Tissue surface tension (TST) is an important mechanical property influencing cell sorting and tissue envelopment. The study by Manning et al. (1) reported on a mathematical model describing TST on the basis of the balance between adhesive and tensile properties of the constituent cells. The model predicts that, in high-adhesion cell aggregates, surface cells will be stretched to maintain the same area of cell–cell contact as interior bulk cells, resulting in an elongated and flattened cell shape. The authors (1) observed flat and elongated cells at the surface of high-adhesion zebrafish germ-layer explants, which they argue are undifferentiated stretched germ-layer progenitor cells, and they use this observation as a validation of their model.
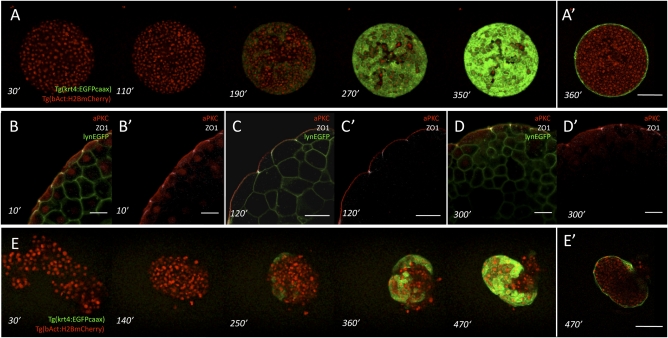
We disagree with the view that the surface cells of germ-layer explants described in ref. 1 are undifferentiated stretched germ-layer progenitor cells and argue that they are instead differentiated simple squamous epithelial enveloping-layer (EVL) cells. Our argument is based on the following observations: (i) we have repeated the preparation of explants as described in ref. 1 and observed EVL cell differentiation on the surface of these explants using Tg(keratin4:EGFPcaax) transgenic embryos expressing EGFP specifically in EVL cells (Fig. 1A); (ii) we have determined apical-basolateral polarization of surface cells from ectoderm explants and found that they are polarized as expected for EVL cells (2) (Fig. 1B); and (iii) we have prepared ectoderm germ-layer progenitor aggregates from dissociated Tg(keratin4:EGFPcaax) transgenic embryos and observed EVL differentiation at the surface, indicating that EVL differentiation occurs de novo (Fig. 1C).
Fig. 1.
Surface cell differentiation in zebrafish germ-layer explants and aggregates. (A and A′) Time course of surface cell differentiation in ectoderm explants from Tg(krt4:EGFPcaax)/Tg(βactin:H2AmCherry) transgenic embryos expressing EGFP (green) in differentiating enveloping cell-layer (EVL) cells and mCherry (red) in all nuclei. Z projections of a representative explant at consecutive time points (A) and a single confocal section (A′) through the explant shown at the last time point. (Scale bar: 100 μm.) Time labels indicate minutes after preparation of the explants from high-to-oblong–stage embryos [3 and 5 h postfertilization (hpf)]. (B–D) Immunostaining of ectoderm explants using antibodies against atypical protein kinase C (aPKC) (apical membrane, red) and Zona Occludens 1 (ZO-1) (tight junctions, white). The plasma membrane is labeled with lynEGFP (green). (Scale bars: 25 μm.) (E and E’) Time course of surface cell differentiation in ectoderm aggregates from Tg(krt4:EGFPcaax)/Tg(βactin:H2AmCherry) transgenic embryos expressing EGFP (green) in differentiating EVL cells and mCherry (red) in nuclei. Z projections of a representative aggregate at consecutive time points (B) and confocal section through the aggregate shown at the last time point (B’). (Scale bar: 100 μm.) Time labels indicate minutes after dissociation of high-to-oblong–stage embryos (3 and 5 hpf).
In line with previous studies (3, 4), we were also unable to confirm the observations reported in ref. 1 regarding the lack of EVL marker gene expression in germ-layer explants and cell exchanges between surface/EVL and bulk cells, which were used to support the claim that surface cells are undifferentiated.
We would like to stress that this is not solely a question of naming cells correctly, but that the reported model (1) is based on the assumption that surface cells have essentially the same properties as bulk cells and can change position with bulk cells, something we were unable to experimentally confirm. One might argue that, because surface cells behave as predicted by the model, they must have the same properties as bulk cells, irrespective of whether they are EVL cells or not. However, because polarized EVL cells are clearly different from bulk cells with respect to the composition and organization of their cytoskeleton and adhesion apparatus (2), the model as such has very little predictive value for zebrafish explants and thus, should not be used to predict the properties of surface cells. Finally, we would like to point out that our criticism is not directed at the model as such, which might be suitable to describe the behavior of aggregates other than zebrafish explants, but instead, it pertains to the use of untenable experimental data to support an otherwise interesting model.
Footnotes
The authors declare no conflict of interest.
References
- 1.Manning ML, Foty RA, Steinberg MS, Schoetz EM. Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc Natl Acad Sci USA. 2010;107:12517–12522. doi: 10.1073/pnas.1003743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zalik SE, Lewandowski E, Kam Z, Geiger B. Cell adhesion and the actin cytoskeleton of the enveloping layer in the zebrafish embryo during epiboly. Biochem Cell Biol. 1999;77:527–542. [PubMed] [Google Scholar]
- 3.Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- 4.Sagerström CG, Gammill LS, Veale R, Sive H. Specification of the enveloping layer and lack of autoneuralization in zebrafish embryonic explants. Dev Dyn. 2005;232:85–97. doi: 10.1002/dvdy.20198. [DOI] [PubMed] [Google Scholar]