Microcapsules (MCs) are versatile systems with applications in areas as diverse as microreactors, catalysis,[1] diagnostics and drug delivery.[2] In these systems self-assembly of lipids and/or polymers can be used to generate several types of nano- and micro- capsules. These include vesicular structures such as liposomes,[3] polymerosomes,[4] colloidosomes,[5] and polyelectrolyte capsules that feature aqueous interiors and exteriors.[6] An alternate motif is provided by emulsions, where additives are used to stabilize the interface between immiscible fluids to produce e.g. oil-in-water emulsions.[7] Through tailoring of the composition and structure of the building blocks MCs of both types can be engineered with well-defined structures, functions and stability.[8] MCs provide excellent delivery vehicles for biomedical applications, featuring high payload-to-carrier ratios and protection of encapsulated materials from degradation.
Nanoparticle-stabilized capsules (NPSCs) present microcapsule structural motifs where the nanoparticles [9] are assembled at the interface of immiscible solvent droplets. The physical properties of NPSCs can be precisely controlled in a modular fashion through the proper choice of nanoparticle precursors and assembly conditions,[10] giving these systems utility in numerous applications.[11] In NPSCs, nanoparticles in the capsule shell serve as modular building blocks, allowing incorporation of the particle properties into the functional capabilities of the microcapsules. When used as delivery vehicles, these oil-in-water emulsion particles are ideally suited for transport of hydrophobic drugs, making these systems complementary to their vesicular counterparts, e.g. liposomes or polymerosomes. The nanoparticle shell of NPSCs imparts additional functional capabilities, for example nanoparticles can serve as antennae leading to controlled release of materials encapsulated in the microcapsules by responding to external stimuli (e.g. magnetic fields or laser light).[12] Additionally, the inherent rigidity of the particles and the capsule shell offer encapsulation vehicles with mechanical advantage as compare to the soft, self-assembled shell made of polymers or lipids.[13] The permeability of the NPSCs can be tailored through variation of the dimension of the colloidal particles, as shown by Duan and coworkers[11c] who fabricated magnetic NPSCs with tunable permeability by assembling various sized magnetite (Fe3O4) nanoparticles at the interfaces of water-in-oil (W/O) droplets.
Capsule size plays a pivotal role for the applications of MCs in catalysis, nanoreactors[14] and sensors,[15] with decreasing size generally providing more effective systems. Likewise, carrier size is critical for application in drug delivery processes: vehicle size controls the perfusion of materials through the endothelium and the diffusion of materials through tissues. For longer circulation times, the size of the carrier should be small enough (<200 nm) to escape capture and subsequent removal by the resident macrophages in the reticuloendothelial system, such as the liver and spleen.[16] In this respect, materials with diameters between 10 and 200 nm are particularly useful due to their enhanced bioavailability and their ability to take advantage of the Enhanced Permeation and Retention (EPR) effect.[17] for NPSCs to be useful in the delivery context, they will need to have a proper size (10≤d≤200 nm) for optimal activity.
A variety of techniques e.g. tuning of particle wettability[11] or lateral crosslinking[18] of the particles at interface have been developed to address the stability of nanoparticles on the capsule shells. However, despite progress in this field, current methods generate NPSCs ranging from several micrometers to millimeters. The fabrication of smaller NPSCs encounters two challenges. First, the confinement of particles on droplets surface is driven by total interfacial energy decrease.[19] The three contributions to the interfacial energy arise from the particle-oil interface (γP/O), the particle-water interface (γP/W), and the oil-water interface (γO/W). Effective decrease in interfacial energy due to the assembly of a single particle at the oil-water interface is given by[20]
| (1) |
where γ and r are interfacial tension between two adjacent phases and effective radius of the particle respectively. Because ΔE depends on r2, the energy decrease is smaller and is comparable to the thermal energy for small particles than for larger ones. Consequently, interfacially confined nanoparticles are more prone towards spatial fluctuations and eventual displacement from the interface. A second issue is that stabilization of capsules relies on the Laplace pressure, i.e. the pressure difference between the inside and outside of the capsules. This effect is caused by the surface tension of the interface between the solvents[21] and is given as
| (2) |
where G is radius of the capsules. It is evident from the Eq. 2 that as capsule size decreases the Laplace pressure exerted to the interfacial nanoparticles increases, making smaller droplets unstable. As a result smaller droplets tend to coalesce in solution. Taken together, as capsule size decreases there is less driving force for the nanoparticle to go to the interface, an effect that is exacerbated by the higher surface area of nanometer-scale capsules.
To generate the nanoscale NPSCs required for delivery applications, we have investigated an alternative strategy that relies upon nanoscale droplet stabilization through supramolecular interactions. Two different supramolecular strategies were synergistically combined to tune interfacial energy and stabilize nanoparticle shell of NPSCs (Figure 1). First, we have engineered the interaction between the nanoparticle that constitute the NPSC shell with the “oil” interior of the capsule to access nanoscale assembiles. Second, the NPSC architecture was stabilized through lateral particle-particle interactions. Utilizing this combined approach we have developed a direct and versatile technique for the creation of NPSCs featuring diameters as small as ~100 nm that are stable in buffer as well as in media and serum.
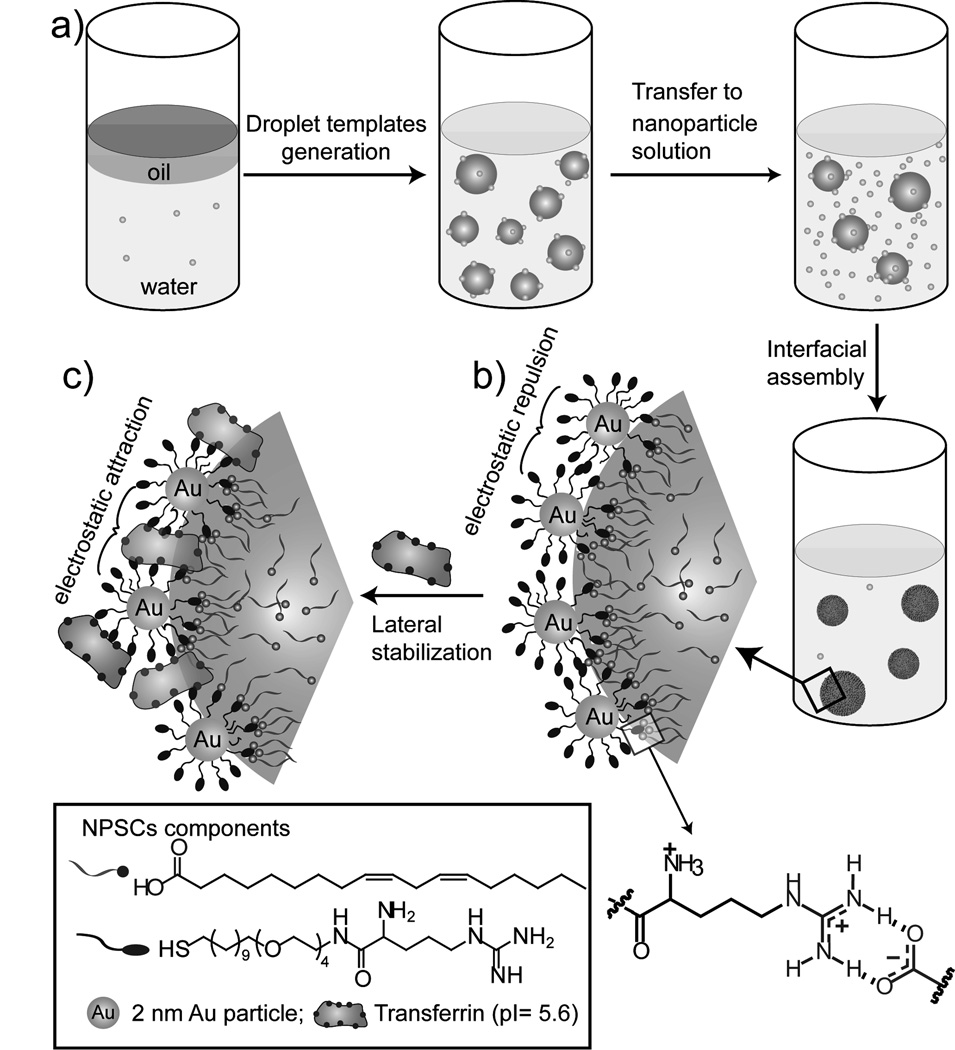
Figure 1. Schematic of the stable nanoscale NPSC fabrication.
a) Generation of nanosized oil-in-water droplets followed by interfacial assembly of arginine functionalized gold nanoparticles. b) Supramolecular interactions between nanoparticle and linoleic acid droplets via arginine-carboxylate interactions. c) Transforming strong particle-particle repulsion to a stabilizing electrostatic attraction by lateral non-covalent cross-linking of particles using transferrin protein.
Our initial efforts to produce stable NPSCs focused on the use of nanoparticle-droplet interactions to stabilize the particle shell. The well-known arginine-carboxylate hydrogen bonding/electrostatic interaction[22] was exploited through use of biocompatible linoleic acid as the “oil”, and arginine-functionalized gold nanoparticles (AuNP-Arg, core diameter ~2 nm) as the nanoparticle shell. Other than the required surface functionality, gold nanoparticles also provide a biocompatible and non-toxic platform,[23] well-suited for delivery applications. The stable nanoscale NPSCs were produced by a three-step procedure. First, ~105 nm template droplets were generated by agitating linoleic acid in phosphate buffer (5 mM, pH 7.4) in the presence of a small amount of AuNP-Arg using a commercial homogenizer. In the second step, the droplets were transferred into solutions of AuNP-Arg. This dispersion process results in essentially instantaneous assembly of the arginine nanoparticles at the droplet surface through arginine-carboxylate hydrogen bonding/electrostatic interactions, generating nanoscale NPSCs. NPSC formation was optimized by varying the nanoparticle/droplet ratio, and the resultant assemblies were characterized by TEM (see Supporting Information Figure S3a–c). The optimized nanoparticle/droplet ratio for successful nanoscale NPSC formation was 2.5µM/0.64 nM (on a per droplet basis). At this optimum ratio, the TEM image shows essentially no free nanoparticles on the sample grid and a dense packing of nanoparticles on the droplets. The average number of AuNP-Arg nanoparticle bound to a single linoleic acid droplet estimated is 4061 (see Supporting Information for the calculation).
Two analogues of linoleic acid (methyl linolate and linoleoyl alchohol) were used as controls to verify that NPSC formation was driven by guanidinium-carboxylate interactions. As shown in Figure 2a, the arginine nanoparticles bind to linoleic acid droplets, forming NPSCs that could be readily pelleted by centrifugation (at a speed of 3000 rpm, 5min). Significantly, no free particle was observed after centrifugation, indicating complete incorporation of AuNP-Arg into the NPSC shell. In contrast, no NPSC formation was observed with the controls. The confinement of AuNP-Arg at linoleic acid droplets surface was also evidenced from encapsulating fluorescent Nile Red dye into the oil droplets followed by nanoparticle assembly and centrifugation. As shown in Figure 2b, Nile Red encapsulated droplets showed intensive red fluorescence while no fluorescence signal was detected from the supernatant of NPSCs after centrifugation, indicating effective binding of nanoparticles to the droplets.
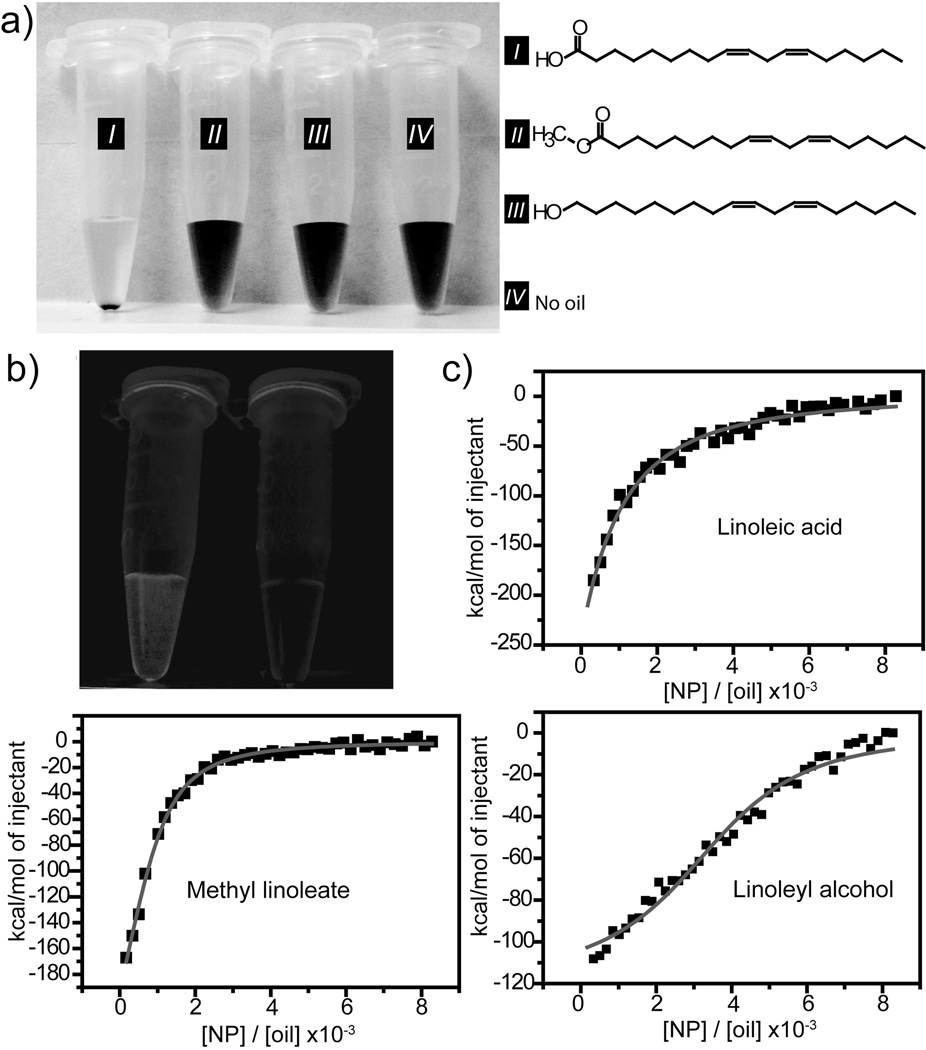
Figure 2. Controlling supramolecular interaction to create NPSCs.
a) NPSC formation with linoleic acid whereas no NPSC assembly was observed using controls. b) Photograph of nile red encapsulated linoleic acid-NPSCs containing vial under UV-light before and after centrifugation of NPSCs. c) ITC titration of AuNP-Arg nanoparticles into a buffered (5 mM, pH 7.4) suspension of each of the three oil droplets. The squares represent the integrated heat changes during complex formation and the lines represent the curve fit to the binding isotherm.
Further evidence of the strong interaction between the AuNP-Arg particles and the linoleic acid droplets was obtained using isothermal titration calorimetry (ITC) study, where AuNP-Arg was titrated into a buffered (5 mM, pH 7.4) suspension of each of the three oil droplets. The ITC results indicated that the interactions between the nanoparticle and oil droplets made from each of the three oils were all exothermic in nature. Significantly, the ΔH for linoleic acid (−17.6 kcal mol−1) was far more exothermic than that observed for methyl linoleate (−0.3 kcal mol−1) and linoleyl alcohol (−0.1 kcal mol−1) (Figure 2c and Supporting Information Figure S4), indicative of the strong interaction between arginine and linoleic acid.
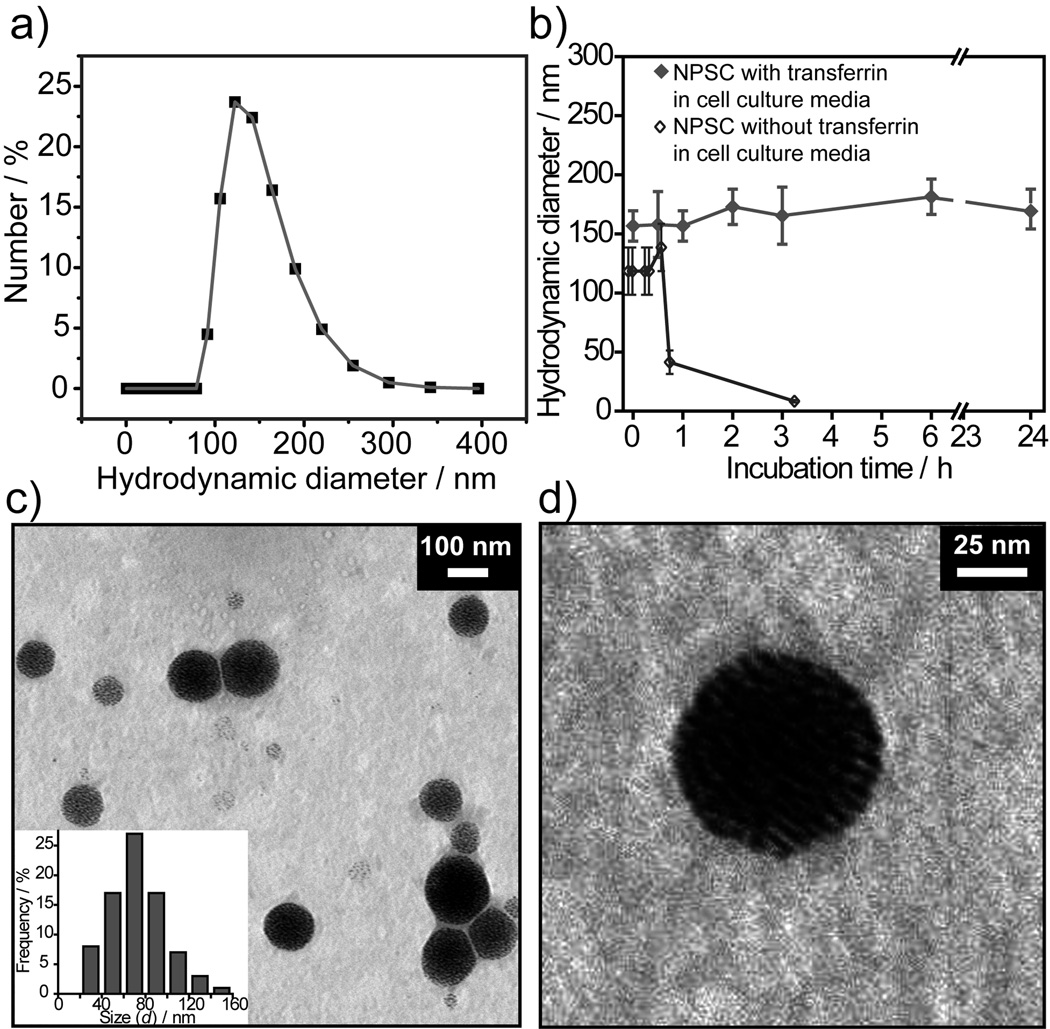
The AuNP-Arg-linoleic acid capsules assemblies were stable in buffer, however degraded within 3 h in cell culture medium, as observed from the DLS measurement of hydrodynamic diameter (Figure 3b). Our hypothesis was that the low stability of NPSCs in physiological conditions arose from destabilization due to particle-particle repulsion between the highly positively charged AuNP-Arg particles (Figure 1b). In recent studies we have shown that nanoparticles can form stable assemblies with enzymes at oil-water interfaces.[24] Incorporation of complimentary negatively charged transferrin (pI= 5.6) proteins into the capsule shell converted the repulsions between AuNP-Arg particles into an attractive interactions, generating capsule stable under physiological conditions as demonstrated by DLS (Figure 3b). The dispersity of linoleic acid-transferrin NPSCs was measured by using DLS (Figure 3a). Hydrodynamic diameter of 122±2 nm was observed from this experiment. The protein stabilized NPSCs were further characterized by using TEM (Figure 3c). The average size distribution (d) determined from TEM image of NPSCs is 75±25 nm. The smaller size observed by TEM is expected, and arises from shrinkage due to drying of the NPSC in the TEM microscope.
Figure 3. Size and shape of NPSCs in different conditions.
a) Hydrodynamic diameter as observed by DLS measurement of protein stabilized NPSCs in phosphate buffer (5 mM, pH 7.4). NPSCs fabricated via assembly of 0.64 nM droplets with 2.5 µM nanoparticle solution followed by lateral crosslinking of particles using transferrin proteins. b) Stability study of linoleic acid-NPSCs with and without transferrin in physiological condition over time. c) TEM image of NPSCs. The inset shows the size distribution determined from TEM image by counting the diameter of 80 individual NPSCs. d) Magnified view of a protein stabilized NPSC.
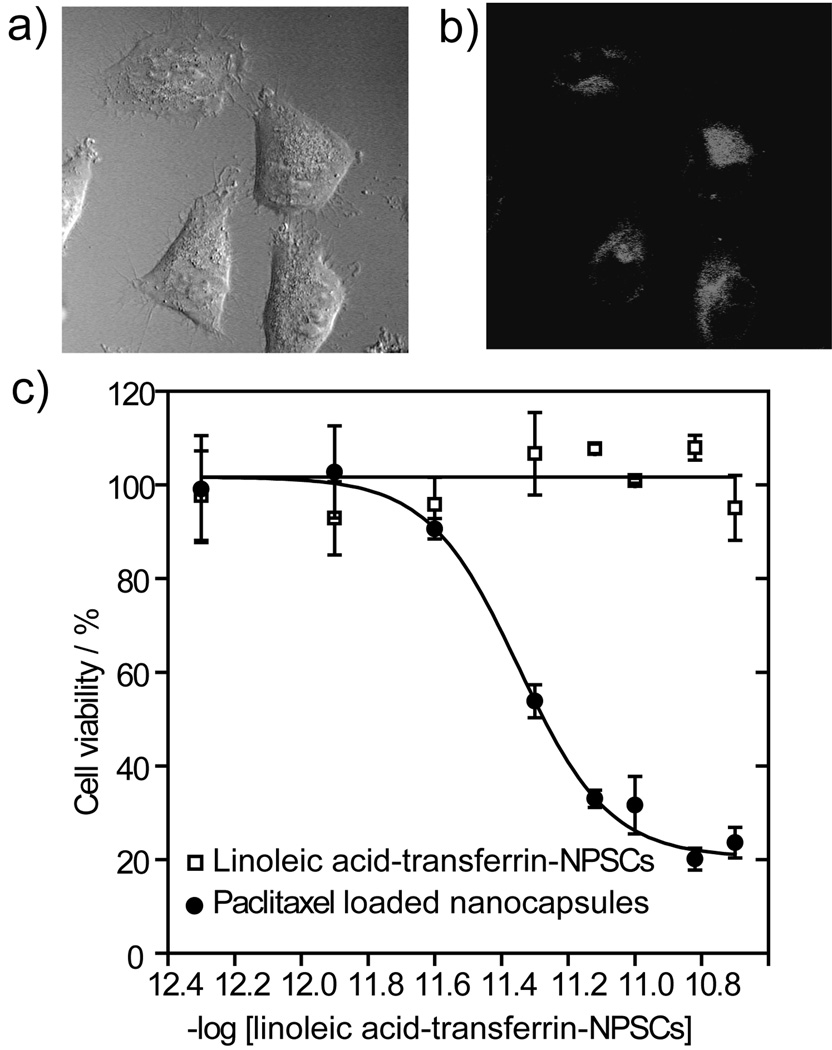
The high stability of the linoleic acid-transferrin NPSCs under physiological conditions makes them potentially useful as delivery vehicles. Preliminary studies of cellular delivery and uptake of NPSCs were performed using a combination of confocal laser scanning microscopy (CLSM) and inductively coupled plasma mass spectrometry (ICP-MS). In these studies, NPSCs loaded with Nile Red fluorophore were incubated with HeLa cells, and payload delivery followed using CLSM. As shown in Figure 4a–b and Supporting Information Figure S6, efficient delivery of Nile Red to the cytosol was observed after 3 h. There are two potential mechanisms for this payload delivery using these NPSCs. In one mechanism material is transferred via membrane fusion from the NPSC to the cell without NPSC uptake. In the second mechanism, the cell takes up the intact particle via an endocytotic mechanism, with payload release occurring inside the cell. To differentiate between these mechanisms, we performed a time point analysis. Analysis of cell fluorescence as determined using fluorescence microscopy indicated that maximal uptake was observed after 3h, with little further increase in fluorescence observed after 6h (Supporting Information Figure S5a). However, very different kinetics were observed for AuNP-Arg uptake using ICP-MS, in which steadily increasing amounts of intracellular Au were observed over 6h (Supporting Information Figure S5b). This observation was consistent with the NPSC-cell fusion mechanism. Further support for a non-endocytotic delivery process comes from fluorophore delivery studies using sodium azide and deoxyribose, well known inhibitors of energy-dependent endocytosis through disruption of cellular ATP production. Time point analysis revealed no significant difference in particle uptake (Supporting Information Figure S7) in the presence of either inhibitor, consistent with fluorophore uptake via a non-endocytotic pathway.
Figure 4. Delivery of Nile red encapsulated linoleic acid-transferrin-NPSCs in HeLa cells.
(a–b) CLSM images of HeLa cell treated with same NPSCs for 3 h (a, bright field, and, b, red channel). (d) Cytotoxicity of paclitaxel loaded linoleic acid-transferrin NPSCs. The cell viabilities were measured by Alamar blue assay after 24 h incubation of paclitaxel loaded linoleic acid-transferrin-NPSCs and carrier with HeLa cells
Having established effective fluorophore delivery, we next investigated the efficacy of these NPSCs as drug delivery systems in cell culture models. Using the Alamar blue viability assay and HeLa cells, we determined that the linoleic acid-transferrin NPSCs featured no non-specific cytotoxicity at the concentrations studied (Figure 4d). Our initial drug candidate for NPSC-mediated delivery was paclitaxel due to its high toxicity and hydrophobicity. Paclitaxel is one of the most effective anticancer drugs for the treatment of a wide variety of cancers, however this highly hydrophobic drug suffers from poor solubility with concomitant issues in biodistribution.[25] We have used linoleic acid-transferrin NPSCs for paclitaxel encapsulation and delivery purposes due to its high stability under physiological conditions and efficient fluorophore delivery efficiency (vide supra). Paclitaxel has a relatively high solubility in linoleic acid (10 mg mL−1), allowing high loading of the NPSCs without substantial processing. Delivery studies indicate that release of paclitaxel from the NPSC is quite efficient, with an IC50 value for the encapsulated drug of 13.5 ng mL−1 (Figure 4d), as compared to 6.5 ng mL−1 for free paclitaxel.
In summary, we have developed a strategy for NPSC fabrication that synergistically combines radial particle-lipid and lateral particle-protein supramolecular interactions to generate capsules of unprecedentedly small size (~100 nm). We envision that further modulation of these interactions through the use of diverse chemical functionality will provide a control over the capsule size and functionality for myriad applications. Cell culture studies of drug delivery into cancer cells using these NPSC carriers demonstrated highly efficient payload delivery with no measurable carrier toxicity. The nanoparticle shell of NPSC provides a platform for future incorporating targeting molecule to realize targeted cancer therapy for animal study, a prospect that is currently under investigation.
Experimental Section
See supporting information for experimental details.
Footnotes
This research was supported by the NIH (GM077173 and EB012246-01) and MRSEC facilities. SSA acknowledges a U. Mass. Graduate School Fellowship. XCY gratefully acknowledges support from China Scholarship Council for State Scholarship Fund.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Xiao-Chao Yang, College of Biomedical Engineering and Medical Imaging, Third Military Medical University, Chongqing, 400030, China; Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Bappaditya Samanta, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Sarit S. Agasti, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Youngdo Jeong, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Zheng-Jiang Zhu, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Subinoy Rana, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
Oscar R. Miranda, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA
Vincent M. Rotello, Department of Chemistry, University of Massachusetts Amherst, 710 North Pleasant Street, Amherst, MA 01003, USA.
References
- 1.Kim KT, Cornelissen JJLM, Nolte RJM, van Hest JCM. Adv. Mat. 2009;21:2787–2791. [Google Scholar]
- 2.a) Zhao Y, Jiang L. Adv. Mater. 2009;21:3621–3638. [Google Scholar]; b) Parthasarathy RV, Martin CR. Nature. 1994;369:298–301. doi: 10.1038/369298a0. [DOI] [PubMed] [Google Scholar]; c) De Geest BG, De Koker S, Sukhorukov GB, Kreft O, Parak WJ, Skirtach AG, Demeester J, De Smedt SC, Hennink WE. Soft Matter. 2009;5:282–291. [Google Scholar]
- 3.Torchilin VF. Nat. Rev. Drug Discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 4.a) Cheng Z, Thorek DLJ, Tsourkas A. Adv. Funct. Mater. 2009;19:3753–3759. doi: 10.1002/adfm.200901253. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Howse JR, Jones RAL, Battaglia G, Ducker RE, Leggett GJ, Ryan AJ. Nature Mater. 2009;8:507–511. doi: 10.1038/nmat2446. [DOI] [PubMed] [Google Scholar]
- 5.Dinsmore AD, Hsu MF, Nikolaides MG, Marquez M, Bausch AR, Weitz DA. Science. 2002;298:1006–1009. doi: 10.1126/science.1074868. [DOI] [PubMed] [Google Scholar]
- 6.a) Peyratout CS, Dähne L. Angew. Chem. Int. Ed. 2004;116:3850–3872. doi: 10.1002/anie.200300568. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2004;43:3762–3783. doi: 10.1002/anie.200300568. [DOI] [PubMed] [Google Scholar]; b) Zelikin AN, Li Q, Caruso F. Angew. Chem. 2006;118:7907–7909. doi: 10.1002/anie.200602779. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:7743–7745. doi: 10.1002/anie.200602779. [DOI] [PubMed] [Google Scholar]
- 7.Subramaniam AB, Abkarian M, Stone HA. Nature Mater. 2005;4:553–556. doi: 10.1038/nmat1412. [DOI] [PubMed] [Google Scholar]
- 8.a) Wang B, Zhang LF, Bae SC, Granick S. Proc. Natl. Acad. Sci. U.S.A. 2008;105:18171–18175. doi: 10.1073/pnas.0807296105. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang LF, Dammann K, Bae SC, Granick S. Soft Matter. 2007;3:551–553. doi: 10.1039/b618172d. [DOI] [PubMed] [Google Scholar]
- 9.Glogowski E, Tangirala R, He J, Russell TP, Emrick T. Nano Lett. 2007;7:389–393. doi: 10.1021/nl062581h. [DOI] [PubMed] [Google Scholar]
- 10.a) Lee D, Weitz DA. Adv. Mater. 2008;20:3498–3503. [Google Scholar]; b) Wang D, Duan H, Mohwald H. Soft Matter. 2005;1:412–416. doi: 10.1039/b511911a. [DOI] [PubMed] [Google Scholar]
- 11.a) Binks B, Murakami R. Nature Mater. 2006;5:865–869. doi: 10.1038/nmat1757. [DOI] [PubMed] [Google Scholar]; b) Nie Z, Petukhova A, Kumacheva E. Nature Nanotech. 2010;5:15–25. doi: 10.1038/nnano.2009.453. [DOI] [PubMed] [Google Scholar]; c) Duan, Wang HD, Sobal NS, Giersig M, Kurth DG, Möhwald H. Nano Lett. 2005;5:949–952. doi: 10.1021/nl0505391. [DOI] [PubMed] [Google Scholar]
- 12.a) Skirtach AG, Javier AM, Kreft O, Köhler K, Alberola AP, Möhwald H, Parak WJ, Sukhorukov GB. Angew. Chem. 2006;118:4728–4733. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:4612–4617. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]; b) Hu SH, Liu DM, Tung WL, Liao CF, Chen SY. Adv. Funct. Mater. 2008;18:2946–2955. [Google Scholar]
- 13.Mueggenburg KE, Lin XM, Goldsmith RH, Jaeger HM. Nature Mater. 2007;6:656–660. doi: 10.1038/nmat1965. [DOI] [PubMed] [Google Scholar]
- 14.a) Vriezema DM, Aragones MC, Elemans JAAW, Cornelissen JJLM, Rowan AE, Nolte RJM. Chem. Rev. 2005;105:1445–1490. doi: 10.1021/cr0300688. [DOI] [PubMed] [Google Scholar]; b) Vriezema DM, Garcia PML, Sancho Oltra N, Hatzakis NS, Kuiper SM, Nolte RJM, Rowan AE, van Hest JCM. Angew. Chem. 2007;119:7522–7526. doi: 10.1002/anie.200701125. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Rehm S, Safont-Sempere MM, Wurthner F. Nature Chem. 2009;1:623–629. doi: 10.1038/nchem.368. [DOI] [PubMed] [Google Scholar]
- 16.Moghimi SM, Hunter AC, Murray JC. Pharmacol. Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 17.a) Brigger I, Dubernet C, Couvreur P. Adv. Drug Deliv. Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]; b) MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Nature Mater. 2009;8:993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]; d) Holowka EP, Sun VZ, Kamei DT, Deming TJ. Nature Mater. 2007;6:52–57. doi: 10.1038/nmat1794. [DOI] [PubMed] [Google Scholar]
- 18.a) Samanta B, Patra D, Subramani C, Ofir Y, Yesilbag G, Sanyal A, Rotello VM. Small. 2009;5:685–688. doi: 10.1002/smll.200801659. [DOI] [PubMed] [Google Scholar]; b) Skaff H, Lin Y, Tangirala R, Breitenkamp K, Böker A, Russell TP, Emrick T. Adv. Mater. 2005;17:2082–2086. [Google Scholar]
- 19.Pieranski P. Phys. Rev. Lett. 1980;45:569–572. [Google Scholar]
- 20.Lin Y, Skaff H, Emrick T, Dinsmore AD, Russell TP. Science. 2003;299:226–229. doi: 10.1126/science.1078616. [DOI] [PubMed] [Google Scholar]
- 21.a) Myers D. Surfaces, Interfaces, and Colloids - Principles and Applications. Ed. 2. New York: Wiley-VCH; 1999. [Google Scholar]; b) Mason TG, Wilking JN, Meleson K, Chang CB, Graves SM. J. Phys.: Condens. Matter. 2006;18:R635–R666. [Google Scholar]
- 22.a) Wright AT, Griffin MJ, Zhong ZL, McCleskey SC, Anslyn EV, McDevitt JT. Angew. Chem. 2005;117:6533–6536. doi: 10.1002/anie.200501137. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:6375–6378. doi: 10.1002/anie.200501137. [DOI] [PubMed] [Google Scholar]; b) Buryak A, Severin K. J. Am. Chem. Soc. 2005;127:3700–3701. doi: 10.1021/ja042363v. [DOI] [PubMed] [Google Scholar]; c) Metanis N, Keinan E, Dawson PEA. J. Am. Chem. Soc. 2005;127:5862–5868. doi: 10.1021/ja050110b. [DOI] [PubMed] [Google Scholar]
- 23.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 24.Samanta B, Yang XC, Ofir Y, Park MH, Patra D, Agasti SS, Miranda OR, Mo ZH, Rotello VM. Angew. Chem. 2009;121:5445–5448. doi: 10.1002/anie.200901590. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2009;48:5341–5344. doi: 10.1002/anie.200901590. [DOI] [PubMed] [Google Scholar]
- 25.a) Rowinsky EK, Donehower RC. N. Engl. J. Med. 1995;332:1004–1014. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Dai WM, Guy RK. Angew. Chem. 1994;106:38–69. [Google Scholar]; Angew. Chem. Int. Ed. 1994;33:15–44. [Google Scholar]