Abstract
The widely used nonobese diabetic (NOD) mouse model of autoimmune (Type 1) diabetes mellitus shares multiple characteristics with the human disease, and studies employing this model continue to yield clinically relevant and important information. Here, we review some of the recent key findings obtained from NOD mouse investigations that have both advanced our understanding of disease pathogenesis and suggested new therapeutic targets and approaches. Areas discussed include antigen discovery, identification of genes and pathways contributing to disease susceptibility, development of strategies to image islet inflammation and the testing of therapeutics. We also review recent technical advances that, combined with an improved understanding of the NOD mouse model’s limitations, should work to ensure its popularity, utility and relevance in the years ahead.
Keywords: antigens, autoimmune diabetes, humanized mice, NOD mice, therapies, Type 1 diabetes
The nonobese diabetic (NOD) mouse model of (autoimmune) Type 1 diabetes first appeared in the scientific literature in 1980 [1]. As it became available to researchers worldwide, this inbred model of spontaneous disease was rapidly embraced and remains a mainstay of current Type 1 diabetes research (FIGURE 1). The model is not without its critics, whose feelings stem primarily from the disappointing realization that while multiple manipulations have been reported to prevent disease in NOD mice [2], this goal has not yet been achieved in people. However, the disease in the mouse model has numerous striking similarities to that in humans, and these serve to explain the continued utility of NOD mice as a research tool. These similarities include common antigenic targets (TABLE 1), the expression of class II MHC molecules (i.e., I-Ag7 and HLA-DQ8) displaying related peptides [3] and genetic polymorphisms affecting shared pathways [4]. Indeed, there are numerous instances where discoveries made in NOD mice were found to parallel the human disease. As just one example, the β-cell protein islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) was first identified as a target of islet-reactive CD8+ T cells in NOD mice [5]. Subsequent work revealed that IGRP peptides are also targeted by CD8+ T cells in patients with Type 1 diabetes [6–8]. In addition to antigen discovery, other areas of utility for the NOD mouse include identification of disease susceptibility genes, testing of therapeutics and identification of new targets, exploration of environmental influences on disease and the development of imaging strategies (FIGURE 2). The purpose of this article is to provide an update on progress made in these areas, with the help of NOD mice, from 2005 to the present day. Special mention will be made of those developments having established or potential applicability to Type 1 diabetes in humans. Recent technical advances, such as the availability of NOD-derived embryonic stem (ES) cells [9,10] and the development of increasingly ‘humanized’ NOD strains [11,12], will also be discussed, as they are likely to further increase both the popularity and the utility of the NOD mouse model in the study of Type 1 diabetes.
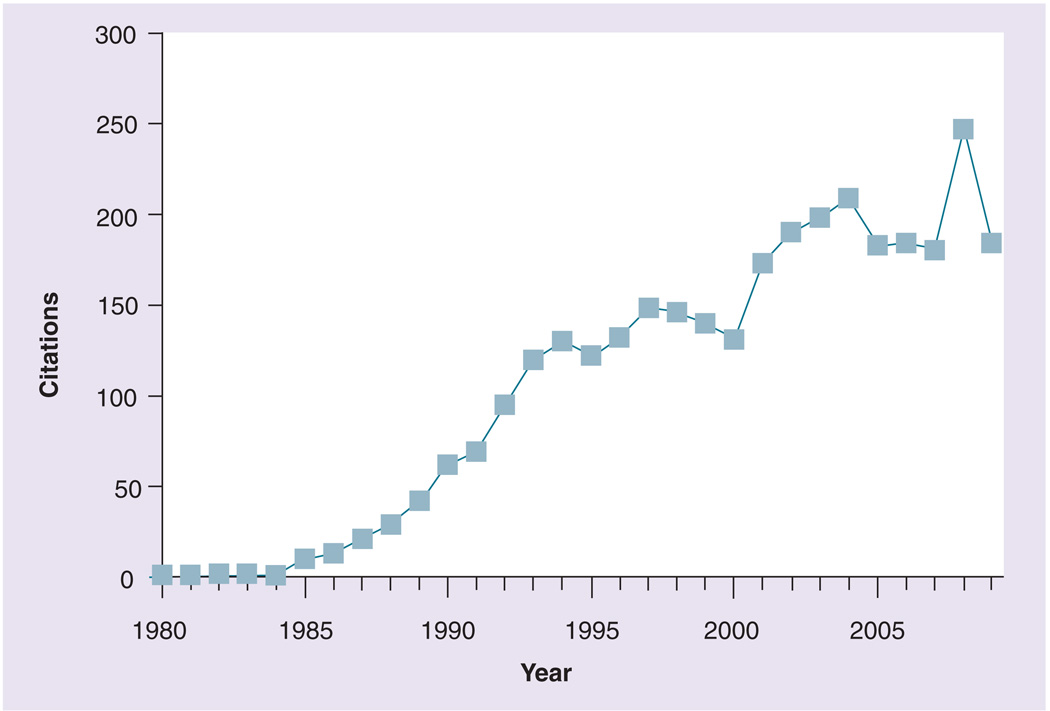
Figure 1. The nonobese diabetic mouse model continues to be a mainstay of Type 1 diabetes research.
The PubMed database was searched using the search string ‘NOD mice, diabetes.’ The plot depicts retrieved citations for each year, excluding review articles.
Table 1.
Original descriptions and discovery strategies for Type 1 diabetes antigens.
| Antigen | Discovery strategy | Human responses | NOD responses | ||
|---|---|---|---|---|---|
| Antibody | T cell | Antibody | T cell | ||
| Insulin | A | [99] | [100] | [101] | [102] |
| GT3 | A | [103] | |||
| HSP-60 | A | [104] | [105] | [106] | [106] |
| GAD65 | A | [107] | [108] | [109] | [109] |
| IAPP | A | [110] | [111] | ||
| CPE (CPH) | E | [112] | [113] | [109] | [109] |
| IA-2 (ICA512) | E | [114] | [115] | [116] | |
| GLUT2 | A | [117] | [118] | [119] | |
| Jun-B | E | [120] | [120] | ||
| GAD67 | A | [121] | [122] | [123] | |
| ICA69 | E | [124] | [125] | [126] | |
| Sulfatide | A | [127] | |||
| Peripherin | A | [109] | [109] | ||
| GD3 | A | [128] | |||
| Imogen38 | E | [129] | |||
| DNA TopII | A | [130] | |||
| Glima38 | A | [131] | |||
| IA-2β (phogrin) | A | [132] | [133] | [134] | |
| GM2–1 | A | [135] | |||
| Osteopontin | R | [136] | |||
| CD38 | A | [137] | |||
| SOX13 | E | [138] | |||
| HIP/PAP | E | [139] | |||
| S100β | A | [30] | [30] | ||
| GFAP | A | [30] | [30] | [30] | [30] |
| HSP-90 | A | [140] | |||
| HSP-70 | A | [141] | [141] | ||
| IGRP | B | [142] | [5] | ||
| REG | A | [143] | |||
| DMK | R | [27] | |||
| Importin β | R | [144] | |||
| REGII | A | [145] | |||
| ZNT8 | C | [28] | |||
| CAII | A | [146] | |||
| LAC | A | [146] | |||
| AMY2A | E | [147] | |||
| HSP10 | E | [148] | |||
| PDX1 | A | [29] | [29] | [29] | |
| CHGA | B | [21] | |||
Antigens are listed in the order of their discovery. The earliest descriptions of NOD or human antibody or T-cell responses are referenced.
The strategy used to initially discover each antigen is denoted as follows: A: Purified candidate antigen challenge; B: Biochemical fractionation; C: Computational prediction; E: Expression cloning; R: Random peptide library.
NOD: Nonobese diabetic.
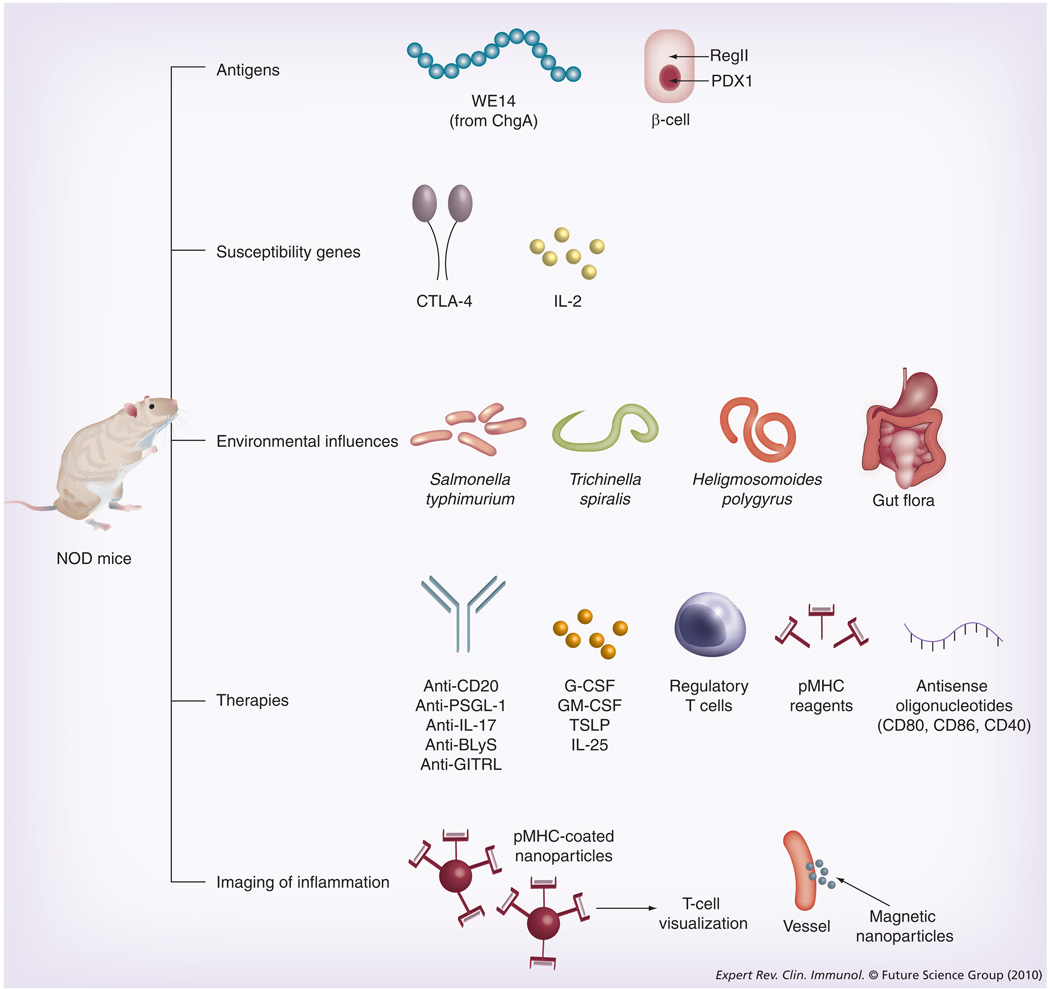
Figure 2. Recent contributions of the nonobese diabetic mouse model to our understanding of Type 1 diabetes have occurred in the indicated areas.
Examples of discoveries covered within this review are shown. See text for details and references. G-CSF: Granulocyte colony-stimulating factor; GM-CSF: Granulocyte-macrophage colony-stimulating factor; NOD: Nonobese diabetic; PDX1: Pancreatic duodenal homeobox 1.
Antigen discovery
Our understanding of disease pathogenesis has been greatly enhanced through the study of Type 1 diabetes antigens in NOD mice (TABLE 1). A large body of evidence now supports a key role for insulin autoreactivity in diabetogenesis [13]. Pathogenic responses to insulin in NOD mice are directed at Ins1/2B9–23 and Ins1/2B15–23 in CD4+ [14] and CD8+ T cells [15], respectively. NOD mice have been generated that are null for Ins1 and Ins2 but express a transgenic Ins2 gene in which InsB16 has been altered from Y to A [16], preventing both binding of InsB15–23 to H-2Kd and activation of InsB9–23-specific CD4+ T cells. The transgenic mice do not develop Type 1 diabetes or any signs of β-cell-specific autoimmunity, suggesting the importance of InsB9–23 and/or InsB15–23 in the development of Type 1 diabetes in NOD mice. Indeed, in NOD mice, establishment of tolerance to insulin can lead to prevention of diabetes [17] as well as remission of established disease [18].
Studies in NOD mice have also suggested structural and antigen-processing characteristics of autoantigens, which may contribute to pathogenicity. InsB9–23 binds weakly to the NOD MHC class II molecule I-Ag7 in two adjacent peptide-binding registers, which are recognized by different subsets of CD4+ T cells [19]. Similarly, a subset of pathogenic InsB9–23-reactive T cells does not respond to exogenous insulin processed by antigen-presenting cells, but does respond if the antigen-presenting cells are in contact with β-cells or have been fed secretory granules purified from β-cells [20]. The use of a monoclonal antibody specific for InsB9–23 but not proinsulin or insulin recently revealed InsB9–23-positive granules in a subset of β-cells [20]. Taken together, these results suggest an antigenic property of InsB9–23, which is specifically conferred by β-cells, possibly reflecting a conformational or posttranslational modification of the peptide. Along these lines, a natural cleavage product of the neuroendocrine protein chromogranin A, WE14, was recently identified as the target of the highly pathogenic NOD-derived CD4+ T-cell clone BDC2.5 [21]. Binding of WE14 to I-Ag7 is atypical in that it is mediated by the C-terminal portion of the peptide-binding groove and seemingly by regions outside of the binding groove of I-Ag7. Whether and how this unusual binding may permit escape of BDC2.5-like T cells during thymic negative selection awaits additional studies. However, some possibilities in this regard include low-affinity binding to I-Ag7 (poor antigen presentation) or inefficient interactions of the WE14/I-Ag7 complex with the T-cell receptor. These studies of insulin and chromogranin A have therefore contributed to a model of diabetes pathogenesis in which autoreactive T cells escape thymic negative selection because recognition of their cognate antigens is compromised due to weakly or atypically bound peptides, and/or due to β-cell-dependent constraints on antigen processing or presentation [22].
Several islet autoantigens targeted in Type 1 diabetes have yet to be identified. For example, CD4+ and CD8+ T-cell clones of unknown specificity that are pathogenic in transfer systems, transgenic mice and retrogenic mice, and are known to recognize islet antigens, have been isolated from NOD mice [23,24]. Moreover, the islet-infiltrating T-cell repertoire is thought to be complex, especially at later stages of inflammation [25], but also antigen specific [26]. In addition, known CD8+ T-cell specificities account for a minority of infiltrating CD8+ T cells [27]. Previous methods for Type 1 diabetes antigen identification have been laborious and slow, relying on biochemical or genetic screens, or on the testing of candidate antigens whose identity was suggested by extensive prior investigations (TABLE 1). A recent bioinformatic search for genes highly and preferentially expressed in islet β-cells identified the Type 1 diabetes antigen ZNT8 [28], suggesting that the computational prediction of Type 1 diabetes antigens is feasible. Indeed, pancreatic duodenal homeobox 1 (PDX1) was another high-ranking potential antigen predicted in this study, which was recently identified independently as a Type 1 diabetes antigen [29]. Analysis of islet transcripts represented in the Unigene database suggests the existence of a large set of islet-specific genes that is enriched for known Type 1 diabetes antigens (FIGURE 3). To our knowledge, every β-cell-specific protein examined to date is a target of either antibody or T-cell responses in either NOD mice or humans, and most are shared (TABLE 1). Some islet-specific, non-β-cell antigens have also been described [30]. If the relationship between islet specificity and antigenicity proves to be robust, hundreds of unidentified islet-specific antigens may await discovery (FIGURE 3). We therefore advocate a systematic examination of islet-specific genes as potential Type 1 diabetes antigens. Of course, the number of potential T-cell and antibody epitopes among these antigens will be considerably larger.
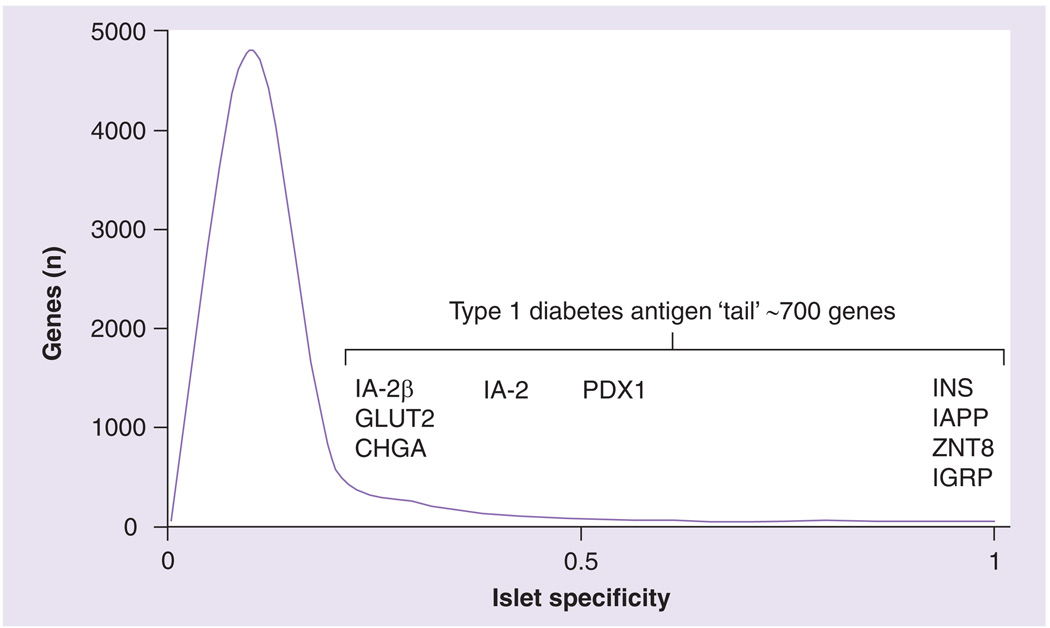
Figure 3. Islet-specific genes remain largely unexplored as Type 1 diabetes antigens.
Islet specificity was calculated as the frequency of a given transcript within the Unigene mouse islets expressed sequence tag library 16013 [178] divided by the cumulative frequency of that transcript in all tissues represented by the Unigene ‘profiles’ dataset excluding whole pancreas [211,212]. The islet library contained 24,059 expressed sequence tags corresponding to 6451 unique genes. Gene bins are 0.1 islet specificity units wide. Known islet-specific Type 1 diabetes antigens are positioned along the smoothed distribution according to their islet specificity values. Approximately 700 genes span the region bounded by IA-2β at the left and INS at the right, suggesting a rich source of as-yet unidentified Type 1 diabetes antigens.
Nonobese diabetic mice provide the only model that can address this scale of antigen discovery efficiently. Importantly, NOD mice are amenable to high-throughput technologies that can interrogate the relevant pathogenic cell types directly. The recent finding that entry and accumulation of T cells in the islets of NOD mice requires the T cells to be specific for islet antigens [26] supports the use of islet-infiltrating cells in antigen discovery efforts. NOD mice can also be used to test the pathogenic hierarchy of candidate antigens by tolerogenic [17], gene ablative and/or mutational approaches [16], while HLA and human antigen transgenic and knock-in mice should help in the translation of these findings to human disease. Finally, retrogenic mice should enable rapid assessment of novel T-cell receptor specificities with respect to pathogenicity as they are discovered [23]. A detailed knowledge of the pathogenic response in NOD mice should inform the development of therapies, clinical trials and prognostic markers for Type 1 diabetes.
Identification of disease susceptibility genes
Nonobese diabetic mice and derivative congenic and recombinant congenic strains have been used extensively for the mapping of autoimmune diabetes susceptibility loci (designated Idd for insulin-dependent diabetes) and nearly 50 such loci have been reported to date [31]. In some cases, a candidate gene that is probably responsible (alone or in part) for the disease-promoting effect of an Idd locus has also been identified [31]. For Idd5.1, one such gene is Ctla4 [32,33]. In NOD mice, a polymorphism that affects RNA splicing results in diminished production of a CTLA-4 isoform that lacks the ligand-binding domain [33] and signals negatively in T cells [34]. Importantly, Ctla4 is also associated with susceptibility to Type 1 diabetes in humans, although the molecular explanation is thought to be different and to involve decreased production of a soluble form of CTLA-4 [33] that is capable of inhibiting T-cell proliferation by binding to CD80 and CD86 [35].
Nonobese diabetic mouse studies greatly facilitated the progress that was recently made in the elucidation of another shared pathway for Type 1 diabetes development in NOD mice and humans, namely, that involving IL-2 and its receptor [4]. While Il2 has long been suspected to be a causal gene for the effect of Idd3 on diabetes development in NOD mice, compelling evidence for this was only recently provided [36]. Sequencing of the Il2 gene from a panel of diabetes-resistant and susceptible mouse strains revealed numerous disease-associated single-nucleotide polymorphisms, although none were nonsynonymous or obviously capable of altering RNA splicing. However, thymocytes, splenocytes and anti-CD3-stimulated splenocytes from NOD mice all contained lower amounts of Il2 mRNA when compared with their counterparts isolated from NOD.B6 Idd3 mice. Reduced IL-2 production correlated with a smaller proportion of Foxp3+CD4+ T cells in the pancreatic lymph nodes and reduced functional competence of CD4+CD25+ regulatory T cells, suggesting a mechanism for the association of Idd3 with diabetes susceptibility. Importantly, susceptibility to Type 1 diabetes in humans has been mapped to the gene encoding IL-2Rα (CD25) [37,38], and defects in regulatory T cells have been reported in Type 1 diabetes patients [39,40]. These results illustrate the utility of NOD mice in the identification of disease susceptibility genes (or affected pathways) with human relevance, and they also highlight the power of the mouse model in clarifying the mechanisms responsible for the observed disease associations.
Exploration of environmental influences
The cumulative diabetes incidence observed for female NOD mice housed at different institutes varies wildly (i.e., from 15 to 100%), suggesting that the environment can influence the development of autoimmune diabetes [41]. While such environmental factors remain largely ill defined, the finding that reduction in microbial load increases diabetes incidence in NOD mice [42] fostered interest in the potential protective nature of microbes (or their components) that continues today. Indeed, deliberate infection of NOD mice with a variety of specific organisms, including Schistosoma mansoni [43], Mycobacterium avium [44], Trichinella spiralis [45], Heligmosomoides polygyrus [45] and Salmonella typhimurium [46], inhibits diabetes development. As reviewed [47], the potential mechanisms responsible for these protective effects include Th2 skewing, increased regulatory T-cell and natural killer T-cell activity, and modulation of antigen-presenting cells towards a diabetes-protective phenotype.
Recently, the seminal observation was made that interaction between the intestinal flora and the innate immune system in NOD mice can influence the development of Type 1 diabetes [48]. In this study, NOD mice genetically deficient in MyD88, an adaptor protein for multiple Toll-like receptors, were found to be resistant to diabetes development and to exhibit altered gut flora. Strikingly, transfer of this altered flora rendered germ-free NOD mice resistant to Type 1 diabetes. Interestingly, a probiotic compound (VSL#3; a mixture of Bifidobacterium and Lactobacillus species and Streptococcus salivarius subsp. thermophilus) [49] and the fungal cell wall constituent zymosan [50] were among the agents tested in NOD mice from 2005 to the present that have been shown to delay or prevent disease (TABLE 2). As knowledge in this area continues to expand, informed application to Type 1 diabetes in humans may be suggested.
Table 2.
Strategies that delay or prevent diabetes in nonobese diabetic mice (2005–present).
| Agent | Details | Diabetes incidence | Ref. | ||
|---|---|---|---|---|---|
|
Control (%) |
Treated (%) |
Age (weeks) |
|||
| G-CSF | 200 µg/kg sc. daily for 5 days at 4, 8, 12 and 16 weeks | 75 | 8 | 30 | [149] |
| Myeloid progenitors expressing proinsulin | Generated in GM-CSF and TGF-β from bone marrow of NOD mice expressing Ins2 under the control of a class II MHC promoter; 2 × 106 cells iv. at 4 weeks | 83 | 33 | 34 | [150] |
| FTY720 | Sphingosine-1-phosphate analog; 1 mg/kg by oral gavage 5-times/week at 23–37 weeks | 78 | 0 | 37 | [151] |
| Altered peptide ligand of IGRP206–214 | 100 µg NRP-I4 ip. every 2 weeks at 4–8 weeks and every 3 weeks thereafter | 60 | 0 | 30 | [152] |
| Anti-PSGL-1 | Apoptosis-inducing anti-PSGL-1 (TAB4); 50 µg ip. at 8, 9, 10, 18, 19 and 20 weeks | 83 | 29 | 27 | [153] |
| GAD65260–279 (an ignored determinant) | Also known as GADp18; 100 µg in IFA at 12 weeks and also 10 days later | 93 | 27 | 45 | [154] |
| Probiotic bacteria | VSL#3 (3 × 1011 viable lyophilized bacteria/g); 3 mg by oral gavage 3-times/week at 4–32 weeks | 81 | 21 | 32 | [49] |
| CXCL10 neutralization | CXCL10 antibodies induced by plasmid DNA vaccination; 100 µg im. by electroporation (50 µg each side) at 4 and 6 weeks | 58 | 26 | 30 | [155] |
| p38α-specific MAPK inhibitor | Indole-5-carboxamide (SD-169); 600 mg/kg in chow at 8–18 weeks | 60 | 10 | 18 | [156] |
| Regulatory T cells | CD4+CD25+CD62L+ cells from NOD.BDC2.5 mice expanded in vitro with antigen-pulsed DCs and IL-2; 5 × 104 cells at 13 weeks | 88 | 33 | 44 | [157] |
| CCL4 | Intradermal gene transfer using EBNA1/oriP-based vector; 1 µg weekly at 9–14 weeks | 78 | 30 | 35 | [158] |
| (S,R)-3-phenyl-4,5-dihydro-5-isoxazole acetic acid | VGX-1027; 20 mg/kg ip. 6-times/week at 12–25 weeks | 81 | 31 | 25 | [159] |
| Cytopiloyne | 25 µg/kg ip. or im. 3-times/week beginning at 4 weeks | 67 | 0 | 30 | [160] |
| Apoptotic β-cells | 1 × 105 UVB-irradiated NIT-1 cells iv. weekly at 10–12 weeks | 100 | 62 | 30 | [161] |
| GM-CSF | 100 ng ip.; 3-times/week for 3 weeks and 2-times/week thereafter | 86 | 21 | 52 | [162] |
| Anti-CD20 | B cell-depleting anti-CD20 (MB20–11); 250 µg iv. at 5, 7 and 9 weeks | 100 | 36 | 40 | [57] |
| Microspheres carrying antisense oligonucleotides | Antisense oligonucleotides to CD40, CD80 and CD86; sc. proximal to pancreatic lymph nodes weekly at 5–13 weeks | 100 | 14 | 40 | [163] |
| Heparanase | 35 µg ip. 2-times/week at 6–14 weeks | 75 | 13 | 22 | [164] |
| TSLP | 500 ng sc. daily for 6 days at 3 weeks | 100 | 25 | 50 | [165] |
| TSLP-treated DCs | 5 × 106 iv. at 3 weeks | 87 | 25 | 45 | [165] |
| Inosine analog | INO-2002; 100 mg/kg by oral gavage daily at 5–30 weeks | 80 | 35 | 30 | [166] |
| GAD65-IgFc DNA (gene gun) | Gold particles coated with 1 µg DNA intradermally weekly at 10–13 weeks | 80 | 50 | 36 | [167] |
| Imatinib (Gleevec®) | 1.5 mg by oral gavage daily at 12–19 weeks | 71 | 25 | 30 | [168] |
| Anti-BLyS (BAFF) | B-cell-depleting anti-BLyS (10F4); 100 µg ip. at 4 weeks and again 5 days later; 15 µg biweekly at 8–25 weeks | 79 | 26 | 40 | [58] |
| Zymosan | Fungal cell wall component; 100 µg ip. 3-times/week every other week at 12–22 weeks | 90 | 40 | 42 | [50] |
| Galectin-1 | 100 µg ip. 3-times/week at 5–36 weeks | 78 | 0 | 37 | [169] |
| IL-25 (IL-17E) | 1 µg sc. daily for 25 days at 10 weeks | 90 | 30 | 26 | [74] |
| Anti-IL-17 | Neutralizing anti-IL-17 (50104); 100 µg ip. every other day for 12 days at 10 weeks | 90 | 40 | 24 | [74] |
| Mesenchymal stem cells (allogeneic) | 105 BALB.B cells ip. or iv. at 4 weeks | 100 | 59 | 36 | [170] |
| BAFF/APRIL blockade | 150 µg BCMA–Fc ip. 2-times/week at 9–15 weeks | 55 | 0 | 50 | [59] |
| Galectin-9 | 100 µg pCR3.1-I-Gal-9 plus 100 µg lipofectamine iv. weekly at 5–8 weeks | 86 | 15 | 40 | [171] |
| GAD65217–236/I-Ag7 dimers | 50 µg iv. for 3 days at 12 and 15 weeks | 100 | 20 | 35 | [172] |
| GAD65290–309/I-Ag7 dimers | 50 µg iv. for 3 days at 12 and 15 weeks | 100 | 10 | 35 | [172] |
| Anti-GITR ligand | Neutralizing anti-GITR ligand (YGL 386.2); 1 mg ip. weekly at 11–14 weeks | 100 | 33 | 32 | [75] |
| Immature DCs pulsed with InsB15–23 and InsB9–23 | Generated in GM-CSF, IL-10 and normal mouse serum; 106 ip. weekly at 5–12 weeks | 83 | 25 | 40 | [173] |
| Humanin | 2 mg/kg ip. daily beginning at 5 weeks | 60 | 30 | 20 | [174] |
| Newborn blood (allogeneic) | C57BL/6 whole blood (25–50 × 106 nucleated cells) iv. at 12 weeks | 80 | 30 | 28 | [175] |
| ECP-treated splenocytes | 2 × 105 cells iv. weekly at 8–12 weeks | 100 | 73 | 30 | [176] |
| Toxin-coupled IGRP206–214/H-2Kd tetramers | Saporin-conjugated tetramers; 34 pmoles iv. per injection; three injections, 6 days apart, beginning at 8 weeks | 100 | 50 | 30 | [177] |
Studies are listed in chronological order. All employed female NOD mice.
APRIL: A proliferation-inducing ligand; BAFF: B-cell-activating factor belonging to the TNF family; BCMA: B-cell maturation antigen; BLyS: B-lymphocyte stimulator; CCL: CC chemokine ligand; CXCL: CXC chemokine ligand; DC: Dendritic cell; EBNA: Epstein–Barr virus nuclear antigen; ECP: Extracorporeal photopheresis; GAD: Glutamic acid decarboxylase; G-CSF: Granulocyte colony-stimulating factor; GITR: Glucocorticoid-induced TNF receptor; GM-CSF: Granulocyte-macrophage colony-stimulating factor; IFA: Incomplete Freund’s adjuvant; IGRP: Islet-specific glucose-6-phosphatase catalytic subunit-related protein; im.: Intramuscularly; ip.: Intraperitoneally; iv.: Intravenously; MAPK: Mitogen-activated protein kinase; NOD: Nonobese diabetic; PSGL: P-selectin glycoprotein ligand; sc.: Subcutaneously; TSLP: Thymic stromal lymphopoietin; UVB: Ultraviolet B.
Testing of therapeutics & identification of new potential targets
In 2005, a comprehensive review of all preventive or therapeutic interventions that had been tested in female NOD mice became available to the scientific community [2]. This heroic effort included all interventions (subject to certain inclusion criteria, such as the availability of diabetes incidence data), regardless of whether they had a favorable, neutral or unfavorable influence on disease course. Importantly, the authors were among the first to highlight the fact that protocol differences (e.g., in terms of relative dose and disease stage) between NOD mouse studies and human trials probably contributed to the disappointing results reported in patients [2], a notion subsequently affirmed by others [51]. As a result of collective extensive experience with the NOD mouse model, the field is far more aware now than it was one or two decades ago of its limitations and of the factors that need to be considered when translating therapies to the human disease. It is now recognized that a regimen that prevents disease in NOD mice when begun at 4 weeks of age will not necessarily be successful in reversing ongoing autoimmunity in a patient. Differences between mouse and human immunology are also better appreciated [52]. Despite its limitations, the NOD mouse model has aided in the development of promising interventions for Type 1 diabetes patients, for example, anti-CD3 treatment. The ability of anti-CD3 to reverse Type 1 diabetes was discovered in NOD mice [53], and subsequent human trials have demonstrated the capacity of anti-CD3 to slow the rate of loss of insulin production and reduce exogenous insulin requirements in patients [54,55].
Given the belief that NOD mouse studies will continue to suggest therapeutic strategies to be considered for translation to patients, we have compiled representative interventions that have recently (reported in 2005 or later) been shown to prevent (TABLE 2) or reverse (TABLE 3) spontaneous Type 1 diabetes in female NOD mice. While not exhaustive, these compilations are intended to illustrate the variety of approaches being pursued. Their examination suggests some general trends. The first is a growing interest in B cells, evidenced by the use of multiple reagents to deplete or manipulate these cells in NOD mice in order to prevent (TABLE 2) or reverse disease (TABLE 3). B-cell-depleting reagents successfully employed include anti-CD20 [56,57], anti-BLyS [58], BAFF/APRIL blockade [59] and cytotoxin-coupled anti-CD22 [60]. Although it has been known for nearly 15 years that B cell-deficient NOD mice do not develop diabetes [61], B cells have been relatively neglected by investigators, as Type 1 diabetes has long been viewed as a strictly T-cell-mediated disease. However, we now know that B cells act as efficient antigen-presenting cells that permit the development of T-cell responses to β-cell antigens such as GAD65 [62]. Thus, their manipulation would seem to be a rational strategy. Indeed, the use of anti-CD20 (rituximab) in new-onset Type 1 diabetes in patients was recently explored [63]. Although as disease was not reversed, further protocol optimization is warranted, some clinical benefit was achieved.
Table 3.
Strategies that reverse new-onset diabetes in nonobese diabetic mice (2005–present).
| Agent | Details | Disease reversal | Ref. | ||
|---|---|---|---|---|---|
| Number | % |
Follow-up (weeks) |
|||
| FTY720 | Sphingosine-1-phosphate analog; 3 mg/kg by oral gavage daily | 5/10 | 50 | 24 | [151] |
| EGF plus gastrin | 1 µg/kg/day EGF plus 3 µg/kg/day gastrin ip.; 2 injections/day for 2 weeks | 5/6 | 83 | 12 | [67] |
| Anti-CD3 plus hInsB24-C4 | 40 µg anti-CD3 F(ab’)2 (145–2C11) iv. on days 0–4 plus 40 µg hInsB24-C4 in. on days 0, 2, 7 and 12 | 17/31 | 55 | 9 | [64] |
| Lisofylline plus exendin-4 | 27 mg/kg/day lisofylline plus 18 nM/day exendin-4 sc. by osmotic mini-pump for 28 days | 5/9 | 56 | 24 | [66] |
| Insulin-coupled fixed splenocytes | 5 × 107 cells iv. | 10/19 | 53 | 25 | [18] |
| Regulatory T cells | CD4+CD25+CD62L+ cells from NOD.BDC2.5 mice expanded in vitro with antigen-pulsed DCs and IL-2; 0.8–1.5 × 106 cells on days 0 and 14 | 4/11 | 36 | 24 | [157] |
| Anti-CD3 plus exendin-4 | 10 µg anti-CD3 (145–2C11) iv. for 5 days plus 0.075 µg exendin-4 ip. for 10 days | 20/45 | 44 | 3 | [65] |
| Anti-human CD20 | NOD/hCD20 mice; B cell-depleting anti-hCD20 (2H7); 500 µg followed by 3 injections of 250 µg at 3-day intervals | 4/14 | 29 | 19 | [56] |
| Microspheres carrying antisense oligonucleotides | Antisense oligonucleotides to CD40, CD80 and CD86; sc. proximal to pancreatic lymph nodes 2-times/week for 25 days | 7/15 | 47 | 8 | [163] |
| α1-antitrypsin (Aralast™) | 2 mg ip. every 3 days; 5 injections | 21/24 | 88 | 39 | [179] |
| Cytotoxin-coupled anti-CD22 | B cell-depleting N-acetyl-calicheamicin dimethyl acid-conjugated anti-CD22 (Cy34.1); 160 µg/kg ip.; 2 injections, 5 days apart | 6/10 | 60 | 14 | [60] |
| Imatinib (Gleevec®) | 1.5 mg by oral gavage daily for 10 weeks | 8/17 | 47 | 35 | [168] |
| Sunitinib (Sutent®) | 2 mg by oral gavage daily for 9 weeks | 5/6 | 83 | 9 | [168] |
| GLP-1 plus gastrin | 100 µg/kg GLP-1 plus 1.5 µg/kg gastrin ip. twice daily for 3 weeks | 7/7 | 100 | 8 | [68] |
| DPP-4 inhibitor plus proton pump inhibitor | 10 mg/kg DPP-4 inhibitor by oral gavage once daily plus 30 mg/kg proton pump inhibitor sc. twice daily for 12 weeks | 6/8 | 75 | 12 | [69] |
| Nanoparticles carrying peptide/MHC complexes | NRP-V7/H-2Kd or MimA2/H-2Db; NRP-V7 and MimA2 are mimotopes of IGRP206–214 and DMK138–146, respectively; iv. 2-times/week until euglycemic for 4 weeks | 8/11 | 73 | 13 | [180] |
Studies are listed in chronological order. All employed female NOD mice except where noted. Follow-up for disease reversal is from time of disease onset. In some studies, mice were treated with insulin that was later withdrawn; see references for details.
DMK: Dystrophia myotonica kinase; DPP: Dipeptidyl peptidase; GLP: Glucagon-like peptide; hIns: Human insulin; IGRP: Islet-specific glucose-6-phosphatase catalytic subunit-related protein; in.: Intranasally; ip.: Intraperitoneally; iv.: Intravenously; NOD: Nonobese diabetic; PDGFR: PDGF receptor; sc.: Subcutaneously.
Examination of TABLE 2 and TABLE 3 also reveals that, in contrast to most recent prevention studies in NOD mice, several reversal studies have employed combination therapies (TABLE 3), including anti-CD3 plus either an insulin peptide [64] or exendin-4 [65]; lisofylline plus exendin-4 [66]; EGF plus gastrin [67]; glucagon-like peptide-1 plus gastrin [68]; and a dipeptidyl peptidase (DPP)-4 inhibitor plus a proton pump inhibitor [69]. Multiple ongoing or planned clinical trials in patients with Type 1 diabetes have also adopted this combination approach [70], for example, GAD65 in combination with the proton pump inhibitor lansoprazole and the DPP-4 inhibitor sitagliptin [201] and autologous cord blood cells in combination with vitamin D and omega-3 fatty acids [202]. When considering the utility of the NOD mouse in suggesting clinical interventions, it is important to note that several of the agents listed in TABLE 2 and TABLE 3 are the subject of ongoing or planned studies in Type 1 diabetes patients or at-risk individuals. These include proton pump and DPP-4 inhibitors [201], mesenchymal stem cells [203], anti-CD3 [204,205], granulocyte colony-stimulating factor (G-CSF) [206], dendritic cells [207], lisofylline [208] and intranasal insulin [209,210].
In addition to the execution of preclinical studies in NOD mice, more ‘basic’ research conducted in this model can help in the identification of molecules, cell types or pathways previously understudied from a Type 1 diabetes perspective that could potentially be targeted for clinical benefit. Examples from the period covered by this review include IL-21 [71–73], Th17 cells [74], and the glucocorticoid-induced TNF receptor [75] and inducible costimulator pathways [76].
Development of imaging strategies
In recent years, the NOD mouse model of Type 1 diabetes has become a valuable tool for the development of imaging protocols that permit the visualization of disease-relevant phenomena such as islet inflammation [77–79] and β-cell death [80]. Non-invasive methods that are being developed to detect islet inflammation include both direct visualization of islet-infiltrating T cells [77,78] and indirect examination of the consequences of inflammation, such as microvascular alterations [79]. As an example of the former, anionic magnetic nanoparticles, which are efficiently internalized by cells, were used to label T cells isolated from the spleens of diabetic NOD mice [77]. Upon transfer of the labeled cells to nondiabetic NOD recipients, their homing to the pancreatic lymph nodes and pancreas was successfully visualized by in vivo MRI for at least 20 days posttransfer. No alterations in T-cell survival or function were noted as a result of the labeling procedure. While suggesting a number of research applications, the need to label diabetogenic T cells ex vivo would appear to limit the eventual translation of this technique to the imaging of spontaneous islet inflammation in patients. By contrast, administration of antigen-coated super-paramagnetic iron oxide nanoparticles to NOD mice allowed visualization of the islet accumulation of naturally arising T cells by in vivo MRI [78]. This advance was made possible by the identification of IGRP206–214/H-2Kd as the target of a prevalent population of diabetogenic T cells in NOD mice [5], as the antigen used to coat the nanoparticles was a mimotope of this peptide/MHC complex. Continuing efforts to identify antigens recognized by islet-reactive T cells in Type 1 diabetes patients has led to the identification of peptides that could potentially be used to move this technique from mouse to man. These include IGRP265–273 and InsB10–18, as both have been identified in multiple studies as T-cell targets in HLA-A*0201-positive individuals [6,7,81–85]. They also have identical sequences in mice and humans and are recognized by islet-infiltrating T cells isolated from HLA-A*0201-transgenic NOD mouse models [11,12,86], supporting the idea that they are disease-relevant. These characteristics should facilitate reagent testing in NOD-based models before translation to patients.
Nonobese diabetic mice have also been used to demonstrate the indirect visualization of islet inflammation as a consequence of inflammation-induced microvascular alterations [79]. Such alterations permit administered magnetic nanoparticles to leak from the vasculature and be taken up by macrophages in the insulitic lesions. The resulting accumulation of nanoparticles allows islet inflammation to be detected by MRI. Importantly, when diabetic NOD mice were treated with a regimen of anti-CD3 that leads to remission in approximately half of all recipients after 2–4 weeks, MRI visualization of islet inflammation permitted early identification (after only 8 days) of animals that would ultimately have a favorable clinical response [79].
Methods to image β-cell death are also being developed, with the NOD mouse model also contributing to this area. When Cy5.5-labeled annexin V was administered to NOD mice between the ages of 3 and 12 weeks, pancreatic accumulation of the probe was observed upon ex vivo near-infrared fluorescence imaging of pancreata obtained from 8- and 12-week-old mice [80]. Although in vivo imaging was not utilized in this study, the authors discussed their expectation that methodological advances will eventually render this technique suitable for noninvasive imaging of β-cell death. The ability to visualize both islet inflammation and β-cell death would present new opportunities to better understand the disease process, permit monitoring of these events in at-risk individuals and those undergoing preventive or reversal therapies, and facilitate the development and testing of strategies to interfere with disease development.
Recent technical advances
The inability to generate NOD ES cells capable of efficient germline transmission has severely hampered the use of gene knock-in technology in NOD mice and has prohibited the direct testing of the diabetes-promoting activity of candidate genes present in Idd loci. In addition, knock-out strains generated using non-NOD ES cells followed by repeated backcrossing to NOD mice may give misleading results due to the contribution of non-NOD DNA flanking the knock-out allele [87,88]. These technical hurdles now appear to have been overcome with the recent publication of two independent reports of the derivation of NOD ES cells exhibiting germline transmission [9,10]. One approach employed a serum-free and feeder cell-free system in which small-molecule inhibitors of differentiation (the glycogen synthase kinase inhibitor Chir99021 and the mitogen-activated protein kinase inhibitor PD0325901) were combined with leukemia inhibitory factor, a regulator of ES cell self-renewal and pluripotency [10]. Multiple ES cell lines were derived from NOD embryos using this strategy and maintained to passage 14 with a normal karyotype and continued expression of the pluripotency factors Nanog and Oct4. Chimeric mice were readily obtained when the NOD ES cells were injected in C57BL/6 blastocysts. Upon mating of the chimeric mice with NOD partners, efficient germline transmission was observed, as determined by coat color and verified by flow cytometric detection of NOD class I and class II MHC molecules and molecular analysis of microsatellite markers. In addition, NOD ES cell-derived female mice developed spontaneous diabetes at a rate and incidence equivalent to that of female NOD mice housed in the same facility. Finally, germline transmission of NOD ES cells that had been genetically modified was also confirmed. Another approach to the generation of NOD ES cell lines also employed small-molecule inhibitors but in a system containing mouse embryonic fibroblasts as feeder cells [9]. One of the lines so derived recently became available from The Jackson Laboratory (Bar Harbor, ME, USA). In addition, bacterial artificial chromosome libraries have recently been prepared for the two commonly used NOD substrains (NOD/ShiLtJ and NOD/MrkTac) [89]. These libraries will greatly facilitate the construction of efficient targeting vectors to be used in conjunction with the newly developed NOD ES cells. It should be noted that the production of gene-targeted mice using NOD-derived ES cells has not yet been reported.
Another recent advance that has increased the utility and the clinical relevance of the NOD mouse model is the development of strains that have been ‘humanized’ to varying degrees. One aspect of this humanization has been the development of NOD mice expressing the human class I MHC molecule HLA-A*0201 [12], which is a Type 1 diabetes-associated allele in patients [90–92], in the absence of murine class I MHC molecules. These NOD.β2m−/−.HHD mice have been used to map epitopes of IGRP and preproinsulin that are recognized by islet-infiltrating HLA-A*0201-restricted CD8+ T cells [12,86]. At least some of these peptides are now known to also be targeted by CD8+ T cells in Type 1 diabetes patients [7,93], thus demonstrating the utility of this HLA-transgenic NOD mouse strain. A further humanized version of this strain, designated NOD.β2m−/−.HHD.Ins2−/−, has recently been described [11]. In these mice, HLA-A*0201 is expressed in the absence of murine class I MHC molecules. In addition, Ins2 deficiency leads to reduced thymic expression of insulin and serves to mimic the situation in humans bearing predisposing alleles of the insulin gene [94,95].
Ideally, a humanized mouse model of Type 1 diabetes would incorporate human immune cells. To this end, Roep and coworkers reported the transfer of human β-cell-specific CD4+ T-cell clones to NOD-scid mice [96]. Human antigen-presenting cells were also transferred, as the mice did not express the human MHC restriction element for the clones in this case. In these experiments, the T cells accumulated in the pancreas, pancreatic lymph nodes and spleen, but not in other sites. While encouraging, only early peri-insulitis could be observed in the recipient mice. Recently, NOD.scid.IL2rγnull mice have emerged as a much improved recipient for the engraftment of human peripheral blood mononuclear cells [97] or CD34+ cells [98]. Multiple laboratories are currently working to incorporate human antigens, human β-cell-specific T cells and HLA class I molecules into this model system.
Expert commentary & five-year view
As we mark the 30th anniversary of the NOD mouse model of Type 1 diabetes, it should be with gratitude for all it has taught us and excitement in anticipation of the secrets it has yet to reveal. NOD mice have contributed to our knowledge in a variety of areas, including antigen discovery and the identification and validation of therapeutic targets. The model is not perfect, and some of its imperfections, such as differences in lifespan compared with humans, have no ready remedies. However, the field is now more aware of the limitations of the model and how best to design investigations that will minimize the impact of these limitations. For example, many protocols are capable of preventing disease in NOD mice if treatment is initiated prior to the onset of insulitis. Yet, over the last few years, many researchers have wisely turned instead to the more clinically relevant situation in which attempts are made to reverse insulitis or even diabetes itself. This logical trend should continue in the future. Disease reversal is likely to require not only attention to the destructive autoimmune process, but also some manipulation of the remaining β-cell mass (e.g., with agents such as glucagon-like peptide-1 and gastrin) to facilitate its recovery and augmentation, and studies in the NOD mouse will continue to be a critical component of these efforts.
Recent advances that should increase the utility of the NOD mouse model include the development of NOD-derived ES cells reported to be capable of efficient germline transmission. It should now be possible to knock out genes directly in the NOD background, eliminating the need for time-consuming and costly backcrossing and preventing artifact results due to the carry-over of genetic material from the original knock-out strain. NOD ES cells will also facilitate the efficient generation of knock-in mice that will aid in the elucidation of the contribution of specific genes to diabetes susceptibility and resistance. One could also envision knock-in of human genes, for example, those encoding autoantigens. Such work could become part of a more general push to increase the clinical relevance of the NOD mouse model by humanizing it in various ways, including the introduction of human MHC molecules and immune cells. These technical advances should significantly enhance the utility and importance of the NOD mouse model and its ability to yield clinically relevant results and information. Cooperation among Type 1 diabetes researchers engaged in mouse, rat and human investigations will also go far to facilitate progress towards an improved understanding of the disease and tangible benefits for patients and at-risk individuals.
Key issues
The nonobese diabetic (NOD) mouse model of Type 1 diabetes, although imperfect, exhibits multiple striking similarities to the disease in humans.
Investigations utilizing NOD mice continue to advance our understanding of disease pathogenesis and to yield information that is applicable to Type 1 diabetes in humans.
Areas of utility for the NOD mouse model include antigen discovery, identification of disease susceptibility genes, testing of therapeutics, exploration of environmental disease influences and the development of imaging strategies.
The availability of NOD-derived embryonic stem cells will now permit knock-in and knock-out strains to be developed directly on the NOD background, thus greatly enhancing the utility of the model.
Increasingly humanized NOD-derived strains, incorporating human MHC molecules, antigens, predisposing factors and even human immune cells, are being developed and probably represent the future direction of research.
Footnotes
Financial & competing interests disclosure
Related research by the authors’ laboratory was supported by the NIH (grant numbers R01 DK064315 and R56 AI088256), the Juvenile Diabetes Research Foundation International, the American Diabetes Association, and the Irma T Hirschl/Monique Weill-Caulier Trust. Rodolfo José Chaparro was supported in part by the NIH Molecular Neuropathology Training Grant NS07098. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Exp. Anim. 1980;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 2.Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23(2):115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Suri A, Walters JJ, Gross ML, Unanue ER. Natural peptides selected by diabetogenic DQ8 and murine I-Ag7 molecules show common sequence specificity. J. Clin. Invest. 2005;115(8):2268–2276. doi: 10.1172/JCI25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Wicker LS, Santamaria P. IL-2 and its high-affinity receptor: genetic control of immunoregulation and autoimmunity. Semin. Immunol. 2009;21(6):363–371. doi: 10.1016/j.smim.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman SM, Evans AM, Han B, et al. Identification of the β cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl Acad. Sci. USA. 2003;100(14):8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP. Identification of novel IGRP epitopes targeted in Type 1 diabetes patients. Clin. Immunol. 2008;127(3):359–365. doi: 10.1016/j.clim.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallone R, Martinuzzi E, Blancou P, et al. CD8+ T-cell responses identify β-cell autoimmunity in human Type 1 diabetes. Diabetes. 2007;56(3):613–621. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 8.Standifer NE, Ouyang Q, Panagiotopoulos C, et al. Identification of novel HLA-A*0201-restricted epitopes in recent-onset Type 1 diabetic subjects and antibody-positive relatives. Diabetes. 2006;55(11):3061–3067. doi: 10.2337/db06-0066. [DOI] [PubMed] [Google Scholar]
- 9. Hanna J, Markoulaki S, Mitalipova M, et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell. 2009;4(6):513–524. doi: 10.1016/j.stem.2009.04.015. • Describes the derivation of nonobese diabetic (NOD) embryonic stem cells capable of germline transmission.
- 10. Nichols J, Jones K, Phillips JM, et al. Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat. Med. 2009;15(7):814–818. doi: 10.1038/nm.1996. • Describes the derivation of NOD embryonic stem cells capable of germline transmission.
- 11.Jarchum I, DiLorenzo TP. Ins2 deficiency augments spontaneous HLA-A*0201-restricted T cell responses to insulin. J. Immunol. 2010;185(2):658–665. doi: 10.4049/jimmunol.0903414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaki T, Marron MP, Mathews CE, et al. HLA-A*0201-restricted T cells from ‘humanized’ NOD mice recognize autoantigens of potential clinical relevance to Type 1 diabetes. J. Immunol. 2006;176(5):3257–3265. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr. Opin. Immunol. 2008;20(1):111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 1995;25(4):1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 15.Wong FS, Karttunen J, Dumont C, et al. Identification of an MHC class I-restricted autoantigen in Type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 1999;5(9):1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of Type 1 diabetes in NOD mice. Nature. 2005;435(7039):220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnamurthy B, Dudek NL, McKenzie MD, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Invest. 2006;116(12):3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fife BT, Guleria I, Gubbels Bupp M, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1–PD-L1 pathway. J. Exp. Med. 2006;203(12):2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J. Immunol. 2007;178(10):6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- 20.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat. Immunol. 2010;11(4):350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stadinski BD, Delong T, Reisdorph N, et al. Chromogranin A is an autoantigen in Type 1 diabetes. Nat. Immunol. 2010;11(3):225–231. doi: 10.1038/ni.1844. • Identifies chromogranin A as the long sought-after antigen for the pathogenic CD4+ T-cell clone BDC2.5.
- 22.Stadinski B, Kappler J, Eisenbarth GS. Molecular targeting of islet autoantigens. Immunity. 2010;32(4):446–456. doi: 10.1016/j.immuni.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Burton AR, Vincent E, Arnold PY, et al. On the pathogenicity of autoantigen-specific T-cell receptors. Diabetes. 2008;57(5):1321–1330. doi: 10.2337/db07-1129. [DOI] [PubMed] [Google Scholar]
- 24.Haskins K. Pathogenic T-cell clones in autoimmune diabetes: more lessons from the NOD mouse. Adv. Immunol. 2005;87:123–162. doi: 10.1016/S0065-2776(05)87004-X. [DOI] [PubMed] [Google Scholar]
- 25.Drexler K, Burtles S, Hurtenbach U. Limited heterogeneity of T-cell receptor Vβ gene expression in the early stage of insulitis in NOD mice. Immunol. Lett. 1993;37(2–3):187–196. doi: 10.1016/0165-2478(93)90030-6. [DOI] [PubMed] [Google Scholar]
- 26.Lennon GP, Bettini M, Burton AR, et al. T cell islet accumulation in Type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity. 2009;31(4):643–653. doi: 10.1016/j.immuni.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieberman SM, Takaki T, Han B, Santamaria P, Serreze DV, DiLorenzo TP. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J. Immunol. 2004;173(11):6727–6734. doi: 10.4049/jimmunol.173.11.6727. [DOI] [PubMed] [Google Scholar]
- 28.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human Type 1 diabetes. Proc. Natl Acad. Sci. USA. 2007;104(43):17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li SW, Koya V, Li Y, et al. Pancreatic duodenal homeobox 1 protein is a novel β-cell-specific autoantigen for type I diabetes. Lab. Invest. 2010;90(1):31–39. doi: 10.1038/labinvest.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winer S, Tsui H, Lau A, et al. Autoimmune islet destruction in spontaneous Type 1 diabetes is not β-cell exclusive. Nat. Med. 2003;9(2):198–205. doi: 10.1038/nm818. [DOI] [PubMed] [Google Scholar]
- 31.Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune Type 1 diabetes: a NOD to similarities and differences to human disease. Semin. Immunopathol. 2010 doi: 10.1007/s00281-010-0204-1. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 32.Araki M, Chung D, Liu S, et al. Genetic evidence that the differential expression of the ligand-independent isoform of CTLA-4 is the molecular basis of the Idd5.1 Type 1 diabetes region in nonobese diabetic mice. J. Immunol. 2009;183(8):5146–5157. doi: 10.4049/jimmunol.0802610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda H, Howson JM, Esposito L, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 34.Vijayakrishnan L, Slavik JM, Illes Z, et al. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells. Immunity. 2004;20(5):563–575. doi: 10.1016/s1074-7613(04)00110-4. [DOI] [PubMed] [Google Scholar]
- 35.Oaks MK, Hallett KM, Penwell RT, Stauber EC, Warren SJ, Tector AJ. A native soluble form of CTLA-4. Cell. Immunol. 2000;201(2):144–153. doi: 10.1006/cimm.2000.1649. [DOI] [PubMed] [Google Scholar]
- 36. Yamanouchi J, Rainbow D, Serra P, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat. Genet. 2007;39(3):329–337. doi: 10.1038/ng1958. • Identifies IL2 as a gene responsible for the diabetes susceptibility conferred by Idd3.
- 37.Lowe CE, Cooper JD, Brusko T, et al. Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in Type 1 diabetes. Nat. Genet. 2007;39(9):1074–1082. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- 38.Vella A, Cooper JD, Lowe CE, et al. Localization of a Type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. Am. J. Hum. Genet. 2005;76(5):773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kukreja A, Cost G, Marker J, et al. Multiple immuno-regulatory defects in type-1 diabetes. J. Clin. Invest. 2002;109(1):131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4+CD25+ T-cells from patients with Type 1 diabetes. Diabetes. 2005;54(1):92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 41.Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world – recent facts and figures. Immunol. Today. 1993;14(5):193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 42.Ohsugi T, Kurosawa T. Increased incidence of diabetes mellitus in specific pathogen-eliminated offspring produced by embryo transfer in NOD mice with low incidence of the disease. Lab. Anim. Sci. 1994;44(4):386–388. [PubMed] [Google Scholar]
- 43.Cooke A, Tonks P, Jones FM, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21(4):169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 44.Bras A, Aguas AP. Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology. 1996;89(1):20–25. doi: 10.1046/j.1365-2567.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune Type 1 diabetes by gastrointestinal helminth infection. Infect. Immun. 2007;75(1):397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raine T, Zaccone P, Mastroeni P, Cooke A. Salmonella typhimurium infection in nonobese diabetic mice generates immunomodulatory dendritic cells able to prevent Type 1 diabetes. J. Immunol. 2006;177(4):2224–2233. doi: 10.4049/jimmunol.177.4.2224. [DOI] [PubMed] [Google Scholar]
- 47.Cooke A. Review series on helminths, immune modulation and the hygiene hypothesis: how might infection modulate the onset of Type 1 diabetes? Immunology. 2009;126(1):12–17. doi: 10.1111/j.1365-2567.2008.03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. • Demonstrates that interaction of the gut flora with the innate immune system can modify susceptibility to Type 1 diabetes.
- 49.Calcinaro F, Dionisi S, Marinaro M, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48(8):1565–1575. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 50.Karumuthil-Melethil S, Perez N, Li R, Vasu C. Induction of innate immune response through TLR2 and dectin 1 prevents Type 1 diabetes. J. Immunol. 2008;181(12):8323–8334. doi: 10.4049/jimmunol.181.12.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on Type 1 diabetes: immune-based therapeutic approaches for Type 1 diabetes. Clin. Exp. Immunol. 2007;148(1):17–31. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J. Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 53.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc. Natl Acad. Sci. USA. 1994;91(1):123–127. doi: 10.1073/pnas.91.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herold KC, Gitelman SE, Masharani U, et al. A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of Type 1 diabetes. Diabetes. 2005;54(6):1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset Type 1 diabetes. N. Engl. J. Med. 2005;352(25):2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 56.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Invest. 2007;117(12):3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiu Y, Wong CP, Bouaziz JD, et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in FcγR effector functions. J. Immunol. 2008;180(5):2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 58.Zekavat G, Rostami SY, Badkerhanian A, et al. In vivo BLyS/BAFF neutralization ameliorates islet-directed autoimmunity in nonobese diabetic mice. J. Immunol. 2008;181(11):8133–8144. doi: 10.4049/jimmunol.181.11.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marino E, Villanueva J, Walters S, Liuwantara D, Mackay F, Grey ST. CD4+CD25+ T-cells control autoimmunity in the absence of B-cells. Diabetes. 2009;58(7):1568–1577. doi: 10.2337/db08-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiorina P, Vergani A, Dada S, et al. Targeting CD22 reprograms B-cells and reverses autoimmune diabetes. Diabetes. 2008;57(11):3013–3024. doi: 10.2337/db08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new ‘speed congenic’ stock of NOD.Igµnull mice. J. Exp. Med. 1996;184(5):2049–2053. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J. Immunol. 1998;161(8):3912–3918. [PubMed] [Google Scholar]
- 63.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N. Engl. J. Med. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bresson D, Togher L, Rodrigo E, et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J. Clin. Invest. 2006;116(5):1371–1381. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of β-cells. Endocrinology. 2007;148(11):5136–5144. doi: 10.1210/en.2007-0358. [DOI] [PubMed] [Google Scholar]
- 66.Yang Z, Chen M, Carter JD, et al. Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem. Biophys. Res. Commun. 2006;344(3):1017–1022. doi: 10.1016/j.bbrc.2006.03.177. [DOI] [PubMed] [Google Scholar]
- 67.Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin increases β-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes. 2005;54(9):2596–2601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- 68.Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes. 2008;57(12):3281–3288. doi: 10.2337/db08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suarez-Pinzon WL, Cembrowski GS, Rabinovitch A. Combination therapy with a dipeptidyl peptidase-4 inhibitor and a proton pump inhibitor restores normoglycaemia in non-obese diabetic mice. Diabetologia. 2009;52(8):1680–1682. doi: 10.1007/s00125-009-1390-z. [DOI] [PubMed] [Google Scholar]
- 70.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in Type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Datta S, Sarvetnick NE. IL-21 limits peripheral lymphocyte numbers through T cell homeostatic mechanisms. PLoS One. 2008;3(9):3118. doi: 10.1371/journal.pone.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of Type I diabetes in the NOD mouse. Proc. Natl Acad. Sci. USA. 2008;105(37):14028–14033. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sutherland AP, Van Belle T, Wurster AL, et al. Interleukin-21 is required for the development of Type 1 diabetes in NOD mice. Diabetes. 2009;58(5):1144–1155. doi: 10.2337/db08-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Emamaullee JA, Davis J, Merani S, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58(6):1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.You S, Poulton L, Cobbold S, et al. Key role of the GITR/GITR ligand pathway in the development of murine autoimmune diabetes: a potential therapeutic target. PLoS One. 2009;4(11):e7848. doi: 10.1371/journal.pone.0007848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hawiger D, Tran E, Du W, et al. ICOS mediates the development of insulin-dependent diabetes mellitus in nonobese diabetic mice. J. Immunol. 2008;180(5):3140–3147. doi: 10.4049/jimmunol.180.5.3140. [DOI] [PubMed] [Google Scholar]
- 77.Billotey C, Aspord C, Beuf O, et al. T-cell homing to the pancreas in autoimmune mouse models of diabetes: in vivo MR imaging. Radiology. 2005;236(2):579–587. doi: 10.1148/radiol.2362040603. [DOI] [PubMed] [Google Scholar]
- 78.Medarova Z, Tsai S, Evgenov N, Santamaria P, Moore A. In vivo imaging of a diabetogenic CD8+ T cell response during Type 1 diabetes progression. Magn. Reson. Med. 2008;59(4):712–720. doi: 10.1002/mrm.21494. [DOI] [PubMed] [Google Scholar]
- 79. Turvey SE, Swart E, Denis MC, et al. Noninvasive imaging of pancreatic inflammation and its reversal in Type 1 diabetes. J. Clin. Invest. 2005;115(9):2454–2461. doi: 10.1172/JCI25048. • Reports a noninvasive method to image islet inflammation and its use in monitoring response to a therapeutic intervention.
- 80.Medarova Z, Bonner-Weir S, Lipes M, Moore A. Imaging β-cell death with a near-infrared probe. Diabetes. 2005;54(6):1780–1788. doi: 10.2337/diabetes.54.6.1780. [DOI] [PubMed] [Google Scholar]
- 81.Martinuzzi E, Novelli G, Scotto M, et al. The frequency and immunodominance of islet-specific CD8+ T-cell responses change after Type 1 diabetes diagnosis and treatment. Diabetes. 2008;57(5):1312–1320. doi: 10.2337/db07-1594. [DOI] [PubMed] [Google Scholar]
- 82.Ouyang Q, Standifer NE, Qin H, et al. Recognition of HLA class I-restricted β-cell epitopes in Type 1 diabetes. Diabetes. 2006;55(11):3068–3074. doi: 10.2337/db06-0065. [DOI] [PubMed] [Google Scholar]
- 83.Pinkse GG, Tysma OH, Bergen CA, et al. Autoreactive CD8 T cells associated with β cell destruction in Type 1 diabetes. Proc. Natl Acad. Sci. USA. 2005;102(51):18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Toma A, Haddouk S, Briand JP, et al. Recognition of a subregion of human proinsulin by class I-restricted T cells in Type 1 diabetic patients. Proc. Natl Acad. Sci. USA. 2005;102(30):10581–10586. doi: 10.1073/pnas.0504230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Unger WW, Pinkse GG, Mulder-van der Kracht S, et al. Human clonal CD8 autoreactivity to an IGRP islet epitope shared between mice and men. Ann. NY Acad. Sci. 2007;1103:192–195. doi: 10.1196/annals.1394.024. [DOI] [PubMed] [Google Scholar]
- 86.Jarchum I, Baker JC, Yamada T, et al. In vivo cytotoxicity of insulin-specific CD8+ T cells in HLA-A*0201-transgenic NOD mice. Diabetes. 2007;56(10):2551–2560. doi: 10.2337/db07-0332. [DOI] [PubMed] [Google Scholar]
- 87.Kanagawa O, Xu G, Tevaarwerk A, Vaupel BA. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-γ receptor loci. J. Immunol. 2000;164(7):3919–3923. doi: 10.4049/jimmunol.164.7.3919. [DOI] [PubMed] [Google Scholar]
- 88.Leiter EH, Reifsnyder PC, Wallace R, Li R, King B, Churchill GC. NOD × 129. H2g7 backcross delineates 129S1/SvImJ-derived genomic regions modulating Type 1 diabetes development in mice. Diabetes. 2009;58(7):1700–1703. doi: 10.2337/db09-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Steward CA, Humphray S, Plumb B, et al. Genome-wide end-sequenced BAC resources for the NOD/MrkTac and NOD/ShiLtJ mouse genomes. Genomics. 2010;95(2):105–110. doi: 10.1016/j.ygeno.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fennessy M, Metcalfe K, Hitman GA, et al. A gene in the HLA class I region contributes to susceptibility to IDDM in the Finnish population. Childhood Diabetes in Finland (DiMe) Study Group. Diabetologia. 1994;37(9):937–944. doi: 10.1007/BF00400951. [DOI] [PubMed] [Google Scholar]
- 91.Nejentsev S, Howson JM, Walker NM, et al. Localization of Type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450(7171):887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robles DT, Eisenbarth GS, Wang T, et al. Identification of children with early onset and high incidence of anti-islet autoantibodies. Clin. Immunol. 2002;102(3):217–224. doi: 10.1006/clim.2001.5171. [DOI] [PubMed] [Google Scholar]
- 93.Enee E, Martinuzzi E, Blancou P, Bach JM, Mallone R, van Endert P. Equivalent specificity of peripheral blood and islet-infiltrating CD8+ T lymphocytes in spontaneously diabetic HLA-A2 transgenic NOD mice. J. Immunol. 2008;180(8):5430–5438. doi: 10.4049/jimmunol.180.8.5430. [DOI] [PubMed] [Google Scholar]
- 94.Pugliese A, Zeller M, Fernandez A, Jr, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for Type 1 diabetes. Nat. Genet. 1997;15(3):293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 95.Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 1997;15(3):289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 96.van Halteren AG, Kardol MJ, Mulder A, Roep BO. Homing of human autoreactive T cells into pancreatic tissue of NOD-scid mice. Diabetologia. 2005;48(1):75–82. doi: 10.1007/s00125-004-1613-2. [DOI] [PubMed] [Google Scholar]
- 97.King M, Pearson T, Shultz LD, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor γ chain gene. Clin. Immunol. 2008;126(3):303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 98.Brehm MA, Cuthbert A, Yang C, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rγnull mutation. Clin. Immunol. 2010;135(1):84–98. doi: 10.1016/j.clim.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 100.Rudy G, Stone N, Harrison LC, et al. Similar peptides from two β cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Mol. Med. 1995;1(6):625–633. [PMC free article] [PubMed] [Google Scholar]
- 101.Pontesilli O, Carotenuto P, Gazda LS, Pratt PF, Prowse SJ. Circulating lymphocyte populations and autoantibodies in non-obese diabetic (NOD) mice: a longitudinal study. Clin. Exp. Immunol. 1987;70(1):84–93. [PMC free article] [PubMed] [Google Scholar]
- 102.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur. J. Immunol. 1994;24(8):1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 103.Gillard BK, Thomas JW, Nell LJ, Marcus DM. Antibodies against ganglioside GT3 in the sera of patients with Type I diabetes mellitus. J. Immunol. 1989;142(11):3826–3832. [PubMed] [Google Scholar]
- 104.Ozawa Y, Kasuga A, Nomaguchi H, et al. Detection of autoantibodies to the pancreatic islet heat shock protein 60 in insulin-dependent diabetes mellitus. J. Autoimmun. 1996;9(4):517–524. doi: 10.1006/jaut.1996.0069. [DOI] [PubMed] [Google Scholar]
- 105.Abulafia-Lapid R, Elias D, Raz I, Keren-Zur Y, Atlan H, Cohen IR. T cell proliferative responses of Type 1 diabetes patients and healthy individuals to human hsp60 and its peptides. J. Autoimmun. 1999;12(2):121–129. doi: 10.1006/jaut.1998.0262. [DOI] [PubMed] [Google Scholar]
- 106.Elias D, Markovits D, Reshef T, van der Zee R, Cohen IR. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc. Natl Acad. Sci. USA. 1990;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 108.DeAizpurua HJ, Honeyman MC, Harrison LC. A 64 kDa antigen/glutamic acid decarboxylase (GAD) in fetal pig pro-islets: co-precipitation with a 38 kDa protein and recognition by T cells in humans at risk for insulin-dependent diabetes. J. Autoimmun. 1992;5(6):759–770. doi: 10.1016/0896-8411(92)90191-r. [DOI] [PubMed] [Google Scholar]
- 109.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 110.Clark A, Yon SM, de Koning EJ, Holman RR. Autoantibodies to islet amyloid polypeptide in diabetes. Diabet. Med. 1991;8(7):668–673. doi: 10.1111/j.1464-5491.1991.tb01675.x. [DOI] [PubMed] [Google Scholar]
- 111.Panagiotopoulos C, Qin H, Tan R, Verchere CB. Identification of a β-cell-specific HLA class I restricted epitope in Type 1 diabetes. Diabetes. 2003;52(11):2647–2651. doi: 10.2337/diabetes.52.11.2647. [DOI] [PubMed] [Google Scholar]
- 112.Castano L, Russo E, Zhou L, Lipes MA, Eisenbarth GS. Identification and cloning of a granule autoantigen (carboxypeptidase-H) associated with type I diabetes. J. Clin. Endocrinol. Metab. 1991;73(6):1197–1201. doi: 10.1210/jcem-73-6-1197. [DOI] [PubMed] [Google Scholar]
- 113.Yang L, Zhou Z, Du T, Tan S, Zhnag Y, Jin P. [Detection of carboxypeptidase H specific T cells in peripheral blood of latent autoimmune diabetic patients with carboxypeptidase antibody positivity by ELISPOT assay] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34(10):1011–1016. [PubMed] [Google Scholar]
- 114.Rabin DU, Pleasic SM, Palmer-Crocker R, Shapiro JA. Cloning and expression of IDDM-specific human autoantigens. Diabetes. 1992;41(2):183–186. doi: 10.2337/diab.41.2.183. [DOI] [PubMed] [Google Scholar]
- 115.Durinovic-Bello I, Hummel M, Ziegler AG. Cellular immune response to diverse islet cell antigens in IDDM. Diabetes. 1996;45(6):795–800. doi: 10.2337/diab.45.6.795. [DOI] [PubMed] [Google Scholar]
- 116.Trembleau S, Penna G, Gregori S, Magistrelli G, Isacchi A, Adorini L. Early Th1 response in unprimed nonobese diabetic mice to the tyrosine phosphatase-like insulinoma-associated protein 2, an autoantigen in Type 1 diabetes. J. Immunol. 2000;165(12):6748–6755. doi: 10.4049/jimmunol.165.12.6748. [DOI] [PubMed] [Google Scholar]
- 117.Inman LR, McAllister CT, Chen L, et al. Autoantibodies to the GLUT-2 glucose transporter of β cells in insulin-dependent diabetes mellitus of recent onset. Proc. Natl Acad. Sci. USA. 1993;90(4):1281–1284. doi: 10.1073/pnas.90.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monetini L, Barone F, Stefanini L, et al. Establishment of T cell lines to bovine β-casein and β-casein-derived epitopes in patients with Type 1 diabetes. J. Endocrinol. 2003;176(1):143–150. doi: 10.1677/joe.0.1760143. [DOI] [PubMed] [Google Scholar]
- 119.Liddi R, Beales PE, Rosignoli G, Pozzilli P. Incomplete Freund’s adjuvant reduces diabetes in the non-obese diabetic mouse. Horm. Metab. Res. 2000;32(6):201–206. doi: 10.1055/s-2007-978622. [DOI] [PubMed] [Google Scholar]
- 120.Honeyman MC, Cram DS, Harrison LC. Transcription factor jun-B is target of autoreactive T-cells in IDDM. Diabetes. 1993;42(4):626–630. doi: 10.2337/diab.42.4.626. [DOI] [PubMed] [Google Scholar]
- 121.Hagopian WA, Michelsen B, Karlsen AE, et al. Autoantibodies in IDDM primarily recognize the 65,000-Mr rather than the 67,000-Mr isoform of glutamic acid decarboxylase. Diabetes. 1993;42(4):631–636. doi: 10.2337/diab.42.4.631. [DOI] [PubMed] [Google Scholar]
- 122.Honeyman MC, Cram DS, Harrison LC. Glutamic acid decarboxylase 67-reactive T cells: a marker of insulin-dependent diabetes. J. Exp. Med. 1993;177(2):535–540. doi: 10.1084/jem.177.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elliott JF, Qin HY, Bhatti S, et al. Immunization with the larger isoform of mouse glutamic acid decarboxylase (GAD67) prevents autoimmune diabetes in NOD mice. Diabetes. 1994;43(12):1494–1499. doi: 10.2337/diab.43.12.1494. [DOI] [PubMed] [Google Scholar]
- 124.Pietropaolo M, Castano L, Babu S, et al. Islet cell autoantigen 69 kD (ICA69). Molecular cloning and characterization of a novel diabetes-associated autoantigen. J. Clin. Invest. 1993;92(1):359–371. doi: 10.1172/JCI116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roep BO, Duinkerken G, Schreuder GM, Kolb H, de Vries RR, Martin S. HLA-associated inverse correlation between T cell and antibody responsiveness to islet autoantigen in recent-onset insulin-dependent diabetes mellitus. Eur. J. Immunol. 1996;26(6):1285–1289. doi: 10.1002/eji.1830260616. [DOI] [PubMed] [Google Scholar]
- 126.Karges W, Hammond-McKibben D, Cheung RK, et al. Immunological aspects of nutritional diabetes prevention in NOD mice: a pilot study for the cow’s milk-based IDDM prevention trial. Diabetes. 1997;46(4):557–564. doi: 10.2337/diab.46.4.557. [DOI] [PubMed] [Google Scholar]
- 127.Buschard K, Josefsen K, Horn T, Fredman P. Sulphatide and sulphatide antibodies in insulin-dependent diabetes mellitus. Lancet. 1993;342(8875):840. doi: 10.1016/0140-6736(93)92697-r. [DOI] [PubMed] [Google Scholar]
- 128.Tiberti C, Dotta F, Anastasi E, et al. Anti-ganglioside antibodies in new onset Type 1 diabetic patients and high risk subjects. Autoimmunity. 1995;22(1):43–48. doi: 10.3109/08916939508995298. [DOI] [PubMed] [Google Scholar]
- 129.Arden SD, Roep BO, Neophytou PI, et al. Imogen 38: a novel 38-kD islet mitochondrial autoantigen recognized by T cells from a newly diagnosed Type 1 diabetic patient. J. Clin. Invest. 1996;97(2):551–561. doi: 10.1172/JCI118448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chang YH, Hwang J, Shang HF, Tsai ST. Characterization of human DNA topoisomerase II as an autoantigen recognized by patients with IDDM. Diabetes. 1996;45(4):408–414. doi: 10.2337/diab.45.4.408. [DOI] [PubMed] [Google Scholar]
- 131.Aanstoot HJ, Kang SM, Kim J, et al. Identification and characterization of glima 38, a glycosylated islet cell membrane antigen, which together with GAD65 and IA2 marks the early phases of autoimmune response in Type 1 diabetes. J. Clin. Invest. 1996;97(12):2772–2783. doi: 10.1172/JCI118732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hawkes CJ, Wasmeier C, Christie MR, Hutton JC. Identification of the 37-kDa antigen in IDDM as a tyrosine phosphatase-like protein (phogrin) related to IA-2. Diabetes. 1996;45(9):1187–1192. doi: 10.2337/diab.45.9.1187. [DOI] [PubMed] [Google Scholar]
- 133.Kelemen K, Gottlieb PA, Putnam AL, Davidson HW, Wegmann DR, Hutton JC. HLA-DQ8-associated T cell responses to the diabetes autoantigen phogrin (IA-2 β) in human prediabetes. J. Immunol. 2004;172(6):3955–3962. doi: 10.4049/jimmunol.172.6.3955. [DOI] [PubMed] [Google Scholar]
- 134.Kelemen K, Crawford ML, Gill RG, Hutton JC, Wegmann D. Cellular immune response to phogrin in the NOD mouse: cloned T-cells cause destruction of islet transplants. Diabetes. 1999;48(8):1529–1534. doi: 10.2337/diabetes.48.8.1529. [DOI] [PubMed] [Google Scholar]
- 135.Dotta F, Gianani R, Previti M, et al. Autoimmunity to the GM2–1 islet ganglioside before and at the onset of Type I diabetes. Diabetes. 1996;45(9):1193–1196. doi: 10.2337/diab.45.9.1193. [DOI] [PubMed] [Google Scholar]
- 136.Fierabracci A, Biro PA, Yiangou Y, et al. Osteopontin is an autoantigen of the somatostatin cells in human islets: identification by screening random peptide libraries with sera of patients with insulin-dependent diabetes mellitus. Vaccine. 1999;18(3–4):342–354. doi: 10.1016/s0264-410x(99)00204-2. [DOI] [PubMed] [Google Scholar]
- 137.Pupilli C, Giannini S, Marchetti P, et al. Autoantibodies to CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) in Caucasian patients with diabetes: effects on insulin release from human islets. Diabetes. 1999;48(12):2309–2315. doi: 10.2337/diabetes.48.12.2309. [DOI] [PubMed] [Google Scholar]
- 138.Kasimiotis H, Myers MA, Argentaro A, et al. Sex-determining region Y-related protein SOX13 is a diabetes autoantigen expressed in pancreatic islets. Diabetes. 2000;49(4):555–561. doi: 10.2337/diabetes.49.4.555. [DOI] [PubMed] [Google Scholar]
- 139.Gurr W, Yavari R, Wen L, et al. A Reg family protein is overexpressed in islets from a patient with new-onset Type 1 diabetes and acts as T-cell autoantigen in NOD mice. Diabetes. 2002;51(2):339–346. doi: 10.2337/diabetes.51.2.339. [DOI] [PubMed] [Google Scholar]
- 140.Qin HY, Mahon JL, Atkinson MA, Chaturvedi P, Lee-Chan E, Singh B. Type 1 diabetes alters anti-hsp90 autoantibody isotype. J. Autoimmun. 2003;20(3):237–245. doi: 10.1016/s0896-8411(03)00035-0. [DOI] [PubMed] [Google Scholar]
- 141.Abulafia-Lapid R, Gillis D, Yosef O, Atlan H, Cohen IR. T cells and autoantibodies to human HSP70 in Type 1 diabetes in children. J. Autoimmun. 2003;20(4):313–321. doi: 10.1016/s0896-8411(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 142.Yang J, Danke NA, Berger D, et al. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J. Immunol. 2006;176(5):2781–2789. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- 143.Shervani NJ, Takasawa S, Uchigata Y, et al. Autoantibodies to REG, a β-cell regeneration factor, in diabetic patients. Eur. J. Clin. Invest. 2004;34(11):752–758. doi: 10.1111/j.1365-2362.2004.01419.x. [DOI] [PubMed] [Google Scholar]
- 144.Ola TO, Biro PA, Hawa MI, et al. Importin β: a novel autoantigen in human autoimmunity identified by screening random peptide libraries on phage. J. Autoimmun. 2006;26(3):197–207. doi: 10.1016/j.jaut.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 145.Gurr W, Shaw M, Li Y, Sherwin R. RegII is a β-cell protein and autoantigen in diabetes of NOD mice. Diabetes. 2007;56(1):34–40. doi: 10.2337/db06-0669. [DOI] [PubMed] [Google Scholar]
- 146.Hardt PD, Ewald N, Brockling K, et al. Distinct autoantibodies against exocrine pancreatic antigens in European patients with Type 1 diabetes mellitus and non-alcoholic chronic pancreatitis. JOP. 2008;9(6):683–689. [PubMed] [Google Scholar]
- 147.Endo T, Takizawa S, Tanaka S, et al. Amylase α-2A autoantibodies: novel marker of autoimmune pancreatitis and fulminant Type 1 diabetes. Diabetes. 2009;58(3):732–737. doi: 10.2337/db08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Takizawa S, Endo T, Wanjia X, Tanaka S, Takahashi M, Kobayashi T. HSP 10 is a new autoantigen in both autoimmune pancreatitis and fulminant Type 1 diabetes. Biochem. Biophys. Res. Commun. 2009;386(1):192–196. doi: 10.1016/j.bbrc.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 149.Kared H, Masson A, Adle-Biassette H, Bach JF, Chatenoud L, Zavala F. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4+CD25+ regulatory T-cells. Diabetes. 2005;54(1):78–84. doi: 10.2337/diabetes.54.1.78. [DOI] [PubMed] [Google Scholar]
- 150.Steptoe RJ, Ritchie JM, Jones LK, Harrison LC. Autoimmune diabetes is suppressed by transfer of proinsulinencoding Gr-1+ myeloid progenitor cells that differentiate in vivo into resting dendritic cells. Diabetes. 2005;54(2):434–442. doi: 10.2337/diabetes.54.2.434. [DOI] [PubMed] [Google Scholar]
- 151.Maki T, Gottschalk R, Ogawa N, Monaco AP. Prevention and cure of autoimmune diabetes in nonobese diabetic mice by continuous administration of FTY720. Transplantation. 2005;79(9):1051–1055. doi: 10.1097/01.tp.0000161220.87548.ee. [DOI] [PubMed] [Google Scholar]
- 152.Han B, Serra P, Amrani A, et al. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat. Med. 2005;11(6):645–652. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- 153.Huang CC, Lu YF, Wen SN, et al. A novel apoptosis-inducing anti-PSGL-1 antibody for T cell-mediated diseases. Eur. J. Immunol. 2005;35(7):2239–2249. doi: 10.1002/eji.200525849. [DOI] [PubMed] [Google Scholar]
- 154.Olcott AP, Tian J, Walker V, et al. Antigen-based therapies using ignored determinants of β cell antigens can more effectively inhibit late-stage autoimmune disease in diabetes-prone mice. J. Immunol. 2005;175(3):1991–1999. doi: 10.4049/jimmunol.175.3.1991. [DOI] [PubMed] [Google Scholar]
- 155.Shigihara T, Shimada A, Oikawa Y, et al. CXCL10 DNA vaccination prevents spontaneous diabetes through enhanced β cell proliferation in NOD mice. J. Immunol. 2005;175(12):8401–8408. doi: 10.4049/jimmunol.175.12.8401. [DOI] [PubMed] [Google Scholar]
- 156.Medicherla S, Protter AA, Ma JY, et al. Preventive and therapeutic potential of p38α-selective mitogen-activated protein kinase inhibitor in nonobese diabetic mice with Type 1 diabetes. J. Pharmacol. Exp. Ther. 2006;318(1):99–107. doi: 10.1124/jpet.105.097857. [DOI] [PubMed] [Google Scholar]
- 157.Tarbell KV, Petit L, Zuo X, et al. Dendritic cell-expanded, islet-specific CD4+CD25+CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 2007;204(1):191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Meagher C, Arreaza G, Peters A, et al. CCL4 protects from Type 1 diabetes by altering islet β-cell-targeted inflammatory responses. Diabetes. 2007;56(3):809–817. doi: 10.2337/db06-0619. [DOI] [PubMed] [Google Scholar]
- 159.Stosic-Grujicic S, Cvetkovic I, Mangano K, et al. A potent immunomodulatory compound, (S,R)-3-Phenyl-4,5-dihydro-5-isoxazole acetic acid, prevents spontaneous and accelerated forms of autoimmune diabetes in NOD mice and inhibits the immunoinflammatory diabetes induced by multiple low doses of streptozotocin in CBA/H mice. J. Pharmacol. Exp. Ther. 2007;320(3):1038–1049. doi: 10.1124/jpet.106.109272. [DOI] [PubMed] [Google Scholar]
- 160.Chang CL, Chang SL, Lee YM, et al. Cytopiloyne, a polyacetylenic glucoside, prevents Type 1 diabetes in nonobese diabetic mice. J. Immunol. 2007;178(11):6984–6993. doi: 10.4049/jimmunol.178.11.6984. [DOI] [PubMed] [Google Scholar]
- 161.Xia CQ, Peng R, Qiu Y, Annamalai M, Gordon D, Clare-Salzler MJ. Transfusion of apoptotic β-cells induces immune tolerance to β-cell antigens and prevents Type 1 diabetes in NOD mice. Diabetes. 2007;56(8):2116–2123. doi: 10.2337/db06-0825. [DOI] [PubMed] [Google Scholar]
- 162.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J. Immunol. 2007;179(6):3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 163.Phillips B, Nylander K, Harnaha J, et al. A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes. Diabetes. 2008;57(6):1544–1555. doi: 10.2337/db07-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bitan M, Weiss L, Zeira M, et al. Heparanase prevents the development of Type 1 diabetes in non-obese diabetic mice by regulating T-cell activation and cytokines production. Diabetes Metab. Res. Rev. 2008;24(5):413–421. doi: 10.1002/dmrr.868. [DOI] [PubMed] [Google Scholar]
- 165.Besin G, Gaudreau S, Menard M, Guindi C, Dupuis G, Amrani A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes. 2008;57(8):2107–2117. doi: 10.2337/db08-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mabley JG, Pacher P, Murthy KG, et al. The novel inosine analogue, INO-2002, protects against diabetes development in multiple low-dose streptozotocin and non-obese diabetic mouse models of Type I diabetes. J. Endocrinol. 2008;198(3):581–589. doi: 10.1677/JOE-07-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Goudy KS, Wang B, Tisch R. Gene gun-mediated DNA vaccination enhances antigen-specific immunotherapy at a late preclinical stage of Type 1 diabetes in nonobese diabetic mice. Clin. Immunol. 2008;129(1):49–57. doi: 10.1016/j.clim.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Louvet C, Szot GL, Lang J, et al. Tyrosine kinase inhibitors reverse Type 1 diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA. 2008;105(48):18895–18900. doi: 10.1073/pnas.0810246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Perone MJ, Bertera S, Shufesky WJ, et al. Suppression of autoimmune diabetes by soluble galectin-1. J. Immunol. 2009;182(5):2641–2653. doi: 10.4049/jimmunol.0800839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Madec AM, Mallone R, Afonso G, et al. Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia. 2009;52(7):1391–1399. doi: 10.1007/s00125-009-1374-z. [DOI] [PubMed] [Google Scholar]
- 171.Chou FC, Shieh SJ, Sytwu HK. Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Eur. J. Immunol. 2009;39(9):2403–2411. doi: 10.1002/eji.200839177. [DOI] [PubMed] [Google Scholar]
- 172.Li L, Yi Z, Wang B, Tisch R. Suppression of ongoing T cell-mediated autoimmunity by peptide-MHC class II dimer vaccination. J. Immunol. 2009;183(7):4809–4816. doi: 10.4049/jimmunol.0901616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Haase C, Yu L, Eisenbarth G, Markholst H. Antigen-dependent immunotherapy of non-obese diabetic mice with immature dendritic cells. Clin. Exp. Immunol. 2010;160(3):331–339. doi: 10.1111/j.1365-2249.2010.04104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoang PT, Park P, Cobb LJ, et al. The neurosurvival factor humanin inhibits β-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59(3):343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jayaraman S, Patel T, Patel V, et al. Transfusion of nonobese diabetic mice with allogeneic newborn blood ameliorates autoimmune diabetes and modifies the expression of selected immune response genes. J. Immunol. 2010;184(6):3008–3015. doi: 10.4049/jimmunol.0903615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xia CQ, Chernatynskaya A, Lai Y, Campbell KA, Clare-Salzler MJ. Experimental extracorporeal photopheresis therapy significantly delays the development of diabetes in non-obese diabetic mice. Clin. Immunol. 2010;135(3):374–383. doi: 10.1016/j.clim.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 177.Vincent BG, Young EF, Buntzman AS, et al. Toxin-coupled MHC class I tetramers can specifically ablate autoreactive CD8+ T cells and delay diabetes in nonobese diabetic mice. J. Immunol. 2010;184(8):4196–4204. doi: 10.4049/jimmunol.0903931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nishimura M, Yokoi N, Miki T, et al. Construction of a multi-functional cDNA library specific for mouse pancreatic islets and its application to microarray. DNA Res. 2004;11(5):315–323. doi: 10.1093/dnares/11.5.315. [DOI] [PubMed] [Google Scholar]
- 179.Koulmanda M, Bhasin M, Hoffman L, et al. Curative and β cell regenerative effects of α1-antitrypsin treatment in autoimmune diabetic NOD mice. Proc. Natl Acad. Sci. USA. 2008;105(42):16242–16247. doi: 10.1073/pnas.0808031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tsai S, Shameli A, Yamanouchi J, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32(4):568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
Websites
- 201. NCT00837759: Novel therapy combining regenerative stimuli and immunomodulation to preserve β-cell function in new onset Type 1 diabetes. http://clinicaltrials.gov/ct2/show/NCT00837759.
- 202. NCT00873925: Cord blood plus vitamin D and omega 3s in T1D. http://clinicaltrials.gov/ct2/show/NCT00873925.
- 203. NCT01068951: Treatment of patients with new onset of Type 1 diabetes with mesenchymal stem cells. http://clinicaltrials.gov/ct2/show/NCT01068951.
- 204. NCT00378508: Anti-CD3 mAb treatment of recent onset Type 1 diabetes. http://clinicaltrials.gov/ct2/show/NCT00378508.
- 205. NCT00678886: Trial of otelixizumab for adults with newly diagnosed Type 1 diabetes mellitus (autoimmune): DEFEND-1. http://clinicaltrials.gov/ct2/show/NCT00678886.
- 206. NCT00662519: Neulasta in Type 1 diabetes. http://clinicaltrials.gov/ct2/show/NCT00662519.
- 207. NCT00445913: Autologous dendritic cell therapy for Type 1 diabetes suppression: a safety study. http://clinicaltrials.gov/ct2/show/NCT00445913.
- 208. NCT00896077: Subcutaneous administration of lisofylline to healthy normal subjects and subjects with Type 1 diabetes. http://clinicaltrials.gov/ct2/show/NCT00896077.
- 209. NCT00223613: Intranasal insulin for prevention of Type 1 diabetes. http://clinicaltrials.gov/ct2/show/NCT00223613.
- 210. NCT00336674: Trial of intranasal insulin in children and young adults at risk of Type 1 diabetes. http://clinicaltrials.gov/ct2/show/NCT00336674.
- 211.cDNA library 16013: Mus musculus. www.ncbi.nlm.nih.gov/UniGene/library.cgi?ORG=Mm&LID=16013.
- 212.Unigene expression profile for Mus musculus. ftp://ftp.ncbi.nih.gov/repository/UniGene/Mus_musculus/Mm.profiles.gz.