Abstract
INTRODUCTION
Clostridium difficile has been an increasing problem in UK hospitals. At the time of this study, there was a high incidence of C. difficile within our trust and a number of patients developed acute fulminant colitis requiring subtotal colectomy. We review a series of colectomies for C. difficile, examining the associated morbidity and mortality and the factors that predispose to acute fulminant colitis.
PATIENTS AND METHODS
This is a retrospective study of patients undergoing subtotal colectomy for C. difficile colitis in an NHS trust over 18 months. Case notes were reviewed for antibiotic use, duration of diarrhoea, treatment, blood results, pre-operative imaging and surgical morbidity and mortality.
RESULTS
A total of 1398 patients tested positive for C. difficile in this period. Of these, 18 (1.29%) underwent colectomy. All were emergency admissions, 35% medical, 35% surgical, 24% neurosurgical and 6% orthopaedic. In the cohort, 29% were aged less than 65 years. Patients had a median of three antibiotics (range, 1–6), for a median of 10 days (range, 0–59 days). Median length of stay prior to C. difficile diagnosis was 13 days. Subtotal colectomy was performed a median of 4 days (range, 0–23 days) after diagnosis. Postoperative mortality was 53% (9 of 17). The median C-reactive protein level for those who died was 302 mg/l, in contrast to 214 mg/l in the survival group. Whilst 62% of all C. difficile cases were medical, the colectomy rate was only 0.7%. In the surgical specialties, the colectomy rates were 3.2% for general surgical, 1.2% for orthopaedic and 8% for neurosurgical patients.
CONCLUSIONS
Colectomy for C. difficile colitis has a high mortality but can be life-saving, even in extremely sick patients. Although heavy antibiotic use is a predisposing factor, this is not an obligatory prerequisite in the development of C. difficile. Neither is it a disease of the elderly, making it difficult to predict vulnerable patients. There are large differences in colectomy rates between specialties and we suggest there may be a place for a surgical opinion in all cases of severe C. difficile colitis.
Keywords: Clostridium difficile, Colitis, Colectomy, Pseudomembranous colitis
Clostridium difficile is the most common cause of hospital-acquired diarrhoea and has been a serious problem in the UK. Infection produces a spectrum of disease, from mild abdominal pain and diarrhoea, to fulminant pseudomembranous colitis. This nosocomial infection associated with antibiotic use, causes more deaths per year than methicillin-resistant Staphylococcus aureus (MRSA). In 2007, there were 49,785 reported cases of C. difficile infection in England.1 The UK Government is now investing £230 million per year in order to cut C. difficile related deaths.2
In 2007, C. difficile infection was documented on 8324 death certificates in England and Wales. This was an increase of 28%, from 6480, in the previous year. It was recorded as the underlying cause of death in 4056 of these cases and mentioned on a further 4268 certificates.3 Our own trust saw a rise in C. difficile related deaths from 13 in 2001 to 158 in 2007.3 This was partly due to an increase in reporting, as C. difficile deaths were not accurately reported until 2007. Nationally, between 2004 and 2007, there was a rising incidence of the 027-ribotype strain of C. difficile and in our trust, a growing number of patients developed fulminant colitis. With an increasing number of these patients requiring subtotal colectomy for pseudomembranous colitis, we reviewed a series of these cases.
Patients and Methods
This study was a retrospective analysis of patients who underwent a subtotal colectomy for fulminant C. difficile colitis in a large hospital trust, over a period of 18 months, (April 2006 to September 2007). The trust encompasses two large acute hospitals, providing a total of about 1250 beds and serving a population of approximately half a million people.
The number of patients with a C. difficile toxin (CDT)-positive stool samples during the 18-month period was established using the microbiology computer database (cytotoxin assay A and B). All subtotal colectomies carried out in this time-frame were identified from theatre log books. Patient Administration System (PAS) computer records were used to ascertain the indication for each of the 298 subtotal colectomies. The case notes of all patients whose surgery was performed for C. difficile or pseudomembranous colitis were then reviewed. Data were collected on antibiotic use, duration of diarrhoea and the diagnosis and management of C. difficile. Blood results were reviewed at admission, diagnosis of C. difficile diarrhoea and at time of surgery. Information on pre-operative imaging and surgical morbidity and mortality was recorded.
Results
A total of 1398 patients tested positive for C. difficile from 1 April 2006 to 30 September 2007. This included both inpatient and community-acquired cases. Of these, 62% were medical patients, 15% renal and ITU, 13% surgical, 6% orthopaedic and 4% neurosurgical and ENT. Of the study cohort, 18 patients (1.29%) underwent subtotal colectomy. Seventeen cases have been reviewed; one patient was excluded due to missing case notes.
Overall, 29% (5) of patients were under 65 years of age and the mean age was 71 years (range, 51–88 years). Thirteen patients (76%) were male and all were emergency admissions. Six (35%) were medical patients, three of whom were admitted with C. difficile diarrhoea; six (35%) were surgical, four (24%) neurosurgical and one (6%) orthopaedic. There was a considerable difference between the colectomy rates of various specialities. Whilst 62% of all C. difficile diarrhoea cases were in medical patients, they accounted for only 35% of the colectomies, with a colectomy rate of only 0.7%. Comparatively, in the surgical specialties, the colectomy rates were 3.2% for general surgical, 1.2% for orthopaedic and 8% for neurosurgical patients. The median length of stay prior to diagnosis of C. difficile was 13.4 days (range, 0–49 days).
There was great variability in antibiotic usage prior to the development of C. difficile. Patients had a median of three antibiotics (range, 1–6), for a median of 10 days (range, 0–59 days; Table 1). Of patients, 71% (12) had been treated with a cephalosporin as part of their antibiotic regimen. Other commonly used antibiotics were quinolones (Moxifloxacin and/or Levofloxacin, n = 4; Ciprofloxacin, n = 3), Co-Amoxiclav (n = 4), and Clarithromycin and Gentamicin, each used in three patients.
Table 1.
Number of different antibiotics received and total number of days of antibiotics, prior to diagnosis of C. difficile
| Patient | Different antibiotics (n) | Duration of antibiotics (days |
|---|---|---|
| A | 3 | 14 |
| B | 3 | 6 |
| C | 3 | 47 |
| D | 1 | 4 |
| E | 3 | 4 |
| F | 2 | 7 |
| G | 2 | 13 |
| H | 0 | 0 |
| I | 1 | 10 |
| J | 2 | 6 |
| K | 2 | 7 |
| L | 3 | 10 |
| M | 4 | 59 |
| N | 4 | 25 |
| O | 3 | 11 |
| P | 3 | 20 |
| Q | 6 | 16 |
| Median | 3 | 10 |
All patients were treated for C. difficile with Metronid-azole, and 24% had oral Vancomycin in addition. Metronidazole was started promptly. It was commenced a median of 2 days after the onset of diarrhoea (range, 0–14 days) and a median of 0 days after a positive stool culture (range 4 days before culture to 7 days after). Ribotyping results were only available for seven patients: four patients were 027 positive, two had 015 and one patient type 106.
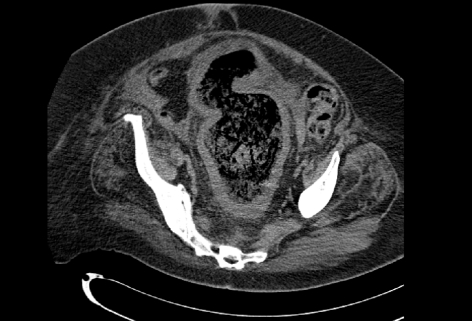
Of the patients, 35% (6) had pre-operative abdominal computerised tomography (CT) showing evidence of colitis. The remainder had either an abdominal X-ray or water-soluble contrast enema, supporting a diagnosis of colitis. CT demonstrated oedematous, thick-walled, dilated bowel with infiltrated pericolic fat (Fig. 1). Other findings included free intra-abdominal fluid (2 of 6) and distended transverse colon measuring 6–8 cm (3 of 6). None showed free gas. Of the eleven patients who did not have a CT, all had abdominal radiographs. Five were reported as significantly dilated bowel consistent with colitis; two consistent with obstruction; two a degree of bowel dilatation and one suggestive of ileus. The two patients with a degree of dilated bowel went on to have a gastrograffin enema. Of these, only one showed oedematous mucosa and colitis, the other confirmed the dilated colon.
Figure 1.
CT scan showing gross distension of rectum and sigmoid, with thick walls and infiltration of perirectal and pericolic fat.
Subtotal colectomy was performed a median of 4 days after diagnosis of C. difficile by stool culture (range, 0–23 days) and all patients had a positive CDT at the time of surgery. The decision to operate was based on the general deterioration of the patient rather than a change in abdominal signs or symptoms; not all patients were peritonitic. Sixteen patients underwent subtotal colectomy and end ileostomy, as recommended in the literature.4 One of these sixteen procedures was performed laparoscopically. One patient had a subtotal colectomy and ileorectal anastomosis, having had a sigmoid colectomy for volvulus 2 months previously.
Postoperative mortality was 53% (9 patients). Death occurred a median of 6 days after surgery (range, 0–22 days). Two patients died on the day of surgery, one of whom suffered a cardiac arrest intra-operatively and was unable to be resuscitated. The mean age of those who died was 75 years (median, 75 years; range, 63–88 years), compared to 67 years (median, 69 years; range, 51–78 years) in those who survived (P = 0.127). Of the seventeen patients reviewed, eight had undergone an operation during this admission, prior to the colectomy. Postoperative mortality differed between this group, at 38% (3 of 8) and the 9 patients in whom the only surgery performed was the colectomy, at 67% (6 of 9).
The median C-reactive protein (CRP) immediately prior to surgery in those who died was 302 mg/l, (mean, 281 mg/l), in contrast to 214 mg/l (mean, 206 mg/l) in the survival group (P = 0.216). Mean albumin levels were similar in both groups of patients at 16.5 g/l in the deceased group and 17.8 g/l in patients who survived, (P = 0.815).
Two patients (12%) required further surgery. One had a repeat laparotomy, washout and laparostomy and one underwent resuturing of a burst abdomen. Excluding four patients who died in the first five postoperative days, wound dehiscence occurred in 38% (5 of 13). Of the five patients whose wounds dehisced, one required return to theatre for resuturing, one healed with a Vac dressing and one was managed with a stoma bag over the open wound but died 2 weeks postoperatively. The other two were superficial dehiscence, requiring non-operative management.
As one would expect, there was significant postoperative morbidity. Five patients developed acute renal failure, two of whom required haemofiltration. Postoperative infections were common, with four patients suffering lower respiratory tract infections, another two developing pseudomonas urinary tract infections and one contracting MRSA in his abdominal wound. Cardiovascular complications also occurred: one myocardial infarction, two pulmonary emboli, two patients developed pulmonary oedema and three had episodes of atrial fibrillation. Two patients also had on-going gastrointestinal problems, due to colitis in their rectal stump. Postoperatively, patients were managed on ITU or HDU, with many requiring inotropic support and nasogastric feeding. Median hospital stay was 37 days (range, 8–141 days). To date, none of the 16 patients with ileostomies have had restoration of bowel continuity.
Discussion
During the 18 months of the study, 1398 patients in our hospital trust developed C. difficile infection. Throughout the same period there were 80,200 cases nationally, in those over 65 years of age. It is indisputable that C. difficile is a significant clinical issue. Fulminant C. difficile colitis requiring surgery occurred in 1.3% of patients, a figure comparable to other case series.5 However, this figure does not account for all patients who were clinically suffering from colitis. We acknowledge that we are unable to deduce the number of patients for whom no surgical opinion was sought, or who were deemed unsuitable candidates for a colectomy, despite progressing to pseudomembranous colitis, or, indeed, who developed colitis but recovered without surgery. We have presented our experience of colectomy as a potential solution for C. difficile colitis, reviewing common factors between this series of patients and reporting their outcomes.
Fulminant C. difficile colitis is not merely a disease of the elderly; a third of patients in our case series were under 65 years of age. Supporting this, a study of 59 intensive care unit patients with C. difficile infection reported an average age of 66.4 years.6 While this does demonstrate that middle-aged patients are at significant risk of C. difficile colitis, the high proportion of under 65-year-olds in our study may reflect their being optimal operative candidates compared to those patients with fulminant colitis that were unable to undergo surgical intervention and, hence, are not accounted for in these figures. The male:female ratio was 3.25:1. It is possible that men are more predisposed to developing fulminant disease, or perhaps that they receive more antibiotic courses. However, it is more probable that they are deemed good candidates for surgery, due to fewer co-morbidities and a better baseline function than their female counterparts. Although there are various characteristics predisposing to C. difficile infection, it can be difficult to identify patients at particular risk. Generally, patients were not responding well to oral therapy and developed systemic toxicity, becoming acidotic, tachycardic and hypotensive. However, very few were peritonitic and, in some, the diarrhoea had resolved. Relapse of C. difficile infection was extremely common, with 24% (4) having suffered from C. difficile previously.
Surgery was performed a median of 4 days after initiation of medical management. This was a reasonable length of time to give patients to respond to oral therapy and is comparable to that found by Greenstein et al.7 at 4.6 days. Eight patients developed C. difficile infection requiring colectomy, while recovering from surgery they had undergone in the same admission. Five patients had received previous abdominal surgery, two neurosurgical operations and one a vascular procedure. The death rate for this group was lower than that of the group who had not had surgery prior to the colectomy, at 38% versus 69%. This may not be significant due to small numbers; however, it is perhaps explained by surgical patients having superior baseline fitness or by prompter surgical intervention in those already under the care of surgical teams. Similarly, there are large differences in colectomy rates between each speciality that warrants further investigation. While patients developing C. difficile under the care of medical teams had a 0.7% chance of undergoing a colectomy, 3.2% of surgical patients proceeded to operative management; a trend mirrored by other studies.8 This suggests that surgery may precipitate a more severe form of the infection or, alternatively, that operative management is more frequently considered within surgical specialties. Medical teams are more familiar with managing ulcerative colitis, where referral for colectomy would be based on serial X-rays and new abdominal signs, particularly peritonism. Many of the patients in this series were not peritonitic, but extremely systemically unwell, with stool-culture proven C. difficile and were not improving on medical therapy. There may be a case for a surgical opinion in all severe cases of C. difficile colitis.
Antibiotic therapy plays a major role in the development of C. difficile infection. At the time of this study, our hospital protocol included many of the predisposing antibiotics, in particular cephalosporins. Equally as culpable were the roles of polypharmacy, with a median of three different antibiotics and the prolonged course of antibiotic therapy that many patients received (median, 10 days). Our findings are supported by those of other studies. Koss et al.4 reported a median antibiotic use of 10 days and found that 57% (8 of 14) of patients had a cephalosporin prior to developing C. difficile. In an attempt to reduce the increasing numbers of C. difficile infections, several measures have been taken by our trust over the last year. One strategy was the rewriting of protocols, such that cephalosporins and quinolones are avoided wherever possible. They have been substituted by Benzylpenicillin, Gentamicin and Metronidazole in surgical patients with intra-abdominal sepsis; Co-Trimoxazole in place of Co-Amoxiclav for lower respiratory tract infections and Trimethoprim in preference to Ciprofloxacin for urinary tract infections. There are, however, still some exceptions where a cephalosporin remains invaluable, such as in the management of bacterial meningitis. A campaign to reduce inappropriate durations of antibiotics saw posters dispersed across all wards promoting the ‘Antibiotic Stop Date Policy’. Pharmacists now insist that a stop date is stipulated on every antibiotic prescription and that courses are regularly reviewed. Cohort wards were opened in both hospitals within the trust in September 2007. Patients were transferred there on diagnosis of positive CDT. Another initiative undertaken by the trust in the fight to overcome this nosocomial infection was the introduction of Actimel®. The probiotic drink containing Lactobacillus casei imunitass® was used for 9 months as prophylaxis against C. difficile in patients receiving antibiotics during their hospital stay. It was commenced with the onset of antibiotic therapy and continued for 2 weeks after the course was finished. The evidence supporting the use of Actimel® was set out by Hickson et al.,9 who carried out a randomised, double-blind, placebo-controlled trial of Lactobacillus in patients taking antibiotics. They found that C. difficile infection was developed by 17% (9 of 53) of patients in the placebo group, but no patients (0 of 56) in the probiotic group. Collectively, these measures have been successful in dramatically reducing the rates of C. difficile in our trust to 365 cases (inpatient and community acquired), from April 2008 to April 2009. The individual step that had the most significant impact on rates was alteration of the antibiotic policy; therefore, Actimel® has now been withdrawn.
Although these measures are paramount, as heavy antibiotic usage does predispose to C. difficile infection, fulminant colitis requiring surgery can still occur with minimal antibiotic treatment. One patient received merely one dose of Cephalosporin at induction, yet developed C. difficile. Another patient, having received no antibiotics whatsoever, contracted C. difficile during a particularly virulent outbreak on a ward. Likewise, Longo et al.10 found that 3% (2 of 67) of patients had no history of antibiotic use. Thus, having antibiotics is not an obligatory prerequisite for the development of C. difficile.
Most cases of C. difficile diarrhoea resolve within a few days with solely medical management, namely oral Metronidazole or Vancomycin.11 For those who do not respond accordingly, one should be suspicious that they may progress to fulminant colitis. During every month of this case series, in our hospital trust alone, one patient underwent a colectomy for C. difficile colitis. Those unfortunate enough to develop fulminant colitis requiring surgery face the prospect of significant postoperative morbidity and over a 50% chance of death.
Mortality is extremely high following colectomy for C. difficile colitis; 53% in our case series. In some studies, 30-day postoperative mortality is akin to ours, such as Dallal et al.8 at 57%. However, other rates vary immensely; Bryn et al.5 found postoperative in-hospital mortality after colectomy for C. difficile colitis to be 34%, while Klipfel et al.12reported a higher rate of 80%.
Fulminant colitis can result in paralytic ileus, toxic mega-colon, colonic perforation and death. Caution is required; patients may not be peritonitic. Failure to recognise symptoms early and promptly refer to surgeons, delays management and may contribute to poor prognosis. Despite the considerable mortality rate, colectomy for C. difficile colitis can be a life-saving procedure, as demonstrated by Lamontagne et al.13 Their study of ICU patients with C. difficile-associated disease, which adjusted for confounding factors, found that those who underwent a colectomy as opposed to solely medical management were less likely to die.
Most of the patients included in our study were profoundly unwell, extremely acidotic, septic and in renal failure. Yet half still survived. One cannot wholly predict the fate of these patients had they not been operated on, but each was reviewed by a consultant colorectal surgeon who felt that, without surgery, death was inevitable; in our opinion, colectomy was their last resort. Several moribund patients improved significantly and fairly rapidly, after removal of their colon. We would suggest that even if a patient has more than one organ failing, if their morbidity is only secondary to C. difficile colitis, they should undergo a colectomy. Patients can be turned down for surgery on the basis of pre-existing co-morbidities and poor baseline function, but not merely for severe sepsis attributable to C. difficile colitis.
References
- 1.Health Protection Agency. Quarterly Reporting Results for Clostridium difficile Infections and MRSA Bacteraemia. London: HPA; 2008. < http://www.hpa.org.uk/web/HPAweb&Page&HPAwebAutoListName/Page/1191942126541>. [Google Scholar]
- 2.Duerden B. C. Diff deaths ‘continue to rise’. BBC News, 28 August 2008 < http://news.bbc.co.uk/1/hi/health/7585668.stm>. [Google Scholar]
- 3.Office for National Statistics. Deaths Involving Clostridium difficile by Communal Establishment in England and Wales, 2001–2007. < http://www.statistics.gov.uk/downloads/theme_health/C_Diff_establishments.xls>. [Google Scholar]
- 4.Koss K, Clark MA, Sanders DSA, Morton D, Keighley MRB, Goh J. The outcome of surgery in fulminant Clostridium difficile colitis. Colorectal Dis. 2006;8:149–54. doi: 10.1111/j.1463-1318.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 5.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008;143:150–4. doi: 10.1001/archsurg.2007.46. [DOI] [PubMed] [Google Scholar]
- 6.Grundfest-Broniatowski S, Quader M, Alexander F, Walsh RM, Lavery I, Milsom J. Clostridium difficile colitis in the critically ill. Dis Colon Rectum. 1996;39:619–23. doi: 10.1007/BF02056938. [DOI] [PubMed] [Google Scholar]
- 7.Greenstein AJ, Bryn JC, Zhang LP, Swedish KA, Jahn AE, Divino CM. Risk fac tors for the development of fulminant Clostridium difficile colitis. Surgery. 2008;143:623–9. doi: 10.1016/j.surg.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Dalall RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–72. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibi otics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longo WE, Mazuski JE, Virgo KS, Lee P, Bahadursingh AN, Johnson FE. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum. 2004;47:1620–6. doi: 10.1007/s10350-004-0672-2. [DOI] [PubMed] [Google Scholar]
- 11.Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am J Gastroenterol. 1997;92:739–50. [PubMed] [Google Scholar]
- 12.Kilpfel AA, Schein M, Fahoum B, Wise L. Acute abdomen and Clostridium difficile colitis: still a lethal combination. Dig Surg. 2000;17:160–3. doi: 10.1159/000018821. [DOI] [PubMed] [Google Scholar]
- 13.Lamontagne F, Labbe AC, Haeck O, Lesur O, Lalancette M, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245:267–72. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]