Abstract
A highly bioactive bone-bonding Ti metal was obtained when Ti metal was simply heat-treated after a common acid treatment. This bone-bonding property was ascribed to the formation of apatite on the Ti metal in a body environment. The formation of apatite on the Ti metal was induced neither by its surface roughness nor by the rutile phase precipitated on its surface, but by its positively charged surface. The surface of the Ti metal was positively charged because acid groups were adsorbed on titanium hydride formed on the Ti metal by the acid treatment, and remained even after the titanium hydride was transformed into titanium oxide by the subsequent heat treatment. These results provide a new principle based on a positively charged surface for obtaining bioactive materials.
Keywords: bioactive Ti metal, apatite formation, surface charge, chemical treatment, dental implant, orthopaedic implant
1. Introduction
In general, synthetic materials implanted into bone defects become encapsulated by a fibrous tissue to isolate them from the surrounding bone. Only a few types of ceramic, based on calcium phosphate, have been shown to bond to living bone without the intervention of fibrous tissue at the interface (Kokubo 2008). These are called bioactive ceramics and they are already used clinically as important bone substitutes. However, they are brittle and have poor fracture toughness, and, hence, cannot be used under load-bearing conditions.
In the orthopaedic and dental fields, metallic materials, such as Ti metal and its alloys, are widely used as various implants because of their high fracture toughness and good biocompatibility. However, they do not bond to living bone. As-polished or as-abraded Ti metal is encapsulated by fibrous tissue that isolates it from the surrounding bone when it is implanted into a bone defect (Yan et al. 1997; Hacking et al. 2002). When a rough texture is produced on its surface by grit blasting or acid etching, Ti can form a direct contact with living bone (Hacking et al. 2002). Therefore, some orthopaedic and dental implants have been subjected to grit blasting and/or acid etching (Coelho et al. 2009). However, this direct contact itself does not bond the implant to bone. To enable Ti metal and its alloys to bond to bone, hydroxyapatite has been coated on their surfaces using various methods (Leeuwenburgh et al. 2008). However, the hydroxyapatite coating is not stable in the living body for long periods.
Early on, we found that a titania gel prepared using a sol–gel method forms a bone-like apatite layer on its surface in an acellular simulated body fluid (SBF) with ion concentrations nearly equal to those of human blood plasma (Li et al. 1994). On the other hand, it has been shown for various bioactive ceramics that a material able to form bone-like apatite on its surface in an SBF generally forms the apatite on its surface also in the living body and bonds to living bone through the apatite layer (Kokubo & Takadama 2006). Based on these findings, it was assumed that even Ti metal could form apatite on its surface in the living body and bond to living bone through this apatite layer if it could be modified with a functional group that was effective for the nucleation of apatite on its surface. As expected, it was shown that Ti metal formed with sodium titanate on its surface by an NaOH solution and a subsequent heat treatment induced the formation of apatite on its surface in a living body, so that it can bond to living bone (Kim et al. 1996; Yan et al. 1997; Nishiguchi et al. 2003). This treatment was applied to a porous Ti metal layer of a total artificial hip joint, and the obtained bioactive joint has been used successfully clinically in Japan since 2007 (Kawanabe et al. 2009).
However, the apatite-forming ability of the NaOH- and heat-treated Ti metal is liable to decrease when it is stored in a humid environment for long periods, since the sodium ions of the sodium titanate are slowly released via exchange with the H3O+ ions in the moisture in the atmosphere. In this study, we show that a highly bioactive Ti metal formed with titanium oxide on its surface, which is stable in a humid environment, is obtained using only a simple heat treatment after a common acid treatment, and that a new principle for obtaining bioactive materials can be proposed based on the mechanism of apatite formation on the bioactive Ti metal.
Several papers have been published on the formation of apatite on the titanium oxide formed on Ti metal after a chemical treatment. Uchida et al. (2002) first reported that Ti metal which formed anatase and rutile on its surface by an NaOH solution, water and a heat treatment induced the formation of apatite on its surface in an SBF. Wang et al. (2002) reported that Ti metal which formed anatase on its surface by an H2O2/HCl solution and a heat treatment induced the formation of apatite on its surface in an SBF. Wu et al. (2004) reported that Ti metal which formed anatase and rutile on its surface by HF/HNO3 and subsequent H2O2/TaCl2 solution treatments induced the formation of apatite on its surface in an SBF. Yang et al. (2004) reported that Ti metal which formed anatase and rutile on its surface by anodic oxidation using a spark discharge in an H2SO4 solution induced the formation of apatite on its surface in an SBF. Lu et al. (2007) reported that Ti metal that was treated with strong nitric acid for a long period induced the formation of apatite on its surface in an SBF, although no titanium oxide was detected on its surface. Lu et al. (2008) reported that acid-etched Ti metal which formed rutile on its surface by a heat treatment induced the formation of apatite on its surface in an SBF. Pattanayak et al. (2009) reported that the apatite-forming ability of Ti metal with rutile and anatase on its surface after soaking in NaOH and HCl solutions, and then a heat treatment, increased with increasing concentration of the HCl solution. However, there is no consensus on the principles governing the apatite-forming ability of titanium oxide on Ti metal.
2. Material and methods
2.1. Preparation of the Ti metal samples
Commercial pure Ti metal (Kobe Steel, Japan, grade 2, O2 content = 0.15 wt%) was cut into rectangular samples (dimensions = 10 × 10 × 1 mm3), and abraded with a no. 400 diamond abrasive plate. All the abraded samples were washed with acetone, 2-propanol and ultrapure water for a period of 30 min each in an ultrasonic cleaner, and then dried overnight in an oven at 40°C.
The samples were soaked in 20 ml of a mixture of 66.3 per cent H2SO4 (w/w) solution (Kanto Chemical Co., Inc., Japan) and 10.6 per cent HCl (w/w) solution (Kanto Chemical Co., Inc.) in a weight ratio of 1 : 1 at 70°C for a period of 1 h in an oil bath shaken at 120 strokes min−1, then gently washed with ultrapure water and dried overnight in an oven at 40°C. This mixed acid solution is the same etchant used for one of the commercial dental implants (Takeuchi et al. 2005).
The acid-treated samples were heated to temperatures in the range 400–800°C at a rate of 5°C min−1 in an Fe–Cr furnace in air. The samples were kept at the desired temperature for a period of 1 h, and then allowed to cool to room temperature at the natural rate of the furnace. As a reference, the abraded samples were heat-treated using the same method without being subjected to an acid treatment.
2.2. Examination of the apatite-forming ability of Ti metal in an SBF
The samples subjected to the acid and heat treatments, and those subjected to only the heat treatment, were soaked in 30 ml of an acellular SBF with ion concentrations nearly equal to those of human blood plasma at 36.5°C (Na+ = 142.0, K+ = 5.0, Mg2+ = 1.5, Ca2+ = 2.5, Cl− = 147.8, 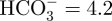 ,
, 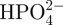 = 1.0 and
= 1.0 and 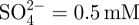 ; Kokubo & Takadama 2006). The SBF was prepared by dissolving reagent grade NaCl, NaHCO3, KCl, K2HPO4 · 3H2O, MgCl2 · 6H2O, CaCl2 and Na2SO4 (Nacalai Tesque Inc., Japan) into ultrapure water, and then buffering the solution at pH = 7.4 using tris-hydroxymethylaminomethane ((CH2OH)3CNH2) and 1 M HCl (Nacalai Tesque Inc.). The samples were removed from the SBF after 1 day, gently washed with ultrapure water and dried in an oven at 40°C. The formation of apatite on the sample surface was examined using scanning electron microscopy (SEM) and thin film X-ray diffraction (TF-XRD) employing the methods described in the next section.
; Kokubo & Takadama 2006). The SBF was prepared by dissolving reagent grade NaCl, NaHCO3, KCl, K2HPO4 · 3H2O, MgCl2 · 6H2O, CaCl2 and Na2SO4 (Nacalai Tesque Inc., Japan) into ultrapure water, and then buffering the solution at pH = 7.4 using tris-hydroxymethylaminomethane ((CH2OH)3CNH2) and 1 M HCl (Nacalai Tesque Inc.). The samples were removed from the SBF after 1 day, gently washed with ultrapure water and dried in an oven at 40°C. The formation of apatite on the sample surface was examined using scanning electron microscopy (SEM) and thin film X-ray diffraction (TF-XRD) employing the methods described in the next section.
To examine the stability of the apatite-forming ability in a humid environment, the acid- and heat-treated samples were kept under an atmosphere of 95 per cent relative humidity at 80°C for a period of one week, and the formation of apatite on the surface in an SBF was examined using SEM and TF-XRD.
2.3. Analysis of the surface of the Ti metal samples
2.3.1. Scanning electron microscopy
The surface and cross-sectional area of the Ti metal samples subjected to both the acid and heat treatments, to the heat treatment alone, and those subsequently soaked in an SBF were coated with a Pt/Pd film and observed under a field-emission scanning electron microscope (FE-SEM; S-4300, Hitachi Co., Japan), using an acceleration voltage of 15 kV.
2.3.2. Thin film X-ray diffraction
The surface of the Ti metal samples subjected to both the acid and heat treatments, to the heat treatment alone, and those subsequently soaked in an SBF were analysed using TF-XRD (model RNT-2500, Rigaku Co., Japan), employing a CuKα X-ray source operating at 50 kV and 200 mA. The glancing angle of the incident beam was set to an angle of 1° against the sample surface.
2.3.3. Surface roughness measurements
The surface roughness of the Ti metal samples subjected to the acid and heat treatments was measured using a surface roughness testing system (Surftest model SV-2000, Mitutoyo Co., Japan) using a stylus with a diameter of 2 μm. Based on the data in the JIS standard 1994, the measuring length, evaluation length and cut-off wavelength used were 2.5, 12.5 and 2.5 mm, respectively. Seven measurements were performed for each sample and averaged.
2.3.4. Scratch resistance measurements
The scratch resistance of the surface layer formed on the Ti metal samples by the acid and heat treatments or the heat treatment alone was measured using a thin film scratch tester (model CSR-2000, Rhesca Co., Japan) employing a stylus with a diameter of 5 μm with a spring constant of 200 g mm−1. Based on the data in the JIS R-3255 standard, the amplitude, scratch speed and loading rate used were 100 μm, 10 μm s−1 and 100 mN min−1, respectively. Eight to 10 measurements were carried out for each sample, and the average value was used in our analysis.
2.3.5. Zeta potential measurements
Titanium metal plates (size = 13 × 33 × 1 mm3) were prepared using the same method described in §2.1, and these were subjected to the acid and heat treatments, or to the heat treatment alone, at various temperatures. The volume of the acid solution was increased to 30 ml in the acid treatment, because the surface area of the sample was larger than that used for the other surface analysis measurements. Thus, the treated Ti metal samples were grounded to allow for leakage of any stray charge, and they were immediately set in a zeta potential and particle size analyser (model ELS-Z1, Otsuka Electronics Co., Japan) using a glass cell for the plate sample. The zeta potentials of the samples were measured under an applied voltage of 40 V in a 10 mM NaCl solution. The dispersant monitoring particles of polystyrene latex (size = 500 nm) were coated with hydroxyl propyl cellulose. Five samples were measured for each experimental condition and the average reported in our analysis.
2.3.6. X-ray photoelectron spectroscopy
The surfaces of the Ti metal samples soaked in an SBF for various periods after the acid and heat treatments, or the heat treatment alone, were analysed using X-ray photoelectron spectroscopy (XPS, ESCA-3300KM, Shimadzu Co., Japan) as a function of the soaking time in the SBF. In our analysis, MgKα radiation (λ = 9.8903 Å) was used as the X-ray source. The XPS take-off angle was set at 45°, which enabled the system to detect photoelectrons to a depth of 5–10 nm from the surface. The binding energies of the measured spectra were calibrated with reference to the C1s peak of the surfactant CH2 groups on the substrate at 284.6 eV.
2.3.7. Radio frequency (RF) glow discharge optical emission spectroscopy
The depth profiles of various elements on the surface of the Ti metal samples subjected to the acid and heat treatments were analysed using RF glow discharge optical emission spectroscopy (GD-OES, GD-Profiler 2, Horiba Co., Japan) under Ar sputtering at an Ar pressure of 600 Pa. A RF electric field with a power of 35 W was applied at a regular interval of 20 ms.
2.4. Examination of the bone-bonding ability of Ti metal
The Animal Research Committee of the Graduate School of Medicine, Kyoto University, Japan, approved our animal studies. Rectangular samples (size = 15 × 10 × 2 mm3) of Ti metal subjected to the acid and heat treatments, or the heat treatment alone, were sterilized with ethylene oxide gas and implanted into metaphyses of the tibiae of mature male Japanese white rabbits weighing 2.8–3.2 kg. The surgical methods used have been described previously (Nakamura et al. 1985). After four weeks' implantation, the rabbits were sacrificed using an overdose of intravenous pentobarbital sodium. The segments of the proximal tibiae metaphyses containing the implanted samples were retrieved. The bone tissue surrounding the implants was removed on both sides and at the ends using a dental burr. Traction was applied vertically to the sample surface at a crosshead speed of 35 mm min−1 using an Instron-type autograph (model 1011, Aikon Engineering Co., Japan). The detaching failure load was recorded when the sample was detached from the bone. Five measurements were performed for each experimental condition and the average value recorded.
Other segments of the tibiae containing the implanted samples were fixed in 10 per cent phosphate-buffered formalin, dehydrated using ethanol, and embedded in polyester resin. Sections with a thickness of 500 μm were cut, bound to a transparent acrylic plate, and ground to a thickness of 40–50 μm. These samples were stained using Stevenel's blue and Van Gieson's picro-fuchsin. A histological evaluation was performed on each stained section using transmitted light microscopy (model Eclipse 80i, Nikon Co., Japan).
3. Results
3.1. Change in the surface structure of the Ti metal after the acid and heat treatments
Figure 1 shows FE-SEM photographs of the surfaces of Ti metal samples as-abraded, and after being heat-treated at 600°C and 800°C. The abraded sample had a smooth surface, but its surface roughness increased a little with increasing temperature of the heat treatment.
Figure 1.
FE-SEM photographs of surfaces of Ti metals as-abraded, and subsequently heat-treated at different temperatures. (a) Before heat treatment, (b) heat-treated at 600°C and (c) heat-treated at 800°C. Scale bars, 1 μm.
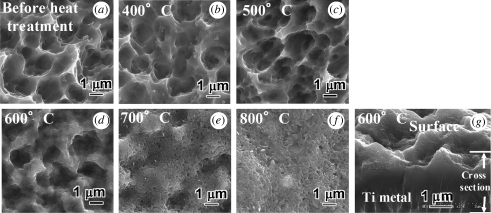
Figure 2 shows FE-SEM photographs of the surfaces of Ti metal samples that had been subjected to the acid treatment and subsequently heat-treated at various temperatures up to 800°C. A cross-sectional view of a sample heat-treated at 600°C is also shown in figure 2. A surface roughness was produced on the Ti metal sample by the acid treatment, and this remained unchanged up to 600°C, but the sample surface roughness decreased above 700°C.
Figure 2.
FE-SEM photographs of surfaces and a cross section of Ti metals as acid-treated, and subsequently heat-treated, at various temperatures. (a) Before heat treatment and heat-treated at (b) 400°C, (c) 500°C, (d) 600°C, (e) 700°C, (f) 800°C and (g) a cross section of Ti metal at 600°C.
According to our surface roughness measurements, the values of Ra and Rz were Ra = 0.99 ± 0.07 and Rz = 8.87 ± 0.94 μm for the samples that had been acid-treated, and Ra = 0.99 ± 0.17 and Rz = 7.33 ± 0.83 μm for the samples that had been heat-treated at 600°C.
The scratch resistance of the Ti metal samples as-abraded and acid-treated were very low, about 1–2 mN. Both of these samples showed a marked increase up to 40–55 mN after the subsequent heat treatments at 600–800°C.
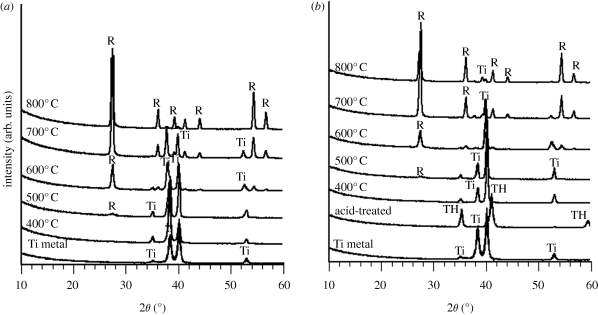
Figure 3a,b shows thin film X-ray diffraction patterns of the surfaces of the Ti metal samples subjected to heat treatment at various temperatures, without and after the acid treatment, respectively. The as-abraded sample consisted of α titanium, whereas the acid-treated sample formed a titanium hydride layer (TiHx, where 0 < x < 2) on its surface. Despite these differences, both of these samples began to precipitate rutile around 500°C on their surfaces, and the rutile content increased with increasing heat-treatment temperature up to 800°C. It is apparent from these results that the increase in surface roughness of the abraded sample, the decrease in surface roughness of the acid-treated sample, and the increase in the scratch resistance of the abraded and acid-treated samples with increasing temperature of the heat treatment are all attributed to the precipitation of the rutile on the surface of the Ti metals.
Figure 3.
TF-XRD patterns of surfaces of Ti metals heat-treated at various temperatures (a) without and (b) after acid treatment. R, rutile; Ti, α titanium; TH, TiHx.
3.2. Change in the apatite-forming ability of Ti metal in an SBF after acid and heat treatments
Figure 4 shows FE-SEM photographs of the surface of Ti metal samples soaked in an SBF for a period of 1 day, after being abraded and subsequently heat-treated at 600 and 800°C. No deposit was observed on these surfaces.
Figure 4.
FE-SEM photographs of surfaces of the Ti metals soaked in SBF for 1 day, after being abraded, and subsequently heat-treated at different temperatures. (a) Before heat treatment, (b) heat-treated at 600°C and (c) heat-treated at 800°C. Scale bars, 2 μm.
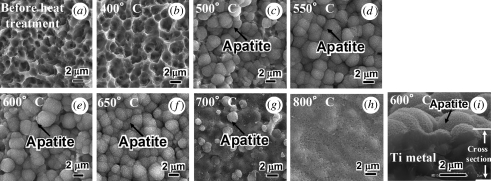
Figure 5 shows FE-SEM photographs of the surfaces of Ti metal samples soaked in an SBF for a period of 1 day, after being treated with acid and subsequently heat-treated at various temperatures up to 800°C. The cross section of a sample soaked in an SBF for a period of 1 day after the acid and heat treatments at 600°C is also shown in figure 5.
Figure 5.
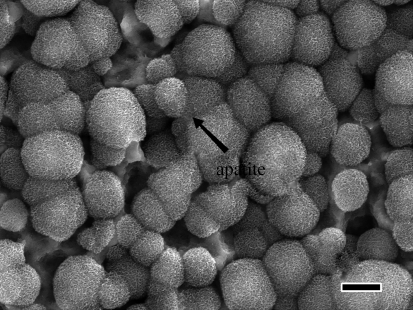
FE-SEM photographs of surfaces and a cross section of Ti metals soaked in SBF for 1 day, after being heat-treated at various temperatures following the acid treatment. (a) Before heat treatment and heat-treated at (b) 400°C, (c) 500°C, (d) 550°C, (e) 600°C, (f) 650°C, (g) 700°C, (h) 800°C and (i) a cross section of Ti metal at 600°C.
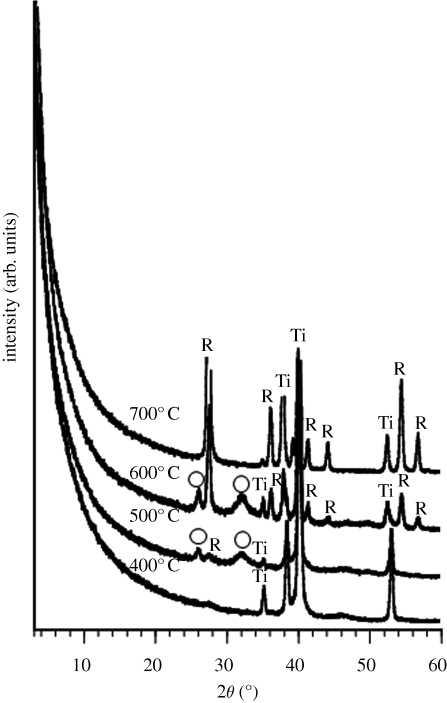
Figure 6 shows TF-XRD patterns of the surfaces of Ti metal samples soaked in an SBF for a period of 1 day, after being treated with acid and subsequently heat-treated at various temperatures. The round particles observed on the surfaces of the Ti metal samples in figure 5 are identified as being apatite from TF-XRD patterns of figure 6, although its detailed structure is not clear from these TF-XRD patterns (Lu & Leng 2004). From figure 5, it can be seen that apatite was deposited actively only on the surface of Ti metal samples that had been heat-treated at temperatures from 500 to 650°C after an acid treatment.
Figure 6.
TF-XRD patterns of the surfaces of Ti metals soaked in SBF for 1 day after being heat-treated at various temperatures following acid treatment. Ti, α titanium; R, rutile; open circle, apatite.
Figure 7 shows an FE-SEM photograph of the surface of a Ti metal sample soaked in an SBF for a period of 1 day that had been kept under an atmosphere of 95 per cent relative humidity at 80°C for a period of one week following an acid and heat treatment at 600°C. The apatite-forming ability of the acid- and heat-treated Ti metal sample was only a little decreased even after it was kept in a humid environment for a period of one week.
Figure 7.
FE-SEM photograph of the surface of Ti metal soaked in SBF for 1 day, after being kept in a humid environment for one week following the acid and heat treatments at 600°C (scale bar, 2 μm).
3.3. Change in the zeta potential of Ti metal after acid and heat treatments
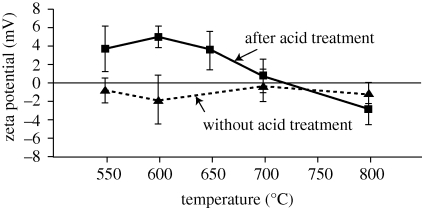
Figure 8 shows the zeta potentials of the surfaces of Ti metal samples heat-treated at various temperatures without and after an acid treatment. The zeta potentials of the samples that had been heat-treated at temperatures lower than 500°C were not able to be measured, since there was no or only a thin insulating titanium oxide layer formed on their surface (see figure 3). The Ti metal samples heat-treated without being subjected to the acid treatment showed low zeta potentials, around zero, irrespective of the temperature of the heat treatment, whereas those heat-treated after the acid treatment showed positive zeta potentials when heat-treated at temperatures in the range 550–650°C.
Figure 8.
Zeta potentials of surfaces of Ti metals heat-treated at various temperatures without and after acid treatment.
3.4. Change in the XPS spectra of Ti metal after acid and heat treatments
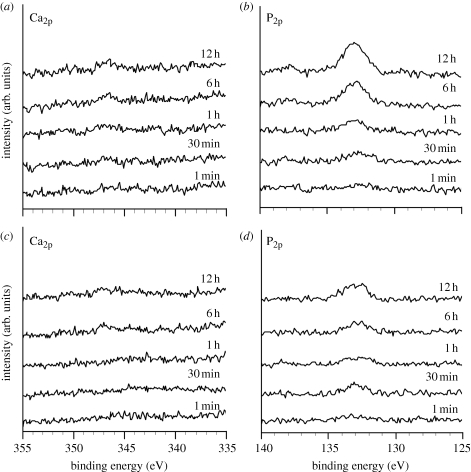
Figure 9 shows the XPS spectra of the surfaces of the Ti metal samples as-abraded and subsequently heat-treated at 600°C as a function of the soaking time in the SBF. The as-abraded sample adsorbed only a small amount of the calcium and phosphate ions, almost simultaneously, on its surface, even after 12 h in the SBF, and this property was unchanged after heat treatment at 600°C.
Figure 9.
(a,b) XPS spectra of the surfaces of Ti metals abraded and (c,d) subsequently heat-treated at 600°C, as a function of soaking time in SBF.
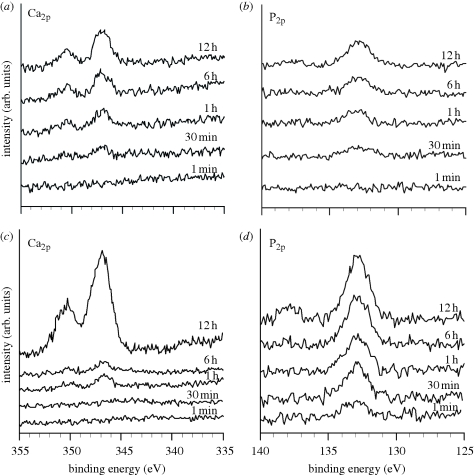
Figure 10 shows the XPS spectra of the surfaces of the Ti metal samples that had been treated with acid and subsequently heat-treated at 600°C as a function of the soaking time in the SBF. The acid-treated sample also adsorbed only a small amount of the calcium and phosphate ions, almost simultaneously, in the SBF, whereas the sample heat-treated at 600°C after the acid treatment initially adsorbed a large amount of phosphate ions preferentially on its surface, and then later adsorbed a large amount of calcium ions.
Figure 10.
(a,b) XPS spectra of surfaces of Ti metals acid-treated and (c,d) subsequently heat-treated at 600°C, as a function of soaking time in SBF.
3.5. Change in the GD-OES spectra of Ti metal after acid and heat treatments
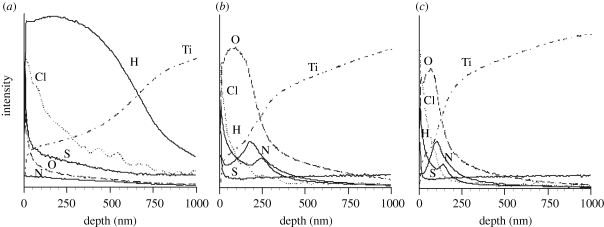
Figure 11 shows the depth profile of the GD-OES spectra of Ti metal samples that were treated with acid, subsequently heat-treated at 600°C, and then kept under an atmosphere of 95 per cent relative humidity at 80°C for a period of one week. Large amounts of H and O, besides Ti atoms, were detected in the surface layer of the samples that were treated with acid and subsequently heat-treated, respectively, because of the presence of titanium hydride and titanium oxide on their surfaces. Besides the presence of H, O and Ti, a large amount of Cl and S atoms was detected in the surface layer of the samples that had been treated with acid, and a considerable amount of these atoms was detected even after the subsequent heat treatment at 600°C and being kept in a humid environment for a period of one week.
Figure 11.
(a) Depth profiles of GD-OES spectra of Ti metals acid-treated, (b) subsequently heat-treated at 600°C and (c) kept in a humid environment.
3.6. Bonding of Ti metal to bone
The as-abraded titanium metal samples and those subsequently heat-treated at 600°C showed a detaching failure load of 1.86 ± 1.72 and 6.12 ± 3.43 N, respectively, when they were implanted into the tibia of a rabbit and subjected to the detaching test at four weeks after implantation. The acid-treated samples and those subsequently heat-treated at 600°C showed a failure load of 4.91 ± 1.94 and 13.3 ± 4.67 N, respectively. The failure load of the abraded sample was only slightly increased by the heat treatment or acid treatment. However, it was increased markedly by a heat treatment after the acid treatment.
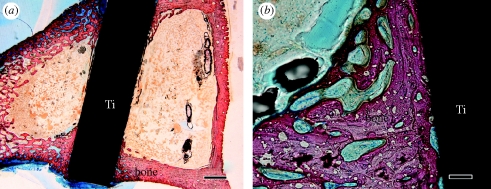
Figure 12 shows the light micrographs of an interface with the living bone of a sample that was heat-treated at 600°C after the acid treatment. Bone had grown along the surface of the Ti metal sample and it was in direct contact with the sample without the intervention of any fibrous tissue.
Figure 12.
Light micrographs of strained sections of acid- and heat-treated Ti metal implanted into a rabbit tibia for four weeks. Scale bars, (a) 1 mm, (b) 100 μm.
4. Discussion
It is apparent from the experimental results described above that a high apatite-forming ability in an SBF is conferred on Ti metal by neither a heat treatment alone nor an acid treatment alone, but is conferred by a heat treatment after an acid treatment. Titanium metal subjected to a heat treatment after an acid treatment to confer a high apatite-forming ability was confirmed to be directly in contact with newly grown bone and formed tight bonding to the bone in the animal experiments. This means that bioactive Ti metal can be obtained by a simple heat treatment after a common acid treatment. The high apatite-forming ability of our prepared Ti metal was confirmed to be maintained, even when kept in a humid environment for a long period. It was also shown that our prepared Ti metal showed a considerably high scratch resistance. Because of these properties, our prepared bioactive Ti metal is believed to be useful for various types of implant in the dental and orthopaedic fields.
It should be noted here that porous bioactive Ti metal with titanium oxide on its surface after NaOH, HCl and heat treatment exhibits high osteoinductivity, which is poor for porous Ti metal with sodium titanate on its surface after NaOH and heat treatment, in addition to the observed osteoconductivity (Takemoto et al. 2006). That is, these samples show active bone formation not only in bone defects but also in muscles. The higher osteoinductivity of the former samples compared with the latter is considered to be attributed to the lack of ions, which, in the latter, are released to form unfavourable conditions for the cells by increasing the pH. In view of this fact, the bioactive Ti metal formed with the titanium oxide on its surface using the present method can also be expected to exhibit a high osteoinductivity. This is now being investigated.
Although detailed results of the animal experiments described in §2.4 will be published elsewhere, the tight bonding of the Ti metal heat-treated after the acid treatment can be attributed to the formation of apatite on its surface in the living body. We are concerned as to the reason why Ti metal forms apatite on its surface in a body environment when it is heat-treated at temperatures in the range 500–650°C after an acid treatment. A rough surface was produced on the Ti metal by the acid treatment, but the surface roughness was unchanged in the temperature range 500–650°C from before heat treatment (see figure 2). Therefore, the formation of apatite on our Ti metal samples cannot be attributed to their surface roughness. The acid-treated Ti metal samples precipitated rutile on their surface when heat-treated at 500–650°C, but the Ti metal samples without an acid treatment also precipitated the same phase in the same temperature range (see figure 3). Therefore, the formation of apatite on our Ti metal samples cannot be attributed to a specific crystalline phase.
The most probable reason for the formation of apatite on our Ti metal samples is their positive surface charge. Figure 8 shows that only the Ti metal samples that were heat-treated at temperatures of 550–650°C after an acid treatment showed a positive zeta potential. Although the zeta potential of Ti metal heat-treated at 500°C after an acid treatment was not measured, it is assumed to have a positive value, since the Ti metal sample heat-treated at 500°C had a titanium oxide layer on its surface, similar to Ti metal samples heat-treated at 550–650°C, even though this layer was very thin (see figure 3). The zeta potentials of the Ti metal samples acid-treated and subsequently heat-treated at temperatures below 400°C also could not be measured, because the insulating titanium oxide layer was not formed on their surfaces (see figure 3). This means that their surfaces are electrically conductive, and hence their zeta potentials are zero.
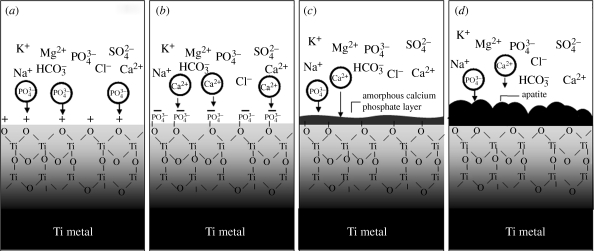
Our question now is to find the reason why the positively charged titanium oxide on Ti metal forms apatite on its surface in the body environment. The XPS spectra of Ti metal shown in figure 10 show that Ti metal heat-treated at 600°C after an acid treatment preferentially adsorbs a large amount of phosphate ions first, and then later it also adsorbs calcium ions. This result indicates that positively charged titanium oxide induces the formation of apatite on its surface by the process shown in figure 13. The positively charged titanium oxide first selectively adsorbs the negatively charged phosphate ions on its surface. As the phosphate ions begin to accumulate, the surface becomes negatively charged, and, hence, combines with the positively charged calcium ions to form calcium phosphate. The calcium phosphate formed eventually transforms into stable crystalline apatite.
Figure 13.
Process of formation of apatite on positively charged Ti metal in SBF. Apatite is formed by the process shown in (a–d).
This process of the formation of apatite on a positively charged titanium oxide layer contrasts with that where sodium titanate is formed on Ti metal after NaOH and heat treatments (Kim et al. 2003). In this case, the sodium titanate releases Na+ ions via exchange with the H3O+ ions in the body fluid to form Ti–OH groups on its surface. The Ti–OH groups formed are negatively charged (Kokubo et al. 1982), since the pH of the surrounding body fluid is increased by the Na+ ions released, and, hence, combines with the positively charged Ca2+ ions in the body fluid to form calcium titanate. As the Ca2+ ions accumulate, the surface becomes positively charged and combines with the negatively charged phosphate ions to form calcium phosphate. Thus, the calcium phosphate formed eventually transforms into stable crystalline apatite. Ever since discovering this apatite-forming process, it was believed that a negatively charged surface is essential for obtaining bioactive materials. However, the present results show that a positively charged surface can also provide bioactive materials by inducing the formation of apatite. This finding provides a new principle for the development of bioactive materials. Generally, Ti metal exhibits surface charge around zero in a body environment independent of heat treatment, as shown for a Ti metal sample without acid treatment in figure 8, and, hence, does not induce apatite formation (see figure 4). However, the present results indicate that Ti metal is negatively charged on its surface in a body environment if it has been subjected to alkali and heat treatments, and it is positively charged on its surface if it has been subjected to acid and heat treatments, and, hence, it can induce apatite formation on its surface in a body environment to give bone-bonding ability.
The reason why Ti metal heat-treated at 500–650°C after an acid treatment is positively charged is interpreted in terms of figure 11. Acid groups, such as chloride and sulphate ions, were adsorbed onto the surface of, and incorporated into, the titanium hydride layer on the surface of the Ti metal formed during the acid treatment, and a considerable amount of these ions remained in the surface layer, even after the titanium hydride was transformed into titanium oxide by the heat treatment at 600°C. These acid groups could be released from the surface to form an acidic environment in the vicinity of the surface when the Ti metal is soaked in an SBF. As a result, the surface would be positively charged, since titanium oxide is positively charged in an acidic environment (Kokubo et al. 1982; Gold et al. 1989; Textor et al. 2001). The Ti metal samples heat-treated at temperatures lower than 400°C after an acid treatment had no insulating oxide layer on their surface, and, hence, their surface was not charged and so did not induce formation of apatite in an SBF. The Ti metal samples heat-treated at temperatures higher than 700°C after the acid treatment decomposed incorporated ions on heat treatment, and, hence, their surface was not positively charged, and so did not induce the formation of apatite. Figure 11 shows that the incorporated ions almost remain on the surface of the Ti metal heat-treated at 600°C after the acid treatment, even after the Ti metal was kept in a humid environment for a period of one week. As a result, the apatite-forming ability of this Ti metal was only a little decreased, even after it was kept in a humid environment.
These results indicate that the requirement for obtaining a positively charged Ti metal that is effective for inducing the formation of apatite on its surface in a body environment is the formation of an electrically insulating oxide layer on its surface, and the adsorption of acid groups on its surface. These requirements are met only by the common acid treatment and subsequent heat treatment at moderately high temperatures. This indicates that the apatite formation of the Ti metal heat-treated after acid treatment does not depend upon the kind of acid solutions, but upon the pH of the solutions. This has already been confirmed experimentally and will be published elsewhere.
In view of this fact, most of the chemical and heat treatments for obtaining bioactive Ti metal by forming a titanium oxide on its surface, which were cited in §1, can be interpreted in terms of the method that forms a layer that can meet these requirements on the surface of the Ti metal.
Zhao et al. (2005, 2008) reported that a plasma-sprayed TiO2 coated layer with a rutile phase showed a high apatite-forming ability in an SBF when treated with an acid solution, such as H2SO4 and HNO3. Kokubo et al. (2008) reported that a titania gel layer coated on polyethylene terephthalate using a sol–gel method formed apatite on its surface in an SBF when it was treated with an HCl solution at 80°C for a period of 8 days. These results can also be interpreted in terms of the formation of a titanium oxide layer containing adsorbed acid groups on its surface to give a positive surface charge in an SBF.
5. Summary
— An ability to form apatite in an SBF was conferred on Ti metal neither by an H2SO4/HCl mixed acid treatment alone nor by a heat treatment alone, but by a heat treatment at 500–650°C after the acid treatment. The apatite-forming ability was hardly decreased, even after the Ti metal was maintained in a humid environment for a long period. The acid- and heat-treated Ti metal showed a high scratch resistance. These are important properties for clinical applications of Ti metal.
— Ti metal that was heat-treated at 600°C after the acid treatment was confirmed to be in direct contact with living bone and tightly bonded to it. This tight bonding was attributed to the formation of apatite on the surface of the Ti metal in a body environment.
— The formation of apatite on Ti metal that had been heat-treated at 500–650°C after an acid treatment was attributed neither to the roughness of the surface nor to the rutile phase that had precipitated on the surface, but to the positive charge on the surface.
— The positive charge on the Ti metal was shown to be formed by the adsorption of acid groups on the surfaces by the acid treatment, and by the formation of a non-conducting titanium oxide layer on the surface by the subsequent heat treatment.
— A new principle for obtaining bioactive materials based on a positively charged surface has been proposed.
Acknowledgements
This work was supported in part by a Grant-in-Aid from the Ministry of Health, Labour, and Welfare of Japan (H21-Trans-Ippan-003) and by a Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (19200039).
Footnotes
One contribution to a Theme Supplement ‘Scaling the heights–challenges in medical materials: an issue in honour of William Bonfield, Part II. Bone and tissue engineering’.
References
- Coelho P. G., Granjeiro J. M., Romanos G. E., Suzuki M., Silva N. R. F., Cardaropoli G., Thompson V. P., Lemons J. E. 2009. Basic research methods and current trends of dental implant surfaces. J. Biomed. Mater. Res. 88B, 579–596. ( 10.1002/jbm.b.31264) [DOI] [PubMed] [Google Scholar]
- Gold J. M., Schmidt M., Steinemann S. G. 1989. XPS study of amino-acid adsorption to titanium surface. Helv. Phys. Acta 62, 246–249. [Google Scholar]
- Hacking S. A., Tanzer M., Harvey E. J., Krygier J. J., Bobyn J. D. 2002. Relative contributions of chemistry and topography to the osseointegration of hydroxyapatite coatings. Clin. Orthopaed. Relat. Res. 405, 24–38. ( 10.1097/00003086-200212000-00004) [DOI] [PubMed] [Google Scholar]
- Kawanabe K., Ise K., Goto K., Akiyama H., Nakamura T., Kaneuji A., Sugimori T., Matsumoto T. 2009. A new cementless total hip arthoplasty with bioactive titanium porous-coating by alkaline and heat treatment: average 4.8-year results. J. Biomed. Mater. Res. 90B, 476–481. ( 10.1002/jbm.b.31309) [DOI] [PubMed] [Google Scholar]
- Kim H. M., Miyaji F., Kokubo T., Nakamura T. 1996. Preparation of bioactive Ti and its alloy via simple chemical surface treatment. J. Biomed. Mater. Res. 32, 409–417. () [DOI] [PubMed] [Google Scholar]
- Kim H. M., Himeno T., Kawashita M., Lee J. H., Kokubo T., Nakamura T. 2003. Surface potential change in bioactive titanium metal during the process of apatite formation in simulated body fluid. J. Biomed. Mater. Res. 67A, 1305–1309. ( 10.1002/jbm.a.20039) [DOI] [PubMed] [Google Scholar]
- Kokubo T. 2008. Bioceramics and their clinical applications. Cambridge, UK: Woodhead Publishing. [Google Scholar]
- Kokubo T., Takadama H. 2006. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27, 2907–2915. ( 10.1016/j.biomaterials.2006.01.017) [DOI] [PubMed] [Google Scholar]
- Kokubo T., Takagi H., Tashiro M. 1982. Alkaline durability of BaO–TiO2–SiO2 glasses. J. Non-Cryst. Solids 52, 427–433. ( 10.1016/0022-3093(82)90317-9) [DOI] [Google Scholar]
- Kokubo T., Ueda T., Kawashita M., Ikuhara Y., Takaoka G. H., Nakamura T. 2008. PET fiber fabrics modified with bioactive titanium oxide for bone substitutes. J. Mater. Sci. 19, 695–702. ( 10.1007/s10856-007-3103-9) [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh S. C. G., Wolke J. G. C., Jansen J. A., de Groot K. 2008. Calcium phosphate coatings. In Bioceramics and their clinical applications (ed. Kokubo T.), pp. 464–484. Cambridge, UK: Woodhead Publishing. [Google Scholar]
- Li P., Ohtsuki C., Kokubo T., Nakanishi K., Soga N., Nakamura T., Yamamuro T., de Groot K. 1994. A role of hydrated silica, titania and alumina in forming biologically active bone-like apatite on implant. J. Biomed. Mater. Res. 28, 7–15. ( 10.1002/jbm.820280103) [DOI] [PubMed] [Google Scholar]
- Lu X., Leng Y. 2004. TEM study of calcium phosphate precipitation on bioactive titanium surfaces. Biomaterials 25, 1779–1786. ( 10.1016/j.biomaterials.2003.08.028) [DOI] [PubMed] [Google Scholar]
- Lu X., Zhao Z., Leng Y. 2007. Biomimetic calcium phosphate coatings on nitric acid treated titanium surfaces. Mater. Sci. Eng. C 27, 700–708. ( 10.1016/j.msec.2006.06.030) [DOI] [Google Scholar]
- Lu X., Wang Y., Yang X., Zhang Q., Zhao Z., Weng L. T., Leng Y. 2008. Spectroscopic analysis of titanium surface functional groups under various surface modification and their behaviors in vitro and in vivo. J. Biomed. Mater. Res 84A, 523–534. ( 10.1002/jbm.a.31471) [DOI] [PubMed] [Google Scholar]
- Nakamura T., Yamamuro T., Higashi S. 1985. A new glass ceramic for bone replacement: evaluation of its bonding to bone tissue. J. Biomed. Mater. Res. 19, 685–698. ( 10.1002/jbm.820190608) [DOI] [PubMed] [Google Scholar]
- Nishiguchi S., Fujibayashi S., Kim H. M., Kokubo T., Nakamura T. 2003. Biology of alkali- and heat-treated titanium implants. J. Biomed. Mater. Res. 67A, 26–35. ( 10.1002/jbm.a.10540) [DOI] [PubMed] [Google Scholar]
- Pattanayak D. K., Kawai T., Matsushita T., Takadama H., Nakamura T., Kokubo T. 2009. Effect of HCl concentrations on apatite-forming ability of NaOH–HCl- and heat-treated titanium metal. J. Mater. Sci. 20, 2401–2411. ( 10.1007/s10856-009-3815-0) [DOI] [PubMed] [Google Scholar]
- Takemoto M., Fujibayashi S., Neo M., Suzuki J., Matsushita T., Kokubo T., Nakamura T. 2006. Osteoinductive porous titanium implants: effect of sodium removal by dilute HCl treatment. Biomaterials 27, 2682–2691. ( 10.1016/j.biomaterials.2005.12.014) [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Saruwatari L., Nakamura H. K., Yang J. M., Ogawa T. 2005. Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface. J. Biomed. Mater. Res. 72A, 296–305. ( 10.1002/jbm.a.30227) [DOI] [PubMed] [Google Scholar]
- Textor M., Sittig C., Frauchiger V., Tosatti S., Brunette D. M. 2001. Properties and biological significance of natural oxide films on titanium and its alloys. In Titanium in medicine (eds Brunette D. M., Tengvall P., Textor M., Thomsen P.), pp. 171–230. Berlin, Germany: Springer. [Google Scholar]
- Uchida M., Kim H.-M., Kokubo T., Fujibayashi S., Nakamura T. 2002. Effect of water treatment on the apatite-forming ability of NaOH-treated titanium metal. J. Biomed. Mater. Res. 63, 522–530. ( 10.1002/jbm.10304) [DOI] [PubMed] [Google Scholar]
- Wang X. X., Hayakawa S., Tsuru K., Osaka A. 2002. Bioactive titania gel layers formed by chemical treatment of Ti substrate with a H2O2/HCl solution. Biomaterials 23, 1353–1357. ( 10.1016/S0142-9612(01)00254-X) [DOI] [PubMed] [Google Scholar]
- Wu J. M., Hayakawa S., Tsuru K., Osaka A. 2004. Low-temperature preparation of anatase and rutile layers on titanium substrates and their ability to induce in vitro apatite deposition. J. Am. Ceram. Soc. 87, 1635–1642. (doi:0.1111/j.1551-2916.2004.01635.x) [Google Scholar]
- Yan W. Q., Nakamura T., Kobayashi M., Kim H. M., Miyaji F., Kokubo T. 1997. Bonding of chemically treated titanium implants to bone. J. Biomed. Mater. Res. 37, 267–275. (doi:0.1002/(SICI)1097-4636(199711)37:2<267::AID-JBM17>3.0.CO;2-B) [DOI] [PubMed] [Google Scholar]
- Yang B., Uchida M., Kim H. M., Zhang X., Kokubo T. 2004. Preparation of bioactive titanium metal via anodic oxidation treatment. Biomaterials 25, 1003–1010. ( 10.1016/S0142-9612(03)00626-4) [DOI] [PubMed] [Google Scholar]
- Zhao X., Liu X., Ding C. 2005. Acid induced bioactive titania surface. J. Biomed. Mater. Res. 75A, 888–894. ( 10.1002/jbm.a.30485) [DOI] [PubMed] [Google Scholar]
- Zhao X., Liu X., You J., Chen Z., Ding C. 2008. Bioactivity and cytocompatibility of plasma-sprayed titania coating treated by sulfuric acid treatment. Surf. Coat. Technol. 202, 3221–3226. ( 10.1016/j.surfcoat.2007.11.026) [DOI] [Google Scholar]