Abstract
T-cell polarization is required for cell migration and cell–cell interactions, cellular behaviors crucial for lymphocyte differentiation. Despite expression of the epithelial polarity network in T cells, neither its contribution to thymocyte polarity nor its requirement during development is known. We report here that depletion of the polarity protein Scribble in hematopoietic progenitor cells results in inefficient T-cell development characterized by a partial developmental block during the early double-negative (DN) stage of differentiation. Scribble-depleted hematopoietic progenitor cells exhibit a delayed transition into late CD44lo/−CD25+ DN3 cells, evidenced by the accumulation of early CD44intCD25+ DN3 cells. As a consequence, a limited cellular expansion and a reduced frequency of intracellular T-cell receptor β–positive DN3 cells are observed among Scribble-deficient differentiating T cells. Moreover, whereas purified Scribble-depleted DN2 and DN3 cells do not exhibit compromised spontaneous motility, T-cell clustering and prolonged homotypic interactions among such cells are reduced. This deficiency correlates with a lack of polarization of the integrin LFA-1 during T-cell migration or on the initiation of T-cell–T-cell interactions. Scribble is therefore a critical contributor to the clustering of immature T cells, an event shown here to be necessary for efficient developmental progression.
A cell's function is typically dependent on its capacity to establish a polarized cellular architecture. Cell polarity is maintained by a network of mutually antagonistic protein complexes, including the Crumbs complex (Crumbs/Pals-associated tight junction protein /protein associated with Lin7), the partition defective complex (Par3/Par6/atypical protein kinase C), and the Scribble complex [Scrib/lethal giant larvae/discs large (Dlg)]. In epithelial cells, depletion of components of the polarity network affects both apico-basal and planar polarity, and also hinder such cellular behaviors as cell migration, cell–cell communication, cell proliferation, and cell differentiation (1).
Although distinct from epithelial cells, lymphoid cells also display extensive polarity. In particular, developing T cells in the thymus exhibit a polarized morphology critical for cell migration and cell–cell interactions (2, 3). In response to chemokine gradients, thymocytes follow a well-defined trajectory through the thymus, adopting a polarized organization including a leading edge and a cell body followed by a uropod (4–6). Such migration through the thymus is required for efficient developmental progression (3). Likewise, T-cell differentiation is dependent on cell–cell interactions characterized by the asymmetrical distribution of surface antigens, which are required for thymocytes to obtain required developmental cues. For example, Notch ligand expression on the thymic epithelium induces Notch signaling events critical for T-lineage commitment and development (7), and E-cadherin homotypic interactions between developing thymocytes are required for the early stages of T-cell development (8). At subsequent stages of development, engagement of the T-cell receptor (TCR) by MHC molecules expressed on the thymic epithelium drives the positive selection of mature T cells (9, 10).
A role for the polarity network in the polarization of thymocytes has not been reported previously. Instead, previous work has focused on the network's role in the regulation of mature T-cell polarization (12). In particular, the polarity protein Scribble has been shown to be required for mature T-cell migration and activation. Scribble is a widely expressed scaffolding protein containing 16 leucine-rich repeats as well as four PDZ domains (13). In mammalian cells, Scribble is localized to the cell–cell junctions of epithelial cells, the leading edges of migrating epithelia and astrocytes, and the presynaptic and postsynaptic compartments of neurons (14–16).
We have addressed the previously unexplored question as to whether the epithelial network is required during T-cell development, by following the differentiation of T cells in the absence of Scribble. We report that Scribble-depleted HPCs demonstrate inefficient T-cell development, resulting in a partial block at the early double-negative (DN) 3 stage of development. Although Scribble-depleted progenitors remain spontaneously motile, they do not engage in T-cell clustering and fail to polarize the integrin lymphocyte function-associated antigen 1 (LFA-1) at sites of T-cell–T-cell interaction. Thus, Scribble contributes to the maintenance of T-cell–T-cell interactions that enable the efficient delivery and reception of cues required for development. As such, a primary component of the epithelial polarity network does indeed contribute to T-lymphocyte differentiation.
Results
Depletion of Scribble Expression in Developing T Cells by RNA Interference.
The Scribble-deficient murine models Circletail and Line 90 are perinatally lethal, which hinders the analysis of lymphopoiesis (17, 18). Moreover, gastroschisis, characterized by externalization of the liver and gut through the open abdominal wall, limits the use of hematopoietic progenitors derived from the fetal livers (FLs) of these mice (17). Likewise, the potential disruption of hematopoieitc niches due to the loss of Scribble in alternate cell lineages calls into question the reliability of any analysis of fetal lymphocyte development. To circumvent these issues, we depleted Scribble gene expression in FL-derived hematopoietic progenitors by RNA interference (RNAi). Depleted progenitors were then differentiated on OP9-DL1 cells, a well-characterized in vitro model of T-cell development (19).
Scribble gene expression was attenuated by the retroviral delivery of three separate RNAi vectors (Scrib1, Scrib2, and Scrib3) encoding Scribble-targeting short hairpin RNA (shRNA). A fourth RNAi vector targeting luciferase was used as a negative control. Expression of GFP from an independent promoter within each vector allowed for the monitoring of transduction efficiency (Figs. S1A and S2A).
Generated viral supernatants were used to transduce enriched early HPCs (HSAlow) isolated from murine FLs on day 14–15 of gestation. At 24 h after transduction, CD117+Sca-1+GFP+ progenitors were FACS-sorted and then cocultured with OP9-DL1 cells. After 5 d and 10 d of culture, CD45+GFP+ cells were FACS-sorted and total cell lysates were generated. The Scrib3 vector maintained stable Scribble depletion and GFP expression, with Scribble silencing of >98% (Fig S1B). In contrast, whereas Scrib1 and Scrib2 vectors depleted Scribble expression (Fig. S2B), GFP expression driven by the Scrib1 and Scrib2 vectors was lost over time. Consequently, all data given herein were derived from the Scrib3 vector, although the same developmental phenotypes were observed in CD45+GFP+Scrib1 knockdown (KD) cells and Scrib2 KD cells isolated from OP9-DL1 cocultures (Fig. S2C).
Depletion of Scribble in FL-Derived Hematopoietic Progenitors Results in Inefficient T-Cell Development.
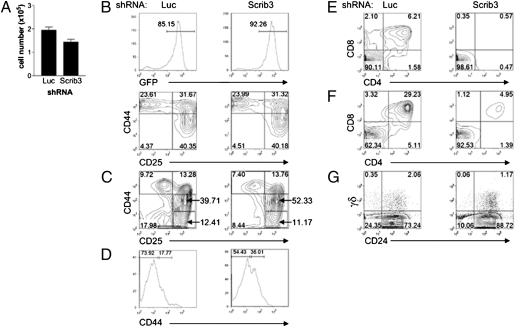
To characterize the developmental effects of Scribble depletion, cells were harvested, counted, and stained for the following surface markers demarking the DN and double-positive (DP) stages of T-cell differentiation: CD25, CD44, CD4, and CD8. On days 5 and 6 of coculture, the cellularity of Scrib3 KD cultures was reduced by an average of 30% (Fig. 1A). At this stage, developmental progression in Scrib3 KD cells was comparable to that in control Luc KD cells, with the majority of cells being CD44high (Fig. 1B). However, by day 13, an accumulation of CD44intCD25+ early DN3 cells was observed in Scrib3 KD cocultures. On average, 10–30% more CD44intCD25+ early DN3 were detected among Scrib3 KD cells compared with those detected among Luc KD cells (Fig. 1C). Such an accumulation suggests compromised DN3 differentiation.
Fig. 1.
Depletion of Scribble gene expression delays T-cell development. (A) Histogram of coculture cellularity on day 5 of culture. The mean ± SEM for culture conditions grown in triplicate are representative of at least three independent experiments. (B and C) The developmental progression of T cells was assessed on day 5 (B) and day 13 (C) of coculture. Representative contour plots are gated on GFP+CD45+ cells and are representative of five independent experiments. (D) After 5–7 d of OP9-DL1 coculture, DN2 (CD45+CD44highCD25+) cells were isolated by cell sorting and plated on fresh OP9-DL1 cells for 5 d. Representative histograms show surface expression levels of CD44 on GFP+CD45+CD25+ cells. Data are representative of four independent experiments. (E) On day 13 of coculture, harvested cells were analyzed for surface expression of CD4 and CD8. Representative contour plots of five independent experiments are shown. (F) After 6 d of culture, cells were harvested and reseeded in the presence of reduced IL-7 concentrations. The contour plots displaying CD4 and CD8 expression are representative of three independent experiments conducted on day 12. (G) Differentiation of γδ T cells was examined on day 11 of coculture. Representative contour plots are gated on GFP+ cells. Numbers in quadrants indicate the percentage of cells within each quadrant.
Given the reduced cellularity of Scrib3 KD cultures and the possible influence of cell density on differentiation, the DN2–DN3 transition was directly compared between equal numbers of Scrib3 and Luc KD DN2 cells. Toward this end, CD44highCD25+ Scrib3 KD and Luc KD DN2 cells were FACS-sorted, plated at equal cell densities on OP9-DL1 cells, and allowed to differentiate. Again, a higher proportion of Scrib3 KD cells were found to be CD44intCD25+ early DN3 cells, confirming a partial block in DN3 differentiation (Fig. 1D). In addition, compromised differentiation of Scrib3 KD cells beyond the CD44intCD25+ early DN3 stage resulted in delayed CD44loCD25− DN4 development and inefficient production of CD4+CD8+ DP cells. The percentage of DP Scrib3 KD cells was an average of 5.3-fold lower than that of control Luc KD DP cells in eight independent experiments (Fig. 1E).
To promote the outgrowth of DP cells, the IL-7 concentration within cultures was reduced (20). On day 6 of coculture, IL-7 concentrations were reduced by fivefold, after which cultures were maintained for 6 more days. Analysis of cultures on day 12 demonstrated that in low IL-7 concentrations, the differentiation of Scrib3 KD DP cells remained less efficient than that of control cells (Fig. 1F).
Scrib3 KD Progenitors Are Not Redirected to the γδ T-Cell Lineage.
In addition to supporting the differentiation of αβ T cells, OP9-DL1 cells also support differentiation of the γδ T-cell lineage (19). Given that the rearrangement of δ and γ loci is initiated at the DN2 stage of development (21), immediately preceding the observed developmental defect, we addressed the possibility that the depletion of Scribble resulted in a preferential commitment to the γδ lineage. Toward this end, after 12 d of culture, cells were stained for CD24 and γδTCR+ to follow the differentiation of γδ T cells. No apparent accumulation of γδ T cells was observed; rather, the percentage of γδ T cells was reduced on average by 1.5- to 2-fold, suggesting that γδ differentiation is affected by loss of Scribble as well (Fig. 1G).
Scribble Is Required for the DN3–DP Developmental Transition.
Although Scribble depletion was found to delay the differentiation of late CD44lo/−CD25+ DN3 cells, whether Scribble is also required for DN3–DP differentiation was unclear. Thus, CD44lo/−CD25+ Scrib3 and Luc KD DN3 cells were FACS-sorted and plated at equal cell densities on OP9-DL1 cells. After 6 d of culture, the cells were harvested, counted, and phenotyped for developmental progression.
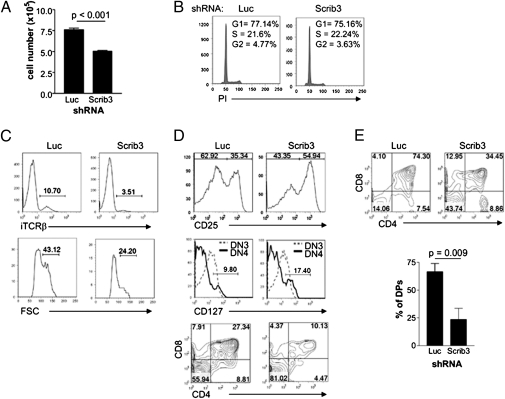
At 6 d postsorting, the cellularity of Scrib3 KD cultures was on average 65% that of control Luc KD cultures (Fig. 2A). However, cell cycle analysis of such cultures did not reveal any discernible difference in cycling rate or apoptosis (Fig. 2B). Thus, the expression of intracellular TCRβ (iTCRβ) and the size of DN3 cells were compared in Scrib3 and Luc KD DN3 cells. The frequency of DN3 cells expressing the iTCRβ chain was threefold lower in the Scrib3 KD DN3 cultures compared with control Luc KD DN3 cultures (Fig. 2C). Analysis of forward scatter showed a lower proportion of iTCRβ+ Scrib3 KD DN3 cells displaying an enlarged morphology typical of β selection-induced proliferation (Fig. 2C). As such, the reduction in cellularity that we noted is likely the result of fewer DN3 cells transitioning through β selection and undergoing proliferation before further differentiation.
Fig. 2.
Depletion of Scribble gene expression delays the DN3–DP transition. (A) FACS-sorted late DN3 (CD44lo/−CD25+) cells were cultured for 6 d. A representative histogram of culture cellularity is shown. Data represent the mean ± SEM of cultures grown in triplicate, repeated in at least three independent experiments. (B) FACS-sorted late DN3 cells cultured for an additional 3 d were analyzed for cellular DNA content by propidium iodide staining. (C) Histograms of iTCRβ in late DN3 cells, along with an analysis of forward scatter of the corresponding iTCRβ+ late DN3 cells. The histograms are gated on GFP+CD44lo/−CD25+ cells and are representative of at least five independent experiments. (D) Phenotyping of FACS-sorted late DN3 cells after 6 d of coculture. Expression of CD25 was examined on GFP+CD44lo/− cells, CD127 expression was examined on both DN3 and DN4 cells, and finally CD4+CD8+ coexpression among GFP+CD45+ cells was determined. Contour plots and histograms are representative of eight independent experiments. (E) Representative CD4/8 FACS profiles, gated on GFP+ cells, of individual 2-deoxyguanosine–treated thymic lobes reconstituted with Luc KD or Scrib3 KD FTs. A graphic representation of the percentage of DP cells among GFP+ cells in four or five individual thymic lobes within one experiment is shown. Data are representative of three independent experiments. All numbers within FACS profiles correspond to the percentage of cells within each gate, quadrant, or region.
Indeed, the reduced iTCRβ expression was correlated with a delay in further development. FACS-sorted late DN3 cells depleted of Scribble exhibited delayed down-regulation of CD25 and IL-7Rα (CD127) expression, indicating compromised differentiation beyond the DN3 stage of development (Fig. 2D). Likewise, the generation of DP cells was typically threefold less efficient in the absence of Scribble (Fig. 2D). This indicates that Scribble is not required solely for DN3 differentiation, but rather is continuously required for the efficient generation of DP cells.
Scribble Is Required for DP Generation in Fetal Thymic Organ Cultures.
The need for Scribble during DP differentiation was confirmed in fetal thymic organ cultures. Day-14.5 fetal thymocytes (FTs) were transduced, generating Luc KD and Scrib3 KD FTs, which were used to reconstitute 2-deoxyguanosine–treated fetal thymic lobes. After 12 d of standard fetal thymic organ culture, lobes were harvested, and the percentage of DP cells among GFP+ cells was determined by flow cytometry. Whereas Luc KD FTs readily differentiated to the DP stage, Scrib3 KD FTs displayed a marked reduction in DP differentiation. In three independent experiments, the average percentage of DP cells was 2.5- to 3-fold higher in Luc KD lobes compared with Scrib3 KD lobes (Fig. 2E).
Loss of Scribble in DN Cells Limits T-Cell Clustering.
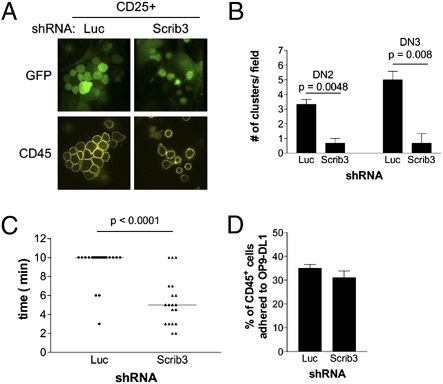
Developing T cells on OP9-DL1 cells are motile and engaged in cell–cell interactions, cellular behaviors that require cell polarization. We thus assessed the migratory and adhesive behaviors of Scrib3 KD cells on OP9-DL1 cells. On day 6 of OP9-DL1 coculture, live cocultures (>80% CD25+) were stained for CD45 expression and immediately imaged. Control Luc KD cells were localized in tightly packed T-cell clusters in which cells were spread onto underlying OP9-DL1 cells. In striking contrast, Scrib3 KD cultures exhibited infrequent, loosely packed clusters (Fig. 3A).
Fig. 3.
T-cell–T-cell clustering is limited in the absence of Scribble. (A) Cluster formation among FACS-purified GFP+CD25+ cells adhered to OP9-DL1 cells. (B) Quantification of clusters formed by FACS-sorted DN2 and DN3 cells. The number of clusters within the center field of view is presented. Data represent the mean ± SEM of cultures grown in triplicate, repeated in three independent experiments. (C) Motility of Luc KD and Scrib3 KD immature T cells. Data show the time period during which a cluster remained intact, with each solid circle (Luc KD) or triangle (Scrib3 KD) representing an imaged cluster. The solid bar represents the mean time of cluster maintenance. (D) Scrib3 and Luc KD cells, previously sorted as CD44highCD25+, were plated on OP9-DL1 cells and allowed to adhere for 16 h. Suspension and adherent cells were counted by flow cytometry after staining for CD45. The average percentage of adherent CD45+ cells from all experiments is shown.
Given the delayed differentiation of DN3 cells, the tendency of purified DN2 and DN3 cells to cluster in the absence of Scribble was compared with that in control cells. DN2 and DN3 cells were purified by FACS and plated in triplicate on subconfluent OP9-DL1 cells to allow quantitation of individual clusters on individual OP9-DL1 cells and avoid the merging of adjacent clusters. Clusters were allowed to form over a 16-h period. A cluster was considered a grouping of three or more T cells attached and spread on to the surface of an individual OP9-DL1 cell. Irrespective of cell density, Luc KD DN2 and DN3 cells formed on average sevenfold more T-cell clusters than Scrib3 KD DN2 or DN3 cells (Fig. 3B).
Time-lapse imaging was then used to evaluate the ability of Scrib3 KD cells to engage in T-cell–T-cell interactions to form clusters. As reported elsewhere, developing T cells on the OP9-DL1 cells were found to be highly motile, spontaneously migrating through the OP9-DL1 monolayer. In addition, extensive motility was observed within individual T-cell clusters. Clustered Luc KD cells displaced themselves within clusters and sampled the surface of adjacent cells (Movie S1). However, despite extensive motility within control clusters, cluster formation was maintained. In three independent experiments, during which more than 20 clusters were imaged over 10 min, >90% of control clusters remained intact (Fig. 3C). In contrast, Scrib3 KD cells polarized and migrated toward one another, but did not remain engaged in prolonged T-cell–T-cell interactions (Movie S2). Rather, Scrib3 KD cells quickly disengaged from one another and did not form clusters, with only 20% of Scrib3 KD clusters remaining intact for 10 min (Fig. 3C).
Given the limited interactions between Scribble-depleted T cells, the adhesion of Scribble-depleted T cells to the underlying OP9-DL1 cells was assessed. FACS-sorted DN2 and DN3 CD25+ cells were plated in triplicate on OP9-DL1 cells and allowed to adhere for 16 h. Suspension and adherent cells were then harvested and counted. Comparable averages of 31% of Scrib3 cells and 35% of Luc KD CD25+ cells were found to be adherent to OP9-DL1 cells at any given time (Fig. 3D), suggesting that the observed clustering defect is not due to a loss of T-cell–stroma interactions.
Presence of WT Differentiating T Cells Does Not Rescue the Scribble-Deficient Phenotype.
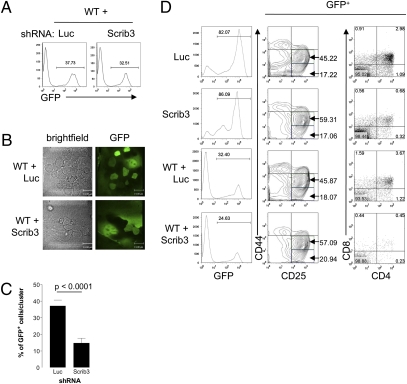
The lack of T-cell clustering in Scribble-deficient progenitors raised the question of whether the presence of WT Scribble-expressing cells could maintain Scribble-deficient cells in cluster formations and rescue efficient DP differentiation. To address this question, Scrib3 KD and Luc KD cKit+Sca-1+GFP+ cells were mixed with uninfected cKit+Sca-1+GFP− cells and allowed to differentiate. Cells were mixed at an approximate Scrib3 KD:WT or Luc KD:WT ratio of 1:3, ensuring an abundance of WT cells to promote clustering (Fig. 4A). The percentage of GFP+ cells within T-cell clusters spread on the underlying OP9-DL1 monolayer was determined between day 8 and day 10 of culture. Clusters of WT cells were first localized by bright-field imaging, after which the presence of GFP+ cells was visualized by fluorescence imaging. In Scrib3 KD + WT cultures, Scrib3 KD cells were detected among clusters of WT immature T cells (Fig. 4B); however, the average percentage of Scrib3 KD GFP+ cells in WT clusters was only a third of the percentage of the total GFP+ cells in the coculture (Fig. 4C), suggesting that Scribble-depleted cells are not retained within clusters of WT cells. In contrast, in Luc KD + WT cultures, the percentage of Luc KD GFP+ cells in WT clusters was comparable to the total percentage of GFP+ cells in the culture, indicating that Luc KD and WT cells engage in comparable clustering behavior (Fig. 4 B and C).
Fig. 4.
The presence of WT T cells does not rescue clustering or efficient T-cell development. (A) FACS-sorted Scrib3 KD and Luc KD GFP+c-Kit+Sca-1+ HPCs were cultured in the presence of threefold excess of uninfected FACS-sorted GFP−c-Kit+Sca-1+ HPCs. Representative histograms of 10,000 events are shown. (B) Bright-field images of mixed cocultures compared with images of GFP fluorescence. (C) The proportion of GFP+ cells within 40 analyzed clusters in Scrib3 KD + WT or Luc KD + WT cocultures between day 8 and day 10 of culture. (D) Cultures were harvested on day 12 of coculture and phenotyped for developmental progression. The contour plots of CD44 and CD25 expression and dot plots of CD4 and CD8 expression shown are representative of three independent experiments. All plots are gated on GFP+ cells, and numbers indicate the percentage of cells within indicated gates or quadrants.
The inability of WT cells to rescue the clustering defect of Scribble-depleted immature T cells was correlated with a continued partial developmental block in Scrib3 KD cells. On day 12 of culture, cells were harvested and analyzed by flow cytometry for developmental progression through the DN subsets, as well as differentiation of DPs. In the presence of WT cells, Scrib3 KD cells still exhibited a delay in CD44lo/−CD25+ DN3 and DP differentiation (Fig. 4D).
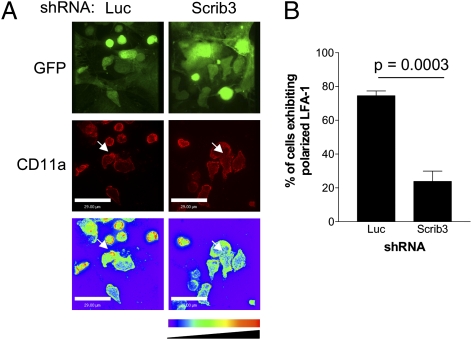
Depletion of Scribble Limits Polarization of LFA-1 During Cell Migration and at Sites of T-Cell–T-Cell Contact.
OP9-DL1 cells secrete stromal-derived factor 1α, a chemokine that stimulates the redistribution of LFA-1 to polarized patches (22). Such cell surface clustering contributes to increased LFA-1 avidity, thereby enhancing T-cell adhesion (23). Thus, the localization of endogenous LFA-1 was compared in control and Scribble-depleted cocultures to determine whether Scribble depletion affects the polarization of this integrin implicated in cell clustering.
Between day 6 and day 10 of coculture, cultures were fixed and stained for surface LFA-1. Individual migrating cells, as well as cells initiating T-cell–T-cell interactions, were scored for LFA-1 polarization. Confocal images of 1-μm optical sections for a total of 10 μm were captured from identified cells. An extended-focus algorithm was used on the confocal stack, after which a look-up table was applied. A cell was scored as having polarized LFA-1 when a twofold higher density of LFA-1 staining was detected at the leading edge. A minimum of 100 Luc and Scrib3 KD cells were scored from three independent experiments. In Luc KD cultures, on average 74.5% of cells exhibited clearly defined polarized LFA-1 at the leading edge and at sites of initial cell–cell contact. In contrast, LFA-1 was more diffuse on the surface of Scrib3 KD cells, with only 23.8% of cells exhibiting a polarized accumulation of LFA-1 (Fig. 5 A and B). This observed difference in polarized LFA-1 redistribution did not result from differential expression levels of LFA-1 or its ligand ICAM-1 on immature T cells, as evidenced by flow cytometry staining (Fig. S3A).
Fig. 5.
Scribble is required for LFA-1 polarization on immature T cells. (A) Localization of LFA-1 on immature T cells was visualized by fixation and surface staining of cocultures by staining for CD11a. Representative extended-focus images of polarized CD25+ immature T cells of either GFP or LFA-1 (Cy-3) are shown. A color look-up table was applied to all images of LFA-1 staining. White arrows identify sites of accumulated LFA-1. (B) The incidence of polarized LFA-1 among >100 cells from three independent experiments.
Chemokine stimulation also induces the conversion of LFA-1 between a low-affinity state and a high-affinity state. An antibody that preferentially binds the low-affinity conformation of LFA-1 (24) was used to detect the surface expression of low-affinity LFA-1 by flow cytometry. Comparable levels of expression were detected on Scrib3 KD and Luc KD CD44+CD25+ DN2 and CD44-/loCD25+ DN3 cells (Fig. S3B), indicating that Scribble does not appear to influence the conversion between LFA-1 affinity states.
Discussion
We have presented evidence suggesting that the polarity protein Scribble plays a crucial role in promoting the cell–cell interactions required for T-cell development. Strikingly, depletion of Scribble in immature T cells did not abolish cell migration, but rather disrupted polarized distribution of surface LFA-1, compromised T-cell clustering, and limited the efficiency of T-cell development.
The developmental block occurring at the DN T-cell stage in Scribble-deficient cells was correlated with a reduced incidence of T-cell–T-cell clustering in DN2 and DN3 cells. Whereas Scribble-deficient immature T cells migrated toward one another, they did not engage in prolonged interactions, as did control cells. Control cells were observed to form stable interactions leading to the establishment of T-cell clusters that remained intact over periods exceeding 10 min. Thus, it can be concluded that in developing T cells, the scaffold Scribble contributes to the establishment and maintenance of T-cell clusters.
Homotypic interactions between immature developing T cells are required to specify lineage commitment and differentiation; for example, T-cell–T-cell adhesion promotes SLAM family member interactions required to drive natural killer T-cell differentiation (25). Similarly, thymocyte–thymocyte interactions promote CD4 cell selection in the presence of MHC II–expressing thymocytes (26). The role of homotypic interactions during the early stages of αβ T-cell development has not been identified, however. Thus, although others have reported clustering of developing T cells on OP9-DL1 cells, our results provide evidence that this behavior is required for the efficient development of DP cells.
A parallel may be drawn with the large clusters of activated T cells that persist on dendritic cells. Homotypic interactions within such clusters enable direct polarized cytokine delivery to promote activation responses and are dependent on LFA-1 activity (27). Within the OP9-DL1 coculture system, LFA-1 is clustered at sites of contact between immature T cells. LFA-1 was not clustered at sites of contact between Scribble-depleted immature T cells, however, suggesting that Scribble is required to orchestrate molecular events directing LFA-1 localization to promote homotypic interactions between developing T cells.
Limited evidence supports a requirement for LFA-1 during the early stages of T-cell development. Thus, it must be considered that the early developmental defect that we have identified does not result solely from lost LFA-1 polarization. Rather, the lack of LFA-1 polarization is likely representative of a polarity defect among multiple molecules that cumulatively contribute to inefficient T-cell development. The identification of these molecules is clearly of importance.
Regardless, the role of Scribble in LFA-1 polarization is itself of interest. The Scrib complex includes Scribble, Dlg, and Lgl, which in epithelial cells act in concert to establish cell polarity. Dlg has been shown to form a dynamic complex with the LFA-1–binding membrane protein PTA-1 and the actin-binding protein 4.1G. This complex is proposed to provide the structural support to enable LFA-1 clustering (28). Thus, examining whether Scribble plays an active role in this pathway is of interest.
The initial characterization of Scribble in mature T cells found that Scribble depletion results in the loss of uropod formation and cell migration. These experiments were done in a T-cell hybridoma and described nondirected migration of cells in culture (12). Thus, although we did not observe similar defects in our system, the two models are not comparable; the OP9-DL1 coculture system that we used requires underlying cells to provide cues that modify cellular behavior. However, the lack of stable T-cell–T-cell interactions among differentiating T cells does parallel evidence that Scribble-depleted mature T cells are unable to form stable conjugates or immunologic synapses (12). Our findings provide insight into the molecular role of Scribble, linking Scribble depletion to limited LFA-1 polarization and adhesion.
The role of Scribble in cell polarity has been described as cell context–specific, with varied functions including the regulation of directed cell migration and stabilization of adherens junctions. Recently however, the Scrib complex has been reported to be recruited to the leading edge of migrating epithelial cells during dorsal closure of the Drosophila embryo, whereas it is relocalized to the lateral membrane to promote the restoration of apico-basal polarity on closure. Through differential localization and binding partners, Scribble apparently acts as a molecular switch, allowing epithelial cells to alternate between an apical-basal adhesive state and a migratory state during epithelial-to-mesenchyme transition (29). In mature T cells, Scribble is localized to the uropod in migrating cells and temporarily relocalizes to sites of contact on interaction with antigen-presenting cells (12). We propose that in T cells, Scribble acts as a similar molecular switch, allowing migrating T cells to transition to an adherent state and engage in homotypic interactions, enabling the acquisition of differentiation signals.
Materials and Methods
Fetal Cell Isolation.
FLs were harvested from CD-1 mice on day 14–15 of gestation. Single-cell suspensions were generated and filtered through 40-μm filters. Antibody and complement lysis was performed to enrich CD24−/lo FL cells.
Retroviral Gene Transfer and In Vitro Differentiation.
Vectors were packaged as recombinant retrovirus and pseudotyped with VSV-G protein to enhance infectivity. Either pSIREN-ZsGreen or pMSCV-miR30 was cotransfected with pCMV-VSV-G into the retroviral packaging cell line Platinum E (30). Viral supernatants were collected at 48 h posttransfection and aliquoted into 24-well plates (1 mL aliquoted per well). Harvested CD24−/lo-depleted FL cells or FTs were resuspended to 1 × 106 cells/mL, and 0.5 × 106 cells were added to each well containing viral supernatant. Infections were supplemented with 10 μg/mL of hexadimethrine bromide (Sigma-Aldrich), 5 μg/mL of mIL-7, 5 μg/mL of hFlt-3L, and 10 μg/mL of mSCF. Plates were then centrifuged at 1,000 × g for 1.5 h at room temperature.
In Vitro T-Cell Differentiation.
At 16 h after transduction, CD24−/lo FL cells were FACS-sorted as c-Kit+Sca-1high GFP+ and plated at 4,000–6,000 cells per well on a 24-well plate containing a confluent layer of OP9-DL1 cells. Hematopoieitc progenitors were differentiated as described originally. In brief, cocultures were maintained in alpha MEM supplemented with 20% FCS, 5 μg/mL of hFlt-3L, and 0.5 μg/mL of mIL-7 and passaged onto fresh confluent OP9-DL1 every 4 d.
Fetal Thymic Organ Culture.
Fetal thymic lobes were isolated from day-14.5 CD-1 embryos, laid on insert filters, and cultured in 1.35 mM deoxyguanosine/DMEM. After 5 d of culture, lobes were rinsed for 2 h in DMEM and then placed in Terasaki plates in which 10,000 transduced FTs per well were aliquoted. The plates were then inverted and incubated for 48 h. Lobes were rescued and cultured on insert filters for 1–2 wk.
Microscopy.
All imaging experiments were conducted on the WaveFX Spinning Disk Confocal System (Quorum Technologies) using a Zeiss inverted Axiovert 200M microscope equipped with an ASI stage (Applied Scientific Instruments). Temperature and CO2 level were maintained at 37 °C and 5%, respectively, using the Live Cell System (Pathology Devices). Samples were imaged using a Zeiss C-Apochromat 63×/1.2 NA water immersion objective lens. Sample preparation is described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank G. Knowles and D. White for expert assistance with cell sorting and Dr. R. LaMotte-Mohs for advice on imaging of OP9-DL1 cocultures. We also thank Dr. Juan Carlos Zúñiga-Pflücker and Dr. G. R. Wiggin for critical reading of this manuscript. This work was supported by grants from the Canadian Institutes for Health Research (MOP-57793 and MOP-6849), the Canadian Cancer Society (018469), Genome Canada through the Ontario Genomics Institute, and the Ontario Research Fund (to T.P.). K.A.P. was supported by a postdoctoral fellowship from the Leukemia and Lymphoma Society of Canada and the Fonds de la Recherche en Santé du Québec.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018224108/-/DCSupplemental.
References
- 1.Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- 2.Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006;7:338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- 3.Takahama Y. Journey through the thymus: Stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 4.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–134. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porritt HE, Gordon K, Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–962. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Pozo MA, et al. The two poles of the lymphocyte: Specialized cell compartments for migration and recruitment. Cell Adhes Commun. 1998;6:125–133. doi: 10.3109/15419069809004468. [DOI] [PubMed] [Google Scholar]
- 7.Radtke F, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 8.Müller KM, Luedecker CJ, Udey MC, Farr AG. Involvement of E-cadherin in thymus organogenesis and thymocyte maturation. Immunity. 1997;6:257–264. doi: 10.1016/s1074-7613(00)80328-3. [DOI] [PubMed] [Google Scholar]
- 9.Bevan MJ. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977;269:417–418. doi: 10.4049/jimmunol.176.1.677. [DOI] [PubMed] [Google Scholar]
- 10.Speiser DE, Lees RK, Hengartner H, Zinkernagel RM, MacDonald HR. Positive and negative selection of T cell receptor V beta domains controlled by distinct cell populations in the thymus. J Exp Med. 1989;170:2165–2170. doi: 10.1084/jem.170.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins ED, Russell SM. Upsides and downsides to polarity and asymmetric cell division in leukemia. Oncogene. 2009;27:7003–7017. doi: 10.1038/onc.2008.350. [DOI] [PubMed] [Google Scholar]
- 12.Ludford-Menting MJ, et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Zeitler J, Hsu CP, Dionne H, Bilder D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol. 2004;167:1137–1146. doi: 10.1083/jcb.200407158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- 15.Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol. 2006;16:2395–2405. doi: 10.1016/j.cub.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Richier L, et al. NOS1AP associates with Scribble and regulates dendritic spine development. J Neurosci. 2010;30:4796–4805. doi: 10.1523/JNEUROSCI.3726-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murdoch JN, et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- 18.Zarbalis K, et al. A focused and efficient genetic screening strategy in the mouse: Identification of mutations that disrupt cortical development. PLoS Biol. 2004;2:e219. doi: 10.1371/journal.pbio.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 20.Balciunaite G, Ceredig R, Fehling HJ, Zúñiga-Pflücker JC, Rolink AG. The role of Notch and IL-7 signaling in early thymocyte proliferation and differentiation. Eur J Immunol. 2005;35:1292–1300. doi: 10.1002/eji.200425822. [DOI] [PubMed] [Google Scholar]
- 21.Capone M, Hockett RD, Jr, Zlotnik A. Kinetics of T cell receptor beta, gamma, and delta rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44(+)CD25(+) pro-T thymocytes. Proc Natl Acad Sci USA. 1998;95:12522–12527. doi: 10.1073/pnas.95.21.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 23.Carman CV, Springer TA. Integrin avidity regulation: Are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003;15:547–556. doi: 10.1016/j.ceb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, et al. LFA-1 affinity regulation is necessary for the activation and proliferation of naive T cells. J Biol Chem. 2009;284:12645–12653. doi: 10.1074/jbc.M807207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griewank K, et al. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi EY, et al. Thymocyte–thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity. 2005;23:387–396. doi: 10.1016/j.immuni.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Sabatos CA, et al. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity. 2008;29:238–248. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ralston KJ, et al. The LFA-1–associated molecule PTA-1 (CD226) on T cells forms a dynamic molecular complex with protein 4.1G and human discs large. J Biol Chem. 2004;279:33816–33828. doi: 10.1074/jbc.M401040200. [DOI] [PubMed] [Google Scholar]
- 29.Bahri S, et al. The leading edge during dorsal closure as a model for epithelial plasticity: Pak is required for recruitment of the Scribble complex and septate junction formation. Development. 2010;137:2023–2032. doi: 10.1242/dev.045088. [DOI] [PubMed] [Google Scholar]
- 30.Morita S, Kojima T, Kitamura T. Plat-E: An efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.