Abstract
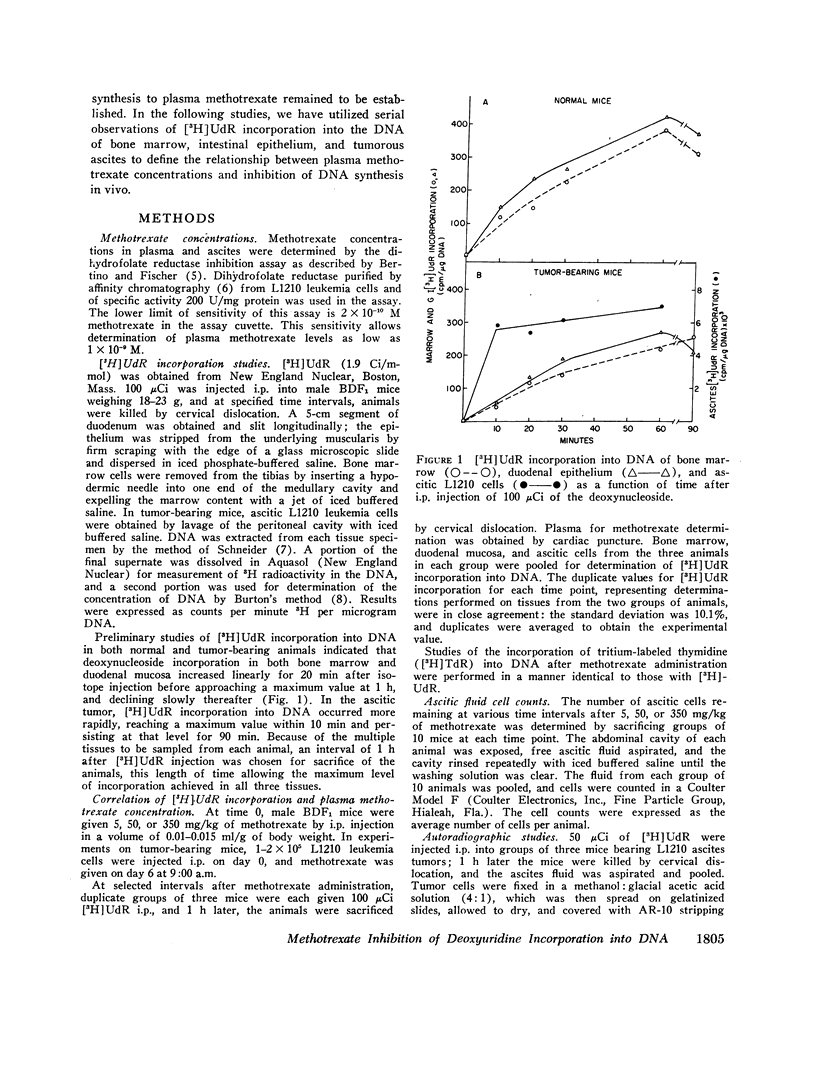
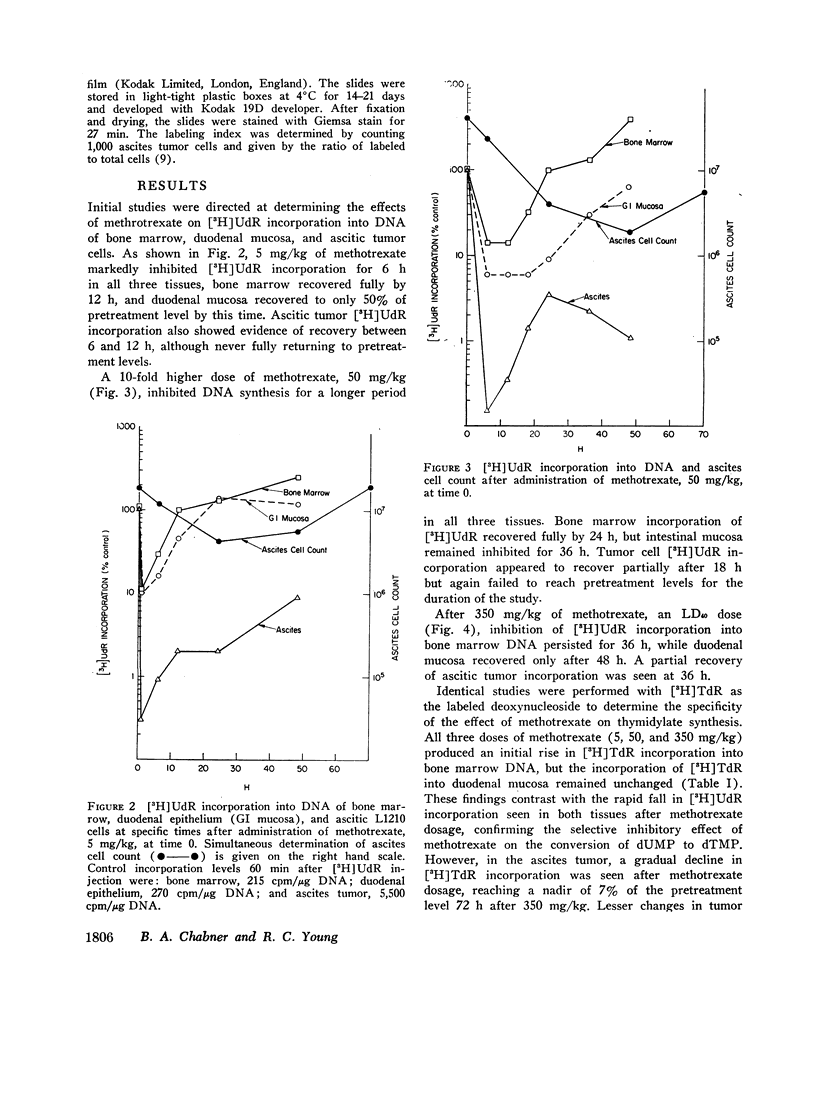
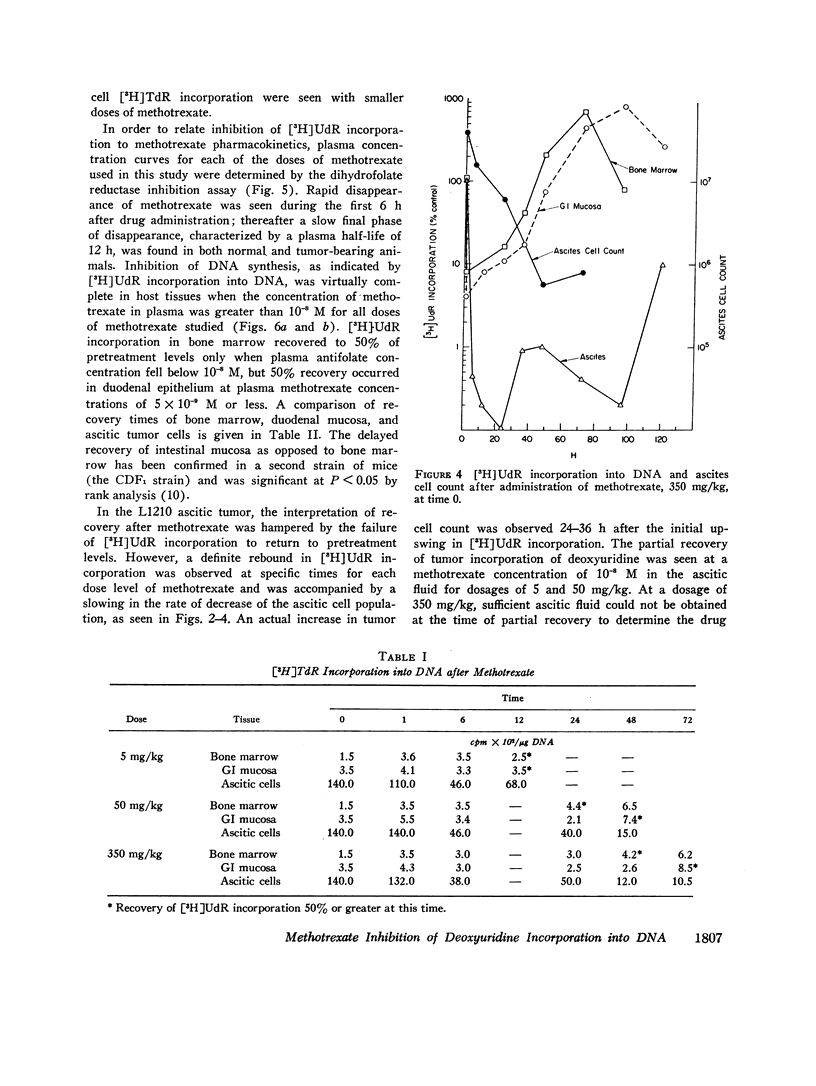
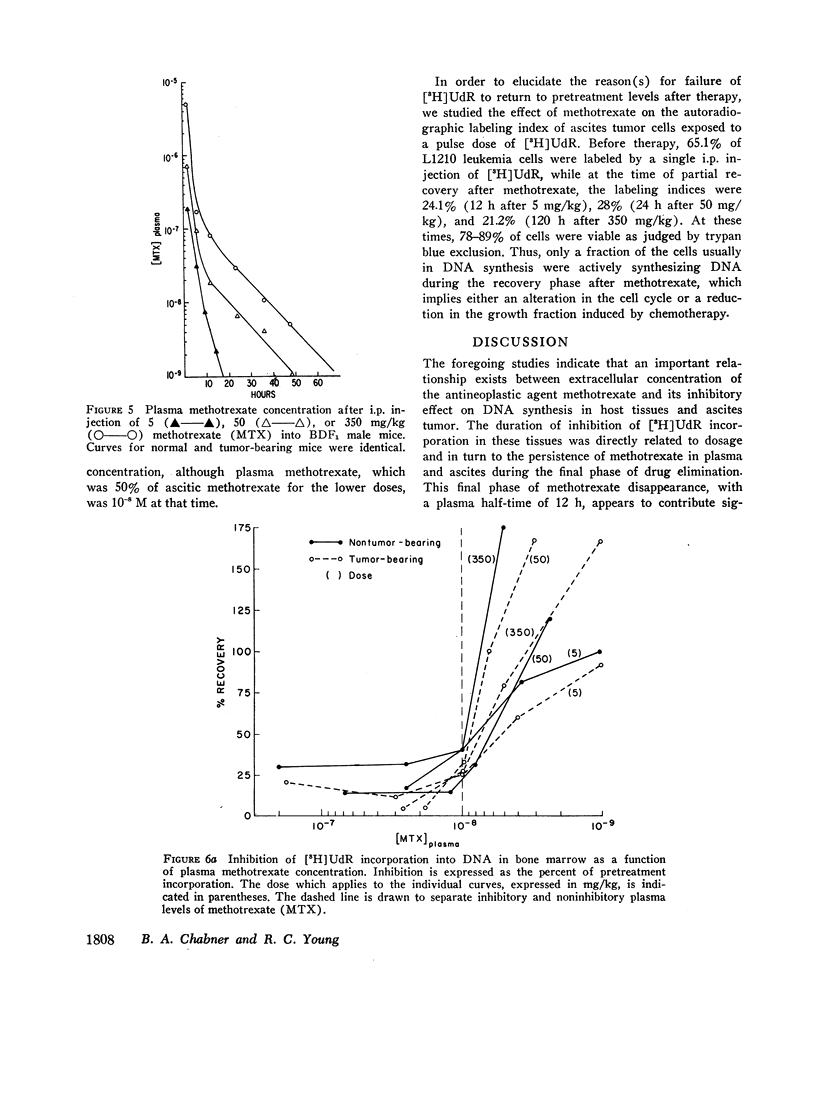
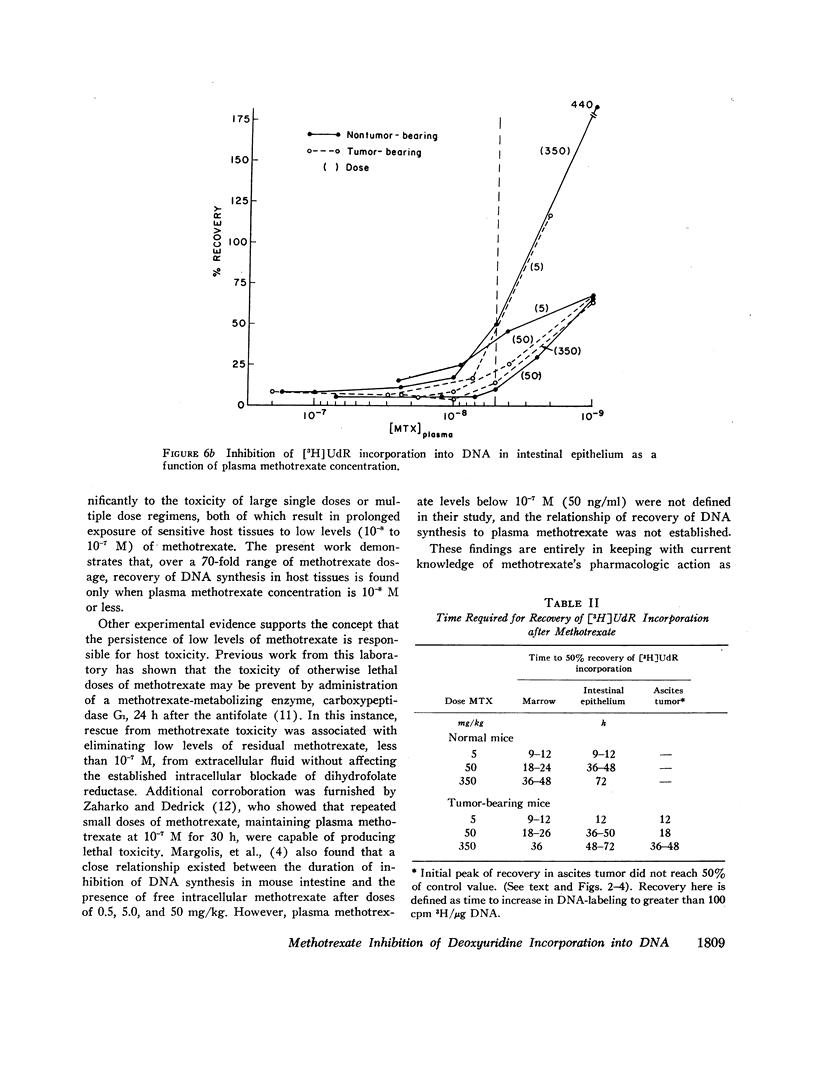
The suppression of DNA synthesis in host and tumor tissues by methotrexate has been monitored in mice by determining the in vivo incorporation of tritium-labeled deoxyuridine ([3H]UdR) into DNA. The duration of inhibition of [3H]UdR incorporation in normal tissues was related to the dose of methotrexate and was a direct function of plasma drug concentration. [3H]UdR incorporation recovered to 50% of pretreatment levels in bone marrow when plasma methotrexate concentration was 10-8 M or less, irrespective of the dose administered, while 50% recovery of DNA synthesis in intestinal epithelium was not observed until plasma methotrexate levels were 5 × 10-9 M or less. Ascitic L1210 leukemia cells did not fully return to pretreatment levels of [3H]UdR incorporation at any time, although a partial recovery of incorporation was noted at methotrexate ascitic fluid concentrations of approximately 10-8 M.
Methotrexate did not suppress the incorporation of tritium-labeled thymidine ([3H]TdR) into bone marrow and duodenal mucosa, confirming the specificity of its action in inhibiting thymidylate synthesis in host tissues. In the ascites tumor a gradual decline in [3H]TdR incorporation was seen after methotrexate, indicating that the tumor tissue depression of [3H]UdR incorporation is not solely due to inhibition of thymidylate synthesis.
These studies indicate that host tissues are inhibited by extremely low concentrations of methotrexate, and indicate the importance of the slow final phase (t½=12 h) of drug elimination from plasma in producing a prolonged exposure of sensitive host tissues to inhibitory drug concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTINO J. R., BOOTH B. A., BIEBER A. L., CASHMORE A., SARTORELLI A. C. STUDIES ON THE INHIBITION OF DIHYDROFOLATE REDUCTASE BY THE FOLATE ANTAGONISTS. J Biol Chem. 1964 Feb;239:479–485. [PubMed] [Google Scholar]
- BERTINO J. R., FISCHER G. A. TECHNIQUES FOR STUDY OF RESISTANCE TO FOLIC ACID ANTAGONISTS. Methods Med Res. 1964;10:297–307. [PubMed] [Google Scholar]
- BERTINO J. R. THE MECHANISM OF ACTION OF THE FOLATE ANTAGONISTS IN MAN. Cancer Res. 1963 Sep;23:1286–1306. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabner B. A., Chello P. L., Bertino J. R. Antitumor activity of a folate-cleaving enzyme, carboxypeptidase G 1 . Cancer Res. 1972 Oct;32(10):2114–2119. [PubMed] [Google Scholar]
- Chabner B. A., Johns D. G., Bertino J. R. Enzymatic cleavage of methotrexate provides a method for prevention of drug toxicity. Nature. 1972 Oct 13;239(5372):395–397. doi: 10.1038/239395b0. [DOI] [PubMed] [Google Scholar]
- Chello P. L., Cashmore A. R., Jacobs S. A., Bertino J. R. Improved purification of tetrahydrofolate dehydrogenase from L1210 leukemia by affinity chromatography. Biochim Biophys Acta. 1972 Apr 7;268(1):30–34. doi: 10.1016/0005-2744(72)90193-3. [DOI] [PubMed] [Google Scholar]
- Divekar A. Y., Vaidya N. R., Braganca B. M. Active transport of aminopterin in Yoshida sarcoma cells. Biochim Biophys Acta. 1967;135(5):927–936. doi: 10.1016/0005-2736(67)90062-4. [DOI] [PubMed] [Google Scholar]
- FERGUSON F. C., Jr, THIERSCH J. B., PHILIPS F. S. The action of 4-amino-N10-methyl-pteroylglutamic acid in mice, rats, and dogs. J Pharmacol Exp Ther. 1950 Mar;98(3):293–299. [PubMed] [Google Scholar]
- Goldman I. D., Lichtenstein N. S., Oliverio V. T. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J Biol Chem. 1968 Oct 10;243(19):5007–5017. [PubMed] [Google Scholar]
- Hryniuk W. M., Bertino J. R. Treatment of leukemia with large doses of methotrexate and folinic acid: clinical-biochemical correlates. J Clin Invest. 1969 Nov;48(11):2140–2155. doi: 10.1172/JCI106181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniuk W. M., Fischer G. A., Bertino J. R. S-phase cells of rapidly growing and resting populations. Differences in response to methotrexate. Mol Pharmacol. 1969 Nov;5(6):557–564. [PubMed] [Google Scholar]
- Hryniuk W. M. Purineless death as a link between growth rate and cytotoxicity by methotrexate. Cancer Res. 1972 Jul;32(7):1506–1511. [PubMed] [Google Scholar]
- Kessel D., Hall T. C., Roberts D. Modes of uptake of methotrexate by normal and leukemic human leukocytes in vitro and their relation to drug response. Cancer Res. 1968 Mar;28(3):564–570. [PubMed] [Google Scholar]
- Kessel D., Hall T. C., Roberts D., Wodinsky I. Uptake as a determinant of methotrexate response in mouse leukemias. Science. 1965 Nov 5;150(3697):752–754. doi: 10.1126/science.150.3697.752. [DOI] [PubMed] [Google Scholar]
- Margolis S., Philips F. S., Sternberg S. S. The cytotoxicity of methotrexate in mouse small intestine in relation to inhibition of folic acid reductase and of DNA synthesis. Cancer Res. 1971 Dec;31(12):2037–2046. [PubMed] [Google Scholar]
- Oliverio V. T., Zaharko D. S. Tissue distribution of folate antagonists. Ann N Y Acad Sci. 1971 Nov 30;186:387–399. doi: 10.1111/j.1749-6632.1971.tb46995.x. [DOI] [PubMed] [Google Scholar]
- Young R. C., DeVita V. T., Perry S. The thymidine-14C and -3H double-labeling technic in the study of the cell cycle of L1210 leukemia ascites tumor in vivo. Cancer Res. 1969 Aug;29(8):1581–1584. [PubMed] [Google Scholar]
- Young R. C., Goldberg D., Schein P. S. Enhanced antitumor effect of cytosine arabinoside given in a schedule dictated by kinetic studies in vivo. Biochem Pharmacol. 1973 Jan 15;22(2):277–280. doi: 10.1016/0006-2952(73)90283-9. [DOI] [PubMed] [Google Scholar]


