There are currently more than 1000 consumer products based on nanomaterials.[1] As a consequence, nanomaterials will inevitably be released into the environment during the manufacture, use, and disposal of these products. Consequently, multiple concerns related to unforeseen health and environmental hazards of nanomaterials have been raised.[2] Many studies on the environmental fate, behavior, bioavailability and toxicity of manufactured nanomaterials have been carried out to address what these hazards might be, but the results are far from conclusive.[3] Currently, carbon-based materials (e.g. fullerenes[4] and carbon nanotubes[4c, 5]), metal and metal oxides (e.g. silver[6] and TiO2[7]) and semiconductor materials (e.g. quantum dots[8]) are the most studied nanomaterials. Investigations show that nanomaterial size,[6, 9] shape,[9] surface chemistry,[4b, 10] and surface area[11] all play a role in determining the toxicity of nanomaterials in model biological and environmental systems.[3a] For example, Chan et al.[9] has demonstrated the size dependence of cellular uptake, and our group[10b] has shown the role of surface charge on uptake.
Many nanomaterials do not have well-defined and well-characterized physical or chemical properties either in the pure form or once added to the systems of interest. As an example, when zebrafish embryos are exposed to silver NPs, Ag ions are released, and this leads to toxic effects that are not necessarily NP related.[6] Such instances challenge efforts to evaluate the roles of physical and chemical properties on the environmental impact of manufactured nanomaterials. Here, we use monolayer-protected gold NPs (AuNPs) as a tool for understanding the influence of surface properties on the environmental impact of nanomaterials. AuNPs have several attributes that make them excellent environmental probes. First, AuNPs can be readily fabricated with controllable sizes ranging from 1 nm to > 100 nm. Second, Au is not a biologically essential element and hence has a low environmental background, allowing facile detection in biological and environmental matrices at concentrations as low as 10 ppt (parts-per-trillion) using inductively coupled plasma mass spectrometry (ICP-MS). Third, when capped with the appropriate surface monolayer, AuNPs do not readily dissociate into ions (e.g. Au+), thereby eliminating any confusion between the nanomaterial and its generated ions.[6, 12] AuNPs are thus well suited for probing the fate, transport, and bioavailability of manufactured nanomaterials in environmental studies.[6, 13]
Previous studies on the environmental impact of nanomaterials have used AuNPs with less well defined surfaces. For example, cationic surfactant stabilized AuNPs (65 nm × 15 nm) were found by Ferry et al. to readily transfer from the water column into the estuarine food web and accumulate in clams and biofilms.[13c] These previous studies, however, did not explore the important role of NP surface properties including charge and hydrophobicity on absorption, distribution, metabolism and excretion in aquatic species (e.g. fish). We report here the use of 2 nm core AuNPs (Figure 1) to probe the bioavailability and clearance of nanomaterials in Japanese medaka (Oryzias latipes). In these studies we observe that surface functionality plays a crucial role in determining the fate of nanomaterials, with hydrophilic particles ingested and rapidly cleared without obvious health impact, whereas hydrophobic particles are accumulated by the fish with toxic effect.
Figure 1.
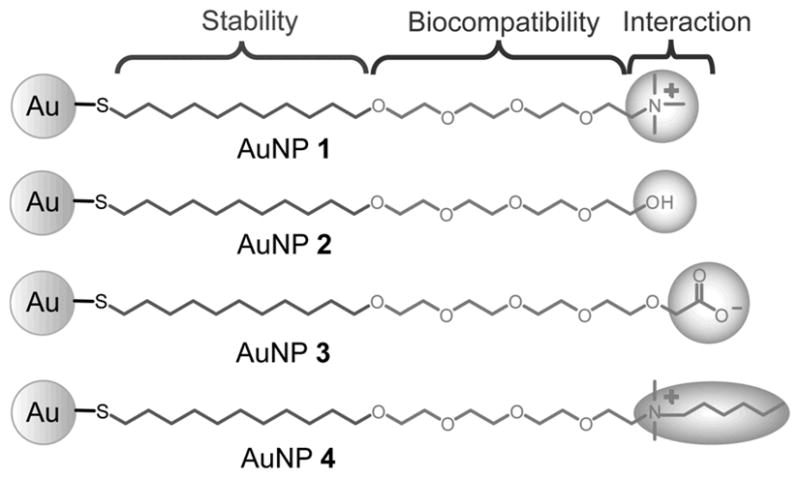
Structural illustrations of gold nanoparticles (AuNPs 1-4).
The 2 nm core AuNPs (Figure 1) used in this study are engineered to probe the effects of surface properties on biosystems. The alkane thiol group provides a hydrophobic layer that stabilizes the core against disassembly in water. The ethylene glycol group improves the biocompatibility and solubility of the AuNPs and prevents non-specific interactions with the alkane interiors. The terminal functional groups determine the surface chemistry on the NP surface and control the interaction with surrounding environments. In addition to the tunability of the particle surfaces, the AuNPs used in this study are resistant to aggregation. The hydrodynamic diameters (DH) of these AuNPs range from 6 to 14 nm (Figure 2a and Figure S2 in Supporting Information) and show little aggregation over a five-day period (DH 10-23 nm, Figure 2a) in the conditioned water where fish were exposed. This resistance to aggregation allows us to more controllably study how surface chemistry affects their biological uptake without the confounding effects of aggregation. We also find that the ζ-potentials change slightly over time in the conditioned water (Figure 2b), further highlighting the well-controlled surface chemistry of these AuNPs.
Figure 2.
Stability to aggregation and ε potentials of AuNPs 1-4 in conditioned water over time. (a) The changes in hydrodynamic diameters (DH) were determined by dynamic light scattering (DLS). (b) The change of AuNPs 1-4 ε-potentials.
The Au concentrations in the tank containing fish were monitored during the course of the exposure experiments (Figure S3 in Supporting Information). The 20 nM solutions of AuNPs 1-4 have similar initial concentrations (~800-1000 ppb Au), but the positively charged NPs, AuNP 1 and AuNP 4, decrease in concentration over time, whereas AuNP 2 and AuNP 3 remain approximately constant. Two explanations for the decrease in levels for the cationic particles are the significant uptake of these AuNPs by the fish (vide infra) and the greater adherence of the two AuNP to biofilms on the surface of the fish tanks.[13c]
Japanese medaka (Oryzias latipes), a freshwater fish species used for ecotoxicological studies,[14] were exposed to 20 nM of AuNPs 1-4 for 24, 72, and 120 h. These experiments indicate that positively-charged AuNPs 1 and 4 are taken up by the fish more readily than the neutral (2) or negatively-charged (3) AuNPs. For hydrophilic AuNPs 1-3, no visible effects were observed on the health of the fish. In contrast, after exposure to hydrophobic AuNP 4, all of the fish died within 24 h. Fish were dissected and Au amounts in six representative organs and one appendage (brain, heart, liver, gonads, gills, intestines and dorsal fin) were quantitatively determined by ICP-MS (Figure 3) at specific time points. The accumulation of Au in the fish shows a time-dependent behavior for AuNPs 1-3. For AuNP 1 and AuNP 3, the Au amount in the fish increases during the first 72 h but decreases at the 120 h time point. In contrast, the Au amount remains low but approximately constant for AuNP 2 during the entire exposure period. These concentration profiles indicate that AuNPs 1 and 3 do not continually accumulate but rather are eventually excreted by the fish over time.
Figure 3.
Total amounts of Au detected in fish exposed to 20 nM concentrations of AuNPs 1-4. The Au amounts are the sum total of the Au found in six organs and one appendage (brain, heart, liver, gonads, gills, intestines and dorsal fin). Error bars correspond to standard error of the mean drawn from the exposure of eight fish (24 h and 72 h) or four fish (120 h). For AuNP 4, all of the fish died in 24 hrs, so no data are available for the 72 and 120 h time points.
Detailed examination of the biodistrbution of the AuNPs in the fish indicates that the intestines are the predominant site of accumulation for AuNPs 1-3. In contrast, AuNP 4 was widely distributed, with substantial quantities observed in the gills, heart, and dorsal fin (Table 1). For AuNPs 1-3, one-way ANOVA analyses indicate that the intestines are the main site of accumulation, with accumulation in the gills significant in some cases (Table 1). At 72 h, all three of AuNPs 1-3 are significantly accumulated in the intestines. Interestingly, significant accumulation levels of AuNP 1 are found in the gills at all time points, but AuNP 2 and AuNP 3 are not accumulated in the gills until 72 and 120 h, respectively. For AuNPs 1-3, no significant amount of Au was found in brain, heart, liver, gonad and dorsal fin at any time period (Table 1 and Tables S1-3 in Supporting Information for additional values).
Table 1.
Biodistribution of AuNPs 1-4 in fish organs at 24, 72, and 120 h.
| Organ | Control [d] | AuNP 1 [d] | AuNP 2 [d] | AuNP 3 [d] | AuNP 4 [d] |
|---|---|---|---|---|---|
| Au [ng/mg] | Au [ng/mg] | Au [ng/mg] | Au [ng/mg] | Au [ng/mg] | |
| Brain [a] | 0.35 ± 0.14 | 1.10 ± 0.49 | 0.27 ± 0.08 | 0.20 ± 0.04 | 1.29 ± 0.15 *** |
| Heart [a] | 2.28 ± 1.11 | 19.0 ± 9.59 | 5.03 ± 3.68 | 1.57 ± 0.77 | 16.0 ± 5.3 * |
| Liver [a] | 0.19 ± 0.09 | 1.12 ± 0.57 | 0.22 ± 0.09 | 0.18 ± 0.04 | 0.99 ± 0.23 * |
| Gonad [a] | 0.13 ± 0.05 | 12.1 ± 11.1 | 0.18 ± 0.08 | 0.09 ± 0.03 | 6.05 ± 3.97 |
| Dorsal fin [a] | 1.35 ± 0.76 | 1.03 ± 0.19 | 3.41 ± 2.69 | 0.76 ± 0.32 | 14.3 ± 3.4 ** |
| Gill [a] | 0.24 ± 0.09 | 5.07 ± 1.38 ** | 0.53 ± 0.11 | 0.16 ± 0.03 | 25.5 ± 5.7 *** |
| Intestine [a] | 0.12 ± 0.05 | 35.2 ± 9.1 ** | 8.15 ± 3.19 * | 7.35 ± 3.68 | 0.86 ± 0.29 * |
| Gill [b] | 0.25 ± 0.12 | 2.17 ± 0.42 *** | 0.63 ± 0.08 ** | 0.33 ± 0.09 | - - - |
| Intestine [b] | 0.08 ± 0.02 | 144 ± 26 *** | 11.2 ± 2.8 ** | 40.9 ± 13.7 ** | - - - |
| Gill [c] | 0.10 ± 0.04 | 1.09 ± 0.08 *** | 0.48 ± 0.09 ** | 0.24 ± 0.03 * | - - - |
| Intestine [c] | 0.03 ± 0.01 | 75.4 ± 21.6 * | 9.95 ± 3.00 * | 13.3 ± 7.3 | - - - |
24 h;
72 h, and
120 h,
The concentration unit refers to ng of Au per mg of organ weight; Means are averaged from eight fish (24 h and 72 h) or four fish (120 h) and accompanied by standard error of the mean (SEM); For AuNP 4, all of the fish died in 24 h, no data available for 72 and 120 h.
P ≤ 0.05
P ≤ 0.01
P ≤ 0.001 through one-way ANOVA between control and AuNP treated groups, see detail p values in Tables S1-4 in Supporting Information.
The results in Table 1 clearly show that the main uptake route for the AuNPs 1-3 is ingestion into the intestine, with gill absorption being a minor route. Neither exposure routes, however, causes significant absorption or transfer of the AuNPs into other organs (brain, heart, liver and gonad), indicating that the AuNPs do not enter the circulatory system of the fish. The variance analyses indicate that AuNP 1 accumulates much more readily than AuNPs 2 or 3. The higher level of AuNP 1 in the gills and intestines can be explained by the physico-chemical nature of the mucus layers that cover the intestine and gill surfaces. This layer covers the epithelial cells of the gill and intestine and consists of glycoproteins enriched in sialic acid, carboxylic acid and sulfated functional groups, essentially making the mucus a viscous solution of polyanions.[15] Thus, cationic AuNP 1 experiences a strong electrostatic attraction with the mucus layer, whereas neutral and anionic particles 2 and 3 are expected to have negligible and repulsive interactions with the mucus layer, respectively. The modest accumulation of AuNPs 2 and 3 may be mediated by cations such as Ca2+, Mg2+, and Na+ that are very abundant in the mucus layer. As no significant accumulation of AuNP 1-3 is found in other organs, one can reasonably conclude that the mucus layer acts as an effective barrier against these very hydrophilic AuNPs.[15]
In contrast to AuNPs 1-3, AuNP 4 showed significant toxicity, killing all the fish within 24 h. Our data (Table 1 and Table S4 in Supporting Information) show that significant levels of Au are found in six tissues (brain, heart, liver, gills, intestines and dorsal fin, P < 0.05) when the fish are exposed to AuNP 4. Unlike AuNPs 1-3, the gills and dorsal fin become the main sites of accumulation, whereas relatively little Au accumulates in the intestine. In addition, significant accumulation of AuNP 4 is found in the brain, heart, and liver, indicating that these AuNP entered the circulatory system of the fish. Like AuNP 1, the positively-charged nature of AuNP 4 presumably facilitates interactions with the mucus layer that covers many tissues. But, unlike AuNP 1, the hydrophobic functionality on the surface of the NP apparently enables favorable interactions with the membranes of the epithelial cells that lie beneath the mucus layer. Such membrane interactions might facilitate the cellular uptake of these NPs as has been observed previously in cell culture experiments,[10b] allowing them to be transported into the circulatory system of the fish. Alternatively, interactions of AuNP 4 with the epithelial cells may cause the cell membranes to be reconstructed,[16] damaging the epithelial layer. Once the epithelium is compromised, the AuNPs accumulated in mucus could more readily enter into the circulatory system through the damaged tissue.[15]
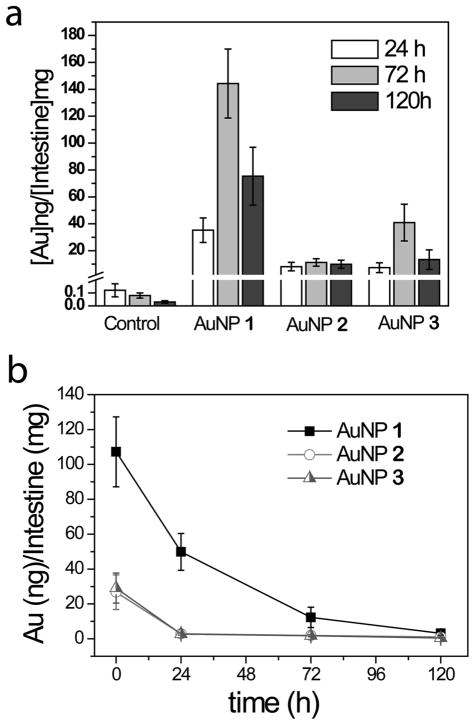
Accumulation data for the intestines reveals that the concentrations for AuNP 1 and 3 drop after reaching a maximum at 72 h (Figure 4a and Table 1). We hypothesized that this might be due to the clearance of the AuNPs from the intestines. To test this hypothesis, we exposed fish to 20 nM of AuNP 1-3 for 3 days and then placed the fish in new tank with AuNP-free water. Then, AuNP concentrations in the intestines were measured at 0, 24, 72 and 120 h (Figure 4b). Clearly, all three AuNPs are readily excreted after the initial accumulation in the intestines. Furthermore, AuNP 2 (half-life ~12 h) and AuNP 3 (half-life ~12 h) are more rapidly excreted than AuNP 1 (half-life ~24 h), consistent with stronger interactions of this cationic AuNP with the negatively-charged mucus layer.
Figure 4.
Clearance of AuNPs 1-3 from fish intestine. (a) Accumulation of AuNPs 1-3 in fish intestines at 24, 72 and 120 h. Error bars correspond to standard error of the mean (SEM) drawn from exposures to eight fish (24 h and 72 h) or four fish (120 h). (b) Clearance of AuNPs 1-3 from fish intestine over time. Error bars correspond to SEM drawn from six fish.
Exposure of Japanese medaka fish to anionic, neutral, and cationic hydrophilic AuNPs results in temporary accumulation in the intestines and, to some degree, the gill, with the cationic particles accumulating to a moderately higher degree. Negligible accumulation in the dorsal fin, brain, heart, gonad, and liver is observed, indicating lack of uptake of the NPs into the circulatory system. None of the NPs with hydrophilic terminal groups (i.e. AuNP 1-3), however, is significantly accumulated, as excretion experiments indicate complete clearance of the AuNPs. These studies indicate that the hydrophilic surface chemistry of AuNPs 1-3 prevents accumulation, facilitates clearance, and hence are promising coatings to minimize environmental impact. On the contrary, cationic and hydrophobic AuNP 4 readily penetrates into the circulatory system of the fish, leading to widespread distribution in the organs of the fish and ultimately fish mortality in less than 24 h. These results suggest that creating nanoparticles with hydrophilic surfaces is a strategy for developing less toxic and more sustainable nanomaterials. This strategy presents the possibility of a “win-win“ methodology that combines more environmentally benign hydrophilic nanomaterials with “green“ water-based processing methods.
Experimental Section
Synthesis and characterization of AuNPs
The surface monolayers and AuNPs were synthesized using methods previously published.[10b, 17] A two-phase synthesis method was first used to synthesize pentanethiol-coated AuNPs with core diameters of 2 nm.[18] After that, the ligand-exchange method[19] was used to obtain AuNP 1, AuNP 3 and AuNP 4. AuNP 2 was synthesized by the single phase synthesis method described previously.[20] The core sizes of the synthesized AuNPs were measured on a JEOL 100S transmission electron microscope. Dynamic light scattering (DLS) and ζ-potential measurements of the AuNPs were made with a Malvern Zetasizer Nano ZS. 1 μM of each AuNP in conditioned water was used to monitor the aggregation and ζ-potential changes over time. Conditioned water was made by mixing reverse osmosis (RO) water with sodium bicarbonate and calcium chloride to achieve a pH of 7.24 and a conductivity of 470 μS.
Fish and fish care
Japanese medaka (Oryzias latipes) were reared and housed on a recirculating flow-through system (Aquatic Habitats, Apopka, FL) in which water temperature was maintained between 21-25 C, pH 6.8-8.0, and conductivity 500-700 μS, with a 16:8 light:dark photoperiod. Fish were fed twice daily with ground Deli Flake dry food (Brine Shrimp Direct; Ogden, UT, USA) and once every other day with live brine shrimp hatched from cysts (Brine Shrimp Direct; Ogden, UT, USA) unless otherwise noted. Rearing and experimental protocols were approved by the University of Massachusetts Internal Animal Care and Use Committee (IACUC).
AuNP exposure experiments
In the first set of experiments, a single dose of 20 nM AuNPs 1-3 was separately added into each of three of 1-L plastic tanks, each containing 800 mL of conditioned water. After aeration for 30-60 minutes with an air-stone, eight fish (four females and four males) were placed into the plastic tank. Fish were fed 0.03 g of dry flake once every 24 h. Four fish (two females and two males) were taken out and dissected after 24 and 72 h, respectively. In a second set of experiments, a single dose of 20 nM AuNPs 1-3 was separately added into three 2.75-L plastic tanks, each containing 1200 mL of conditioned water. After aeration, 12 fish (six females and six males) were placed into the tank. Four fish (two females and two males) were taken out and subjected to dissection after 24, 72, and 120 h, respectively. In these experiments, fish were also fed 0.03 g of dry flake once every 24 h. No mortality was observed during the two sets of experiments. Upon removal, each fish was euthanized using 4% Tricaine (MS-222), and a whole body weight was measured. Six representative organs and one appendage, including brain, gill, heart, liver, gonads, intestines and dorsal fin, were extracted and weighed. The gold amounts in the organs were later determined by ICP-MS.
In a third set of experiments, a single dose of 20 nM AuNP 4 was added to a 2.75-L plastic tank containing 1200 mL of conditioned water. After aeration, 12 fish (six females and six males) were placed into the tank. After 24 h of exposure, all 12 fish were found dead. The same experiment was repeated, and the same result was observed. In a fourth experiment, a single dose of 20 nM AuNP 4 was added to a 2.75-L plastic tank containing 800 mL of conditioned water. After aeration, eight fish were added, and again all fish died within 24 h. A total of 8 fish from AuNP 4 exposure were dissected, and Au amounts in the collected organs were measured by ICP-MS.
For each of the experiments described above, control experiments were carried out under the same conditions except no AuNPs were added to the tank water. No mortality was observed during any control experiment. Fish from the control experiments were also dissected, and Au amounts in the collected organs were also measured by ICP-MS.
For the clearance experiments, a single dose of 20 nM AuNPs 1-3 was separately added into three of 2.75-L plastic tanks, each containing 1200 mL of conditioned water and 12 fish. After three days, three fish were taken out and subjected to dissection, and the time was set as 0 h in Fig. 5b. The remaining fish were then removed from the AuNP-containing water placed into a clan tank with AuNP-free conditioned water. In addition, the tank water was changed every 24 hrs during the five-day period. At 24, 72 and 120 h intervals, three fish were taken out and dissected each time. All the organs were digested and measured by ICP-MS. The clearance experiments were performed in duplicate. No mortality was observed during any of the clearance experiments.
Sample digestion and measurement
Each organ was digested overnight using a mixture of 3 mL of HNO3 and 1 mL of H2O2. On the next day, ~2 mL of aqua regia was added, and then the sample was allowed to react for another 1-2 h. Aqua regia is highly corrosive and must be used with extreme caution! A hot plate (~100 °C) was used to reduce the above digested solution to less than 1 mL. The concentrated sample solution was then diluted to 10 mL with 0.5 mL aqua regia and de-ionized water. The gold in the sample solution was measured on a Perkin-Elmer Elan6100 ICP mass spectrometer. The instrument was operated with a 1200 W RF power and the nebulizer Ar flow rate was optimized around 0.9 -1.0 L/min.
Supplementary Material
Footnotes
This work was supported by the Office of Naval Research (N000140510501), the NIH (GM077173), and the NSF Center for Hierarchical Manufacturing (DMI-0531171). The authors wish to thank Prof. Julian F. Tyson for the access to the ICP-MS instrumentation.
Supporting Information is available on the WWW under http://www.small-journal.com or from the author.
Contributor Information
Zheng-Jiang Zhu, Department of Chemistry, University of Massachusetts, Amherst, MA 01003 (USA).
Rachel Carboni, Department of Veterinary and Animal Sciences, University of Massachusetts, Amherst, MA 01003 (USA).
Michael Quercio, Department of Veterinary and Animal Sciences, University of Massachusetts, Amherst, MA 01003 (USA).
Bo Yan, Department of Chemistry, University of Massachusetts, Amherst, MA 01003 (USA).
Oscar R. Miranda, Department of Chemistry, University of Massachusetts, Amherst, MA 01003 (USA)
Prof. Douglas L. Anderton, Department of Sociology, University of Massachusetts, Amherst, MA 01003 (USA)
Prof. Kathleen F. Arcaro, Email: karcaro@vasci.umass.edu, Department of Veterinary and Animal Sciences, University of Massachusetts, Amherst, MA 01003 (USA)
Prof. Vincent M. Rotello, Email: rotello@chem.umass.edu, Department of Chemistry, University of Massachusetts, Amherst, MA 01003 (USA)
Prof. Richard W. Vachet, Email: rwvachet@chem.umass.edu, Department of Chemistry, University of Massachusetts, Amherst, MA 01003 (USA)
References
- 1.The Nanotechnology Consumer Products Inventory’. published online at http://www.nanotechproject.org/inventories/consumer/
- 2.a) Colvin VL. Nature Biotechnol. 2003;21:1166. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]; b) Service RF. Science. 2005;310:1609. doi: 10.1126/science.310.5754.1609. [DOI] [PubMed] [Google Scholar]; c) Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Nature. 2006;444:267. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]; d) Nel A, Xia T, Madler L, Li N. Science. 2006;311:622. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]; e) Behra R, Krug H. Nature Nanotechnol. 2008;3:253. doi: 10.1038/nnano.2008.113. [DOI] [PubMed] [Google Scholar]; f) Handy RD, Owen R, Valsami-Jones E. Ecotoxicology. 2008;17:315. doi: 10.1007/s10646-008-0206-0. [DOI] [PubMed] [Google Scholar]; g) Auffan M, Rose J, Bottero JY, Lowry GV, Jolivet JP, Wiesner MR. Nature Nanotechnol. 2009;4:634. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]; h) Owen R, Handy R. Environ Sci Technol. 2007;41:5582. doi: 10.1021/es072598h. [DOI] [PubMed] [Google Scholar]; i) Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P. Environ Sci Technol. 2006;40:4336. doi: 10.1021/es062726m. [DOI] [PubMed] [Google Scholar]
- 3.a) Handy RD, von der Kammer F, Lead JR, Hassellov M, Owen R, Crane M. Ecotoxicology. 2008;17:287. doi: 10.1007/s10646-008-0199-8. [DOI] [PubMed] [Google Scholar]; b) Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao AJ, Quigg A, Santschi PH, Sigg L. Ecotoxicology. 2008;17:372. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]; c) Farre M, Gajda-Schrantz K, Kantiani L, Barcelo D. Anal Bioanal Chem. 2009;393:81. doi: 10.1007/s00216-008-2458-1. [DOI] [PubMed] [Google Scholar]
- 4.a) Oberdorster E. Environ Health Persp. 2004;112:1058. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao YJ, Sitharaman B, Wilson LJ, Hughes JB, West JL, Colvin VL. Nano Lett. 2004;4:1881. [Google Scholar]; c) Lin SJ, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC. Small. 2009;5:1128. doi: 10.1002/smll.200801556. [DOI] [PubMed] [Google Scholar]
- 5.Smith CJ, Shaw BJ, Handy RD. Aquat Toxicol. 2007;82:94. doi: 10.1016/j.aquatox.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Ofek BI, Ralph MA, Valerie EF, Darin YF. Small. 2009;5:1897. [Google Scholar]
- 7.Federici G, Shaw BJ, Handy RD. Aquat Toxicol. 2007;84:415. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Hardman R. Environ Health Persp. 2006;114:165. doi: 10.1289/ehp.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chithrani BD, Ghazani AA, Chan WCW. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 10.a) Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Bioconjugate Chem. 2004;15:897. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]; b) Zhu ZJ, Ghosh PS, Miranda OR, Vachet RW, Rotello VM. J Am Chem Soc. 2008;130:14139. doi: 10.1021/ja805392f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain S, Boland S, Baeza-Squiban A, Hamel R, Thomassen LCJ, Martens JA, Billon-Galland MA, Fleury-Feith J, Moisan F, Pairon JC, Marano F. Toxicology. 2009;260:142. doi: 10.1016/j.tox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Bhabra G, Sood A, Fisher B, Cartwright L, Saunders M, Evans WH, Surprenant A, Lopez-Castejon G, Mann S, Davis SA, Hails LA, Ingham E, Verkade P, Lane J, Heesom K, Newson R, Case CP. Nature Nanotechnol. 2009;4:876. doi: 10.1038/nnano.2009.313. [DOI] [PubMed] [Google Scholar]
- 13.a) Renault S, Baudrimont M, Mesmer-Dudons N, Gonzalez P, Mornet S, Brisson A. Gold Bull. 2008;41:116. [Google Scholar]; b) Tedesco S, Doyle H, Blasco J, Redmond G, Sheehan D. Comp Biochem Phys C. 2010;151:167. doi: 10.1016/j.cbpc.2009.10.002. [DOI] [PubMed] [Google Scholar]; c) Ferry JL, Craig P, Hexel C, Sisco P, Frey R, Pennington PL, Fulton MH, Scott IG, Decho AW, Kashiwada S, Murphy CJ, Shaw TJ. Nature Nanotechnol. 2009;4:441. doi: 10.1038/nnano.2009.157. [DOI] [PubMed] [Google Scholar]
- 14.Hinton DE, Kullman SW, Hardman RC, Volz DC, Chen PJ, Carney M, Bencic DC. Mar Pollut Bull. 2005;51:635. doi: 10.1016/j.marpolbul.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Handy RD, Henry TB, Scown TM, Johnston BD, Tyler CR. Ecotoxicology. 2008;17:396. doi: 10.1007/s10646-008-0205-1. [DOI] [PubMed] [Google Scholar]
- 16.a) Leroueil P, Hong S, Mecke A, Baker J, Orr B, Banaszak Holl M. Acc Chem Res. 2007;40:335. doi: 10.1021/ar600012y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang B, Zhang L, Bae SC, Granick S. Proc Natl Acad Sci USA. 2008;105:18171. doi: 10.1073/pnas.0807296105. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Verma A, Stellacci F. Small. 2010;6:12. doi: 10.1002/smll.200901158. [DOI] [PubMed] [Google Scholar]; d) Ginzburg VV, Balijepalli S. Nano Lett. 2007;7:3716. doi: 10.1021/nl072053l. [DOI] [PubMed] [Google Scholar]
- 17.Hong R, Emrick T, Rotello VM. J Am Chem Soc. 2004;126:13572. doi: 10.1021/ja0461163. [DOI] [PubMed] [Google Scholar]
- 18.Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. J Chem Soc, Chem Commun. 1994:801. [Google Scholar]
- 19.Templeton AC, Wuelfing WP, Murray RW. Acc Chem Res. 2000;33:27. doi: 10.1021/ar9602664. [DOI] [PubMed] [Google Scholar]
- 20.Kanaras AG, Kamounah FS, Schaumburg K, Kiely CJ, Brust M. Chem Commun. 2002:2294. doi: 10.1039/b207838b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.