Abstract
The D2/AKT1/GSK-3β signaling pathway has been involved in the downstream intracellular effects of dopamine, in the pathophysiology of cognitive deficits and related brain activity in schizophrenia, as well as in response to treatment with antipsychotics. Polymorphisms in the D2 (DRD2 rs1076560) and AKT1 (AKT1 rs1130233) genes have been associated with their respective protein expression and with higher-order cognition and brain function, including attention. Given the strong potential for their relationship, we investigated the interaction of these polymorphisms on multiple molecular and in vivo phenotypes associated with this signaling pathway. We measured AKT1 and GSK-3β proteins and phosphorylation in human peripheral blood mononuclear cells, functional MRI cingulate response during attentional control, behavioral accuracy during sustained attention, and response to 8 wk of treatment with olanzapine in a total of 190 healthy subjects and 66 patients with schizophrenia. In healthy subjects, we found that the interaction between the T allele of DRD2 rs1076560 and the A allele of AKT1 rs1130233 was associated with reduced AKT1 protein levels and reduced phosphorylation of GSK-3β, as well as with altered cingulate response and reduced behavioral accuracy during attentional processing. On the other hand, interaction of these two alleles was associated with greater improvement of Positive and Negative Syndrome Scale scores in patients with schizophrenia after treatment with olanzapine. The present results indicate that these functional polymorphisms are epistatically associated with multiple phenotypes of relevance to schizophrenia. Our results also lend support to further investigation of this downstream molecular pathway in the etiology and treatment of this disorder.
A large series of experimental data indicate that dopamine D2 receptors and schizophrenia are tightly related. First, these receptors are privileged targets of antipsychotic drugs, which antagonize their activity (1). Second, previous reports have suggested association between psychosis and relatively greater D2 density in striatum, even though change is moderate (2). Third, clinical symptoms and cognitive deficits have been associated with abnormal D2 signaling (3–12). The relationship between D2 receptors and cognitive deficits in schizophrenia is also supported by previous models postulating that relatively excessive D2 signaling may lead to lower cortical signal-to-noise ratio and reduced filtering of information, as well as blocking of distracting inputs (10–12). These brain processes contribute to different higher-order cognitive functions and are strongly involved in top-down modulation of attention (13, 14). Consistent with these models, previous data have suggested that attentional behavior is affected by D2 genetic variation (15). Furthermore, deficits in attentional processing are centrally implicated in schizophrenia (16, 17). In fact, patients with schizophrenia performing attentional tasks have abnormal activity in the cingulate cortex, a brain region tightly linked to attentional processing (14) and modulated by D2 receptors (18, 19), as well as by genetic variants possibly affecting D1/D2 ratio stimulation (10, 20).
Two isoforms of the D2 receptor are known. The D2 long isoform (D2L) is mainly postsynaptic and is a target for haloperidol, and the D2 short (D2S) isoform is mainly presynaptic and serves as an autoreceptor regulating dopamine synthesis and release (21). These two isoforms are coded by the D2 receptor gene (DRD2–11q23) with a mechanism of alternative splicing acting at exon 6. In a previous study (15), we have characterized a functional SNP within DRD2 at intron 6 (rs1076560 − guanine > timine − G > T) associated with relative expression of the two isoforms in the frontal cortex. In particular, the T allele shifts splicing from D2S to D2L, decreasing the D2S/D2L ratio relative to the G allele. This SNP has also been associated with behavior and brain activity during cognitive and emotion processing in healthy humans and in patients with schizophrenia (15, 22, 23). More specifically, the T allele has been associated with less efficient prefronto-striatal activity during working memory (23) and with putatively greater levels of striatal dopamine (24). DRD2 has also been weakly associated with diagnosis of schizophrenia (25).
Downstream of D2 receptors, different molecular pathways have been identified: the classic cAMP-PKA pathway and another cAMP-independent pathway that includes the serine/threonine protein kinase AKT1, which phosphorylates to inhibit another protein kinase, GSK-3β (reviewed in ref. 26). The specific relationship between D2 receptor signaling and AKT1 has been elucidated by data indicating that D2 stimulation by dopamine inhibits AKT1 signaling through dephosphorylation via the β-arrestin 2/phosphatase PP2A complex (27, 28) (for review, see refs. 26 and 29). Consistent with their preferential postsynaptic localization, another experiment has also indicated that knock-out of D2L receptors is sufficient to reduce activity of this pathway (28). Moreover, other studies in mice have demonstrated that D2 but not D1 agonists impair performance at the T maze and prepulse inhibition of startle in AKT1-deficient mice (30, 31). Importantly, AKT1 levels in lymphoblasts and in prefrontal cortex of patients with schizophrenia are reduced (31, 32). Furthermore, clozapine, a D2 antagonist antipsychotic, increases AKT1 and GSK-3β phosphorylation, as well as total cellular and intranuclear levels of β-catenin (33), a crucial factor for gene expression that is inhibited by GSK-3β activity (26).
The gene coding for AKT1 (14q32.32) has also been associated with schizophrenia (31, 32, 34–37). Importantly, the A allele in a synonymous SNP in AKT1 (rs1130233 − G > A) is associated with reduced AKT1 protein levels in lymphoblasts (38, 39), reduced cognitive performance, inefficient prefronto-striatal activity during cognition (39), as well as with diagnosis of schizophrenia (39).
Because genetic variation does not directly cause behavioral phenotypes but rather impacts on neuronal features that influence neural systems-level processing, we investigated the possible impact of DRD2 rs1076560 and AKT1 rs1130233 on a series of progressively more complex and distal phenotypes in healthy subjects and in patients with schizophrenia. In particular, given the known reciprocal relationship between D2 and AKT1 and their effects on GSK-3β activity (26), as the more proximal phenotype, we evaluated the interaction between DRD2 and AKT1 SNPs on AKT1 and GSK-3β protein levels and phosphorylation in human blood cells. Because this experiment suggested functional epistatic downstream effects of these two polymorphisms, we performed further specific analyses. Given the earlier involvement of anterior cingulate in the pathophysiology of schizophrenia, the expression of D2 receptors in anterior cingulate (40) and the involvement of D2 signaling in attentional processing (15), we evaluated cingulate physiology during attentional control as well as behavioral accuracy during sustained attention. Finally, given the strong involvement of D2 receptors and the recent implication of AKT1 (33) in determining response to antipsychotics, we also evaluated response to treatment with olanzapine in patients with schizophrenia.
Results
Association of DRD2 rs1076560 and AKT1 rs1130233 Genotypes with AKT1 and GSK-3β Protein Levels and Phosphorylation in Peripheral Blood Mononuclear Cells of Healthy Humans.
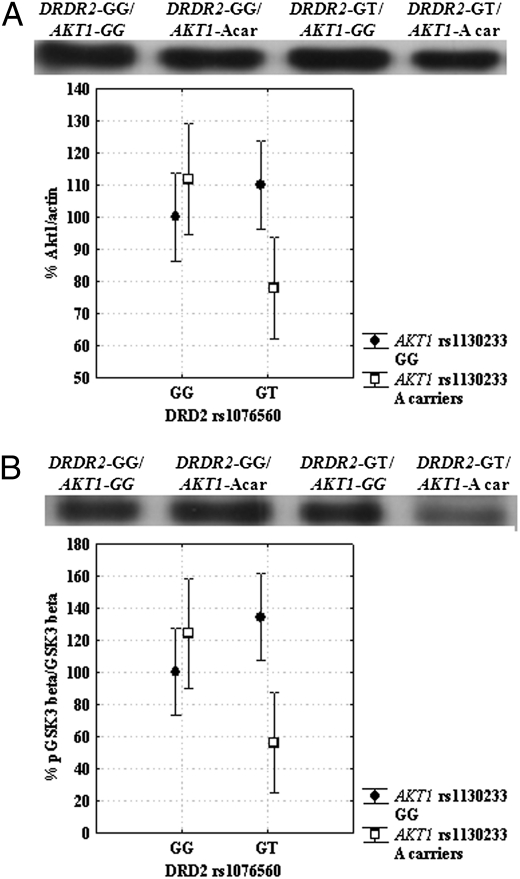
Factorial ANOVA indicated a statistical trend for a main effect of DRD2 rs1076560 (F1,23 = 2.6; P = 0.1) and of AKT1 rs1130233 (F1,23 = 2.0; P = 0.1) on AKT1 protein, with DRD2 GT subjects and AKT1 A carriers having reduced mean levels. Furthermore, there was an interaction between DRD2 and AKT1 polymorphisms (F1,23 = 8.9; P = 0.006). Posthoc analysis revealed that DRD2 GT/AKT1 A carriers subjects had lower AKT1 protein levels relative to other genotypes groups (all P < 0.04) (Fig. 1A). On the other hand, no significant association between genotypes and AKT1 phosphorylation at Ser473 was found (all P > 0.2) other than a strong trend for an effect of AKT1 rs1130233 (F1,23 = 3.6; P = 0.07), with reduced AKT1 phosphorylation in A carriers.
Fig. 1.
Western blots and graphs showing interaction between DRD2 rs1076560 and AKT1 rs1130233 on AKT1 expression (A) and GSK-3β phosphorylation (B). DRD2 GT/AKT1 A carriers subjects had lower AKT1 expression and GSK-3β phosphorylation relative to all other genotypes groups. See text for statistics.
To examine potential downstream effects of these two polymorphisms, further analysis was performed to investigate association of DRD2 and AKT1 genetic variants on GSK-3β protein levels and phosphorylation. Factorial ANOVA revealed no genotype effects on GSK-3β protein levels (all P > 0.4). Analysis on GSK-3β phosphorylation at Ser9 indicated no effects of DRD2 rs1076560 (F1,23 = 1.3; P = 0.3), a strong trend for a main effect of AKT1 rs1130233 (F1,23 = 3.5; P = 0.07), and a significant interaction (F1,23 = 12.4; P = 0.001). Posthoc analysis indicated reduced phosphorylation of Ser9 GSK-3β in DRD2 GT/AKT1 A carrier subjects relative to all other genotypes groups (all P < 0.04) (Fig. 1B).
Association of DRD2 rs1076560 and AKT1 rs1130233 Genotypes with Cingulate Cortex Activity During Attentional Control in Healthy Subjects.
In the functional MRI (fMRI) sample (Table 1), genotype groups were matched in terms of gender, age, handedness, and IQ (all P > 0.1). No genotype effects were present on variable attentional control (VAC) behavioral data (all P > 0.05) (Table S1), thus allowing us to compare brain responses without this potential confound.
Table 1.
Demographics (± SD) of the samples included in the experiments performed
| fMRI | Cognitive behavior | Olanzapine study | |
| n | 73 | 176 | 66 |
| Sex | 45 F | 108 F | 14 F |
| Age | 24.5 ± 4.5 | 25.6 ± 6.1 | 28.3 ± 7.2 |
| Handedness | 0.6 ± 0.5 | 0.7 ± 0.5 | |
| IQ | 110.1 ± 12.3 | 108.7 ± 3.3 | |
| TIB | 102.4 ± 8.3 | ||
| PANSS at baseline | |||
| Total | 103.33 ± 21.3 | ||
| Positive | 25.11 ± 6.2 | ||
| Negative | 26.12 ± 10.1 | ||
| General psychopathology | 52.11 ± 12.2 | ||
| N | |||
| DRD2 GG/ AKT1 GG | 30 | 74 | 30 |
| DRD2 GG/ AKT1 A carriers | 29 | 56 | 24 |
| DRD2 T carriers/ AKT1 GG | 7 | 28 | 7 |
| DRD2 T carriers/ AKT1 A carriers | 7 | 18 | 5 |
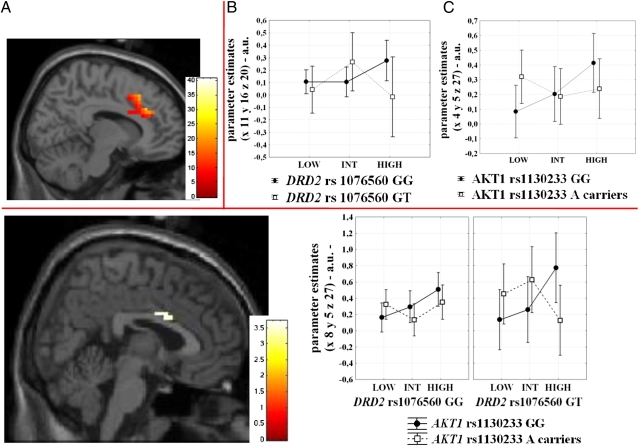
Imaging analysis revealed a main effect of load in a cingulate cluster extending bilaterally (local maxima in x = 8; y = 30; z = 26, BA32, k = 112, Z = 7.43) (Fig. 2), although no main effect of DRD2 rs1076560 or of AKT1 rs1130233 was found. However, several interactions between genotypes and load were present. There was a significant interaction between DRD2 genotype and load (x = 11; y = 16; z = 20, BA24, k = 7, Z = 3.27), and between AKT1 genotype and load (x = 4; y = 5; z = 27, BA24, k = 28, Z = 4.30) on cingulate activity. Of note, an interaction between DRD2 rs1076560, AKT1 rs1130233, and load was also found in this brain area (x = 8; y = 5; z = 27, BA24, k = 10, Z = 3.65) (Fig. 2). Posthoc analysis on parameter estimates extracted from this cluster was performed to illustrate load-dependent differences among genotype groups. This investigation revealed that cingulate responses at the higher attentional control load were greater than those at the intermediate attentional level in all groups (all P < 0.02) but in DRD2 GT/AKT1 A carriers, who displayed reduced activity at the higher load (P = 0.008) (Fig. 2). Furthermore, between-group differences in cingulate activity were also evident at the higher attentional load when comparing DRD2 GT/AKT1 A carriers vs. DRD2 GT/AKT1 GG (P = 0.02) and DRD2 GG/AKT1 GG subjects (P = 0.09) (Fig. 2).
Fig. 2.
(Upper) (A) Sagittal section of the brain illustrating the effect of load in the cingulate cortex during performance of the VAC (local maxima: x = 8; y = 30; z = 26). Color bar represents t values. (B and C) Graphs illustrating parameter estimates extracted from the clusters in the cingulate cortex showing the DRD2 rs1076560 by load (local maxima: x = 11; y = 16; z = 20) (B) and the AKT1 rs1130233 by load (local maxima: x = 4; y = 5; z = 27) (C) interaction. (Lower) Sagittal section of the brain showing the cingulate cluster associated with the DRD2 rs1076560 by AKT1 rs1130233 by load interaction during performance of the VAC task, and relative parameter estimates to illustrate load dependent differences between genotype groups. A drop in cingulate activity at the greater attentional load was present in DRD2 GT/AKT1 A carriers. See text for statistics. Color bar represents t values.
Association of DRD2 rs1076560 and AKT1 rs1130233 Genotypes with Performance During Sustained Attention in Healthy Subjects.
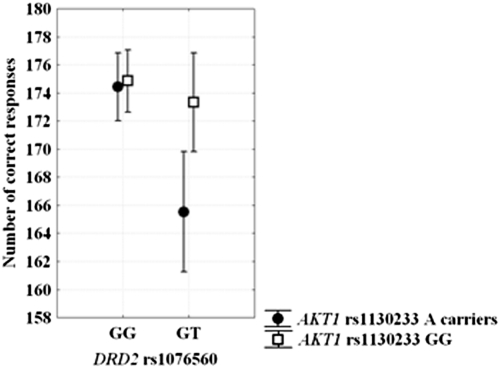
In the Continuous Performance Test (CPT) sample, there were no genotype effects on demographics (all P > 0.1). ANOVA on correct responses at the CPT revealed a main effect of DRD2 rs1076560 (F1,154 = 10.2; P = 0.001), a main effect of AKT1 rs1130233 (F1,154 = 6.3; P = 0.01), and an interaction between DRD2 and AKT1 genotypes (F1,154 = 5.1; P = 0.02). Posthoc analysis indicated that DRD2 GT/AKT1 A carriers have reduced number of correct responses relative to all other genotype groups (all P < 0.007) (Fig. 3). No significant effects were found on reaction time data (all P > 0.2).
Fig. 3.
Graph showing DRD2 rs1076560 by AKT1 rs1130233 interaction on the number of correct responses at the CPT. DRD2 GT/AKT1 A carriers had lower accuracy relative to all other genotypes groups. See text for statistics.
Association of DRD2 rs1076560 and AKT1 rs1130233 Genotypes with Response to Treatment with Olanzapine in Patients with Schizophrenia.
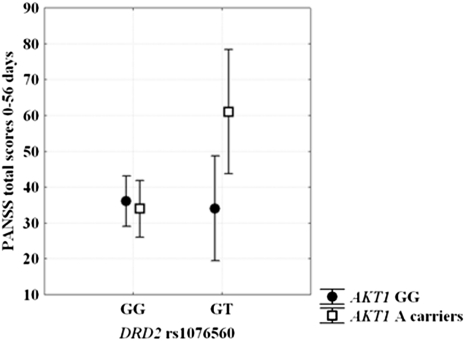
Genotype groups were matched in terms of demographics, mean olanzapine dose (mg 21.1 ± 7.5), and baseline Positive and Negative Syndrome Scale (PANSS) scores (all P > 0.1). ANOVA on the difference between PANSS total scores at 56 and 0 d of olanzapine treatment indicated a main effect of DRD2 rs1076560 (F1,62 = 3.99; P = 0.05), a main effect of AKT1 rs1130233 (F1,62 = 3.94; P = 0.05), and an interaction between DRD2 and AKT1 genotypes (F1,62 = 5.42; P = 0.02). Posthoc analysis revealed greater difference in symptom scores in DRD2 GT/AKT1 A carriers relative to all other groups (all P < 0.02) (Fig. 4). Exploratory ANOVAs were also performed on PANSS subscales. Negative symptoms scores revealed a main effect of DRD2 rs1076560 (F1,62 = 7.39; P = 0.008) and a DRD2 by AKT1 genotype interaction (F1,62 = 3.76; P = 0.05). Posthoc analysis indicated greater improvement in DRD2 GT/AKT1 A carriers relative to all other groups (all P < 0.05). General psychopathology symptoms scores revealed an interaction between DRD2 and AKT1 genotypes (F1,62 = 4.38; P = 0.04), with greater improvement in DRD2 GT/AKT1 A carriers relative to other genotype groups (P < 0.04). No other statistically significant effects were found on PANSS scores.
Fig. 4.
Modulation by DRD2 rs1076560 and AKT1 rs1130233 of response to 8 wk of olanzapine treatment. DRD2 GT/AKT1 A carriers showed greater PANSS total scores improvement relative to all other genotypes groups. See text for statistics.
CPT scores were available for 61 patients with schizophrenia (DRD2 GG/AKT1 GG n = 29; 23 DRD2 GG/AKT1 A carriers n = 23; DRD2 GT/AKT1 GG n = 5; DRD2 GT/AKT1 A carriers n = 4). ANOVA indicated a trend for an effect of DRD2 rs1076560 (F1,57 = 2.3; P = 0.1), a main effect of AKT1 rs1130233 (F1,57 = 4.8; P = 0.03), and an interaction between DRD2 and AKT1 genotypes approaching significance (F1,57 = 3.5; P = 0.06). Further exploratory posthoc analysis showed that DRD2 GT AKT1 A carrier subjects have greater improvement in the number of CPT correct responses relative to other genotype groups (all P < 0.05) (Fig S1).
Discussion
The present results consistently suggest epistatic effects of DRD2 rs1076560 and AKT1 rs1130233 genotypes on multiple, progressively more distant and complex phenotypes. In particular, we found in peripheral blood mononuclear cells (PBMCs) of healthy humans that these genetic variants interact in conferring individual variability in AKT1 protein levels and phosphorylation of GSK-3β. Furthermore, interaction of these two genotypes is associated with cingulate activity and behavior during attentional tasks in healthy subjects, as well as with response to 8 wk of olanzapine treatment in patients with schizophrenia in terms of symptoms scores and, to a limited extent, attentional behavior.
Our molecular results suggest specific effects of DRD2 rs1076560 and AKT1 rs1130233 genotypes on the cAMP-independent D2 signaling cascade, which is a crucial pathway for intraneuronal transduction of dopamine signaling (41). More specifically, consistent with knowledge that phosphorylation of AKT1 is a process concerted between D2 receptors with the β-arrestin 2/phosphatase PP2A complex, we did not find association between DRD2 and AKT1 genotypes and AKT1 phosphorylation at Ser473. On the other hand, our data indicate that DRD2 GT/AKT1 A carrier individuals have reduced AKT1 protein levels. Other studies (39) have also demonstrated that the A allele of AKT1 rs1130233 is associated with reduced expression of AKT1 relative to the G allele. Furthermore, previous reports have indicated greater relative measures of D2L ratio in DRD2 rs1076560 T allele subjects (15), suggesting relative greater D2L signaling in these individuals compared with GG. Therefore, genetically determined increase in D2L signaling in DRD2 rs1076560 T allele subjects may determine molecular mechanisms leading to a further relative decrease in AKT1 protein within a genetic context already favoring lower expression of this protein (the AKT1 rs1130233 A allele). Importantly, we have demonstrated these molecular effects in PBMC rather than in neurons, and this is a limitation of the present results. However, even considering the physiological difference between lymphocytes and neurons, some speculative inferences are possible based on previous studies and on the present findings. In particular, the previously demonstrated relative greater expression of D2L in brain tissue of DRD2 rs1076560 T carriers (15), together with data indicating that D2L knock-out mice display greater AKT1 activity in the brain (28), support relevance of the genetic interaction for D2 signaling transduction in the neuron, where D2L is the main mediator of D2 signaling at the postsynaptic level (21).
Our results on genetic interaction between D2 and AKT1 are in line with and further substantiated by the effect of phosphorylation of GSK-3β. GSK-3β is an important molecular target downstream of AKT1 and is involved in a series on mechanisms of gene expression, including inactivation of β-catenin (26, 42). Previous studies have indicated that AKT1 signaling inhibits GSK-3β activity via phosphorylation (43). Indeed, we found that DRD2 GT/AKT1 A carrier individuals also have reduced phosphorylation of GSK-3β, although no difference was evident on GSK-3β protein levels. Therefore, all these results together further suggest downstream functional effects of the interaction between DRD2 rs1076560 and AKT1 rs1130233 genotypes on GSK-3β within the cAMP independent pathway. GSK-3β phosphorylation may in turn affect regulation of gene-expression mechanisms of neurodevelopment and synaptic growth, which may be altered in schizophrenia (44). Consistently, previous studies have found reduced AKT1 levels and phosphorilated GSK-3β (31) in patients with schizophrenia. Our results suggest that these earlier findings may be also because of the interaction between genetic variation in DRD2 and AKT1 genes, previously associated with schizophrenia phenotypes (23, 39). Further studies addressing these effects at the neuronal level are needed to confirm these speculations.
Our fMRI data in healthy subjects also indicate a specific interaction between DRD2 rs1076560, AKT1 rs1130233, and load on cingulate activity during attentional control processing. The relationship between increasing load of attentional control and activity in anterior cingulate is linear, increasing from lower to higher loads (14, 17). This relationship was not evident in DRD2 GT/AKT1 A carrier individuals whose activity in anterior cingulate dropped off from the intermediate to the higher level of attentional control. This pattern of response is strongly reminiscent of the results we have recently reported in patients with schizophrenia, in whom a similar drop of cingulate responses was present at the high attentional control load (17).
Behavioral results during attentional processes as elicited by the CPT were consistent with these physiological data. Here, DRD2 GT/AKT1 A carrier subjects had reduced accuracy relative to all other genotype configurations, further supporting the detrimental role of the interaction between these genetic variants for attentional processes in healthy subjects. Previous data have indicated that relatively greater D2 signaling is associated with less filtering of information flow and blocking of distracting inputs (10). Thus, greater relative D2 postsynaptic signaling possibly associated with lower D2S/L ratio in DRD2 rs1076560 T carriers may interact with genetically determined lower expression of AKT1 rs1130233, also previously associated with cognitive inefficiency (39), in determining altered processing of attentional inputs, which indeed characterizes schizophrenia (16).
As suggested by previous models (10), the relationship between genetic modulation of D2 signaling and cognitive processing may also relate to clinical symptoms of schizophrenia. In particular, the net effect of relatively predominant D2 vs. D1 stimulation is of reducing inhibition of neuronal network activity, which may lead to easier access of inputs into cognitive buffers. More specifically, greater D2 signaling may be associated with reduced filtering of information, with reduced blocking of distracting inputs and with multiple network representations, thus overloading cortical processing with too much information. This physiological state may lead to less optimal cognitive processing as well as to possible coexistence of multiple cognitive representations, both internally generated or driven by environmental stimuli (10). Consistently, other models have also attributed a role to dopamine in conferring salience to internal representations or external stimuli (45). Altered dopamine signaling may drive to altered attribution of salience to these stimuli or representations; antipsychotic treatment targeting D2 receptors may dampen such aberrant physiology associated with dopamine signaling (45).
Relevance of the impact of DRD2 rs1076560 and AKT1 rs1130233 variants for schizophrenia is further suggested by our data on their interaction on response to antipsychotic treatment. In this case, DRD2 GT/AKT1 A carrier individuals with schizophrenia had better response after 8 wk of olanzapine monotherapy. This effect was statistically significant in term of PANSS total, negative symptoms, and general psychopathology scores. These results are in line with the notion that olanzapine blocks D2 signaling and with data showing that second generation antipsychotics activate AKT1 (33) or mimic AKT1 activity increasing GSK-3β phosphorylation (46). These results are also consistent with a similar, albeit weaker, effect found on the CPT number of correct responses.
Interestingly, patients with schizophrenia carrying the two “risk” alleles (DRD2 T and AKT1 A) had better response to treatment with olanzapine, a beneficial effect that would seem at odds with the effects of these two alleles in healthy subjects. This finding can be interpreted in two ways, which are not mutually exclusive. First, patients with this genotype configuration may respond better because their dopamine cAMP-independent pathway is more profoundly altered in terms of dopamine D2 signaling (24), and thus there is more “room” for improvement by treatment with a drug which specifically acts on it (33). Second, it is possible that genetic variants interact with dysregulated levels of dopamine in patients, determining an effect which is not immediately derived by studying healthy subjects only (47, 48). Both these explanations are speculative and have to be treated with caution. Nonetheless, to our knowledge, this a unique demonstration in humans of the involvement of the D2-AKT1 signaling pathway in modulating the effect of antipsychotic treatment.
Previous theories have hypothesized that dopamine dysregulation may characterize the pathophysiology of schizophrenia. More specifically, dopamine levels may be reduced in the cortex (especially prefrontal) but they may be increased in the striatum (44). In an earlier longitudinal study we have reported that treatment with olanzapine in patients with schizophrenia is associated with attenuated improvement in subjects carrying the COMT Valine allele (49, 50). In the present study, we report that the same treatment protocol is associated with greater improvement in patients with schizophrenia carrying the two risk alleles in DRD2 and AKT1. In other words, the risk allele is associated with poorer response when evaluating a gene controlling cortical dopamine (COMT), although it is associated with better response when the genes in question are more expressed in the striatum (DRD2, AKT1). These apparently incongruent findings may be reconciled if examined in the context of the hypothesized dysregulation/imbalance between cortical and subcortical dopamine.
In conclusion, the present results suggest that the interaction between genetic factors conferring risk for impairment in the D2-AKT1 signaling pathway may be relevant for the understanding of correlates of the pathophysiology of schizophrenia at the molecular, neuronal networks, and behavioral level. These aspects should also be taken into account to disambiguate mechanisms associated with individual response to antipsychotic treatment in schizophrenia.
Materials and Methods
A total of 190 healthy subjects and 66 patients with schizophrenia were included in this study (see SI Materials and Methods for inclusion and exclusion criteria). All subjects underwent one or more of the below described procedures. Furthermore, the subjects were genotyped for DRD2 rs1076560 and AKT1 rs1130233, as specified in SI Materials and Methods.
Association of DRD2 rs1076560 and AKT1 rs1130233 Genotypes with AKT1 and GSK-3β Protein Levels and Phosphorylation in PBMC of Healthy Humans.
Based on previous literature indicating D2 expression in T cells and on relevance of D2 receptor signaling for T-cell normal metabolism and function (51, 52), we explored the potential impact of DRD2 and AKT1 genetic variants on cAMP independent D2 signaling cascade in human PBMC. Blood samples were drawn from 29 healthy individuals (18 females, mean age ± SD 26.8 ± 4.8; DRD2 GG/AKT1 A carriers n = 7; DRD2 GG/AKT1 GG n = 8; DRD2 GT/AKT1 A carriers n = 6; DRD2 GT/AKT1 GG n = 8) from the larger group enrolled in this study. AKT1, P-Ser473-AKT1, GSK-3β, P-Ser9-GSK-3β were quantified as specified in SI Materials and Methods. Factorial ANOVA was then used for statistical analysis on averaged and normalized proteins values.
Association of DRD2 rs1076560 and AKT1 rs1130233 Genotypes with Cingulate Cortex Activity During VAC in Healthy Subjects.
Seventy-three healthy subjects (Table 1) were enrolled to evaluate the association of DRD2 rs1076560 and AKT1 rs1130233 with brain activity during VAC processing. All subjects underwent fMRI while performing the VAC task, which elicits increasing demand for attentional control and which was identical to that published in previous studies (14, 15, 17, 20). This task allows investigation of brain activity during three levels of attentional control (low, intermediate, high), which were obtained manipulating both the relative directions of arrows with different sizes and the related cue words (SI Materials and Methods).
Functional MRI was performed on a GE Sigma 3T scanner (SI Materials and Methods). Analysis was completed using Statistical Parametric Mapping 5 (SPM5 -http://www.fil.ion.ucl.ac.uk/spm). After single-subject processing (SI Materials and Methods), a random-effects ANOVA was performed to investigate the main effect of increasing level of attentional control, of DRD2 rs1076560 genotype, of AKT1 rs1130233 genotype, and their interaction. Based on previous data demonstrating association of cingulate activity with other dopamine genes using the present task (20), as well as on findings of abnormal activity in this brain region in patients with schizophrenia during attentional processes (17), we focused our analyses on cingulate blood-oxygen level-dependent (BOLD) responses (see SI Materials and Methods for cluster localization). We used a statistical threshold of P < 0.001, minimum cluster size (k) = 5, with further family-wise error small-volume correction at P < 0.05 applied on the activated clusters, using the cingulate as the volume of interest as defined by the WFU_PickAtlas (http://fmri.wfubmc.edu/cms/software#PickAtlas) (see SI Materials and Methods for clusters localization). To further explore load dependent differences between genotype groups, posthoc analysis with Fisher's test outside of SPM was also used on BOLD responses extracted from the cluster showing significant genotypes by load interaction using MarsBar (http://marsbar.sourceforge.net/). ANOVAs and χ2 were used to compare demographics and behavioral data. Fisher's test was used for posthoc analyses.
Association of DRD2 rs1076560 and AKT1 rs1130233 Genotypes with Performance During Sustained Attention in Healthy Subjects.
One-hundred seventy-six healthy subjects (Table 1), partially overlapping (n = 59) with those included in the fMRI study, performed the A-X version of the CPT, which is a measure of selective attention and context processing (53) (SI Materials and Methods). Performance data were recorded as the number of correct responses and reaction time. ANOVAs and χ2 were used to compare demographics and behavioral data. Fisher's test was used for posthoc analyses.
Association of DRD2 rs1076560 and AKT1 rs1130233 Genotypes with Response to Treatment with Olanzapine in Patients with Schizophrenia.
Sixty-six patients with schizophrenia with current exacerbation of symptoms requiring hospitalization (Table 1) and who had been drug-free for at least 1 wk or 1 mo if under depot medication, were treated for 8 wk with olanzapine monotherapy (50). Titration was allowed for the first 4 wk. Then, the dose was kept constant until 8 wk of treatment. Symptoms were assessed at study entry (day 0) and at day 56 (8 wk) with the PANSS by a trained psychiatrist, who was blind to genotype. The CPT (see above) was also administered after 7 and 56 d of treatment to investigate the effect of olanzapine treatment on behavior associated with sustained attention.
ANOVA and χ2 were used as appropriate to compare demographics and mean dose of olanzapine. The difference between PANSS total scores at 56 and 0 d as well as between CPT scores at 56 and 7 d of olanzapine treatment was entered into factorial ANOVA with DRD2 and AKT1 genotypes as predictors. Further exploratory factorial ANOVAs were performed on PANSS subscales. Fisher's test was used for all posthoc analyses.
Supplementary Material
Acknowledgments
We thank Riccarda Lomuscio and Rita Masellis for help in data acquisition, all the people who participated to this study, and Paolo Stratta and Alessandro Rossi for making the computerized version of the Continuous Performance Test available to us. This study was supported in part by a National Alliance for Research on Schizophrenia and Depression Young Investigator award (to G.B.) and a research award (to A.B.) by Fondazione Cassa di Risparmio di Puglia. A.U. represents the Mariano Scippacercola Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.R.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013535108/-/DCSupplemental.
References
- 1.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 2.Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 3.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 4.Kellendonk C, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Drew MR, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knable MB, Weinberger DR. Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol. 1997;11:123–131. doi: 10.1177/026988119701100205. [DOI] [PubMed] [Google Scholar]
- 7.Martinot JL, et al. Central D2 receptors and negative symptoms of schizophrenia. Br J Psychiatry. 1994;164:27–34. doi: 10.1192/bjp.164.1.27. [DOI] [PubMed] [Google Scholar]
- 8.Abi-Dargham A, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta MA, Montgomery AJ, Kitamura Y, Grasby PM. Dopamine D2 receptor occupancy levels of acute sulpiride challenges that produce working memory and learning impairments in healthy volunteers. Psychopharmacology (Berl) 2008;196:157–165. doi: 10.1007/s00213-007-0947-0. [DOI] [PubMed] [Google Scholar]
- 10.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- 13.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 14.Blasi G, et al. Differentiating allocation of resources and conflict detection within attentional control processing. Eur J Neurosci. 2007;25:594–602. doi: 10.1111/j.1460-9568.2007.05283.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci USA. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weickert TW, et al. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 17.Blasi G, et al. Nonlinear response of the anterior cingulate and prefrontal cortex in schizophrenia as a function of variable attentional control. Cereb Cortex. 2010;20:837–845. doi: 10.1093/cercor/bhp146. [DOI] [PubMed] [Google Scholar]
- 18.Glickstein SB, Desteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb Cortex. 2005;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- 19.Aalto S, Brück A, Laine M, Någren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: A positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blasi G, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usiello A, et al. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- 22.Blasi G, et al. Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. J Neurosci. 2009;29:14812–14819. doi: 10.1523/JNEUROSCI.3609-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertolino A, et al. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain. 2009;132:417–425. doi: 10.1093/brain/awn248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolino A, et al. Genetically determined measures of striatal D2 signaling predict prefrontal activity during working memory performance. PLoS ONE. 2010;5:e9348. doi: 10.1371/journal.pone.0009348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen NC, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 26.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Beaulieu JM, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Lai WS, et al. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc Natl Acad Sci USA. 2006;103:16906–16911. doi: 10.1073/pnas.0604994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 32.Thiselton DL, et al. AKT1 is associated with schizophrenia across multiple symptom dimensions in the Irish study of high density schizophrenia families. Biol Psychiatry. 2008;63:449–457. doi: 10.1016/j.biopsych.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang UG, et al. The effects of clozapine on the GSK-3-mediated signaling pathway. FEBS Lett. 2004;560:115–119. doi: 10.1016/S0014-5793(04)00082-1. [DOI] [PubMed] [Google Scholar]
- 34.Schwab SG, et al. Further evidence for association of variants in the AKT1 gene with schizophrenia in a sample of European sib-pair families. Biol Psychiatry. 2005;58:446–450. doi: 10.1016/j.biopsych.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Norton N, et al. Association analysis of AKT1 and schizophrenia in a UK case control sample. Schizophr Res. 2007;93:58–65. doi: 10.1016/j.schres.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Bajestan SN, et al. Association of AKT1 haplotype with the risk of schizophrenia in Iranian population. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:383–386. doi: 10.1002/ajmg.b.30291. [DOI] [PubMed] [Google Scholar]
- 37.Ikeda M, et al. Association of AKT1 with schizophrenia confirmed in a Japanese population. Biol Psychiatry. 2004;56:698–700. doi: 10.1016/j.biopsych.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 38.Harris SL, et al. Detection of functional single-nucleotide polymorphisms that affect apoptosis. Proc Natl Acad Sci USA. 2005;102:16297–16302. doi: 10.1073/pnas.0508390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan HY, et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–2208. doi: 10.1172/JCI34725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB. Dopamine D2 receptors in the cerebral cortex: Distribution and pharmacological characterization with [3H]raclopride. Proc Natl Acad Sci USA. 1989;86:6412–6416. doi: 10.1073/pnas.86.16.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arguello PA, Gogos JA. A signaling pathway AKTing up in schizophrenia. J Clin Invest. 2008;118:2018–2021. doi: 10.1172/JCI35931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao Y, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beaulieu JM, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 45.Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- 47.Bertolino A, Blasi G. The genetics of schizophrenia. Neuroscience. 2009;164:288–299. doi: 10.1016/j.neuroscience.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 48.Law AJ, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertolino A, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 50.Bertolino A, et al. COMT Val158Met polymorphism predicts negative symptoms response to treatment with olanzapine in schizophrenia. Schizophr Res. 2007;95:253–255. doi: 10.1016/j.schres.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 51.Pacheco R, Prado CE, Barrientos MJ, Bernales S. Role of dopamine in the physiology of T-cells and dendritic cells. J Neuroimmunol. 2009;216:8–19. doi: 10.1016/j.jneuroim.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Basu B, et al. D1 and D2 dopamine receptor-mediated inhibition of activated normal T cell proliferation is lost in jurkat T leukemic cells. J Biol Chem. 285:27026–27032. doi: 10.1074/jbc.M110.144022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stratta P, Daneluzzo E, Bustini M, Prosperini P, Rossi A. Processing of context information in schizophrenia: relation to clinical symptoms and WCST performance. Schizophr Res. 2000;44:57–67. doi: 10.1016/s0920-9964(99)00142-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.