Abstract
Our long-term objective is to develop methods to form, in the jaw, bioengineered replacement teeth that exhibit physical properties and functions similar to those of natural teeth. Our results show that cultured rat tooth bud cells, seeded onto biodegradable scaffolds, implanted into the jaws of adult rat hosts and grown for 12 weeks, formed small, organized, bioengineered tooth crowns, containing dentin, enamel, pulp, and periodontal ligament tissues, similar to identical cell-seeded scaffolds implanted and grown in the omentum. Radiographic, histological, and immunohistochemical analyses showed that bioengineered teeth consisted of organized dentin, enamel, and pulp tissues. This study advances practical applications for dental tissue engineering by demonstrating that bioengineered tooth tissues can be regenerated at the site of previously lost teeth, and supports the use of tissue engineering strategies in humans, to regenerate previously lost and/or missing teeth. The results presented in this report support the feasibility of bioengineered replacement tooth formation in the jaw.
Keywords: tooth tissue engineering, dental stem cells, mandibular model
INTRODUCTION
Recent advances in materials science and tissue engineering have created opportunities for the development of methods to bioengineer tissue and organ replacements, including highly mineralized craniofacial and dental tissues. Current methods to repair and/or replace dental tissues principally use synthetic materials, whose physical and mechanical properties are quite distinct from those of naturally formed teeth. The need for alternative therapies is evident in recent reports by the United States Department of Health and Human Services (USDHHS, 2000) and the NIDCR Strategic Plan 2003–2008 (NIDCR, 2003), which reveal startling statistics about the high incidence of tooth loss and edentulism.
The recent identification of post-natal dental stem cell (DSC) populations suitable for dental tissue engineering applications suggests that bioengineering approaches can be used to regenerate a variety of dental tissues and whole teeth (Kaigler and Mooney, 2001; Young et al., 2002, 2005a,b; Duailibi et al., 2004, 2006; Modino and Sharpe, 2005; Yelick and Vacanti, 2006; Cai et al., 2007; Lin and Yelick, 2007, 2008). Adult and juvenile human dental pulp can form dentin, cementum, bone, nerve, vascular endothelium (Gronthos et al., 2000, 2006; Shi et al., 2001; Braut et al., 2003; Miura et al., 2003; Shi and Gronthos, 2003; Hu et al., 2006; Huang et al., 2006), and reparative dentin (Nakashima et al., 2004). Human DSCs have been used for periodontal ligament (PDL) repair (Seo et al., 2004; Gronthos et al., 2006), gene delivery techniques have been used in PDL, dentin, and alveolar bone regeneration (Nakashima and Reddy, 2003; Jin et al., 2004; Trubiani et al., 2006), and embryonic stem cells have been shown to express tooth initiation genes (Ohazama et al., 2004; Yen and Sharpe 2006).
Approaches to mesenchymal DSC enrichment include in vitro differentiation models (Kikuchi et al., 1994; Cortes et al., 2006) and analyses of DSC progenitors in transgenic mice (Braut et al., 2003; Mina and Braut, 2004). Characterization of epithelial DSCs has been more difficult, largely due to the fact that epithelial DSCs are no longer present in erupted teeth, and has focused on rodent models exhibiting continuously growing incisor and/or molar tooth eruption (Kikuchi et al., 1994; Tummers and Thesleff, 2003; Harada and Ohshima, 2004; Kawano et al., 2004).
The recent demonstration of bioengineered whole tooth crowns from pig and rat tooth bud cells provided the first evidence that post-natal DSCs could be used for whole-tooth tissue engineering applications (Young et al., 2002, 2005a,b; Duailibi et al., 2004, 2006). Here we tested whether dental-cell-seeded scaffolds grown in the jaw could form organized tooth crowns, similar to identical constructs grown in the omentum.
MATERIALS & METHODS
Animal Husbandry
Lewis rats (Charles River Laboratories, Wilmington, MA, USA) were housed in The Forsyth Institute Animal Facility (Boston, MA, USA), in accordance with National Institutes of Health (NIH) IACUC guidelines, Protocol # 01-009, and animal assurance #A3051-1.
Fabrication of Polymer Scaffolds
Polyglycolate/poly-L-lactate (PGA/PLLA) and poly-L-lactate-co-glycolate (PLGA) tooth scaffolds of uniform size (1 × 5 × 5 mm3) were prepared as previously described (Young et al., 2002; Duailibi et al., 2004). Briefly, PGA fiber mesh (Albany International Research Co., Mansfield, MA, USA) containing 3% PLLA (Sigma-Aldrich, St. Louis, MO, USA) was packed into a negative tooth mold in chloroform, lyophilized, and sanitized. PLGA scaffolds were generated with 85:15 w/w molar ratio PLGA solutions in chloroform and packed with sodium chloride crystals, which were then lyophilized, soaked in distilled water, and sanitized.
Tooth Bud Cell Cultures, Scaffold Fabrication, and Scaffold Seeding
Rat tooth bud cells were prepared from harvested four-day post-natal (dpn) rat tooth buds, and seeded onto polymer scaffolds as previously described (Duailibi et al., 2004). Briefly, tooth buds were harvested, minced into small pieces, digested with collagenase and dispase, filtered to generate single-cell suspensions, and cultured in vitro. Three different enzyme concentrations were tested for optimized dental cell yield and viability: 0.2 mg/mL collagenase/0.1 mg/mL dispase; 0.4 mg/mL collagenase/0.2 mg/mL dispase; and 0.8 mg/mL/0.4 mg/mL dispase. At confluence, cells were trypsinized, washed, and statically seeded onto scaffolds.
Mandibular Implant Model
In parallel to our published rat omental dental implant studies (Duailibi et al., 2004), an identical set of experimental and control scaffolds was generated and implanted into adult rat M1 mandibular tooth extraction sockets. Experimental implants were placed on the right side, positive and/or negative control implants were placed on the left side, and all were grown for 12 wks. Adult Lewis rat hosts were pre-anesthetized with Isoflorane, anesthetized with 87 mg/Kg Ketamine and 13 mg/Kg Xylazine, the oral cavity was disinfected with chlorhexidine (0.12%), and M1 molars including roots were carefully extracted. Continuous saline irrigation was used to ensure maintenance of a continuous blood supply to the extraction site. We created a gingival flap by making a 3-mm incision just proximal to the third molar and extending to the mesial point of the first molar tooth extraction socket. Implants were placed into the empty alveolar cavity, and the gingival mucosa was sutured with simple points and Chromo gut 5-0. Rat hosts were fed powdered food for at least 1 wk post-surgery, until the wound had healed. Full healing was determined by clinical examination under isofluorane anesthesia, with an air jet to confirm complete healing of the mucosal border. Implants were grown in the jaw for 12 wks.
Experimental and Control Mandibular Implant Groups
Control groups consisted of: 7 intact, positive control 4 dpn molar tooth buds; 5 negative control unseeded PGA/PLLA scaffolds; and 5 negative control unseeded PLGA scaffolds. Experimental groups consisted of 8 cell-seeded PGA/PLLA and 8 cell-seeded PLGA scaffold implants.
Radiographic Analysis
Right-side experimental and left-side control hemi-mandibles were removed and examined radiographically by means of a Hewlett-Packard Faxitron (Model 43855 TO-2, Hewlett-Packard, Palo Alto, CA, USA) at 40 Kv and 3 mA for 30 min at a focal distance of 40 cm, and Kodak high-speed SO-253 holographic film (Kodak, Rochester, NY, USA), as previously described (Duailibi et al., 2004). Radiographic films were digitally imaged with a Zeiss Axiocam digital camera (Carl Zeiss, Oberkochen, Germany) and imported into Adobe Photoshop.
Histological Analysis
Control and experimental hemi-mandibles were fixed in 5% formalin for 24 hrs, washed extensively with distilled water, decalcified in equal parts 45% formic acid and 20% sodium citrate for 30 days, and embedded in paraffin. Specimens were sectioned at 6-µm intervals, stained with hematoxylin and eosin (H&E) or Goldner’s Trichrome Stain, and examined under a Leica DMRE compound microscope (Leica, Heidelberg, Germany) and a Zeiss digital Axiocam camera.
Immunohistochemical Analysis
Immunohistochemical analyses were performed as previously described (Duailibi et al., 2004), with the following antibodies: polyclonal rabbit anti-pig amelogenin (AM, 1:8000), kindly provided by Dr. Jim Simmer; polyclonal mouse anti-pig dentin sialophosphoprotein (DSPP, 1:4000), kindly provided by Dr. Larry Fisher; mouse anti-human periostin (PER, 1:200), kindly provided by Dr. Lan Bo Chen; and mouse anti-porcine vimentum (VM, 1:1600, #sc-6260, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Each of these antibodies has demonstrated cross-reactivity with rat dental proteins. Natural rat tooth tissues were used as positive control tissues. Negative controls lacked primary antibody, since we used only secondary antibodies.
RESULTS
Optimization of 4-dpn Rat Tooth Bud Cell Preparation
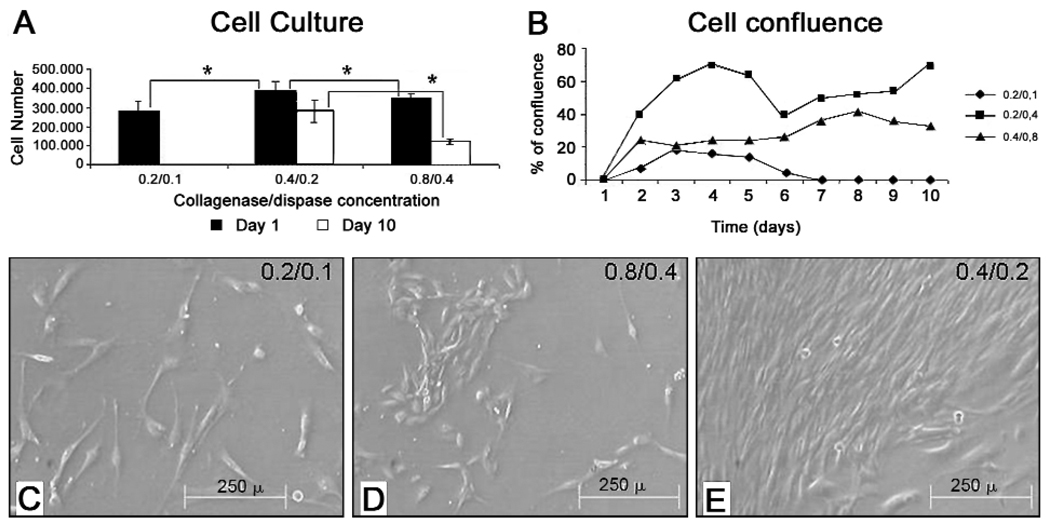
Immature tooth buds were harvested from 4-day post-natal rats and used to generate primary cell cultures. Three different type I collagenase and dispase I enzyme concentrations were tested for optimized cell yield and viability after 10 days in culture (Table). Five independent experiments were performed, for a total of 15 experimental samples. Total cell counts were determined daily from days 0 to 10, and percent confluence was recorded. Immediately after digestion, cell numbers obtained with 0.2 mg/mL collagenase/0.1 mg/mL dispase were less than those obtained with the higher concentrations, indicating incomplete digestion (Figs. 1A, 1C). Although similar cell numbers were obtained with the 0.4 mg/mL collagenase/0.2 mg/mL dispase, and the 0.8 mg/mL/0.4 mg/mL dispase enzyme concentrations, after 10 days in culture, greater viable cell yields were obtained with the 0.4 mg/mL collagenase/0.2 mg/mL dispase enzyme concentration (Figs. 1A, 1B, 1D, 1E), suggesting that the higher enzyme concentration was too harsh. The highest cell yields at day 10 were obtained with 0.4/0.2 mg/mL collagenase/dispase I (Table, Figs. 1A, 1E). Cell proliferation analyses revealed that cells prepared with this enzyme concentration consistently grew better, and achieved greater confluence after 10 days in culture (Figs. 1B, 1E). Statistical analysis by the Wilcoxon test revealed significant differences among the 3 enzyme concentrations (Table, Appendix Table).
Table 1.
Radiographic Analyses of Dental Implants
| PLGA* | PGA | |||||||
|---|---|---|---|---|---|---|---|---|
| + | − | Total | % + | + | − | Total | % + | |
| Tooth bud controls | 7 | 0 | 7 | 100 | 7 | 0 | 7 | 100 |
| Scaffold alone | 0 | 5 | 5 | 0 | 0 | 5 | 5 | 0 |
| Cell-seeded scaffold | 4 | 4 | 8 | 50 | 5 | 3 | 8 | 63 |
| Total | 11 | 9 | 20 | 55 | 12 | 8 | 20 | 57 |
| X2Calculated = 11.92* (p < 0.001); X2Critic = 5.99 | X2Calculated = 12.34* (p < 0.001); X2Critic = 5.99 | |||||||
Both PGA and PLGA scaffolds supported the formation of bioengineered dental tissues in the jaw, and at similar frequencies. (+) indicates mineralized tissue formation, and (−) indicates lack of mineralized tissue formation. These results demonstrate that cell-seeded, and not unseeded, scaffolds resulted in mineralized dental tissue formation.
Figure 1. Dental cell proliferation in culture.
(A) Analysis of cell number at day 0, and after 10 days in culture, revealed that enzyme concentrations of 0.4 mg/mL collagenase and 0.2 mg/mL dispase resulted in the highest cell yields. (B) Growth curves for digested tooth bud cell preparations confirmed that tooth buds digested with 0.4 mg/mL type I collagenase and 0.2 mg/mL dispase I exhibited the highest cell number after 10 days in culture. (C) Digital images of cultured tooth bud cells, prepared with the 0.4 mg/mL collagenase and 0.2 mg/mL dispase enzyme concentrations, after 2, 4, and 10 days in culture. Five independently isolated cell preparations were examined for each type of analysis (Table). Wilcoxon’s test revealed highly significant values of P = 0.0003. Scale bar is 2 mm.
Harvesting and Radiographic Analysis of Jaw Implants
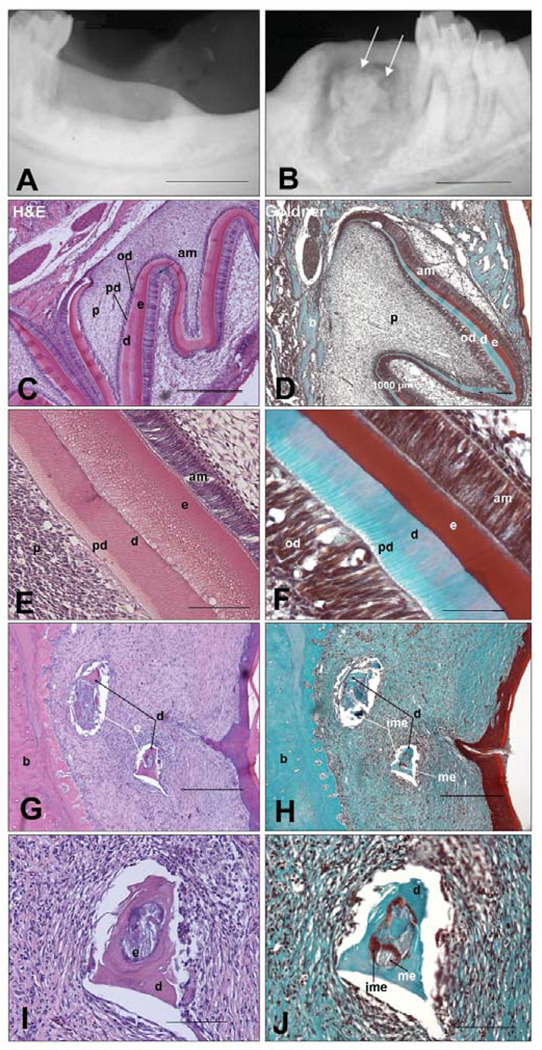
Morphological and gross inspection of all harvested control and experimental mandibles revealed a smooth oral gingival surface at the implant site. Radiographic analyses revealed the absence of radiopaque mineralized tissue formation in all of the empty scaffold negative control implants (Fig. 2A), and, in contrast, the formation of radiopaque, mineralized tissues in all PGA/PLLA and PLGA cell-seeded implant sites, indicating that similar amounts of mineralized tissue were generated with either type of scaffold (Fig. 2B, arrows). Radio-translucent areas were evident around the bioengineered mineralized tissues, indicative of possible inflammatory response to scaffold degradation, and/or lack of integration of bioengineered tooth structures with surrounding alveolar bone. The apparent uneven nature of the bioengineered mineralized tissues, as revealed in the radiographs, is consistent with the observed formation of small tooth structures throughout the scaffold implant, as opposed to a single large tooth. All positive control 4-dpn tooth bud implants formed mineralized tooth tissues (Figs. 2C, 2D).
Figure 2. Radiographic and histological analysis of harvested and sectioned 12-week experimental and control implants.
(A) Negative control implant site. (B) Localized radiopaque areas (arrows) indicate mineralized tissue formation at the cell-seeded implant site. (C,E) H&E-stained positive control implanted 4-dpn tooth buds exhibited well-formed tooth structures, with organized crown and root structures. (D,F) Goldner’s stained 4-dpn tooth bud implants revealed characteristic blue-stained dentin, and brown-stained immature enamel. (G,I) H&E-stained bioengineered dental tissues present in jaw implants. (H,J) Goldner’s stained sections revealed blue-stained dentin, brown-stained immature enamel, and gray-stained mature enamel tissues. Unseeded, negative control scaffolds did not form dental tissues (data not shown). All 16 of the experimental cell-seeded implants and all of the 14 implanted tooth bud controls produced radiopaque dental tissues. None of the negative control unseeded scaffolds produced radiopaque mineralized tissue. Abbreviations: am, ameloblasts; b, bone; d, dentin; e, enamel; ime, immature enamel; me, mature enamel; od, odontoblasts; p, pulp; pd, pre-dentin. Scale bars are 100 µm.
Histological and Immunohistochemical Analysis of Bioengineered Rat Tooth Tissues
Histological analysis of positive control implanted 4-dpn tooth buds revealed organized, mineralized tooth structures containing predentin (pd), dentin (d), enamel (e), odontoblast (od), and ameloblast (am) layers (Figs. 2C, 2E). Histological analysis of experimental implants (Figs. 2G, 2I) revealed distinct dentin (d, black arrows) and demineralized enamel (e, white arrows) tissues. Negative control unseeded implants did not form mineralized dental tissues (data not shown).
Goldner’s trichrome, which stains newly formed enamel red/brown, mature enamel (me) gray, and dentin (d) blue (Figs. 2D, 2F), reveals distinct dentin (d), mature enamel (me), immature enamel (ime), and surrounding alveolar bone (b) in dental implants (Figs. 2H, 2J). These results demonstrated that, although bioengineered mandibular implant dental tissues were not as organized as those grown in the omentum, distinct dentin, enamel, and alveolar bone tissues were present.
Immunohistochemical Analyses of Dentin- and Enamel-expressed Proteins
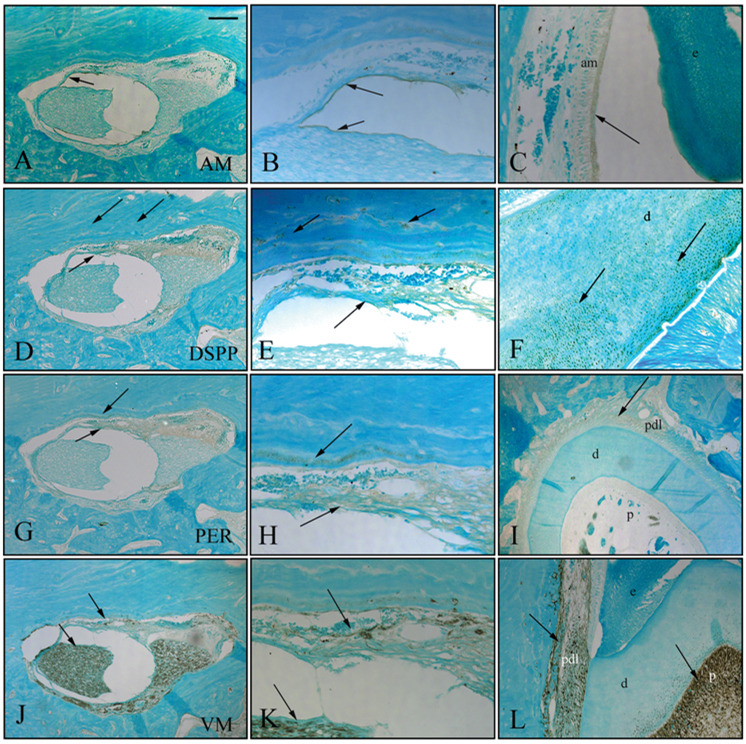
The expression of amelogenin (AM), dentin sialo-phosphoprotein (DSPP), periostin (PER), and vimentum (VM) was analyzed for further characterization of bioengineered dental tissues. AM was detected in an epithelial cell layer resembling inner and outer dental epithelium (Figs. 3A, 3B). DSPP was detected in differentiated odontoblasts, and in mineralized bioengineered dentin tissue (Figs. 3D, 3E, arrows). PER was detected in bioengineered periodontal ligament-like tissue at the outer portion of the implant, adjacent to mineralized bone (Figs. 3G, 3H, arrows). Vimentum (VM) was detected in pulp-like tissues at the center of the implant, and in surrounding PDL-like tissues (Figs. 3J, 3K, arrows). All antibodies exhibited positive staining in control tooth tissues (Figs. 3C, 3F, 3I, 3L, arrows). These results indicated that bioengineered dental tissues expressed enamel, dentin, and periodontal ligament proteins in an appropriate manner.
Figure 3. Immunohistochemical (IHC) analysis of mandibular implant bioengineered tooth tissues. Bioengineered dental tissues were positive for amelogenin (AM).
(A,B), dentin sialophosphoprotein (DSPP) (D,E), periostin (PER) (G,H), and vimentum (VM) (J,K). Corresponding immunostaining of natural tooth tissues is shown (C,F,I,L). All isotype control immunostaining was negative. Three experimental PGA and three experimental PLGA cell-seeded scaffold implants were analyzed by IHC. Two negative control ‘scaffold alone’ controls and 2 positive control tooth bud implants were also analyzed by IHC. Abbreviations: AM, amelogenin; DSPP, dentin sialophosphoprotein; PER, periostin; VM, vimentum. Scale bars = 200 µm.
DISCUSSION
The objective of this study was to characterize bioengineered dental tissues grown in the jaw for comparison with identical implants grown in the omentum, as a clinically relevant dental implant model useful for human tooth replacement therapies. The implant site was the fresh extraction socket of adult rat, mandibular M1 teeth. Enzymatic digestion conditions for 4-dpn rat molar tooth bud dissociation were defined, guided by prior published reports (Kikuchi et al., 1994; Cortes et al., 2006). Identical experimental and control scaffolds were implanted either in the jaw or in the omentum of adult rat hosts and allowed to grow for 12 wks.
As previously reported, bioengineered rat omental dental implants generated numerous small tooth crowns (Duailibi et al., 2004), similar to bioengineered pig omental implant crowns (Young et al., 2002). The M1 molar extraction site also supported the formation of bioengineered dental tissues. Consistent with our results, a similar study performed in dogs demonstrated that a canine first molar tooth bud transplanted into a fresh tooth extraction socket formed dentin and bone at 24 wks, although no enamel or tooth roots formed (Honda et al., 2006).
Comparison of bioengineered dental tissues grown in the mandible vs. the omentum revealed both similarities and differences. Both implant sites supported the formation of bioengineered dentin, enamel, pulp, and periodontal tissues. In general, omental implant dental tissues appeared more organized than those grown in the mandible. These results suggest that further modification of the mandibular implant model is required to optimize tooth formation. It is possible that a healed extraction socket could reduce inflammatory responses, which may have deleteriously affected dental implant growth. It is also likely that alternative scaffold materials and designs would improve bioengineered dental tissue formation in the jaw, based on extensive evidence for the influence of scaffold pore size, interconnectivity, and degradation rate on cell viability, proliferation, and differentiation (Kim et al., 2007). Alternative scaffold materials and design will also facilitate the formation of one large bioengineered tooth of predetermined size and shape, as opposed to the many small teeth currently observed in our dental implants.
In summary, our results showed that cultured 4-day post-natal rat tooth bud cells seeded onto PGA/PLLA and PLGA scaffolds and implanted and grown in the mandible can form organized bioengineered dental tissues containing dentin, enamel, pulp, and PDL. Bioengineered mandibular implant tooth tissues expressed characteristic enamel and dentin proteins, and exhibited morphological and histological appearances typical of naturally formed dentin and enamel. Based on these studies, and on our previously published results, we anticipate that similar use of human DSCs harvested from immature teeth, such as wisdom teeth, will eventually be used in clinically relevant replacement tooth therapies in humans. Although methods for the reliable generation of bioengineered teeth of predetermined size and shape remain to be defined, the results presented here confirm and extend our earlier studies demonstrating the formation of bioengineered tooth tissues in the omentum (Young et al., 2002, 2005a,b; Duailibi et al., 2004, 2006, and extend these studies to regenerating teeth in the functionally appropriate location of the jaw.
Advances indicated by this study include introducing the mandibular implant model for bioengineered dental tissue formation, demonstrating the therapeutic potential of this approach for human tooth regeneration. Limitations include the fact that bioengineered tooth tissues generated in a fresh tooth extraction site in the mandible appear somewhat less organized than those grown in the omentum. We anticipate that further modification of this procedure will eventually result in reliable methods to bioengineer dental tissues in the jaw.
ACKNOWLEDGMENTS
We thank members of the Vacanti laboratory for their expertise and assistance. We acknowledge the support and advice of Dr. Lydia Masako Ferreira, Full Professor of the Plastic Surgery Department at the Universidade Federal of Sã Paulo (UNIFESP), Brazil, and CAPES for support of MTD and SED and FAPESP grant #04/08924-8, and MCT/CNPq 552178/05-5. We especially thank Justine Dobeck, Nadia Mohammed, and Elka Pravda for expert technical assistance, and Dr. Jean Eastcott and Subbiah Yoganathan for expert rat husbandry and care. This work was supported by NIH/NIDCR grant DE016132 (PCY).
APPENDIX
Appendix Table.
Supplement to Table. Cell Yield and Viability after Tooth Bud Tissue Digestion
| Tooth Bud Cell Digestion and Culture over 10 Days | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coll/Disp | % Confluence | ||||||||||||
| Flask | Concentration | ICN | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | FCN |
| 1 | 0.2 mg / 0.1 mg | 350,000 | Fl. | 20 | 30 | 40 | 40 | 10 | 0 | - | - | - | none |
| 2 | 0.2 mg / 0.1 mg | 250,000 | Fl. | Fl. | 5 | 10 | 10 | 0 | - | - | - | - | none |
| 3 | 0.2 mg / 0.1 mg | 200,000 | Fl. | 10 | 20 | 10 | 0 | - | - | - | - | - | none |
| 4 | 0.2 mg / 0.1 mg | 310,000 | Fl. | Fl. | 20 | 10 | 10 | 0 | - | - | - | - | none |
| 5 | 0.2 mg / 0.1 mg | 270,000 | Fl. | Fl. | 10 | 10 | 20 | 10 | 0 | - | - | - | none |
| M | 276,000 | none | |||||||||||
| SD | 57,271 | none | |||||||||||
| 6 | 0.4 mg / 0.2 mg | 350,000 | Fl. | 40 | 70 | 70 | 60 | 40 | 50 | 50 | 50 | 70 | 245,000 |
| 7 | 0.4 mg / 0.2 mg | 450,000 | Fl. | 40 | 50 | 60 | 50 | 40 | 50 | 50 | 50 | 60 | 350,000 |
| 8 | 0.4 mg / 0.2 mg | 320,000 | Fl. | 40 | 70 | 80 | 70 | 40 | 50 | 50 | 50 | 70 | 210,000 |
| 9 | 0.4 mg / 0.2 mg | 380,000 | Fl. | 40 | 50 | 60 | 70 | 40 | 50 | 50 | 50 | 70 | 290,000 |
| 10 | 0.4 mg / 0.2 mg | 430,000 | Fl. | 40 | 70 | 80 | 70 | 40 | 50 | 60 | 70 | 80 | 320,000 |
| M | 386,000 | 283,000 | |||||||||||
| SD | 54,129 | 56,302 | |||||||||||
| 11 | 0.8 mg / 0.4 mg | 350,000 | Fl. | 40 | 30 | 30 | 30 | 40 | 50 | 50 | 50 | 50 | 140,000 |
| 12 | 0.8 mg / 0.4 mg | 350,000 | Fl. | 10 | 10 | 30 | 30 | 40 | 50 | 40 | 30 | 20 | 100,000 |
| 13 | 0.8 mg / 0.4 mg | 340,000 | Fl. | 20 | 10 | 10 | 20 | 10 | 10 | 40 | 30 | 30 | 130,000 |
| 14 | 0.8 mg / 0.4 mg | 380,000 | Fl. | 40 | 40 | 30 | 30 | 30 | 50 | 50 | 30 | 30 | 120,000 |
| 15 | 0.8 mg / 0.4 mg | 310,000 | Fl. | 10 | 10 | 20 | 10 | 10 | 20 | 30 | 40 | 30 | 90,000 |
| M | 346,000 | 116,000 | |||||||||||
| SD | 25,100 | 20,736 | |||||||||||
Five independently isolated 4-dpn tooth bud cell preparations were generated with each of 3 different collagenase/dispase enzyme concentrations, and grown in culture for 10 days. Initial and final cell counts were used to determine optimized enzyme concentrations of 0.4 mg/mL collagenase and 0.2 mg/mL dispase, which were subsequently used in all experimental implants. Abbreviations: Fl, floating cells; M, median; P, Wilcoxon test; SD, standard deviation; Col/Disp, collagenase/dispase; FCN, final cell number; ICN, initial cell number. N = 15, P = 0.0003.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.iadrjournals.org/cgi/content/full/87/8/745/DC1.
REFERENCES
- Braut A, Kollar EJ, Mina M. Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a col1a1-2.3-GFP transgene. Int J Dev Biol. 2003;47:281–292. [PubMed] [Google Scholar]
- Cai J, Cho SW, Kim JY, Lee MJ, Cha YG, Jung HS. Patterning the size and number of tooth and its cusps. Dev Biol. 2007;304:499–507. doi: 10.1016/j.ydbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Cortes O, Garcia C, Perez L, Boj J, Alcaina A. Pulp cell cultures obtained with two different methods for in vitro cytotoxicity tests. Eur Arch Paediatr Dent. 2006;7:96–99. doi: 10.1007/BF03320822. [DOI] [PubMed] [Google Scholar]
- Duailibi MT, Duailibi SE, Young CS, Vacanti JP, Bartlett JD, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- Duailibi SE, Duailibi MT, Vacanti JP, Yelick PC. Prospects for tooth regeneration. Periodontol 2000. 2006;41:177–187. doi: 10.1111/j.1600-0757.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vivo and in vitro. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Mrozik K, Shi S, Bartold PM. Ovine periodontal ligament stem cells: isolation, characterization, and differentiation potential. Calcif Tissue Int. 2006;79:310–317. doi: 10.1007/s00223-006-0040-4. [DOI] [PubMed] [Google Scholar]
- Harada H, Ohshima H. New perspectives on tooth development and the dental stem cell niche. Arch Histol Cytol. 2004;67:1–11. doi: 10.1679/aohc.67.1. [DOI] [PubMed] [Google Scholar]
- Honda MJ, Ohara T, Sumita Y, Ogaeri T, Kagami H, Ueda M. Preliminary study of tissue-engineered odontogenesis in the canine jaw. J Oral Maxillofac Surg. 2006;64:283–289. doi: 10.1016/j.joms.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Hu B, Nadiri A, Kuchler-Bopp S, Perrin-Schmitt F, Peters H, Lesot H. Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 2006;12:2069–2075. doi: 10.1089/ten.2006.12.2069. [DOI] [PubMed] [Google Scholar]
- Huang GTJ, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–1073. doi: 10.1016/j.joen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–526. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaigler D, Mooney D. Tissue engineering’s impact on dentistry. J Dent Ed. 2001;65:456–462. [PubMed] [Google Scholar]
- Kawano S, Saito M, Handa K, Morotomi T, Toyono T, Seta Y, et al. Characterization of dental epithelial progenitor cells derived from cervical-loop epithelium in a rat lower incisor. J Dent Res. 2004;83:129–133. doi: 10.1177/154405910408300209. [DOI] [PubMed] [Google Scholar]
- Kikuchi H, Sawada T, Yanagisawa T. Isolation of dental papilla from young rat lower incisor by three step digestion. Bull Tokyo Dent Coll. 1994;35:183–187. [PubMed] [Google Scholar]
- Kim HJ, Kim UJ, Leisk GG, Bayan C, Georgakoudi I, Kaplan DL. Bone regeneration on macroporous aqueous-derived silk 3-D scaffolds. Macromol Biosci. 2007;7:643–655. doi: 10.1002/mabi.200700030. [DOI] [PubMed] [Google Scholar]
- Lin Y, Yelick PC. Methods to investigate craniofacial growth and development. Chapter 35, Dental and craniofacial tissue engineering. In: Mao JJ, Mikos A, Giordana V, editors. Translational approaches in tissue engineering and regenerative medicine. Boston/London: Artech House, Blackwell Publishing; 2007. [Google Scholar]
- Lin Y, Yelick PC. Dental tissue engineering. In: Atala A, Lanze R, Neram R, Thompson JA, editors. Principles of regenerative medicine. Burlington, MA: Academic Press; 2008. pp. 1286–1297. [Google Scholar]
- Mina M, Braut A. New insight into progenitor/stem cells in dental pulp using Col1a1-GFP transgenes. Cells Tissues Organs. 2004;176:120–133. doi: 10.1159/000075033. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modino SAC, Sharpe PT. Tissue engineering of teeth using adult stem cells. Arch Oral Biol. 2005;50:255–258. doi: 10.1016/j.archoralbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Reddy AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Iohara K, Ishikawa M, Ito M, Tomokiyo A, Tanaka T, et al. Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11) Hum Gene Ther. 2004;15:1045–1053. doi: 10.1089/hum.2004.15.1045. [DOI] [PubMed] [Google Scholar]
- National Institute of Dental and Craniofacial Research. Strategic plan–2003–2008. J Am Coll Dent. 2003;70:43–55. [PubMed] [Google Scholar]
- Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. BONE. 2001;29:532–539. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- Trubiani O, Orsini G, Caputi S, Piatelli A. Adult mesenchymal stem cells in dental research: a new approach for tissue engineering. Int J Immunopathol Pharmacol. 2006;19:451–460. doi: 10.1177/039463200601900301. [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development. 2003;130:1049–1057. doi: 10.1242/dev.00332. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Rockville, MD: NIDCR, NIH; Oral health in America: a report of the Surgeon General. 2000
- Yelick PC, Vacanti JP. Bioengineered teeth from tooth bud cells. Dent Clin North Am. 2006;50:191–203. doi: 10.1016/j.cden.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Yen AH, Sharpe PT. Regeneration of teeth using stem cell-based tissue engineering. Expert Opin Biol Ther. 2006;6:9–16. doi: 10.1517/14712598.6.1.9. [DOI] [PubMed] [Google Scholar]
- Young CS, Honda M, Terada S, Vacanti JP, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- Young CS, Kim SW, Qin C, Baba O, Butler WT, Taylor RR, et al. Developmental analysis and computer modeling of bioengineered teeth. Arch Oral Biol. 2005a;50:259–265. doi: 10.1016/j.archoralbio.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Young CS, Abukawa H, Ariscan R, Ravens M, Troulis MJ, Kaban LB, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005b;11:1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]