loss to follow-up) (figure 1).
Figure 1. Flow chart of patients in care, first time defaulters and treatment interrupters.
1 Defaulting treatment was defined as having stopped all ART drugs for more than 30 days. This category included patients who subsequently returned to care and restarted ART (treatment interrupters) and patients who had not returned to care at the time of censoring (loss to follow-up).
2 Loss to follow-up was defined as stopping ART for more than 30 days and not returning to care at the time of censoring.
3 Treatment interruption was defined as a patient-initiated episode of more than 30 days of stopping ART and subsequently resuming treatment.
Treatment interruption was defined as a patient-initiated episode of more than 30 days of stopping ART (same definition as defaulting) but who subsequently resumed treatment (figure 1).
Patients lost to follow-up were those who stopped ART for more than 30 days and had not returned to care at the time of censoring (figure 1).
Study design
In-programme data on death, transfers outs and loss to follow-up were collected prospectively. Death on ART was defined as any death within three months of drug refill. If the exact date of death was not recorded it was estimated to be the 15th of the month following the last clinic appointment.
Patients who had stopped ART for more than 30 days and resumed therapy were identified using the pharmacy dispensing data. The electronic pharmacy dispensing system records each time medication is dispensed to a patient. Treatment interruption was verified through folder reviews.
The first endpoint was the time from ART initiation to the first time at which all drugs were stopped for a period of at least 30 days (default). Follow-up of patients on continuous therapy was censored at the date of death, date of transfer, or study end (31 December 2009).
The second endpoint was treatment resumption, defined as the time from defaulting treatment for the first time to the time of restarting ART. Follow-up of patients for whom therapy was not resumed was censored at the date of death, date of transfer, date of migration, or study end. For a proportion of these patients (48%) vital status, date of death, date of transfer and date of migration was determined by home visits.
Statistical analysis
All analyses were carried out using Stata version 10.0 (Stata Corp. LP, College Station, TX, United States of America). Frequency tables were produced for all categorical baseline characteristics. For continuous baseline characteristics the median and interquartile ranges were reported. Standard survival analysis methods, including Kaplan-Meier estimates and Poisson regression models were used to analyze the rate and determinants of defaulting therapy and of treatment resumption after defaulting treatment for the first time. The proportional hazards assumption for potential interaction between each variable and time was tested using the likelihood ratio test. A univariate Poisson regression model was used to determine risk for time-to-event outcomes for each exposure variable. Multivariate models were built through backwards elimination. Sensitivity analyses were conducted excluding individuals with unascertained vital status. All reported p values are exact and 2-tailed, and for each analysis p<0.05 was considered significant.
Ethical approval
The study was approved by the University of Cape Town Ethics Committee and the London School of Hygiene and Tropical Medicine Ethics Committee. Written informed consent was obtained from all patients at enrolment.
Results
Patient characteristics
A total of 1154 patients were included in the analysis (table 1) and the median time of follow-up was 1.45 years (IQR: 0.48-3.24). The majority of patients were young women (65.2%) and residents in the township (95.5%). Prior to treatment initiation the majority of patients were in WHO clinical stage 3 (51.1%) and 4 (25.1%) and median CD4 count was 122 cells/μl (IQR: 54-190). The number of patients initiating ART per year doubled from 137 in 2004 to 279 in 2006 and declined thereafter.
Table 1.
Baseline characteristics of patients (n=1154) who enrolled in the ART programme between 2004 and 2009
| Variable | N (%) | Median (IQR) | |
|---|---|---|---|
|
| |||
| Gender | Women | 752 (65.2) | |
| Men | 402 (34.8) | ||
|
| |||
| Age (years) | 31.9 (27.3; 37.5) | ||
|
| |||
| Residents in the study township | Yes | 1102 (95.5) | |
| No | 40 (3.5) | ||
| Unknown | 12 (1.0) | ||
|
| |||
| Transferred in from another ART service |
No | 1046 (90.6) | |
| Yes | 108 (9.4) | ||
|
| |||
| Year of initiating ART | 2004 | 137 (11.9) | |
| 2005 | 242 (21.0) | ||
| 2006 | 279 (24.2) | ||
| 2007 | 153 (13.3) | ||
| 2008 | 155 (13.4) | ||
| 2009 | 188 (16.3) | ||
|
| |||
| WHO clinical stage1 | 1 | 106 (9.3) | |
| 2 | 166 (14.5) | ||
| 3 | 585 (51.1) | ||
| 4 | 287 (25.1) | ||
|
| |||
| Baseline CD4 (cell/μl)2 | 122 (54; 190) | ||
10 missing values
114 missing values
A total of 291 patients defaulted treatment at least once (figure 1). Among these, 96 resumed therapy (treatment interruption) while 195 did not resume therapy during follow-up (lost to follow-up). Of the 96 individuals resuming therapy, 75 individuals had one episode of treatment interruption, 19 had two and 2 had three. The median time patients failed to receive ART was 228 days (IQR: 126-409) during the first episode of treatment default and 194 days (IQR 121-278) during the second episode. Thirty five patients who had stopped treatment underwent re-screening that included clinical assessment, laboratory tests and adherence counseling and yet did not resume therapy during the period of the study.
Subsequent analyses investigated first episode of treatment interruption by analyzing the time to stopping treatment for the first time and resuming therapy thereafter (non-shaded cells in figure 1).
Factors associated with the probability of defaulting treatment
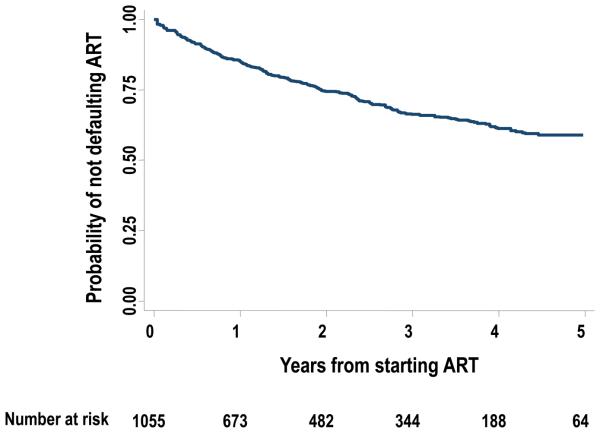
The overall rate of treatment default for the first time was 12.8/100 person years (PYs) (95% CI 11.4-14.4). The Kaplan-Meier estimate of the probability of defaulting treatment for at least 30 days was 14.9% (95% CI 12.7-17.4) by 1 year, 25.6% (95% CI 22.7-28.8) by 2 years and 41.0% (95% CI 37.0-45.3) by 5 years from ART initiation (figure 2).
Figure 2.
Kaplan Meier plot showing the probability of not defaulting antiretroviral therapy (ART) from the time of initiating ART up to the end of the 5th year of treatment
Factors associated with increased risk of defaulting therapy in univariate analysis were male gender, higher baseline CD4 count, recency of ART initiation and shorter duration on ART (table 2). Defaulting rate was highest in the first six months of ART (18.2/100 PYs, 95% CI 14.7-22.5) but decreased thereafter and had more than halved after two years (8.8/100 PYs, 95% CI 7.0-11.0).
Table 2.
Risk factors for defaulting treatment
| Variable | Number defaulting treatment |
Person years at risk |
Rate of default of treatment per 100PYs (95% CI) |
Unadjusted HR of default of treatment (95% CI) |
P valiue |
Adjusted HR of default of treatment (95% CI) |
P value |
|
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gender | Women | 172 | 1544 | 11.1 (9.6-12.9) | 1 | 1 | ||
| Men | 115 | 692 | 16.6 (13.9-20.0) | 1.49 (1.17-1.89) | <0.01 | 1.51 (1.18-1.93) | <0.01 | |
|
| ||||||||
| Age (years) | ≤30 | 196 | 1473 | 13.3 (11.6-15.3) | 1 | |||
| >30 | 91 | 762 | 11.9 (9.7-14.7) | 0.90 (0.70-1.15) | 0.40 | |||
|
| ||||||||
| Residents in the study township |
Yes | 271 | 2168 | 12.5 (11.1-14.1) | 1 | |||
| No | 10 | 65 | 15.4 (8.3-28.7) | 1.23 (0.66-2.32) | 0.52 | |||
|
| ||||||||
| Transferred from another ART service |
No | 268 | 2110 | 12.7 (11.3-14.3) | 1 | |||
| Yes | 19 | 127 | 15.0 (9.6-23.5) | 1.18 (0.74-1.88) | 0.48 | |||
|
| ||||||||
| WHO stage | 1 or 2 | 57 | 499 | 11.4 (8.8-14.8) | 1 | |||
| 3 or 4 | 209 | 1608 | 13.0 (11.4-14.9) | 1.14 (0.85-1.53) | 0.37 | |||
|
| ||||||||
| Baseline CD4 count (cells/μ) |
≤100 | 89 | 823 | 10.8 (8.8-13.3) | 1 | 1 | ||
| 101-200 | 103 | 716 | 14.4 (11.9-17.3) | 1.33 (1.00-1.77) | 0.05 | 1.32 (0.99-1.76) | 0.06 | |
| >200 | 73 | 530 | 13.8 (10.9-17.3) | 1.27 (0.83-1.73) | 0.13 | 1.39 (1.02-1.91) | 0.04 | |
|
| ||||||||
| Year of initiating ART1 |
2004 | 24 | 434 | 5.5 (3.7-8.2) | ||||
| 2005 | 76 | 703 | 10.8 (8.6-13.5) | |||||
| 2006 | 83 | 599 | 13.9 (11.2-17.2) | 1.36 (1.24-1.48) | <0.01 | 1.30 (1.17-1.44) | <0.01 | |
| 2007 | 47 | 247 | 19.0 (14.3-25.3) | |||||
| 2008/2009 | 57 | 253 | 22.6 (17.4-29.3) | |||||
|
| ||||||||
| Time on ART | <6months | 84 | 462 | 18.2 (14.7-22.5) | 1 | 1 | ||
| 6months-2year | 130 | 939 | 13.8 (11.7-16.4) | 0.76 (0.58-1.00) | 0.05 | 0.86 (0.65-1.15) | 0.31 | |
| >2years | 73 | 834 | 8.8 (7.0-11.0) | 0.48 (0.35-0.66) | <0.01 | 0.69 (0.48-0.98) | 0.04 | |
p value for test for departure from linear trend 0.35
Gender, baseline CD4 count, time on ART, and date of initiation remained significantly associated with defaulting in the multivariate model. Men were 1.51 (95% CI 1.18-1.93) times more likely to default treatment compared to women, as were those patients with a higher baseline CD4 count. The adjusted hazard of defaulting treatment increased by 1.30 (95% CI 1.17-1.44) for each calendar year. Patients on treatment for more than 2 years had a lower hazard of 0.69 (95% CI 0.48-0.98) of defaulting compared to patients during the first 6 months of treatment. Similar results were found in a sensitivity analysis that excluded individuals whose vital status could not be ascertained.
Factors associated with the probability of resuming therapy
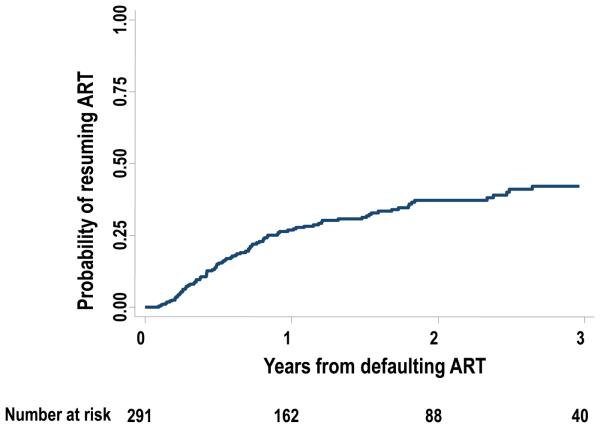
A total of 291 patients defaulted treatment at least once. The overall rate of treatment resumption after defaulting treatment for the first time was 21.4/100 PYs (95% CI 17.5-26.2) (figure 3). The Kaplan-Meier cumulative estimate of the probability of treatment resumption was 26.7% (95% CI 21.7-32.7) in the first year, 37.1% (95% CI 31.1-43.9) in the second year and 42.1% (95% CI 35.2-49.7%) in the third year after stopping treatment.
Figure 3.
Kaplan Meier plot showing the probability of resuming antiretroviral therapy (ART) from the time of defaulting therapy up to 3 years after defaulting treatment
In univariate analysis a greater likelihood of resuming ART was associated with older age and shorter time since defaulting (table 3); gender, residency, calendar year of defaulting and CD4 count nearest to the time of defaulting was not associated with resuming treatment.
Table 3.
Risk factors for resuming treatment after defaulting
| Variable | Number resuming treatment |
Person years at risk |
Rate of restarting treatment per 100PYs (95% CI) |
Unadjusted IRR of restarting treatment (95% CI) |
P value |
Adjusted IRR of restarting treatment (95% CI) |
P value |
|
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gender | Women | 61 | 253 | 24.1 (18.7-31.0) | 1 | 1 | ||
| Men | 32 | 182 | 17.6 (12.5-24.9) | 0.73 (0.48-1.12) | 0.15 | 0.67 (0.43-1.04) | 0.07 | |
|
| ||||||||
| Age (years) | ≤30 | 26 | 174 | 15.0 (10.2-22.0) | 1 | 1 | ||
| >30 | 67 | 261 | 25.7 (20.2-32.6) | 1.72 (1.09-2.70) | 0.02 | 1.80 (1.13-2.86) | 0.01 | |
|
| ||||||||
| Residents | Yes | 87 | 405 | 21.5 (17.4-26.5) | 1 | |||
| No | 5 | 14 | 34.5 (14.4-82.9) | 1.60 (0.65-3.96) | 0.30 | |||
|
| ||||||||
| CD4 count at time of defaulting (cells/μ) |
≤200 | 36 | 155 | 23.4 (16.8-32.4) | 1 | |||
| > 200 | 57 | 280 | 20.3 (15.7-26.3) | 0.87 (0.57-1.32) | 0.52 | |||
|
| ||||||||
| Year of defaulting treatment |
2004/2005 | 9 | 60 | 15.0 (17.8-28.9) | 1 | |||
| 2006 | 23 | 122 | 18.8 (12.5-28.3) | 1.25 (0.58-2.70) | 0.57 | |||
| 2007 | 29 | 127 | 22.7 (15.8-32.6) | 1.51 (0.72-3.19) | 0.28 | |||
| 2008 | 32 | 93 | 21.4 (13.8-33.2) | 1.42 (0.65-3.13) | 0.38 | |||
| 2009 | 12 | 31 | 38.5 (21.9-67.8) | 2.57 (1.08-6.09) | 0.03 | |||
|
| ||||||||
| Time off ART | <1 year | 68 | 222 | 31.6 (24.2-38.9) | 1 | 1 | ||
| >1year | 25 | 213 | 11.7 (7.9-17.4) | 0.38 (0.24-0.61) | <0.01 | 0.40 (0.25-0.63) | <0.01 | |
In multivariate analysis men were less likely to resume treatment compared to women (IRR 0.67, 95% CI 0.43-1.04, p=0.07); whereas patients >30 years old were more likely to restart treatment (IRR 1.80, 95% CI 1.13-2.89). The likelihood of resuming treatment decreased significantly beyond one year of defaulting treatment (IRR 0.40, 95% CI 0.25-0.63).
Of the 96 patients resuming therapy, 86 had a CD4 count measurement while receiving therapy and before the treatment interruption; the majority of these (80) responded to ART with an increase in CD4. Patients who resumed therapy were found to have a median CD4 count (150.5 cells/μl, IQR: 73-266) comparable to their baseline CD4 count prior to initiating therapy (138.5 cells/μl, IQR: 73-188). The median time between the measurement of CD4 count and resuming therapy was 13 days (IQR 0-28 days).
Excluding individuals with unascertained vital status revealed similar results with regards to parameter estimates, but the association with male gender became non-significant (HR 0.81, 95% CI 0.52-1.26, p=0.35).
Discussion
To our knowledge, this is the first study from sub-Saharan Africa to report on unstructured treatment interruptions in a routine programme setting. Our analysis shows that treatment interruption is a common phenomenon. The probability of ART defaulters to resume therapy within 3 years was 42%. Most ART cohorts report on loss to follow-up defined as not attending the clinic for more than 3 months,8 and assume that loss to follow-up is an irreversible event. Our study shows that patients who fulfil the widely used definition of loss to follow-up at one time point might resume therapy later. In this cohort, the median duration of the first treatment interruption was 7.5 months.
The median CD4 count of those resuming therapy was similar to their initial CD4 count prior to starting treatment, which underscores the potentially negative impact of interruption leading to a reversal in immunological recovery made while on treatment. Data from industrialised settings suggest that treatment interruption has detrimental effects on CD4 count, viral load suppression, and clinical progression.11, 12, 19 Programmes that report patient attrition and the number of patients in care will not account for the potential that up to 14% of patients in care have interrupted treatment at least once.
We were able to determine risk factors for defaulting ART and factors associated with resuming therapy. Male gender, high baseline CD4 count, recency of ART initiation and the first 6 months of treatment were associated with a higher risk of defaulting. Treatment resumption was more likely in women, patients more than 30 years old and within the first year of stopping therapy.
Our finding that men were at higher risk of defaulting treatment and less likely to resume treatment is consistent with studies showing that HIV-infected men are less likely to access treatment25, 26, have an increased risk for loss to follow-up in the pre-treatment period27, present with more advanced stages of HIV disease28 and have a higher mortality risk on ART.2, 9, 29-33 Strategies to diagnose HIV in men earlier and to link and to retain them in care might include: i) extending clinic hours into evenings and weekends, ii) training male health care staff and counsellors, iii) offering additional adherence sessions to men and iv) initiating male support groups.
Individuals initiating treatment in more recent years were more likely to default, suggesting that programmatic factors might influence retention in care. A study including data from 15 treatment cohorts from Africa, Asia and South America showed that early patient losses were increasingly common when programs were scaled up.6 Increasing cohort size in an environment of scarce human resources for health has been suggested to influence both the scale-up capacity and the long-term retention in ART programs.34 In the study clinic resources and staffing were further reduced when enrolment for the NIH-funded study finished in 2007. In contrast, year of defaulting was not associated with resumption of treatment, suggesting that patient tracing was less influenced by cohort size (although this would vary according to tracing procedures).
Treatment defaulting was more likely in patients with less advanced immunodeficiency at baseline. This may be explained by the fact that individuals who default treatment and stay alive do so because they feel better on treatment, a phenomenon that has been reported by other studies.35 This finding is particularly important in view of the 2009 WHO guidelines recommending ART initiation at CD4 counts below 350 cells/ μl36 and when considering initiation of ART regardless of CD4 count as proposed in the ‘test and treat’ strategy.37 Initiating ART at the time of HIV diagnosis will result in increased numbers of relatively immunocompetent individuals on ART who may have a higher risk of defaulting treatment. Specific interventions aimed at these individuals need to be developed to ensure optimal retention in care.
This study has several limitations. First, ascertainment of vital status for treatment defaulters was incomplete, which may have led to a misclassification of deaths as defaulters. However, sensitivity analysis excluding individuals with unascertained vital status did not influence our overall findings. Second, resumption of therapy was not ascertained in patients who moved to other communities, possibly resulting in underestimation of treatment resumption. Third, the clinical and immunological consequences of treatment interruption were not analysed due to lack of laboratory data, in particular the lack of capacity to perform routine viral load, and the small number of individuals resuming therapy. However it has been shown in industrialised settings that treatment interruption impacts negatively on CD4 count, viral load suppression and clinical progression.11, 12, 19
We consider that the main finding of this study that a considerable proportion of treatment defaulters return to care is likely to be generalisable to similar settings. Nevertheless, risk factors for defaulting and resuming therapy might differ with regards to eligibility criteria and resources available for patient tracing.
A strength of this study is that the relatively large sample size and follow up time. This allows for an assessment of risk factors for defaulting and treatment interruption that in turn allows for several proposals to be made to limit defaulting and treatment interruption in similar programme settings. In particular, interventions to keep patients in care should be targeted at men, patients with higher CD4 counts and during the first 6 months of ART. Moreover, the finding that the probability of resuming therapy was highest in the first year following treatment defaulting suggests that efforts to bring patients back into care might be most successful early into defaulting treatment.
Acknowledgements
The authors gratefully acknowledge the dedicated staff of the ART clinic and the Desmond Tutu HIV Centre in particular Dr Philip Ginsberg and Carl Morrow.
Disclosure of funding: KK and SDL are funded by the Wellcome Trust, London, UK. JL is funded by the Consortium to Respond Effectively to the AIDS TB Epidemic, who received funding from the Bill and Melinda Gates foundation. RW is funded in part by the National Institutes of Health (NIH) through a CIPRA grant 1U19AI53217-01 and RO1 grant (A1058736-01A1).
Footnotes
Conflict of Interest Statement
No conflicts of interest to declare.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Towards universal access: scaling up priority HIV/AIDS interventions in the health sector : progress report 2009. World Health Organization; Geneva, Switzerland: 2009. Available from: http://www.who.int/hiv/pub/tuapr_2009_en.pdf last accessed 16/04/2010. [Google Scholar]
- 2.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006 Nov;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachega JB, Mills EJ, Schechter M. Antiretroviral therapy adherence and retention in care in middle-income and low-income countries: current status of knowledge and research priorities. Curr Opin HIV AIDS. Jan;5(1):70–77. doi: 10.1097/COH.0b013e328333ad61. [DOI] [PubMed] [Google Scholar]
- 4.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007 May;12(5):687–694. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 5.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. Aids. 2003 Jun 13;17(9):1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 6.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008 Jul;86(7):559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007 Oct 16;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. Aids. 2008 Oct 1;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bisson GP, Gaolathe T, Gross R, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3(3):e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holkmann Olsen C, Mocroft A, Kirk O, et al. Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med. 2007 Mar;8(2):96–104. doi: 10.1111/j.1468-1293.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 12.Touloumi G, Pantazis N, Antoniou A, Stirnadel HA, Walker SA, Porter K. Highly active antiretroviral therapy interruption: predictors and virological and immunologic consequences. J Acquir Immune Defic Syndr. 2006 Aug 15;42(5):554–561. doi: 10.1097/01.qai.0000230321.85911.db. [DOI] [PubMed] [Google Scholar]
- 13.Taffe P, Rickenbach M, Hirschel B, et al. Impact of occasional short interruptions of HAART on the progression of HIV infection: results from a cohort study. Aids. 2002 Mar 29;16(5):747–755. doi: 10.1097/00002030-200203290-00010. [DOI] [PubMed] [Google Scholar]
- 14.d'arminio Monforte A, Cozzi-Lepri A, Phillips A, et al. Interruption of highly active antiretroviral therapy in HIV clinical practice: results from the Italian Cohort of Antiretroviral-Naive Patients. J Acquir Immune Defic Syndr. 2005 Apr 1;38(4):407–416. doi: 10.1097/01.qai.0000147529.57240.b0. [DOI] [PubMed] [Google Scholar]
- 15.Seminari E, De Silvestri A, Boschi A, Tinelli C. CD4+ guided antiretroviral treatment interruption in HIV infection: a meta-analysis. AIDS Rev. 2008 Oct-Dec;10(4):236–244. [PubMed] [Google Scholar]
- 16.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 17.Mugyenyi P, Walker A, Hakim J, et al. Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts < 200 cells/microl. Aids. 2008 Jan 11;22(2):237–247. doi: 10.1097/QAD.0b013e3282f2d760. [DOI] [PubMed] [Google Scholar]
- 18.Skiest DJ, Morrow P, Allen B, et al. It is safe to stop antiretroviral therapy in patients with preantiretroviral CD4 cell counts >250 cells/microL. J Acquir Immune Defic Syndr. 2004 Nov 1;37(3):1351–1357. doi: 10.1097/00126334-200411010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Poulton MB, Sabin CA, Fisher M. Immunological changes during treatment interruptions: risk factors and clinical sequelae. Aids. 2003 Jan 3;17(1):126–128. doi: 10.1097/01.aids.0000042587.93174.83. [DOI] [PubMed] [Google Scholar]
- 20.Sungkanuparph S, Kiertiburanakul S, Apisarnthanarak A, Malathum K, Watcharananan S, Sathapatayavongs B. Rapid CD4 decline after interruption of non-nucleoside reverse transcriptase inhibitor-based antiretroviral therapy in a resource-limited setting. AIDS Res Ther. 2007;4(26):26. doi: 10.1186/1742-6405-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007 May 11;21(8):965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 22.Bansi LK, Benzie AA, Phillips AN, et al. Are previous treatment interruptions associated with higher viral rebound rates in patients with viral suppression? AIDS. 2008 Jan 30;22(3):349–356. doi: 10.1097/QAD.0b013e3282f4709a. [DOI] [PubMed] [Google Scholar]
- 23.Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS ONE [Electronic Resource] 2008;3(7):e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007 Jan 1;175(1):87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remien RH, Chowdhury J, Mokhbat JE, Soliman C, Adawy ME, El-Sadr W. Gender and care: access to HIV testing, care, and treatment. J Acquir Immune Defic Syndr. 2009 Jul 1;51(51):S106–110. doi: 10.1097/QAI.0b013e3181aafd66. Suppl 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muula AS, Ngulube TJ, Siziya S, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7(63):63. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amuron B, Namara G, Birungi J, et al. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9(290):290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009 Jul;14(7):722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacPherson P, Moshabela M, Martinson N, Pronyk P. Mortality and loss to follow-up among HAART initiators in rural South Africa. Trans R Soc Trop Med Hyg. 2009 Jun;103(6):588–593. doi: 10.1016/j.trstmh.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Zachariah R, Harries K, Moses M, et al. Very early mortality in patients starting antiretroviral treatment at primary health centres in rural Malawi. Trop Med Int Health. 2009 Jul;14(7):713–721. doi: 10.1111/j.1365-3156.2009.02291.x. [DOI] [PubMed] [Google Scholar]
- 31.Sieleunou I, Souleymanou M, Schonenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009 Jan;14(1):36–43. doi: 10.1111/j.1365-3156.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 32.Manosuthi W, Chaovavanich A, Tansuphaswadikul S, et al. Incidence and risk factors of major opportunistic infections after initiation of antiretroviral therapy among advanced HIV-infected patients in a resource-limited setting. J Infect. 2007 Nov;55(5):464–469. doi: 10.1016/j.jinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Nachega JB, Hislop M, Dowdy DW, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006 Sep;43(1):78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 34.Assefa Y, Van Damme W, Hermann K. Human resource aspects of antiretroviral treatment delivery models: current practices and recommendations. Curr Opin HIV AIDS. Jan;5(1):78–82. doi: 10.1097/COH.0b013e328333b87a. [DOI] [PubMed] [Google Scholar]
- 35.Dahab M, Charalambous S, Hamilton R, et al. “That is why I stopped the ART”: patients' & providers' perspectives on barriers to and enablers of HIV treatment adherence in a South African workplace programme. BMC Public Health. 2008;8(63):63. doi: 10.1186/1471-2458-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapid advice: Antiretroviral therapy for HIV infection in adults and adolescents. World Health Organization; Geneva: 2009. Available from: http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf accessed March 2010. [PubMed] [Google Scholar]
- 37.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]