Abstract
Introductions or invasions of nonnative organisms can mediate major changes in the trophic structure of aquatic ecosystems. Here we document multitrophic level impacts in a spatially extensive system that played out over more than a century. Positive interactions among exotic vertebrate and invertebrate predators caused a substantial and abrupt shift in community composition resulting in a trophic cascade that extended to primary producers and to a nonaquatic species, the bald eagle. The opossum shrimp, Mysis diluviana, invaded Flathead Lake, Montana, the largest freshwater lake in the western United States. Lake trout had been introduced 80 y prior but remained at low densities until nonnative Mysis became established. The bottom-dwelling mysids eliminated a recruitment bottleneck for lake trout by providing a deep water source of food where little was available previously. Lake trout subsequently flourished on mysids and this voracious piscivore now dominates the lake fishery; formerly abundant kokanee were extirpated, and native bull and westslope cutthroat trout are imperiled. Predation by Mysis shifted zooplankton and phytoplankton community size structure. Bayesian change point analysis of primary productivity (27-y time series) showed a significant step increase of 55 mg C m−2 d−1 (i.e., 21% rise) concurrent with the mysid invasion, but little trend before or after despite increasing nutrient loading. Mysis facilitated predation by lake trout and indirectly caused the collapse of kokanee, redirecting energy flow through the ecosystem that would otherwise have been available to other top predators (bald eagles).
Keywords: invasive species, top predators, food web, Mysis diluviana, lake trout
Atrophic cascade occurs when reciprocal effects of predators on prey alter the abundance, biomass, or productivity of a population, community, or trophic level across more than one link in the food web (1–3). The theory of cascading trophic interactions (CTI) predicts that introduction of piscivores to a planktivore-dominated lake will reduce planktivore abundance, increase zooplankton biomass, promote larger zooplankton species, and reduce algal biomass (4, 5). Opposite effects may occur if piscivores are eliminated in the process. In either case, the strong interactor is the species that mediates instability or radical, permanent change in the food web (6). However, the intensity of CTI may be modified by nutrient supply: food web effects may decline with increasing nutrient loading due to higher algal growth rates and decreased edibility (7); or conversely, food web effects may be weak in ultraoligotrophic lakes, owing to naturally low food quantity for grazers (8). Indeed, it has been argued that lake productivity determines food chain length; thus, CTI are often induced by increased nutrient loading (9), although responses have been documented over a wide range of nutrient conditions (10). Documenting biocomplexity (11) of lakes in relation to long-term consequences of biotic invasions, nutrient loading, and climate forcing is a fundamental, contemporary problem in limnology. Second only to land transformation, biotic invasions are considered the most important cause of extinction, often driving losses in biological diversity of native species and populations (12).
The data demands for understanding trophic cascades are high because the predicted interactions can be complex, nonlinear, and involve transitions between alternative states, often over long time periods (13, 14). Particularly complex trophic interactions have been described in a lake after peacock bass introduction (15), on islands after the introduction of feral pigs and subsequent interactions with invasive golden eagles (16), and in an ocean as a consequence of a century of industrial whaling (17). Here we present analysis of a sequence of invasion-associated transitions involving CTI as revealed in a multidecadal data set for a large lake ecosystem. Of particular interest in this study was how certain species became strong interactors in the ecosystem. Are strong interactors context dependent, that is, does the particular assemblage of species and the interactions of those species determine whether a top predator becomes a strong interactor? In Flathead Lake, lake trout were a top predator at low densities for many decades but were not a strong interactor until the community changed (the arrival of the nonnative opossum shrimp, Mysis).
Flathead Lake is a glacially modified, graben or fault block lake; mean depth is 44 m, maximum 116 m. It is the largest freshwater lake (500 km2 surface area) in the western United States outside of Alaska. The lake is fed by the 18,290-km2 watershed of the Flathead River (mean flow 286 m3 s−1; lake residence time 2.2 y) that drains west from the Continental Divide of the Rocky Mountains in Montana and British Columbia to the Columbia River. Thirty-three percent of the catchment is in Glacier National Park and adjacent roadless wilderness areas, 28% is managed forest lands in Montana and British Columbia, and the rest is intermountain prairie farmland with rapidly expanding urban and exurban zones. The lake is oligotrophic and with few pollutants, although management of nutrient loading to the lake is a long-standing issue in the context of land use changes and wildfires. The piedmont valley that contains the lake basin has some 85,000 residents, with many more seasonal tourists (18, 19). The lake has never supported a substantial commercial fishery. Sport fisheries have been large at times, targeting species that were abundant.
We examined the 120-y record of food web structure and dynamics in relation to invasions and external drivers. Historic Flathead Lake ecosystem data come from a suite of published papers and archives at the Flathead Lake Biological Station. Contemporary (1977–2008) time series data come from measurements of biophysical variables in the lake and chemistry variables in the primary tributaries and aerosol deposition, taken at least monthly. Examination of the data reveals four distinguishable periods: (i) native period, pre-1920, when native species dominated the fish community although numerous nonnative fishes had been introduced; (ii) Kokanee period, 1920–1984, when many nonnative fish species first appeared and nonnative kokanee expanded to a large population size, replacing cutthroat trout as the predominant angler catch; the temporal coverage of the data are too sparse to resolve the rates of these changes; (iii) Mysis explosion period, 1985–1988, when the population of introduced opossum shrimp grew rapidly and then declined to less than half the peak density while the kokanee population crashed; and (iv) Mysis–lake trout period, 1989 to the present, when a new community dominated by the roles of Mysis and lake trout seems to have stabilized.
The available data provide considerable detail about the transitions from ii to iii to iv. Information about the transition from i to ii and about the dynamics within ii was limited.
Results
Native Period (Pre-1920).
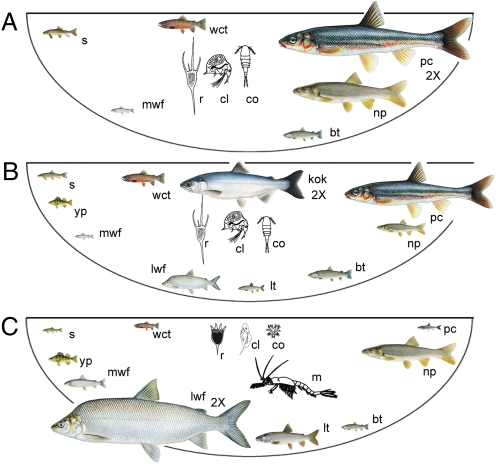
Only 10 fish species are known to have been native to the lake, and most are adfluvial, meaning that they rear in the lake but spawn in tributaries (Table S1). As far as can be determined from the early records (1890–1920), the pristine food web was diatom-based, with the most common crustacean zooplankton being large-bodied Epischura and Daphnia species and the smaller Diacyclops (20, 21). The most abundant piscivores were the northern pikeminnow and the bull trout, whereas abundant peamouth and westslope cutthroat trout occupied intermediate trophic levels (22, 23). Cutthroat trout was predominant in angler catches during this period (23) (SI Discussion). Although 14 species of nonnative fishes were introduced from 1890 to 1920 (19, 24), gill net surveys revealed a native fishery (Fig. 1) with only the incidental collection of a single nonnative species, largemouth bass (Table S1).
Fig. 1.
Food web of Flathead Lake, emphasizing three of the trophic levels (piscivores, planktivores, and herbivores) altered by the introduction of nonnative fishes and an opossum shrimp, M. diluviana. Dominant fish and zooplankton species are shown in the native community (A; 1915–1916), after more than a half century of nonnative fish introductions (B; 1981, 1983), and the present-day community after the introduction of Mysis (C; 1996–2005). Organisms are not drawn to scale, although size of fish roughly represents abundance during each period, with “2×” denoting species approximately twice as abundant as shown. wct, westslope cutthroat trout; bt, bull trout; mwf, mountain whitefish; np, northern pikeminnow; pc, peamouth chub; s, longnose and largescale suckers; lt, lake trout; lwf, lake whitefish; kok, kokanee; yp, yellow perch; m, Mysis; r, rotifers; cl, cladocerans; co, copepods. See also Table S1 for detailed information. Fish illustrations by Joe Tomelleri and zooplankton illustrations by Diane Whited.
Kokanee Period (1920–1984).
In the 1920s, anglers began to report nonnative fishes, and in 1926–1928 lake whitefish and kokanee were caught in netting surveys (22, 25). By 1940 kokanee replaced cutthroat trout as the dominant catch of anglers. Kokanee are landlocked sockeye salmon (the Flathead Lake stock came from the Bonneville, OR hatchery), and they began spawning very successfully in two groundwater upwelling zones on the lake shoreline. In later years as the population grew, Flathead kokanee established other spawning sites, notably the outlet of McDonald Lake in Glacier National Park, and colonized most of the accessible valley bottom lakes upstream of Flathead Lake (24). Similar to the marine-anadromous life history, juvenile kokanee emerge from the gravel spawning sites and migrate immediately to the lake, where they mature as pelagic planktivores.
Fish species compositions in comparable gill netting data (sunken, shoreline sets) contrasting 1915–1916 with 1951–1956 were similar (Table S1), although there was a reduction in native peamouth chub and expansion of nonnative lake whitefish and yellow perch over this interval. Pygmy whitefish and kokanee catches near shore do not accurately index population size, owing to the pelagic distribution of kokanee and the deep water distribution of pygmy whitefish; kokanee were abundant in the fall net sets of the 1950s because those sets were made in near shore spawning areas. Creel surveys from 1962 and 1981 showed that kokanee were the dominant angler catch (77% and 92%) (SI Discussion). During 1979–1983, the kokanee population (three to four age classes) was estimated at 1.6–2.3 million, according to acoustic counts in relation to standardized purse seining throughout the lake (SI Discussion). During 1980–1985, high kokanee spawner abundance drew congregations of bald eagles (peak density exceeding 600) that gathered to feed on the spawning run at the McDonald Creek spawning site (Fig. 2).
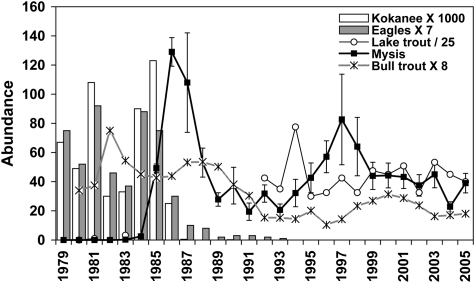
Fig. 2.
Kokanee, eagle, Mysis, lake trout, and bull trout abundance history in Flathead Lake. Data are annual peak numbers of kokanee spawners from Flathead Lake and bald eagles (from weekly eagle and biweekly kokanee spawner counts) at the McDonald Creek index site, lake trout catches (mean catch per unit effort for standardized net sets; note the near detection numbers for 1981 and 1983, no sampling was done in 1982 and 1984–1991), and bull trout redd counts of adfluvial spawners (total annual numbers obtained in standardized, annual census of a suite of spawning streams upstream from Flathead Lake) plotted in relation to abundance of Mysis (mean numbers per m2 with 95% confidence intervals from standardized, annual census at 40 sites lake-wide). To obtain specific densities from the figure, the values shown must be multiplied or divided as indicated in the legend. Bull trout catches in standardized gill net sets declined from one to two fish per net before mysids to less than 0.5 per net after mysids. Updated from Spencer et al. (24).
Standardized gill netting was initiated in 1981, and species composition of fishes other than kokanee was then similar to the 1950s catch data (Table S1), indicating a relatively stable state in the fish assemblage throughout the kokanee period. Note that native cutthroat trout remained at low but measurable densities, nonnative lake whitefish continued to expand, and nonnative lake trout remained at low densities.
All crustacean zooplankton species reported in the native period, except one, remained present and abundant in the kokanee period (Table S2). Whether the available zooplankton forage was more effectively used by kokanee at the expense of cutthroat trout cannot clearly be demonstrated for lack of accurate density data for zooplankton species before 1972; zooplankton were likely a prominent prey item for Flathead Lake cutthroat trout in late fall and winter (26). Phytoplankton again were reported as mainly diatoms, but 1978 autoradiographic analyses showed that the majority of the annual primary productivity (PP) was by nanoplankton of <10 μm (27). Monitoring of PP began in 1977, and values increased from 76 to 92 g C m−2 y−1 by 1985. At the conclusion of the kokanee period, the fish assemblage was predominantly pelagic: with kokanee as zooplankton consumers and bull trout as the top predator, with the other fishes mostly littoral (Table S1), but with expansion in the profundal zone of nonnative lake whitefish and to a lesser degree predatory lake trout.
Mysid Explosion Period (1985–1988).
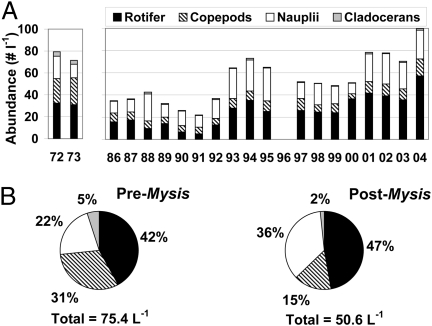
The kokanee period ended abruptly in the mid-1980s with the establishment and rapid expansion of the glacial-relict opossum shrimp, Mysis diluviana (Fig. 2). Mysis was transferred by fisheries managers from Waterton Lake, Alberta, where M. diluviana is native along with lake trout and other fauna of Canadian Shield lakes, to five lakes upstream of Flathead Lake from 1968 to 1976. The intention was to promote kokanee populations by increasing forage, an action based on erroneous interpretations of the results of such introductions elsewhere (28). Mysids reside on the lake bottom during the day and migrate at night to the upper water column, where they feed on large cladoceran zooplankton; whereas kokanee, being obligate visual feeders, consumed cladocerans during daytime. The kokanee sport fishery collapsed the year after peak mysid abundance, along with the eagle–kokanee spawner relationship at McDonald Creek (24) (Fig. 2), and the large-bodied zooplankton (cladoceran and copepod) forage base in Flathead Lake markedly declined (Fig. 3). Zooplankton abundance and biomass declined by half during the Mysis expansion period. Within 2 y of its peak abundance, the Mysis population retreated to less than half of the peak level.
Fig. 3.
(A) Zooplankton abundance by taxonomic group before (1972–1973) and after Mysis (1986–2004). (B) Mean percentage abundance in taxonomic groups of periods before and after Mysis. Pre-Mysis data from Potter (29).
Mysid–Lake Trout Period (1989–Present), the Mysis Reorganization of the Food Web.
After retreat from the initial explosion, Mysis levels fluctuated about what looks to be a new equilibrium, averaging perhaps one third the initial peak level. Kokanee never recovered, bull trout declined, and lake trout came to be the dominant top predator. Bald eagles dispersed after the collapse of their primary prey, kokanee (24). The decline in eagle numbers observed during this study cannot be attributed to the widespread reproductive failures caused by dichlorodiphenyltrichloroethane and its metabolites recognized by the late 1960s. After listing as an endangered species in Montana, the number of nesting pairs increased steadily by an average of 14.5% per year from 1980 to 1990, and the wintering population seemed stable or slightly increasing by 1991 (30).
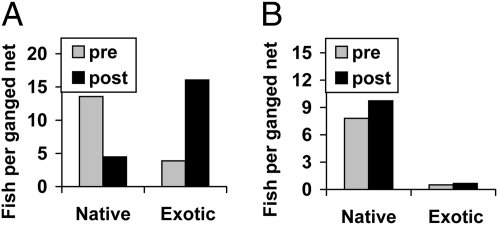
The standardized gill netting before and after mysid expansion clearly showed a remarkable transformation of the Flathead Lake fish community (Fig. 1 and Tables S1 and S3). In surface catches the proportion of natives changed (Fig. 4): cutthroat and bull trout were greatly reduced after Mysis, whereas peamouth and northern pikeminnow increased. In deep water catches, the nonnatives expanded dramatically (Fig. 4): lake trout increased 19-fold and lake whitefish fivefold, largely at the expense of bull trout. Pelagic kokanee were not effectively surveyed by shoreline gill netting, but kokanee clearly were very abundant before Mysis and completely disappeared subsequently. Kokanee represented 92% of the angler catch in 1981, but none of the catch by 1992 (SI Discussion). The most dramatic change in the littoral fish community after Mysis was the large decline in the abundance of the native peamouth chub in sinking net sets; however, the increase observed in floating net sets suggests that some may have simply shifted to the upper water column.
Fig. 4.
Summary of native and exotic fish in Flathead Lake before (1981 and 1983) and after (1996–2005) Mysis establishment. Average number of native and exotic fishes caught in standardized net sets using (A) sinking and (B) floating ganged gill nets (Table S3 shows species composition).
Cladocerans and adult copepods and copepodites represent a smaller share of the zooplankton community after Mysis (Fig. 3). Mysid prey choice trials showed clear selectivity for the large, slow-moving cladocerans over other smaller, faster zooplankton (31). In the lake, cladocerans were reduced by 78% overall, and Bosmina longirostris, which dominated cladoceran abundance before Mysis, declined by 92%. Conversely, Daphnia thorata now dominates cladoceran numbers, increasing in the epilimnion every summer, apparently adapted to warmer temperatures that allow it to avoid predation by the cold-adapted mysids (31). Daphnia rosea and Scapholeberis kingi disappeared. Copepod adults and copepodites also declined by 65%. Leptodiaptomus ashlandi replaced Diacyclops bicuspidatus thomasi as the dominant copepod; Diacyclops declined by 92% after Mysis. L. ashlandi was the only copepod that benefited from the mysid-mediated food web changes. The largest copepod, Epischura nevadensis, declined by 95% after Mysis. The smaller species of zooplankton have increased after Mysis (P < 0.00001 for rotifers and P = 0.001 for copepod nauplii, n = 196), exceeding pre-Mysis densities (Fig. 3), and several rotifer species doubled in abundance after Mysis. The two most abundant rotifers switched in terms of the dominant species before and after Mysis. Filinia terminalis has not recovered from food web changes in the lake (declined 87%), and four rotifer species quantified in 1972–1973 have disappeared.
Cascading changes in the food web after Mysis were not restricted to herbivores and consumers. An unusually large vernal bloom of blue-green algae (Cyanophyceae) occurred in 1988 immediately after mysids vastly reduced the large zooplankton; Cyanophyceae constituted 17.2% of total phytoplankton biomass in 1988, compared with a range of 1.1–3.0% for all years after Mysis. Reduced grazing may have shifted internal nutrient cycling in a way that stimulated blue-greens; however, changes were not limited to the Cyanophyceae. The highest photic zone phytoplankton biomass (0.97 mL m−3) and chlorophyll a (2.9 μg L−1) ever measured in Flathead Lake occurred in 1988, and the highest annual PP measurement was recorded that year (123 g C m−2 yr−1). Numerous other changes in the phytoplankton community were evident during the period of record (19): increase or decrease in biomass and/or density of eight phytoplankton families (all P < 0.05, n = 147), increase in percentage phytoplankton biomass in 10- to 30-μm size fraction (P = 0.01, n = 147), decrease in percentage phytoplankton biomass in >50-μm size fraction (P = 0.0008, n = 147), decrease in 0–30 m chlorophyll a (P = 0.004, n = 230), and increase in PP (P = 0.0001, n = 287).
Discussion
Explanation of CTI in the Transition Mediated by the Mysid Expansion.
What were the mechanisms of the trophic cascade, and how far through the food web did it reverberate in the most recent major community shift? At least four elements of CTI are apparent in the record.
First, mysids became a strong interactor and functionally replaced kokanee as the dominant open water planktivore, but within several years mysids had facilitated dominance by lake trout. Thus the old exotic predator (lake trout) that had been present at low densities for 80 y became a strong interactor in this community only after a second exotic predator (Mysis) of midtrophic level status had invaded. The recent invader, Mysis, facilitated ecological dominance by lake trout by eliminating a recruitment bottleneck for lake trout and lake whitefish, which effectively use them in their juvenile stage. Before mysids, deep water forage was limited to benthos that were available only at very low densities (32). Now, the abundant mysids are released from brood chambers concurrently with emergence of young of the year lake trout. Indeed, recent diet analyses showed that mysids are the main prey item for lake trout up to 60 cm total length and are one of the primary food items for age 2–7 y (>20-cm length) lake whitefish (Fig. S1). Lake trout greater than 60 cm total length eat primarily a fish diet. These and larger size classes of lake trout prey on essentially all fishes in the lake that mouth gape will allow (prey length = 40–50% predator length), including remaining native westslope cutthroat trout, pygmy whitefish, and early stages of bull trout (Fig. S1), thus creating a predator gauntlet for native juveniles emigrating from tributaries to the lake. Bioenergetic modeling corroborated diet interpretations suggesting that increasing lake trout predation was the primary agent of kokanee mortality, with mysids promoting higher recruitment of lake trout (33) (SI Discussion and Tables S4–S6).
Second, the trophic cascade shifted zooplankton community structure from large to smaller forms (Fig. S2). The three largest size fractions (0.4–0.7, 0.7–1.1, and 1.1–2.4 mm) decreased in relative proportion, whereas the smaller size fractions (0.0–0.2 and 0.2–0.4 mm) increased. Altered size structure of zooplankton influences grazing efficiency on phytoplankton (34) and has potential implications for nutrient cycling (35, 36). The demise of kokanee clearly is associated with the changes in zooplankton mediated by the mysids and predation by the expanding lake trout, but indirect effects reverberating from nutrient–PP interactions probably contributed.
Third, we looked for influences of the mysids on PP in relation to nutrient loading over the period 1977–2004. Exploratory regression analysis (SI Materials and Methods) ruled out a dominant role for a cultural eutrophication hypothesis, because significant trends in P and N loading ran in opposite directions (SI Materials and Methods) and bioassays had established colimitation by N and P in the lake (37, 38).
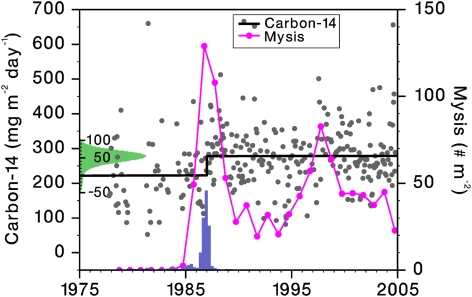
A Bayesian change point analysis was conducted on the time series of deseasonalized, light-corrected PP, fitting two independent linear trajectories allowing one step change between them, with the location (date) of the step also treated as a free parameter. This analysis, as expected from exploratory regression analyses on the pre- and post-Mysis segments (SI Materials and Methods), did not resolve well the before- and after-break slopes (Fig. S3), but it did resolve the change point clearly, showing the following: a 95% probability that the step was an increase; an 88% probability that the step was larger than 25 mg C m−2 d−1 (i.e., a >9% jump); the most probable (posterior mode) step size was 55 mg C m−2 d−1 (i.e., a 21% jump); a 93% probability that the change point or step was before 1988; and a 76% probability that the change occurred in the period 1986–1987, approximately the time when mysid numbers peaked (Fig. 5).
Fig. 5.
Step increase in primary production caused by mysid-mediated CTI. Time series of September Mysis abundance (magenta) and deseasonalized, light-corrected PP (gray dots) for the entire period of record, with regression line (black) from respective posterior modes of the Bayesian change point analysis fitting two independent lines with one step between them. Main y axis PP scale preserves the original long-term mean. x axis histogram shows the posterior distribution for the time (date) position of the step. y axis (rotated) histogram shows the posterior distribution for the height of the step above the beginning of the step. The y axis scale labeling for the y axis histogram (secondary scale) is in the same units as the main PP y axis, but with the origin moved to line up with the bottom of the step.
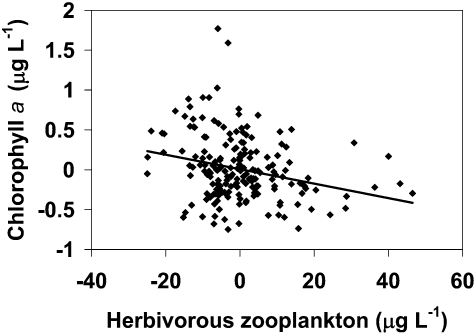
Finally, the shift in zooplankton and nutrients along with the shift in PP suggests that increasing nitrogen might be stimulating algal production, but enhanced grazing by the abundant small size classes of zooplankton after Mysis maintains phytoplankton biomass (as indexed by chlorophyll a concentration) substantially lower than would occur in the absence of mysid predation on zooplankton. Others have shown that consumers can impede the effects of nutrient enrichment on primary producers (10). Indeed, deseasonalized herbivorous zooplankton biomass in Flathead Lake was negatively correlated with deseasonalized chlorophyll a (Fig. 6) in the post-Mysis period. To be certain this relationship was not trend driven, both deseasonalized variables were detrended, and the relationship was still significant (P = 0.0002, n = 168).
Fig. 6.
Detrended, deseasonalized chlorophyll a regressed against detrended, deseasonalized herbivorous zooplankton biomass in Flathead Lake (1987–2004) (P = 0.0002).
The Flathead story documents complex trophic interactions: an introduced invertebrate planktivore displaced piscine planktivores and facilitated ecological dominance by an introduced piscivore (which had been present for decades) that further depressed other piscine planktivores and piscine piscivores. This facilitation of the old invader (lake trout) by the recent invader (Mysis) eventually promoted a rapid shift in community structure, resulting in a trophic cascade affecting phytoplankton, zooplankton, fish, and a nonaquatic species. A top predator like lake trout may or may not be a strong interactor in a lake ecosystem, depending on the community and the species interactions therein. In Flathead Lake, lake trout were a top predator at low densities for many decades but were not a strong interactor until Mysis invaded and the community changed. It remains to be seen how much detailed quantitative knowledge of species-specific parameters would have been needed to predict this particular chain of events. Native species also can have strong effects on food web and ecosystem dynamics. Understanding complex species interactions in native communities as well as those with recent biotic invasions is needed to predict ecological community responses in a changing world.
Lake Trout Period—A Persistent State?
The mysid expansion was a period of strong interaction that accelerated food web change initiated by introduction of exotic fishes. No one knows how far back the native period began; the kokanee period would likely have continued, were it not for the Mysis invasion. The introduction of nonnative species to Flathead Lake has altered the energy flow from pelagic in the early 1900s (zooplankton → peamouth and westslope cutthroat trout → bull trout) and mid-1900s (zooplankton → kokanee and small lake whitefish → bull trout) to a bentho-profundal pathway (zooplankton and benthos → Mysis and small lake whitefish → lake whitefish and small lake trout → lake trout).
Extirpation of some of the native fishes (bull and cutthroat trout) in the near future seems possible (and is decidedly problematic for fisheries managers, given Endangered Species Act threatened status for bull trout) owing to this persistent change in food web structure. Recovery of bull and cutthroat trout will be difficult given strong food web control by the expansive lake trout population. On the other hand, water quality remains near the pristine or native state relative to most lakes with significant numbers of people in the catchment. Understanding trophic cascades requires that long-term data sets be formalized by robust models because of the extreme complexity of interactions. A fully integrative ecosystem model of Flathead Lake is needed to examine emergent properties of these complex interactions. One important challenge is to determine the tipping point for what might be the next ecosystem state as the community continues on its internally driven dynamics and as external drivers such as climate change and direct human intervention (a lake trout reduction program is underway, for example) further force the system.
Materials and Methods
Monthly or more frequent measurements of limnological variables in Flathead Lake and the major tributaries and precipitation chemistry were made from 1977 to 2005 by the Flathead Lake Biological Station (18, 19). Loading of nitrogen (N) and phosphorus (P) to Flathead Lake was determined from measurements of N and P forms from time-series collection on the major tributaries to the lake and the airshed and daily river flow and precipitation volume. Data on species composition and abundance of fishes were obtained annually from standardized gill net sets (SI Materials and Methods) in 1981, 1983, and 1996–2005. Bull trout abundance was determined from standardized redd counts of adfluvial spawners in tributaries used by Flathead Lake fish and collected annually since 1981 (SI Materials and Methods). From 1985 to 2004, duplicate or triplicate 50 m to surface vertical hauls of zooplankton were made approximately monthly at the midlake deep site with a 29-cm-diameter, 64-μm-mesh Wisconsin style net, preserved (4% CaCO3 neutralized formalin), and enumerated using Sedgewick-Rafter cells (19). Monthly or more frequent phytoplankton samples were obtained at the midlake deep site from a 0- to 30-m tubular composite integrating sampler, preserved (1% Lugol's iodine), and enumerated using Utermohl chambers. Mysid numbers were standardized to sampling on moonless nights in September. Vertical hauls were made from near bottom to the surface at approximately 40 sites with a 1-m-diameter, 500-μm-mesh closing net. Monthly or more frequent PP estimates were determined from 4-h in situ incubation of NaH14CO3 in light and dark bottles throughout the lighted zone (0–30 m) (39, 40). Deseasonalizing for statistical analysis was accomplished by fitting a cubic smoothing spline to an overlay of the data for all of the years. The data were grouped into 1-, 3-, 5- and 7-d pools and wrapped around the end of the year for 65 d to encourage continuity at the year ends. The smoothing factor was selected so as to minimize the cross-validation jackknife residuals. Seasonality in each of the variables was removed by subtracting the spline model. The results were examined for linear trends using simple linear regression analysis. Analysis of the pattern of change over time in PP was further examined with a Bayesian change point model. Full methods and associated references are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank two anonymous reviewers for their guidance; T. Stuart and D. Potter for their early studies of Flathead Lake; T. Weaver for redd counts; numerous Flathead Lake Biological Station staff for their assistance in the field, analytical laboratory support, database management, and zooplankton enumeration; and numerous scientists from the Washington Cooperative Fish and Wildlife Unit (SI Acknowledgments) for fish diet analyses and food web bioenergetic modeling. Funding for data analysis and manuscript preparation was provided by the Jessie M. Bierman Professorship, Flathead Lake Biological Station.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013006108/-/DCSupplemental.
References
- 1.Carpenter SR, Cole JJ, Kitchell JF, Pace ML. Trophic cascades in lakes: Lessons and prospects. In: Terborgh J, Estes J, editors. Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature. Washington, DC: Island Press; 2010. pp. 55–69. [Google Scholar]
- 2.Hansson LA, et al. Biomanipulation as an application of food-chain theory: Constraints, synthesis and recommendations for temperate lakes. Ecosystems. 1998;1:558–574. [Google Scholar]
- 3.Pace ML, Cole JJ, Carpenter SR, Kitchell JF. Trophic cascades revealed in diverse ecosystems. Trends Ecol Evol. 1999;14:483–488. doi: 10.1016/s0169-5347(99)01723-1. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter SR, Kitchell JF, Hodgson JR. Cascading trophic interactions and lake productivity. Bioscience. 1985;35:634–639. [Google Scholar]
- 5.Eby LA, Roach WJ, Crowder LB, Stanford JA. Effects of stocking-up freshwater food webs. Trends Ecol Evol. 2006;21:576–584. doi: 10.1016/j.tree.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 6.de Ruiter PC, Neutel A-M, Moore JC. Energetics, patterns of interaction strengths, and stability in real ecosystems. Science. 1995;269:1257–1260. doi: 10.1126/science.269.5228.1257. [DOI] [PubMed] [Google Scholar]
- 7.McQueen DJ. Manipulating lake community structure: Where do we go from here? Freshw Biol. 1990;23:613–620. [Google Scholar]
- 8.Elser JJ, Goldman CR. Zooplankton effects on phytoplankton in lakes of contrasting trophic status. Limnol Oceanogr. 1991;36:64–90. [Google Scholar]
- 9.Persson L, Andersson G, Hamrin SF, Johansson L. In: Complex Interactions in Lake Communities. Carpenter S, editor. New York: Springer; 1988. pp. 45–65. [Google Scholar]
- 10.Carpenter SR, et al. Trophic cascades, nutrients and lake productivity: Whole-lake experiments. Ecol Monogr. 2001;71:163–186. [Google Scholar]
- 11.Michener WK, et al. Defining and unraveling biocomplexity. Bioscience. 2001;51:1018–1023. [Google Scholar]
- 12.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth's ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 13.Persson L, et al. Gigantic cannibals driving a whole-lake trophic cascade. Proc Natl Acad Sci USA. 2003;100:4035–4039. doi: 10.1073/pnas.0636404100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daskalov GM, Grishin AN, Rodionov S, Mihneva V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc Natl Acad Sci USA. 2007;104:10518–10523. doi: 10.1073/pnas.0701100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaret TM, Paine RT. Species introduction in a tropical lake: A newly introduced piscivore can produce population changes in a wide range of trophic levels. Science. 1973;182:449–455. doi: 10.1126/science.182.4111.449. [DOI] [PubMed] [Google Scholar]
- 16.Roemer GW, Coonan TJ, Garcelon DK, Bascompte J, Laughrin L. Feral pigs facilitate hyperpredation by golden eagles and indirectly cause the decline of the island fox. Anim Conserv. 2001;4:307–318. [Google Scholar]
- 17.Springer AM, et al. Sequential megafaunal collapse in the North Pacific Ocean: An ongoing legacy of industrial whaling? Proc Natl Acad Sci USA. 2003;100:12223–12228. doi: 10.1073/pnas.1635156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanford JA, Ellis BK. In: Rocky Mountain Futures: An Ecological Perspective. Baron JS, editor. Washington, DC: Island Press; 2002. pp. 269–284. [Google Scholar]
- 19.Ellis BK. PhD dissertation. Missoula, MT: Univ of Montana; 2006. Alternate states in a large oligotrophic lake: A retrospective analysis of nutrient loading and food web change. [Google Scholar]
- 20.Forbes SA. A preliminary report on the aquatic invertebrate fauna of the Yellowstone National Park, Wyoming, and of the Flathead region of Montana. Bull US Fish Comm. 1893;11:207–258. [Google Scholar]
- 21.Elrod MJ. Limnological investigations at Flathead Lake, Montana and vicinity, July 1899. Trans Am Microsc Soc. 1901;22:68–80. [Google Scholar]
- 22.Elrod MJ. The fishes of Flathead Lake Montana. Montana Wild Life. 1929;11:6–9. [Google Scholar]
- 23.Evermann BW. A reconnaissance of the streams and lakes of western Montana and northwest Wyoming. Bull U S Fish Comm. 1893;11:3–60. [Google Scholar]
- 24.Spencer CN, McClelland BR, Stanford JA. Shrimp stocking, salmon collapse and eagle displacement: Cascading interactions in the food web of a large aquatic ecosystem. Bioscience. 1991;41:14–21. [Google Scholar]
- 25.Bickford WM. Biennial Report of the Montana Fish and Game Commission 1925–1926, ed Montana State Fish and Game Commission. Helena, MT: Independent Publishing; 1927. pp. 31–33. [Google Scholar]
- 26.Brunson RB, Pennington RE, Bjorklund RG. On a fall collection of native trout (Salmo clarkii) from Flathead Lake, Montana. Proc Mont Acad Sci. 1952;12:63–67. [Google Scholar]
- 27.Ellis BK, Stanford JA. Comparative photoheterotrophy, chemoheterotrophy and photolithotrophy in a eutrophic reservoir and an oligotrophic lake. Limnol Oceanogr. 1982;27:440–454. [Google Scholar]
- 28.Northcote TG. In: in Mysids in Fisheries: Hard Lessons from Headlong Introductions. Nesler TP, Bergerson EP, editors. Bethesda, MD: American Fisheries Society Symposium 9; 1991. pp. 5–16. [Google Scholar]
- 29.Potter DS. PhD dissertation. Missoula, MT: Univ of Montana; 1978. The zooplankton of Flathead Lake: An historical review with suggestions for continuing lake resource management. [Google Scholar]
- 30.Flath DL, Hazlewood RM, Harmata AR. Status of the bald eagle (Haliaeetus leucocephalus) in Montana: 1990. Proc Mont Acad Sci. 1991;51:15–32. [Google Scholar]
- 31.Spencer CN, Potter DS, Bukantis RT, Stanford JA. Impact of predation by Mysis relicta on zooplankton in Flathead Lake, Montana, USA. J Plankton Res. 1999;21:51–64. [Google Scholar]
- 32.Newell RL, Andersen DL, Hanzel DA. Bottom fauna as an indicator of lake topology in Flathead Lake, Montana. Northwest Sci. 1978;52:119–126. [Google Scholar]
- 33.Beauchamp DA, Wahl D, Johnson BM. In: Analysis and Interpretation of Inland Fisheries Data. Guy CS, Brown MJ, editors. Bethesda, MD: American Fisheries Society; 2007. pp. 765–842. [Google Scholar]
- 34.Cyr H, Curtis JM. Zooplankton community size structure and taxonomic composition affects size-selective grazing in natural communities. Oecologia. 1999;118:306–315. doi: 10.1007/s004420050731. [DOI] [PubMed] [Google Scholar]
- 35.Sterner RW, Elser JJ, Hessen DO. Stoichiometric relationships among producers, consumers and nutrient cycling in pelagic ecosystems. Biogeochemistry. 1992;17:49–67. [Google Scholar]
- 36.Wen YH, Peters RH. Empirical models of phosphorus and nitrogen excretion rates by zooplankton. Limnol Oceanogr. 1994;39:1669–1679. [Google Scholar]
- 37.Spencer CN, Ellis BK. Co-limitation by phosphorus and nitrogen, and effects of zooplankton mortality, on phytoplankton in Flathead Lake, Montana, USA. Verh Int Ver Theor Angew Limnol. 1990;24:206–209. [Google Scholar]
- 38.Spencer CN, Ellis BK. Role of nutrients and zooplankton in regulation of phytoplankton in Flathead Lake (Montana, USA), a large oligotrophic lake. Freshw Biol. 1998;39:755–763. [Google Scholar]
- 39.Theodorsson P, Bjarnason JO. The acid-bubbling method for primary productivity measurements modified and tested. Limnol Oceanogr. 1975;20:1018–1019. [Google Scholar]
- 40.Wetzel RG, Likens GE. Limnological Analyses. New York: Springer-Verlag; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.