Abstract
It has long been argued that modern human mothers give birth to proportionately larger babies than apes do. Data presented here from human and chimpanzee infant:mother dyads confirm this assertion: humans give birth to infants approximately 6% of their body mass, compared with approximately 3% for chimpanzees, even though the female body weights of the two species are moderately convergent. Carrying a relatively large infant both pre- and postnatally has important ramifications for birthing strategies, social systems, energetics, and locomotion. However, it is not clear when the shift to birthing large infants occurred over the course of human evolution. Here, known and often conserved relationships between adult brain mass, neonatal brain mass, and neonatal body mass in anthropoids are used to estimate birthweights of extinct hominid taxa. These estimates are resampled with direct measurements of fossil postcrania from female hominids, and also compared with estimates of female body mass to assess when human-like infant:mother mass ratios (IMMRs) evolved. The results of this study suggest that 4.4-Myr-old Ardipithecus possessed IMMRs similar to those found in African apes, indicating that a low IMMR is the primitive condition in hominids. Australopithecus females, in contrast, had significantly heavier infants compared with dimensions of the femoral head (n = 7) and ankle (n = 7) than what is found in chimpanzees, and are estimated to have birthed neonates more than 5% of their body mass. Carrying such proportionately large infants may have limited arboreality in Australopithecus females and may have selected for alloparenting behavior earlier in human evolution than previously thought.
Keywords: climbing, hominin, Homo, cooperative breeding
Human mothers give birth to relatively large neonates (1–5). In catarrhine primates, there is a strong allometric relationship between the mass of the mother and the mass of an infant, with a R2 of 0.98 and slope of 0.69 (6) (Fig. S1). From this linear regression, it is expected that humans should give birth to infants that are 2 to 2.2 kg (2, 7). However, humans are exceptional, and have newborns weighing 50% more than expected, averaging more than 3 kg (Table S1). Birthing larger infants not only causes obstetric difficulties, but also introduces the energetic and biomechanical challenge of transporting a relatively large, helpless newborn. This is particularly the case for pretechnological, upright walking hominids, some of which had reduced pedal grasping abilities. Thus, it has generally been argued that many of the uniquely human life history features, such as birthing large helpless infants, extended juvenile period, extended lifespan, and shorter interbirth interval may have emerged with the more technologically adept Homo erectus (4, 5, 8).
Previous work has shown that there is a strong allometric relationship (R2 = 0.97; m = 0.73) between the size of the brain as an adult and the size of the brain at birth in catarrhine primates (9). This relationship has been used to predict the size of the brain at birth in extinct hominid species, a model that has since been independently supported with fossil evidence (10, 11) (Table S2). Neonatal brain mass estimates can in turn be used to generate estimates of neonatal body mass (NBM) because of the isometric relationship between brain and body mass at birth across anthropoids (R2 = 0.92; m = 0.94) (6). In fact, it has been argued that neonatal primates are all born with 12% of their body mass consisting of brain tissue (2, 12, 13). However, this 12% “rule” does not apply to apes. At birth, apes possess a brain that is 10% of body mass whereas a newborn human's brain is on average 12.3% of body mass (Table S3). Given that brain mass at birth can be estimated from adult cranial capacities in fossil hominids (9), NBM estimates for extinct hominid taxa can then be calculated by using an ape model (10%) or a human model (12.3%).
This study calculates a range of NBMs in extinct hominid taxa and presents these data relative to direct measures of femoral head diameter (FHD) and the width of the ankle joint in female Australopithecus specimens (Table S4). To avoid the inherent error of predictions by regression, these ratios are compared with resampled distributions of chimpanzee NBMs (n = 50) with both female FHD (n = 46) and tibial dimensions (n = 20) as explained in Materials and Methods. Calculated NBMs are also compared with the estimated body mass of adult female hominids (14–16). Infant:mother mass ratios (IMMRs) are calculated by using average female and neonatal masses from human populations spanning the globe (n = 18). Additionally, and more importantly, extinct hominid IMMRs are compared with data from actual infant:mother dyads for chimpanzees (n = 47) and modern humans (n = 2,607) rather than solely from population means.
In this study, two hypotheses are tested. The first is a more rigorous test of the long-held hypothesis that humans birth proportionately heavier infants than chimpanzees do, and that the chimpanzee condition is the primitive one for hominids. The second hypothesis tested is that Australopithecus possessed a primitive, chimpanzee-like IMMR.
Results and Discussion
Chimpanzees give birth to infants that are 3.3% the mass of the mother [95% confidence interval (CI), 3.0–3.5%; Table 1]. These data from the Yerkes National Primate Research Center (YNPRC; Atlanta, GA) are consistent with two other chimpanzee populations (Table S1), although more reliable given that the YNPRC data sample actual infant:mother dyads rather than comparing population averages. Infant:mother dyad data are not available for gorillas, although a sample of infant masses (n = 107) (17) compared with mean female body mass in captivity (18) yields an IMMR of 2.7% (95% CI. 2.6–2.8%; Table 1). This lower IMMR in gorillas is expected given that NBM scales with negative allometry (Fig. S1).
Table 1.
IMMRs in modern apes and humans
| Species | n | Mean IMMR, % (95% CI) | Source |
| Gorilla gorilla* | 107 | 2.7 (2.6–2.8) | 17, 18 |
| Pan troglodytes | 47 | 3.3 (3.0–3.5) | YNPRC |
| H. sapiens | 2,607 | 6.1 (6.1–6.1) | Cebu Longitudinal Health and Nutrition Survey |
*Unlike the chimpanzee and human data, the gorilla data are not derived from actual infant:mother dyads and may not be as reliable.
A large sample (N = 2,607) of modern human infant:mother dyads from the Cebu (Philippines) Longitudinal Health and Nutrition Survey yields an IMMR of 6.1% (95% CI, 6.05–6.13%; Table 1). Data from 18 human populations for which both infant and mother's mass averages and SDs are available (total, N = 11,317) demonstrate that humans have infants that are 5.7% of female body mass, with populations ranging from a low of 4.8% to a high of 6.5% (Table S1). There is no overlap between the 95% CIs of any modern human population and those of the great apes. This result is consistent with previous studies suggesting that humans birth exceptionally heavy neonates (1, 2, 5). Occasional reports that humans and chimpanzees have more equivalent IMMRs (e.g., ref. 19) are based on mixed datasets in which masses of neonatal chimpanzees birthed in captivity are combined with small body mass estimates of female chimpanzees in the wild.
NBM in Extinct Hominids.
Ardipithecus ramidus, a 4.4 Myr hominid, is best represented by the female partial skeleton ARA-VP-6/500 (20). Application of an ape growth model yields a 1.3-kg infant, whereas a human model predicts a 1.0-kg infant. Compared directly with a range of possible FHDs estimated from the acetabulum of ARA-VP-6/500 (equation in ref. 16 using acetabulum values reported in ref. 21), the NBM1/3/FHD ratio in Ardipithecus is in the chimpanzee range (Fig. 1A). However, the range of values for both the cranial capacity and the acetabulum of ARA-VP-6/500 yields a ratio with possible values so large as to render this comparison uninformative (Fig. 1A). However, the dimensions of a complete talus (ARA-VP-6/500–023) result in an NBM1/3/ankle width ratio in the low end of the chimpanzee range (Fig. 1B). Using the body mass estimate for ARA-VP-6/500 of 50 kg (24), Ardipithecus would have birthed infants that were 2.1% to 3.2% of the mother's body mass, within the range of modern African apes (Table 2 and Fig. 2). These data support the hypothesis that a low IMMR is the primitive condition for the African hominids.
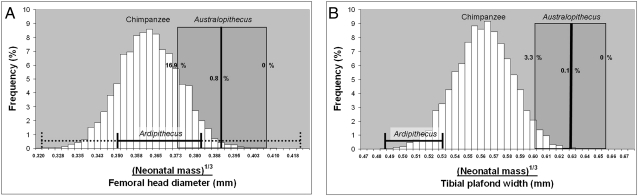
Fig. 1.
NBM1/3 was resampled with replacement and divided by resampled FHDs (A) or tibial plafond widths (B) of female chimpanzees 5,000 times to obtain a distribution of NBM1/3/postcranial dimension means. These data were compared with extinct hominid estimates of NBM1/3 divided by direct measurements of the FHD or the acetabulum (A) using an ape model. Australopithecus had significantly larger NBM1/3/FHD than modern chimpanzees. Ardipithecus has small chimpanzee-like values although the large range of cranial capacity (dark line) and femoral head (dotted line) estimates renders this comparison uninformative. The difference between Australopithecus and chimpanzees is even more extreme in the ankle joint (B), and in this case the Ardipithecus value (from the talus) is more secure and falls within the chimpanzee distribution. In each figure, the dark line is the average, with the shaded box illustrating the 95% CI for the estimate of NBM1/3 divided by the femoral head (A) or the ankle width (B). Recently, data from an undescribed skull and postcrania (two femora, a tibia and talus) from A. afarensis were published (22, 23). Incorporation of these data into this study would barely alter the likelihood of sampling the Australopithecus ratio from a chimpanzee data set from 0.8% to 1.5% for the femoral head and from 0.1% to 0.02% for the ankle joint. However, these results should be considered preliminary until full descriptions of these new fossils are published.
Table 2.
Calculation of IMMR in fossil hominins
| Ape model |
Human model |
Intermediate model |
||||||||
| Species | Age (Myr) | Mean adult brain mass ± SD, cc* | Mean adult female mass, g | Mean neonatal brain mass ± SD, g* | Mean NBM ± SD, g | IMMR (×100) | Mean NBM ± SD, g | IMMR (×100) | Mean NBM ± SD, g | IMMR (×100) |
| Ar. ramidus† | 4.4 | 300 (280–350) | 50,000 | 126.5 (120.4–141.3) | 1,265.0 (1065.0–1600.1) | 2.1–3.2 | 1,028.4 (865.8–1,300.9) | 1.7–2.6 | 1,129.4 (950.9–1,428.7) | 1.9–2.9 |
| A. afarensis | 3.0–3.7 | 455.6 ± 79.4 | 29,000 | 170.2 ± 21.2 | 1,702.1 ± 211.9 | 5.2–6.7 | 1,383.8 ± 172.3 | 4.2–5.4 | 1,519.7 ± 189.2 | 4.6–6.0 |
| A. africanus | 2.0–2.7 | 463.3 ± 50.6 | 31,000 | 172.5 ± 13.4 | 1,724.6 ± 133.8 | 4.9–6.3 | 1,402.1 ± 108.8 | 4.0–5.1 | 1,539.8 ± 119.5 | 4.4–5.6 |
| P. boisei | 1.4–2.4 | 498.4 ± 34.2 | 34,000 | 181.8 ± 9.0 | 1,818.3 ± 90.1 | 4.7–6.1 | 1,478.3 ± 73.3 | 3.8–4.9 | 1,623.5 ± 80.5 | 4.2–5.4 |
| P. robustus | 1.4–2.0 | 530.0 ± 0 | 32,000 | 190.1 ± 0 | 1901.0 ± 0 | 5.2–6.8 | 1,545.0 ± 0 | 4.2–5.5 | 1,697.3 ± 0 | 4.7–6.0 |
| H. habilis | 1.7–1.9 | 612.2 ± 63.2 | 32,000 | 210.6 ± 15.7 | 2,105.6 ± 157.1 | 5.8–7.5 | 1,711.9 ± 127.8 | 4.7–6.1 | 1,880.0 ± 140.3 | 5.2–6.7 |
| Dmanisi | 1.8 | 670.0 ± 74.3 | 40,200 | 224.6 ± 17.7 | 2,246.2 ± 176.8 | 4.9–6.4 | 1,826.2 ± 143.7 | 4.0–5.2 | 2,005.6 ± 157.8 | 4.4–5.7 |
| H. erectus | 0.7–1.78 | 886.0 ± 107.4 | 46,000 | 274.2 ± 23.8 | 2,742.2 ± 238.7 | 5.2–6.8 | 2,229.4 ± 194.1 | 4.2–5.5 | 2,448.4 ± 213.2 | 4.7–6.1 |
| Atapuerca | 0.53 | 1,245.0 ± 134.3 | 60,600 | 350.0 ± 27.0 | 3,500.4 ± 269.5 | 5.0–6.6 | 2,845.9 ± 219.1 | 4.1–5.4 | 3,125.4 ± 240.6 | 4.5–5.9 |
| Skhul-Qafzeh | 0.09 | 1,498.4 ± 106.0 | 58,400 | 399.8 ± 20.5 | 3,998.2 ± 205.4 | 6.0–7.9 | 3,250.6 ± 167.0 | 4.8–6.4 | 3,569.8 ± 183.4 | 5.3–7.0 |
| Pan troglodytes | — | 384.2 ± 39.8 | 53,600 ± 2,200 | 152.8 ± 16.6 | 1,766 ± 369.1 | 3.3 | — | — | — | — |
| H. sapiens | — | 1,336.1 ± 127.7 | 57,100 ± 8,300 | 373.8 ± 100.6 | — | — | 3,111 ± 461.4 | 5.7 | — | — |
*Based on average of conversion of adult brain (cc) to brain (g) using refs. 25 and 26. Calculated using regression equation in ref. 9 and adult cranial capacities listed in Table S4. Extant data are weighted averages from sources listed in Tables S1, S3, and S6.
†Because Ardipithecus is represented by only a single skull, the values in parentheses are based on the full range of estimates of 280–350 cc.
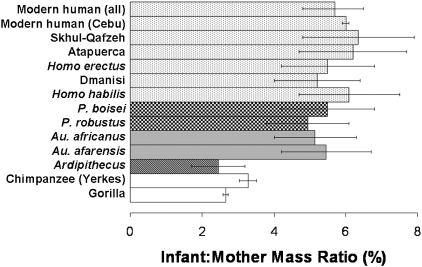
Fig. 2.
IMMRs for modern chimpanzees, humans, and fossil hominids. Notice that Ardipithecus has a small, ape-like ratio of infant:mother mass. However, members of the genus Australopithecus have near modern-human IMMRs of more than 5% (range, 4.0–6.7%). These data suggest that Pliocene hominids were already birthing proportionately heavy neonates. These human-like ratios remain nearly constant in Paranthropus, early Homo, and H. sapiens. For Ardipithecus, column length represents the value obtained by using a 300-cc brain and 50-kg female, and the bars show the 95% CI of values calculated by using a range of cranial capacities from 280 cc to 350 cc. For all other fossil taxa, column lengths represent mean values and the bars are the entire range of values calculated by using both ape and human models of brain development. For modern human (all), column length is the mean and the bars the full range of IMMRs for 18 modern human populations (Table S1). For humans (Cebu), chimpanzees (YNPRC), and gorillas, the column lengths represent mean values and the bars are the 95% CI.
Data for Australopithecus, however, do not support the hypothesis of an apelike IMMR in hominids by the late Pliocene. Based on 12 adult crania (Table S5), the neonatal brain mass estimate is 170 g, and the NBM is estimated to be approximately 1.7 kg by using an ape model of brain development (Table 2). This ape model is likely to be correct for Australopithecus given the evidence that a more human-like pattern of prenatal brain growth may not have been achieved until H. erectus (9, 27) or even later (28). Furthermore, the large neonatal brain in humans is supported in part by increased levels of infant body fat, thought to be related to a high quality diet not adopted by hominids until the genus Homo (29). Compared directly with the FHD of presumed female specimens from Ethiopia (Australopithecus afarensis) and South Africa (Australopithecus africanus; Table S4), Australopithecus infants are proportionately large compared with chimpanzee values (Fig. 1A). In fact, only 0.8% of the resampled chimpanzee values exceeded the average ratio in Australopithecus, and only the very lowest neonatal body size estimates for Australopithecus yielded NBM1/3/FHD ratios that could be sampled from a population of modern chimpanzees. Even then, only 16.9% of the 5,000 resampled combinations yielded such values. Only if an intermediate model (i.e., an average of human and chimpanzee brain development) is used does the ratio in Australopithecus become more chimpanzee-like. Even under these unlikely conditions of prenatal brain growth, the possibility of sampling the Australopithecus NBM1/3/FHD average from a chimpanzee population is still only 12.7%. The femoral head of Australopithecus, however, may be small because of the exceptionally long lever arm of the hip abductors and the resulting small joint reaction force at the hip (30). Therefore, another weight-bearing joint, the ankle, was examined.
Compared with the mediolateral width of the ankle joint (tibia or talus), Australopithecus newborns were quite heavy (Fig. 1B). The average NBM1/3/ankle width ratio in Australopithecus can be sampled from a chimpanzee population only 0.1% of the time, and only 3.3% of the time even when the smallest NBM estimates are used. If the intermediate human/chimpanzee model is used, the likelihood of sampling an Australopithecus NBM1/3/ankle width ratio from a modern chimpanzee dataset is only 2.4%. These data, acquired by comparing a range of NBM estimates to direct measures of female Australopithecus postcrania, suggest that the IMMR in Australopithecus was, on average, significantly higher than what is found in modern chimpanzees.
Given that the FHD and the mediolateral width of the ankle joint have been used to estimate body mass in female hominids (14), it is not surprising that IMMRs based on female body mass estimates are much higher in Australopithecus than in Ardipithecus, chimpanzees, or gorillas (Fig. 2). Applying an ape model of brain development and an average female body mass estimate of 29 kg (14, 24), A. afarensis has an IMMR of between 5.2% and 6.7%. A. africanus exhibits a similarly large ratio (4.9–6.3%). To have a chimpanzee-like IMMR of 3.3%, Australopithecus females would have to have been larger than 50 kg, meaning that mass estimates of Australopithecus females, like “Lucy,” would have to have been miscalculated by approximately 70%.
When the near-modern human range of IMMR had been reached by Australopithecus, it remained relatively stable (Fig. 2). IMMRs are Australopithecus-like in Paranthropus boisei and Paranthropus robustus and in early members of the genus Homo (Fig. 2). However, ratios of postcranial dimensions and NBM estimates are more difficult with early Pleistocene taxa because of small sample sizes and the challenge of definitively assigning isolated postcrania to one of several coexisting hominid taxa. For example, the female body mass in Homo habilis is based in part on OH 8, which may be from P. boisei and not H. habilis (31), and OH 62, which does not preserve any weight-bearing joints (32). The ideas presented in this study should therefore be revisited when taxonomically unambiguous female postcrania from the Pleistocene are recovered.
Early Homo may be best represented by the remains from Dmanisi, Georgia. Neonates of this population are calculated to have been approximately 2.0 kg, with 225-g brains. Body size estimates of the Dmanisi females are approximately 40 kg (33), and thus the infants would have been approximately 5% of the mother's body mass. Because H. erectus is a widespread, evolving lineage, it is necessary to subdivide this species into spatiotemporal, although not necessarily taxonomic, units. Estimates of female body mass have been recently revised to 46 kg based on early Pleistocene postcrania tentatively assigned to H. erectus (16). Crania from this time period yield an NBM estimate of 2.5 kg and an IMMR of approximately 5.5%. Of particular interest to the question of IMMR in H. erectus is the female pelvis BSN49/P27 from Gona, Ethiopia (10), which is remarkable in its small size, estimated to be from a female of only 33.2 kg (16). Homo erectus neonates from the same geological age (900 Kyr to 1.4 Myr) are predicted to have had a cranial capacity of 287.4 cc (Table S2). This neonatal brain estimate suggests an NBM of 2.3 kg, and a 6.7% to 7.0% IMMR using a human model. An ape model yields a highly unlikely 2.8-kg infant and an IMMR in excess of 8%. If the Gona pelvis is from H. erectus, then this species was at least occasionally birthing relatively heavy infants on the high end of the modern human IMMR range. Alternatively, the Gona pelvis may not be from H. erectus, and may instead be from P. boisei (16). If so, calculations of neonatal brain volume in P. boisei of 186 cc (Table 2) together with a birth canal that could accommodate an approximately 300-cc brain (10), suggests that birth would have been a relatively easy process in paranthropines. The Gona pelvis thus presents two equally interesting, but exclusive, possibilities: either the Gona pelvis provides evidence that H. erectus was at least occasionally birthing proportionately heavy infants with an IMMR on the high end of the modern human range, or P. boisei has a relatively voluminous birth canal that allowed for an easy birth process. Whichever scenario proves to be correct, the data presented here suggest that H. erectus possessed a high IMMR. Hominids continued to birth proportionately large infants through the middle Pleistocene to Homo sapiens (Table 2 and Fig. 2).
The hypothesis that Australopithecus had a chimpanzee-like IMMR is not supported by the data presented in this study. By 3.2 Myr and perhaps earlier, females of the genus Australopithecus were giving birth to relatively large infants, approximately 5% to 6% of their own body mass, indicative perhaps of a grade shift from an Ardipithecus-like ancestor (Fig. S1). The findings of this study are supported both by comparing NBM estimates to female body mass estimates, and by comparing them directly to measures of female Australopithecus postcrania. Importantly, even the very lowest estimates of the IMMR for Australopithecus (4.0%), calculated by using the lowest NBM estimates and a modern human brain development model, fall outside the 95% CI for modern apes. To further test the validity of the methods used in this study, chimpanzee NBMs were “calculated” from adult chimpanzee cranial capacities, instead of using chimpanzee newborn masses directly. This was done to mimic the procedure being used to calculate Australopithecus NBM from adult cranial capacity. These estimates of body mass were resampled with chimpanzee femoral head and ankle dimensions and the results mirror those illustrated in Fig. 1 (Fig. S2).
An important caveat is that hominid body masses are based not only on a small number of often taxonomically ambiguous fossil specimens, but also contain large CIs (14–16). However, body mass estimates of female hominids would have to have been grossly and systematically underestimated from Australopithecus right through to late Pleistocene H. sapiens for the ratios calculated in this study to be more chimpanzee-like than human-like. Additionally, these results are corroborated by direct comparisons made on the postcranial skeletons of Australopithecus, which showed significantly larger [NBM]1/3/FHD and [NBM]1/3/tibial plafond width ratios than the resampled range calculated for modern chimpanzees.
The surprising finding that Pliocene hominids were birthing proportionately heavy infants can be explained by two fundamental differences between Australopithecus and modern chimpanzees. First, Australopithecus had both relatively and absolutely larger brains than modern chimpanzees (Table S6). Because of the slightly larger adult brains of Australopithecus, these hominids would have had infants with larger brains than the infants of chimpanzees given the strong correlation between neonatal and adult brain mass (9), and therefore slightly larger bodies as demonstrated in this study. Second, Australopithecus females were smaller than female chimpanzees. Australopithecus females are estimated to have been between 29 and 33 kg (14, 15), below the 34 to 46 kg range reported for wild female chimpanzees (34) and the 44 to 61 kg range of captive female chimpanzees (18) (Table S1). Direct comparisons of adult cranial capacity to femoral head and ankle dimensions reveal significant differences between female chimpanzees and Australopithecus (Fig. S2). These two known differences between Australopithecus and modern chimpanzees result in a significant IMMR difference that aligns the Australopithecus more with the modern human condition than with the African ape condition. Only later, in the genus Homo, did both brain size and body size increase. However, because these occurred more or less concurrently, the IMMR remained unchanged (Table 2 and Fig. 2).
Implications for Large Neonates in Human Evolution.
These findings have several important implications for reconstructing early hominid locomotion, social systems, obstetrics, and energetics. First, chimpanzees are skilled and frequent tree climbers. The females have little difficulty ascending a vertical substrate even while carrying an infant because of the relatively small size of the infant, and because of the grasping halluces keeping both the infant attached to the mother and the mother to the tree (Fig. S3). The postcranial anatomy of A. afarensis and A. africanus is largely inconsistent with frequent and skilled tree climbing (e.g., refs. 35–37), although these hominids may have occasionally taken refuge in trees and there may be more locomotor diversity in the genus. The results of this study suggest that females of these two Australopithecus species were transporting proportionately large infants, a situation that would have rendered arboreality a more dangerous activity. This is further exacerbated by the absence of a grasping toe in A. afarensis (38, 39), the elimination of dorsal riding as an option for infant hominids (40), and the possibility that body hair was thinning by 3.3 Myr ago (41). With a limited capacity to grasp, Australopithecus infants may have been parked (42) or actively carried by their bipedal mothers, at times leaving these females with only a single arm free for climbing.
Carrying infants without technological assistance is energetically expensive for humans (43, 44) and nonhuman primates alike (45). Carrying an infant, without the help of a sling, has been found to increase energetic costs during locomotion in human females by 16% (43), and thus the costs of carrying an infant may have also reduced the amount of traveling done by female Australopithecus (46). In addition to infant carrying postnatally, having a proportionately large infant would have resulted in carrying costs during pregnancy itself, and anatomical changes in the lumbar spine of modern human females and of female A. africanus may reflect this (47). These data also suggest that Australopithecus females were birthing infants that were near the pelvic outlet capacity, as inferred from reconstructions of the A.L. 288–1 Lucy pelvis (48). The hominid NBMs calculated in this study are larger than some previous estimates (49), but smaller than others (7), making the results presented here generally consistent with data used to characterize birth in hominids. Mediolaterally broad outlets in hominid pelves (10, 48) indicate that the modern mechanism of rotational birth may have evolved quite recently (50, 51). However, given such congruence between neonatal head and body size, and pelvic proportions in Australopithecus, birth may still have been a challenging physiological event (3, 48), perhaps requiring the assistance of helpers (52), especially if shoulder rotation during asynclitic birth occasionally caused occiput anterior orientation of the newborn head (53).
The data presented in this study help reconstruct Australopithecus as a primarily ground-dwelling hominid whose strikingly small females carried proportionately large infants. If additional fossil specimens of Ardipithecus confirm a relatively large female (∼40–50 kg), the body size dimorphism present in Australopithecus may be the result of female body mass reduction, rather than an increase in male body mass. Small body mass in females may have had important consequences for the social structure of Australopithecus.
By applying Australopithecus brain mass (n = 12) to the equation provided by Garwicz et al. (54), it can be inferred that Australopithecus babies would have been relatively immobile and unable to walk on their own for the first 6 to 7 mo of their lives. The NBM predicted in this study may serve as a proxy for overall relative body size in the first year of life given that, by 1 y, chimpanzees still weigh only 8.6% the mass of the mother (n = 9; infant:mother dyads from YNPRC), whereas human babies are approximately 14% of the mother's mass (55). During this first year, a female Australopithecus would have faced the challenge of obtaining nutrients to sustain herself and to breast-feed a growing infant, and would have benefited from the help of pair-bonded males (56–58), older children or siblings (59), or a combination of all these (60). If having proportionately large infants led to alloparenting or an increase in male parental care in Australopithecus, it would be in stark contrast to behavior exhibited by female apes who rarely will share their infants with other members of the group during the first several months following birth (reviewed in ref. 60). The contribution of grandmothers would probably have been negligible in Australopithecus given the likelihood of female transfer and evidence that extended lifespans did not evolve until H. erectus (61) or even later in Pleistocene H. sapiens (62). There are comparative data to support the connection between a large IMMR and increased male parental care and/or alloparenting. It has been shown in primates, for instance, that birthing of relatively large neonates is correlated with shared postpartum care (63). However, this study focused on primates with male transfer, and the relationship between IMMR and alloparenting is not supported when phylogenetic controls are used (64). Yet, comparative data from carnivores indicate that taxa that produce larger litters engage in more cooperative care of the pups (60; using data from ref. 65). Increased parental care is also correlated with litter size in some rodents (66) and clutch size in some species of fish (67) and birds (68). Allomothering in primates allows for fast postnatal growth, a reduced weaning age, and therefore a reduced interbirth interval (64, 69), life history features that distinguish humans from the other great apes. The data presented in this study therefore suggest that the increased levels of shared infant care critical to infant survival in modern humans could have its roots in the genus Australopithecus, rather than our own genus Homo.
Conclusion
Although many life history changes first appear in the genus Homo, human-like patterns of infant:mother mass first evolved in the Pliocene genus Australopithecus. These surprising results help reconstruct the paleobiology of Australopithecus as a primarily terrestrial hominid perhaps with more shared parental care than what is found in modern great apes.
Materials and Methods
Data from 415 chimpanzee births were obtained from the YNPRC. Forty-seven of these included mass data for both the infant (within 2 wk of birth) and mother. The average chimpanzee neonatal mass (1,733 g) is near the value found in other studies (17, 18, 70). Although adult female wild chimpanzees are not as heavy as those reported in this study (34), immature wild chimpanzees are also smaller than laboratory-born chimpanzees of a similar developmental age (71), consistent with findings that birth weight is correlated with mother's mass in humans (72) and nonhuman primates (73). Mixed datasets obtained from different sources should be avoided when calculating the IMMR, and thus until neonatal birthweights of wild chimpanzees are known, these captive data are a reasonable alternative. Human neonatal and adult female masses were obtained from the literature (Table S1) and used to calculate the IMMR. Data on specific infant:mother dyads (N = 2,607) were obtained from the Cebu Longitudinal Health and Nutrition Survey (http://www.cpc.unc.edu/projects/cebu).
Adult hominid cranial capacities (n = 61) from literature sources (Table S5) were used to calculate neonatal cranial capacities using the following regression equation:
Ranges of neonatal brain size were calculated from the 95% CI of this regression equation (9). These cranial capacities were converted to masses using conversions from Ruff et al. (25) and Hofman (26), which, together with the 95% CI, generated a range of likely neonatal brain masses (Table 2). NBMs were calculated using an ape model (brain 10% of body mass at birth), a human model (brain 12.3% of body mass at birth), and an intermediate model (average of human and chimpanzee models). These brain:body percentages were calculated using data from YNPRC and published sources (Table S3). IMMRs were calculated for extinct hominid species by dividing the range of NBMs by estimates of female body mass by using data from the literature on hominid adult female body masses (14–16, 74). Because of the population-level differences in modern humans (Table S1), effort was made to examine specific fossil assemblages (Dmanisi, Atapuerca, Skhul-Qafzeh), although these sites may still be sampling from a large time frame. Species for which only single cranial capacities are available (e.g., Australopithecus sediba) were not included in this analysis, with the exception of Ar. ramidus. The entire range of IMMRs, using a chimpanzee model and a human model, was calculated for each fossil taxon and reported in Table 2 and Fig. 2. Because there is great uncertainty in calculating female body masses of extinct hominids, NBM estimates were also compared with direct measurements of postcrania in chimpanzees and in Australopithecus by using resampling statistics.
Resampling statistics have been used in a variety of paleoanthropological studies to best handle the inherent problem of small sample sizes in the fossil record (e.g., refs. 9, 62, 75). A. afarensis and A. africanus have statistically indistinguishable adult cranial capacities (Table 2; Table S6) and nearly identical postcranial dimensions (Table S4), and were thus treated collectively. Twelve Australopithecus crania (Table S5) were used to calculate NBM estimates as previously described. These were compared directly with postcranial fossils (Table S4) at the University of Witwatersrand and the Transvaal Museum (A. africanus) and casts of A. afarensis at the Cleveland Museum of Natural History and Harvard Peabody Museum. The FHD was measured as the maximum superoinferior or anteroposterior dimension of the head. The tibial plafond width was measured as the mediolateral dimension of the talar facet at the midpoint of the joint, and similarly the mediolateral width of the midpoint of the talar trochlea was measured. Acetabulum dimensions were converted to FHDs by using the equation in the publication of Ruff (16). Fossil tali were converted to tibial dimensions by using an reduced major axis regression generated from 79 associated human tibiae and tali measured for another study (76) (y = 0.9887 × +0.7606). The mediolateral width is a reasonably proxy for body size because size standardized values of this measurement are not phylogenetically or functionally informative in humans or apes (76). The smaller half of the Australopithecus femoral sample was assumed to be female. In two samples of modern human femora of known sex but from different populations, the smallest 93.3% (n = 28 of 30) were female, and 89% of the smaller halves of the tibia were from the female. The mean FHD of the presumed females (n = 7) was divided into the cube root of the Australopithecus NBM estimates generated as described earlier. The same approach was used for the ankle joint, although the smallest 58% was used to be sure that half of the specimens from Hadar were included as female (Table S4).
Chimpanzee NBMs were obtained from the YNPRC (n = 50), and the cube root was taken of each. FHD was taken on adult female chimpanzees (n = 46) at the Cleveland Museum of Natural History, American Museum of Natural History, and Harvard Museum of Comparative Zoology; mediolateral width of the tibial plafond was taken on adult female chimpanzees (n = 20) from those same skeletal collections. Resampled distributions were calculated in the following manner: 12 NBM1/3 were resampled with replacement and the mean calculated. Seven chimpanzee FHDs were randomly resampled with replacement and a mean was calculated. These two means were used to calculate a single NBM1/3/FHD number. This process was then repeated 5,000 times and a distribution of chimpanzee NBM1/3/FHD means was compiled (Fig. 1A). The mean NBM1/3/FHD for Australopithecus (ranges determined by 95% CI of neonatal body mass) was then directly compared with address the likelihood of sampling an Australopithecus-like mean from the range of chimpanzee mean values. The same process was repeated using the tibial plafond width (n = 7) rather than the FHD (Fig. 1B).
Supplementary Material
Acknowledgments
I thank S. Hrdy for helpful discussions and for the inspiration to conduct this study. I am grateful to W. Hopkins and J. Schaeffer for access to chimpanzee birth data from the Yerkes National Primate Research Center, and to E. Miller for help finding the Cebu Longitudinal Health and Nutrition Survey data. Thanks to L. Jellema, J. Chupasko, J. Spence, Y. Haile-Selassie, M. Morgan, and D. Pilbeam for access to skeletal and cast collections. B. Zipfel and the University of Witwatersrand fossil access committee permitted study of Au. africanus postcranial fossils, as did S. Potze and F. Thackeray at the Transvaal Museum. Thank you to Z. Alemseged for comments on a draft of this paper. I am quite grateful to the editor and anonymous reviewers whose insightful critiques helped make this a stronger contribution; and to E., B., and J. DeSilva for making me wonder about Australopithecus infants and mothers. Part of this research was supported by a grant from the Leakey Foundation.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003865108/-/DCSupplemental.
References
- 1.Leutenegger W. Newborn size and pelvic dimensions of Australopithecus. Nature. 1972;240:568–569. doi: 10.1038/240568a0. [DOI] [PubMed] [Google Scholar]
- 2.Dienske H. In: Primate Ontogeny, Cognition and Social Behavior. Else JG, Lee PC, editors. Cambridge: Cambridge Univ Press; 1986. pp. 145–154. [Google Scholar]
- 3.Rosenberg KR. The evolution of modern human childbirth. Yearb Phys Anthropol. 1992;35:89–124. [Google Scholar]
- 4.Smith BH, Tompkins RL. Toward a life history of the Hominidae. Annu Rev Anthropol. 1995;24:257–279. [Google Scholar]
- 5.Vasey N, Walker AC. In: Meat-Eating and Human Evolution. Stanford CB, Bunn HT, editors. New York: Oxford University Press; 2001. pp. 332–349. [Google Scholar]
- 6.Leutenegger W. In: Primate Brain Evolution: Methods and Concepts. Armstrong E, Falk D, editors. New York: Plenum; 1982. pp. 85–95. [Google Scholar]
- 7.McHenry HM. Behavioral ecological implications of early hominid body size. J Hum Evol. 1994;27:77–87. [Google Scholar]
- 8.Stanley SM. An ecological theory for the origin of Homo. Paleobiol. 1992;18:237–257. [Google Scholar]
- 9.DeSilva JM, Lesnik JJ. Brain size at birth throughout human evolution: A new method for estimating neonatal brain size in hominins. J Hum Evol. 2008;55:1064–1074. doi: 10.1016/j.jhevol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Simpson SW, et al. A female Homo erectus pelvis from Gona, Ethiopia. Science. 2008;322:1089–1092. doi: 10.1126/science.1163592. [DOI] [PubMed] [Google Scholar]
- 11.Ponce de León MS, et al. Neanderthal brain size at birth provides insights into the evolution of human life history. Proc Natl Acad Sci USA. 2008;105:13764–13768. doi: 10.1073/pnas.0803917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacher GA. In: Primate Brain Evolution: Methods and Concepts. Armstrong E, Falk D, editors. New York: Plenum; 1982. pp. 97–112. [Google Scholar]
- 13.Robson SL. Breast milk, diet, and large human brains. Curr Anthropol. 2004;45:419–424. [Google Scholar]
- 14.McHenry HM. Body size and proportions in early hominids. Am J Phys Anthropol. 1992;87:407–431. doi: 10.1002/ajpa.1330870404. [DOI] [PubMed] [Google Scholar]
- 15.Robson SL, Wood B. Hominin life history: Reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruff C. Body size and body shape in early hominins - implications of the Gona pelvis. J Hum Evol. 2010;58:166–178. doi: 10.1016/j.jhevol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Smith RJ, Leigh SR. Sexual dimorphism in primate neonatal body mass. J Hum Evol. 1998;34:173–201. doi: 10.1006/jhev.1997.0190. [DOI] [PubMed] [Google Scholar]
- 18.Leigh SR, Shea BT. Ontogeny of body size variation in African apes. Am J Phys Anthropol. 1996;99:43–65. doi: 10.1002/(SICI)1096-8644(199601)99:1<43::AID-AJPA3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Dufour DL, Sauther ML. Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. Am J Hum Biol. 2002;14:584–602. doi: 10.1002/ajhb.10071. [DOI] [PubMed] [Google Scholar]
- 20.White TD, et al. Ardipithecus ramidus and the paleobiology of early hominids. Science. 2009;326:75–86. [PubMed] [Google Scholar]
- 21.Lovejoy CO, Suwa G, Spurlock L, Asfaw B, White TD. The pelvis and femur of Ardipithecus ramidus: The emergence of upright walking. Science. 2009;326:71e1–71e6. [PubMed] [Google Scholar]
- 22.Kimbel WH, Rak Y. The cranial base of Australopithecus afarensis: New insights from the female skull. Philos Trans R Soc Lond B Biol Sci. 2010;365:3365–3376. doi: 10.1098/rstb.2010.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reno PL, McCollum MA, Meindl RS, Lovejoy CO. An enlarged postcranial sample confirms Australopithecus afarensis dimorphism was similar to modern humans. Philos Trans R Soc Lond B Biol Sci. 2010;365:3355–3363. doi: 10.1098/rstb.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovejoy CO, Suwa G, Simpson SW, Matternes JH, White TD. The great divides: Ardipithecus ramidus reveals the postcrania of our last common ancestors with African apes. Science. 2009;326:100–106. [PubMed] [Google Scholar]
- 25.Ruff CB, Trinkaus E, Holliday TW. Body mass and encephalization in Pleistocene Homo. Nature. 1997;387:173–176. doi: 10.1038/387173a0. [DOI] [PubMed] [Google Scholar]
- 26.Hofman MA. Encephalization in hominids: Evidence for the model of punctuationalism. Brain Behav Evol. 1983;22:102–117. doi: 10.1159/000121511. [DOI] [PubMed] [Google Scholar]
- 27.DeSilva JM, Lesnik JJ. Chimpanzee neonatal brain size: Implications for brain growth in Homo erectus. J Hum Evol. 2006;51:207–212. doi: 10.1016/j.jhevol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Coqueugniot H, Hublin J-J, Veillon F, Houët F, Jacob T. Early brain growth in Homo erectus and implications for cognitive ability. Nature. 2004;431:299–302. doi: 10.1038/nature02852. [DOI] [PubMed] [Google Scholar]
- 29.Leonard WR, Robertson ML, Snodgrass JJ, Kuzawa CW. Metabolic correlates of hominid brain evolution. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:5–15. doi: 10.1016/s1095-6433(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 30.Lovejoy CO, Heiple KG, Burstein AH. The gait of Australopithecus. Am J Phys Anthropol. 1973;38:757–779. doi: 10.1002/ajpa.1330380315. [DOI] [PubMed] [Google Scholar]
- 31.Gebo DL, Schwartz GT. Foot bones from Omo: Implications for hominid evolution. Am J Phys Anthropol. 2006;129:499–511. doi: 10.1002/ajpa.20320. [DOI] [PubMed] [Google Scholar]
- 32.Johanson DC, et al. New partial skeleton of Homo habilis from Olduvai Gorge, Tanzania. Nature. 1987;327:205–209. doi: 10.1038/327205a0. [DOI] [PubMed] [Google Scholar]
- 33.Lordkipanidze D, et al. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature. 2007;449:305–310. doi: 10.1038/nature06134. [DOI] [PubMed] [Google Scholar]
- 34.Smith RJ, Jungers WL. Body mass in comparative primatology. J Hum Evol. 1997;32:523–559. doi: 10.1006/jhev.1996.0122. [DOI] [PubMed] [Google Scholar]
- 35.Latimer B. In: Origin(s) of Bipedalism in Hominids. Coppens Y, Senut B, editors. Paris: Centre National Center for Scientific Research; 1991. pp. 169–176. [Google Scholar]
- 36.Lovejoy CO. The natural history of human gait and posture. Part 2. Hip and thigh. Gait Posture. 2005;21:113–124. doi: 10.1016/j.gaitpost.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 37.DeSilva JM. Functional morphology of the ankle and the likelihood of climbing in early hominins. Proc Natl Acad Sci USA. 2009;106:6567–6572. doi: 10.1073/pnas.0900270106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latimer B, Lovejoy CO. Hallucal tarsometatarsal joint in Australopithecus afarensis. Am J Phys Anthropol. 1990;82:125–133. doi: 10.1002/ajpa.1330820202. [DOI] [PubMed] [Google Scholar]
- 39.McHenry HM, Jones AL. Hallucial convergence in early hominids. J Hum Evol. 2006;50:534–539. doi: 10.1016/j.jhevol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Amaral LQ. Mechanical analysis of infant carrying in hominoids. Naturwissenschaften. 2008;95:281–292. doi: 10.1007/s00114-007-0325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed DL, Light JE, Allen JM, Kirchman JJ. Pair of lice lost or parasites regained: The evolutionary history of anthropoid primate lice. BMC Biol. 2007;5:7. doi: 10.1186/1741-7007-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Falk D. Prelinguistic evolution in early hominins: Whence motherese? Behav Brain Sci. 2004;27:491–503. doi: 10.1017/s0140525x04000111. [DOI] [PubMed] [Google Scholar]
- 43.Wall-Scheffler CM, Geiger K, Steudel-Numbers KL. Infant carrying: The role of increased locomotory costs in early tool development. Am J Phys Anthropol. 2007;133:841–846. doi: 10.1002/ajpa.20603. [DOI] [PubMed] [Google Scholar]
- 44.Watson JC, Payne RC, Chamberlain AT, Jones RK, Sellers WI. The energetic costs of load-carrying and the evolution of bipedalism. J Hum Evol. 2008;54:675–683. doi: 10.1016/j.jhevol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Altmann J, Samuels A. Costs of maternal care: Infant-carrying in baboons. Behav Ecol Sociobiol. 1992;29:391–398. [Google Scholar]
- 46.Kramer PA. In: From Biped to Strider. The Emergence of Modern Human Walking. Meldrum J, Hilton CE, editors. New York: Plenum; 2004. pp. 101–116. [Google Scholar]
- 47.Whitcome KK, Shapiro LJ, Lieberman DE. Fetal load and the evolution of lumbar lordosis in bipedal hominins. Nature. 2007;450:1075–1078. doi: 10.1038/nature06342. [DOI] [PubMed] [Google Scholar]
- 48.Tague RG, Lovejoy CO. The obstetric pelvis of A.L. 288-1 (Lucy) J Hum Evol. 1986;15:237–255. [Google Scholar]
- 49.Blumenberg B. Allometry and evolution of tertiary hominoids. J Hum Evol. 1984;13:613–676. [Google Scholar]
- 50.Ruff CB. Biomechanics of the hip and birth in early Homo. Am J Phys Anthropol. 1995;98:527–574. doi: 10.1002/ajpa.1330980412. [DOI] [PubMed] [Google Scholar]
- 51.Weaver TD, Hublin J-J. Neandertal birth canal shape and the evolution of human childbirth. Proc Natl Acad Sci USA. 2009;106:8151–8156. doi: 10.1073/pnas.0812554106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trevathan WR. The evolution of bipedalism and assisted birth. Med Anthropol Q. 1996;10:287–290. doi: 10.1525/maq.1996.10.2.02a00100. [DOI] [PubMed] [Google Scholar]
- 53.Trevathan W, Rosenberg K. The shoulders follow the head: Postcranial constraints on human childbirth. J Hum Evol. 2000;39:583–586. doi: 10.1006/jhev.2000.0434. [DOI] [PubMed] [Google Scholar]
- 54.Garwicz M, Christensson M, Psouni E. A unifying model for timing of walking onset in humans and other mammals. Proc Natl Acad Sci USA. 2009;106:21889–21893. doi: 10.1073/pnas.0905777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adair LS, et al. Size at birth, weight gain in infancy and childhood, and adult blood pressure in 5 low- and middle-income-country cohorts: When does weight gain matter? Am J Clin Nutr. 2009;89:1383–1392. doi: 10.3945/ajcn.2008.27139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovejoy CO. The origin of man. Science. 1981;211:341–350. doi: 10.1126/science.211.4480.341. [DOI] [PubMed] [Google Scholar]
- 57.Lovejoy CO. Reexamining human origins in light of Ardipithecus ramidus. Science. 2009;326:74e1–74e8. [PubMed] [Google Scholar]
- 58.Gettler LT. Direct male care and hominin evolution: Why male-child interaction is more than a nice social idea. Am Anthropol. 2010;112:7–21. [Google Scholar]
- 59.Bogin B. Patterns of Human Growth. Cambridge: Cambridge Univ Press; 1999. [Google Scholar]
- 60.Hrdy SB. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge: Belknap Press of Harvard University; 2009. [Google Scholar]
- 61.O'Connell JF, Hawkes K, Blurton Jones NG. Grandmothering and the evolution of Homo erectus. J Hum Evol. 1999;36:461–485. doi: 10.1006/jhev.1998.0285. [DOI] [PubMed] [Google Scholar]
- 62.Caspari R, Lee SH. Older age becomes common late in human evolution. Proc Natl Acad Sci USA. 2004;101:10895–10900. doi: 10.1073/pnas.0402857101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harvey PH, Clutton-Brock TH. Life history variation in primates. Evolution. 1985;39:559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 64.Mitani JC, Watts D. The evolution of non-maternal caretaking among anthropoid primates: Do helpers help? Behav Ecol Sociobiol. 1997;40:213–230. [Google Scholar]
- 65.Gittleman JL. Carnivore life history patterns: Allometric, phylogenetic, and ecological association. Am Nat. 1986;127:744–771. [Google Scholar]
- 66.Cantoni D, Brown RE. Paternal investment and reproductive success in the California mouse, Peromyscus californicus. Anim Behav. 1997;54:377–386. doi: 10.1006/anbe.1996.0583. [DOI] [PubMed] [Google Scholar]
- 67.Sargent RC. Paternal care and egg survival both increase with clutch size in the fathead minnow, Pimephales promelas. Behav Ecol Sociobiol. 1988;23:33–37. [Google Scholar]
- 68.Komdeur J, Wiersma P, Magrath M. Paternal care and male mate-attraction effort in the European starling is adjusted to clutch size. Proc Biol Sci. 2002;269:1253–1261. doi: 10.1098/rspb.2002.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross C. In: Primate Life Histories and Socioecology. Kappeler PM, Pereira MP, editors. Chicago: Univ of Chicago Press; 2003. pp. 266–284. [Google Scholar]
- 70.Hamada Y, Udono T. Longitudinal analysis of length growth in the chimpanzee (Pan troglodytes) Am J Phys Anthropol. 2002;118:268–284. doi: 10.1002/ajpa.10078. [DOI] [PubMed] [Google Scholar]
- 71.Kimura T, Hamada Y. Growth of wild and laboratory born chimpanzees. Primates. 1996;37:237–251. [Google Scholar]
- 72.Niswander KR, Singer J, Westphal M, Jr, Weiss W. Weight gain during pregnancy and prepregnancy weight. Association with birth weight of term gestation. Obstet Gynecol. 1969;33:482–491. [PubMed] [Google Scholar]
- 73.Leutenegger W. Neonatal-maternal weight relationship in macaques: An example of intrageneric scaling. Folia Primatol (Basel) 1977;27:152–159. doi: 10.1159/000155782. [DOI] [PubMed] [Google Scholar]
- 74.McHenry HM, Coffing K. Australopithecus to Homo: Transformations in body and mind. Annu Rev Anthropol. 2000;29:125–146. [Google Scholar]
- 75.Green DJ, Gordon AD, Richmond BG. Limb-size proportions in Australopithecus afarensis and Australopithecus africanus. J Hum Evol. 2007;52:187–200. doi: 10.1016/j.jhevol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 76.DeSilva JM. Vertical climbing adaptations in the ape ankle and midfoot. Implications for locomotion in Miocene catarrhines and Plio-Pleistocene hominins. PhD dissertation. Ann Arbor: Univ of Michigan; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.