Abstract
The applications of molecular surface chemistry in heterogeneous catalyst technology, semiconductor-based technology, medical technology, anticorrosion and lubricant technology, and nanotechnology are highlighted in this perspective. The evolution of surface chemistry at the molecular level is reviewed, and the key roles of surface instrumentation developments for in situ studies of the gas–solid, liquid–solid, and solid–solid interfaces under reaction conditions are emphasized.
Keywords: surface science, nanotechnology, heterogeneous catalysis, in situ techniques, technological application
Surfaces and interfaces define a boundary between a material and its surrounding environment and influence interactions with that environment. At the molecular level, the surface atoms have a different chemical environment, that is, fewer nearest neighbors, from that in the bulk. As a consequence, these surface atoms with changed atomic and electronic structures exhibit high chemical reactivity. This property makes surfaces and interfaces a favored medium for chemical and biological processes in nature and in technological applications (1).
Modern surface chemistry is about molecular-level understanding and the control of surface chemical reactions. Over the past decades, various surface science techniques have been developed and a vast amount of knowledge about surface chemistry has been accumulated (1–6). The fundamental knowledge that is accumulated provides a foundation for the development of many industrial technologies that produce items including chemicals and fuels (7), semiconductor devices (8), and biomedical devices (9). Technological advances, in turn, necessitate the further development of new surface characterization techniques with higher spatial, temporal, and energy resolution.
This perspective begins with an overview of several applications of surface chemistry that have an enormous economic impact on our society. Advances in the fundamental understanding of molecular processes on surfaces over the past decades are then reviewed with an emphasis on the development of the molecular-level surface techniques. We highlight new scientific insights provided by in situ experimental and theoretical techniques capable of characterizing surfaces and interfaces under working conditions.
Selected Technological Applications of Surface Chemistry
Throughout modern industrialization, surface chemistry plays an indispensable role in various industrial technologies for chemical and energy conversion, information processing, heath care, and material and environmental protection. The paramount importance of surface chemistry is reflected in the tremendous economic impact made by these technologies. The roles of surface chemistry in several major industrial technologies are discussed here (Table S1). The major challenges for surface chemistry in further technological developments are highlighted.
Heterogeneous Catalyst Technology.
A catalyst is an entity that accelerates a chemical reaction without being consumed in the process. This ability is usually referred to as the activity of a catalyst. For a chemical reaction with multiple possible products, a catalyst may promote the production of a specific product, which is referred to as the selectivity of a catalyst. Heterogeneous catalytic reactions occur on the surface of solid catalysts and involve elementary surface chemical processes such as adsorption of reactants from a reaction mixture, surface diffusion and reaction of adsorbed species, and desorption of reaction products. The acceleration of a chemical reaction is due to the high reactivity of surface atoms that facilitates bond breaking and bond rearrangement of adsorbed molecules.
In the late 1800s, the large-scale synthesis of simple but important bulk chemicals, including sulfuric acid, ammonia, and nitric acid, were among the first industrial processes based on heterogeneous catalysis (7). After these early successes, many complex catalytic processes evolved in the chemical industry. These processes include the synthesis of methanol from carbon monoxide and hydrogen, the oxidation of ethylene to ethylene oxide for the manufacture of ethylene glycol antifreeze, the dehydrogenation of butane to butadiene for the production of synthetic rubber, and the polymerization of ethylene and propylene to produce plastics (7).
In the middle of the 1900s, the large-scale industrial applications of heterogeneous catalysis emerged in the petroleum industry with the development of catalytic cracking processes for naphtha refining. Since then, the applications of zeolites as cracking catalysts, the supported metal clusters as reforming catalysts, in the petroleum industry have allowed large-scale production of high quality fuels and bulk chemicals (7). These developments have helped to lay out the foundation of our current fast-growing, petroleum-based economy (10).
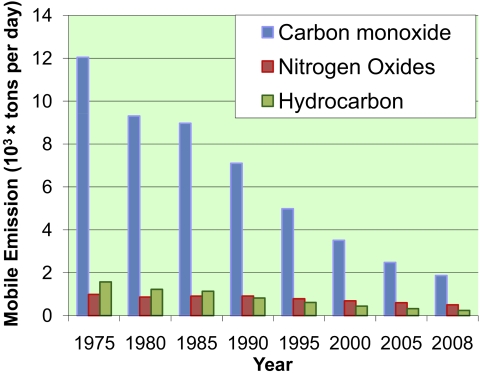
The development of catalytic processes for pollution control and prevention also accompanied the fast-growing economy. One of many successful examples of pollution control is the three-way catalytic converter that reduces the pollutant emission from automobiles (11, 12). Fig. 1 shows that, despite a twofold increase in the number of automobiles, mobile pollutant emissions in the Los Angeles area have continuously decreased since the three-way catalytic converter was introduced in 1975.
Fig. 1.
Annual average of toxic mobile emissions in Los Angeles County from 1975 to 2008. (Source: California Environmental Protection Agency, Air Resources Board. http://www.arb.ca.gov/app/emsinv/emssumcat.php, last date access: 08/10/2010.).
In the 21st century, emphasis shifted to catalytic selectivity. It is desired to produce one specific product out of many other thermodynamically possible compounds that are undesirable waste byproducts (green chemistry) (13, 14). To identify the molecular factors that control selectivity, it is required to monitor surface chemical processes under reaction conditions (15). Innovations in catalytic processes are playing a key role in green chemistry by reducing or eliminating the use and generation of hazardous substances in chemical conversion processes and in the development of renewable energy and chemical feedstocks (16, 17). An example is the commercialization of the hydrogen peroxide to propylene oxide process for making propylene oxide, a polyurethane raw material (18). In this process, propylene is oxidized by hydrogen peroxide over a Ti-silicalite catalyst. The use of toxic reagents, such as chlorine, and the production of coproducts, such as chlor–alkali complexes, tert-butyl alcohol, or styrene, are avoided.
Many challenging catalytic processes are under intensive research and development for: (i) efficient water and CO2 splitting to produce fuels by photocatalysts (19–23), (ii) efficient conversion of fuels to electricity by electrochemical fuel cells (24, 25), (iii) selective conversion of biomass to fuels and bulk chemicals (26–31), (iv) selective production of fine chemicals and pharmaceutical compounds by heterogeneous catalysts (32), and (v) greenhouse gas (e.g., CO2 and CH4) reduction and utilization in fuel and chemical synthesis (32, 33). To achieve the optimal catalytic properties (efficient sunlight and electrical energy conversion, and high activity and selectivity for chemical conversion), much of this research is heavily relying on controlling the structure and composition of catalyst systems on a nanometer or molecular scale (nanocatalysis) (34). For example, the catalytic activity and selectivity of a transition metal nanocatalyst can be tuned by controlling the size and shape of the nanocatalyst (35–38).
Semiconductor-Based Technology.
The transistor and the integrated circuit shaped the semiconductor industry and the modern information-driven world. A transistor is a semiconductor device used to amplify or switch an electronic signal, and an integrated circuit contains millions of transistors etched out of a small semiconductor surface. Integrated circuits are in many modern electronic devices such as computers and cell phones.
Extensive research on the effect of electron surface states led to the creation of the first point-contact germanium transistor in 1947 (39, 40). After the invention of transistors, advances in epitaxial growth of silicon thin films, chemical vapor deposition, and surface etching techniques made the industrial large-scale manufacture of silicon-based integrated circuits possible (41, 42). Since then, the number of transistor that can be placed inexpensively on an integrated circuit has doubled approximately every 2 y, improving the performance and lowering the manufacturing cost of integrated circuits (43). New 18-nm manufacturing processes are expected to arrive in 2018. These processes include the following: the growth and deposition of sub–10-nm thin films of low-k insulating materials (for example, SiOC) and high-k gate dielectric materials (for example, SiOxNy); the conformal etching of deposited thin films with high aspect ratios; high-spatial-resolution patterning with new lithography techniques; the doping of the sub–10-nm silicon channels; and the deposition of new wiring materials with low resistance and high reliability (for example, Cu) (8, 44–46). The development of these new manufacturing processes leads to a broad range of surface and interface chemistry issues.
Unlike the silicon semiconductor, compound semiconductors such as GaAs, GaN, and InGaN are optically active materials. Their tunable optical response through composition control makes them extremely useful for optoelectronics applications (47). Other advantages of compound semiconductors over silicon include the following: higher electron mobility and electron velocity, low noise, and higher breakdown voltage, which make them ideal for radio frequency communication devices such as cell phones and wireless local area network cards (48). However, their compound character often introduces formidable surface chemistry challenges in large-scale manufacturing processes (8, 49). For example, after nearly 30 y of intensive research, the discovery of a metal–organic chemical vapor deposition process (MOCVD) for growing device-quality GaN thin films finally enabled the commercialization of the GaN-based technology (50). In this heteroepitaxial growth process, a thin AlN buffer layer is deposited on the substrate before the GaN growth. The essential role of this buffer layer is to serve as a template for nucleation and to promote lateral growth of the GaN film due to the decrease in interfacial free energy between the film and the substrate.
Medical Technology.
In the late 1940s, along with the development of biomaterials for medical implants such as intraocular lenses, hip joint replacement, and blood contact devices, researchers began exploring biological reactions at surfaces (51). It was indicated by many observations that the biocompatibility of a material is correlated with the surface properties of the material (52–55). For example, when a foreign material comes into contact with blood, it will rapidly adsorb various proteins onto its surface. This process is usually referred to as nonspecific protein adsorption. The adsorbed protein layer determines subsequent platelet adhesion, which plays a major role in thrombogenesis on foreign surfaces (56). To prevent thrombogenesis, many surface modification strategies to achieve protein-resistant (antifouling) surface have been developed (57, 58).
Surface chemistry processes for producing antifouling coatings and immobilization of biological recognition elements are pervasive in many commercialized medical applications, including implant biomaterials, chromatography separations, and biosensors (59–60). For example, the glucose biosensor is one of the most commercialized biosensor technologies (9). It is at the heart of the hand-held devices used by millions of diabetic patients for monitoring glucose levels. In a glucose biosensor, an enzyme layer of glucose oxidase immobilized on an oxygen electrode selectively catalyzes the oxidation of glucose in a blood sample (61). Then, the concentration of the reaction product, H2O2, is monitored by either an electrochemical or an optical transducer to detect the glucose concentration in the blood sample (60, 62). One of major challenges in the development of the new generation of enzyme-based biosensors is to find new enzyme immobilization approaches to maintain the activity of the immobilized enzyme for a long time period.
At the more fundamental level, a detailed understanding of how surface properties control the biological activity at biointerfaces is the key to engineering new biomaterials for advanced biological applications. Advances in surface analysis make it possible to characterize the surface chemistry and structure of increasingly complex biointerfaces (51, 63, 64). For example, the PEG-terminated self-assembly monolayers have been studied by using surface-specific optical spectroscopy techniques to unveil the molecular structural origin associated with their antifouling properties (65, 66).
Anticorrosion Technology.
In many practical applications, high reactivity of surfaces may cause undesirable chemical (corrosion) or mechanical (wear and friction) processes, which lead to material and energy waste. Depending on the materials (metal, oxide, and polymer) and the surrounding environment (gas, corrosive liquid, temperature, and radiation), there are many surface chemical processes that can lead to the progressive corrosion of material surfaces. On a nonnoble metal surface in an oxidative moist environment, severe corrosion usually occurs through an electrochemical process (67).
Anticorrosion technology uses various surface chemical processes to coat a material with a protective layer. Most of the protective layers consist of metal oxides (e.g., chromium oxide on stainless steels) or polymers (e.g., Teflon on pans) that are inert in the environment of their specific applications. A protective layer usually serves as a kinetic barrier to slow down corrosion process (68–70). The long-term stability of the protective layers is the major concern in many applications. For example, due to corrosion, the aluminized-Teflon coating on the Hubble Space Telescope has been severely degraded after 19 y of exposure to atomic radicals and UV radiation in low Earth orbit (70).
The design of protective layers with enhanced stability requires surface chemistry investigations on the structures, growth, and breakdown mechanisms of the protective layers. Many surface analysis tools are used to elucidate the chemical composition and morphology of the protective layers, as well as the kinetics of their growth and breakdown in various corrosive environments (68, 69, 71, 72). These research findings have provided useful information for designing new protective layers with enhanced stability. Several current challenges in this field include the following: (i) the need for detection techniques with high spatial resolution to investigate protective layers that are a few nanometers thick and the corrosion processes that occur locally on the defect sites on the thin layer; and (ii) the development of model systems that simulate real corrosion processes where the corrosive environment may evolve as the corrosion progresses, or where the harsh corrosive environments are hard to reproduce in laboratories (73, 74).
Lubricant Technology.
A lubricant is a material confined between two hard surfaces in relative motion. Its existence helps reduce the friction and wear at the interfaces (75). Most of the fluid-base lubricants consist of viscous base stocks (e.g., paraffinic-, aromatic-, and naphthenic-hydrocarbon–type molecules with carbon number ranging from 14 to 40+) and additives to enhance performance under heavy-duty and high temperature conditions (76). This type of lubricant is widely used in internal combustion engines, vehicle and industrial gear boxes, compressors, turbines, and hydraulic systems. Solid lubricants are layered materials such as graphite, tungsten disulfide, and molybdenum disulfide (77). These solids are used in high-temperature gas turbines and space applications where fluid-based lubricants are not stable because of vaporization. Other lubricants of great technological importance include ultra-thin lubricant films coated on a computer hard disk surface to prevent wear by the disk head, biocompatible lubricants used in artificial joints, and organic monolayer lubricants in microelectro–mechanical systems (78–81).
The terminological introduction of tribology, the science of friction, wear, and lubrication, in the late 1960s reflected the urgent need for scientific focus on molecular mechanisms of lubrication for the development of lubricant technology. In past decades, many tribological studies have focused on properties of thin lubricant layers that hold two sliding surfaces apart (82–86). Now, it is known that, under low sliding speeds and/or high loading conditions, a “boundary lubrication” process occurs. In this process, an active lubricant is a thin film only several molecular layers thick. A strongly adsorbed and oriented monolayer is formed on each of the slide surfaces, which protects the surfaces against wear. The shear plane resides within the thin lubricant film, and the low shear strength between lubricant molecular layers reduces the friction.
The development of environmentally benign lubricants is a major driving force for advances in tribology (85). A fluid-based lubricant is a complex mixture. Detailed understanding of how various components function in the lubrication process may assist the discovery of more benign alternatives. Solid lubricants are usually more environmentally friendly than fluid-based lubricants. However, severe performance degradation in the presence of humidity or oxygen limits the applications for using solid lubricants exposed to the Earth's atmosphere (87, 88). The surface chemistry of their degradation is an important research subject for improving their chemical stability.
Natural joints in humans are the most complex and sophisticated lubrication systems. Their performance is far superior to any current artificial joint (89). Their working mechanism is poorly understood. Further tribological investigations on the molecular level are needed to improve the performance of artificial joints.
Surface Chemistry at the Molecular Level
As many technologies develop, there is constant calling for molecular-level understandings of surface chemical processes in complex environments. It took almost 50 y for surface chemistry to develop into a molecular-level science, which is now capable of in situ characterization of a variety of surface properties and providing fundamental understanding to aid in the design and engineering of large-scale chemical processes in technological applications (90, 91).
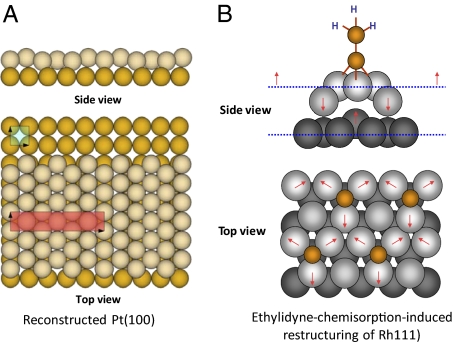
The revolutionary development of surface science at the molecular level began in the 1960s when techniques for preparation of clean single crystal surfaces and characterization of surface structure and chemical compositions in ultrahigh vacuum, such as low energy electron diffraction (LEED), Auger electron spectroscopy (AES), and X-ray photoelectron spectroscopy (XPS), became available to the research community (5). Throughout the 1960s to the early 1990s, thousands of surface structures were studied. A dynamic view of surfaces emerged from the many observations of thermal- and chemisorptions-activated surface reconstructions. In many cases, massive reconstruction on clean surfaces can lead to a surface structure totally different from that projected from bulk structure (For example, a reconstructed Ir(100) (92, 93) is shown in Fig. 2A). Chemisorption can lead to not only the restructuring of adsorbates but also a significant reconstruction of single crystal surfaces [an example of ethylidyne-chemisorption–induced reconstruction of Rh(111) (94) is shown in Fig. 2B].
Fig. 2.
(A) The structure of the reconstructed Ir(100) crystal face obtained by low energy electron diffraction (LEED). The surface layer assumes hexagonal packing that induces bucking (the 5x1 unit cell is indicated by a red box). The second layer retains its bulk-terminated square unit cell indicated by a light blue box. (B) Ethylidyne-chemisorption–induced restructuring of Rh (111). The red arrows indicate the expansion of metal atoms around the adsorption sites and the bulking up of metal atoms in the second layer underneath the adsorption sites.
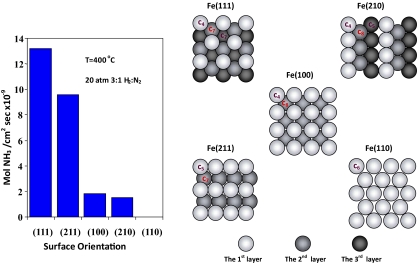
The next big step taken after surface structural techniques became widely available was the establishment of correlations between surface structure and chemical properties of technological importance. Early examples are the studies of ammonia synthesis over various Fe crystal surfaces by an experimental apparatus capable of isolating a single crystal from an ultra-high vacuum system after the surface is characterized by surface structural techniques (95). Catalytic activity measurements of the isolated crystal surfaces at high pressures allow the determination of structure–activity correlations. It was found that certain Fe crystal faces have significantly higher catalytic activity for ammonia synthesis than other Fe crystal faces (Fig. 3) (96). The high catalytic activity was attributed to the existence of surface active sites with specific atomic arrangements (96). These findings, the investigations of elementary reaction steps (97), and the effects of catalyst supports and additives (98, 99) provided the molecular-level understandings for improving industrial ammonia synthesis processes. More importantly, this example highlighted that molecular-level studies on well controlled model systems (the surface science approach; refs. 1 and 2) are capable of extracting the essence of complex surface chemical processes in industries, which were previously believed impossible to obtain.
Fig. 3.
The activities of ammonia synthesis over different Fe crystal faces (Right) and the structures of corresponding crystal faces (Left). It was suggested that the surface atoms with high coordination number (C7 and C8) are the active sites for ammonia synthesis.
The surface structural studies were followed by extensive experimental and theoretical investigations of the nature of chemical bonding at surfaces and interfaces (6). Chemsorption bonding trends across the periodic table were recognized by using the accumulation of data from adsorption energy measurements (100). Detailed electronic structures of surface chemical bonds were obtained by spectroscopic techniques such as ultraviolet photoelectron spectroscopy, X-ray photoelectron spectroscopy, and X-ray emission spectroscopy (5). Theoretical techniques, especially the density functional theory (DFT)-based computational simulations, were developed to rationalize the experimental findings and to provide a consistent framework for understanding surface chemical bonding (101–103). For example, in the d-band model, the surface bonding strength on transition metal surfaces has been correlated with the local electron density in the d-band of the bond site (104). Along with the experimental data, the d-band model provides answers to fundamental questions such as why bulk gold is noble while small gold clusters are not (105, 106) and why surface defect sites (steps and kinks) are more chemically active (107).
The detailed molecular-level studies of surface elementary processes (adsorption, diffusion, and reaction of adsorbed species) were enabled by molecular beam technique in the early 1970s (108, 109). These studies have yielded information about energy transfer in gas-surface collision processes (110) and helped discern the activities of various surface sites for a given reaction (111). For some simple surface reactions, it was even possible to obtain the kinetic parameters (e.g., sticking probabilities and activation energies) of elementary surface reaction steps (112). The invention of scanning tunneling microscopy in 1981 and its later development transformed surface chemistry into a truly atomic-level science (113, 114). For example, by monitoring an individual adsorbate in motion, the surface diffusion rate of a single molecule or a cluster was obtained based on statistics of single molecular events (115). By in situ imaging of the evolution of coadsorbed surface intermediates under reaction conditions, the active sites for many catalytic processes were unveiled (116).
At present, a vast number of experimental and theoretical techniques have been developed for surface chemistry research and are serving as the research and development and quality-control workhorses in industry. The in situ techniques are specifically of great importance and are under intensive development. These techniques (Table 1) feature the capability to access molecular information at complex gas–solid, liquid–solid, and solid–solid interfaces under practical working conditions. Three of these in situ techniques developed in Berkeley are briefly introduced here along with scientific insights recently obtained from their application in heterogeneous catalysis.
Table 1.
Commonly used in situ techniques for the molecular-level surface characterization
| Techniques | Properties characterized | |
| Transmission electron microscopy (TEM) | Surface/interface structure, the size, shape, and crystallinity of nanocrystals | |
| X-ray diffraction (XRD) | Structure of micro- and mesoporous materials | |
| Surface X-ray diffraction (SXRD) | Surface structure | |
| Ambient pressure X-ray photoelectron spectroscopy (AP-XPS) | Surface chemical composition | |
| Small Angle X-ray scattering (SAXS) | Characteristic distances of ordered nanomaterials | |
| X-ray emission spectroscopy (XES) | Electronic structure of surfaces | |
| Near-edge X-ray absorption fine structure (NEXAFS) | Surface chemical composition | |
| Extended X-ray Absorption fine structure (EXAFS) | Chemical composition and bonding environment of nanoparticles | |
| Polarization-modulated reflection–absorption infrared spectroscopy (PM–RAIRS) | Surface reaction intermediates | |
| Surface enhanced Raman spectroscopy (SERS) | Surface reaction intermediates | |
| Sum frequency generation spectroscopy (SFG) | Surface reaction intermediates | |
| High pressure scanning tunneling microscopy (HP-STM) | Surface morphology and electronic structure | |
| Atomic force microscopy (AFM) | Surface morphology, tribological properties, and work function |
Ambient Pressure X-Ray Photoelectron Spectroscopy (AP-XPS).
AP-XPS (117, 118) can be operated at total reactant pressure up to 10 torr. The key component that makes this technique different from conventional XPS is the differentially pumped electrostatic lens system (Fig. S1) that refocuses the photoelectrons from the sample surface into the object plane of a 1 standard electron energy analyzer working in high-vacuum. The kinetic energy of the photoelectrons can be tuned by varying the energy of the X-ray source. By tuning the kinetic energy of the photoelectrons to an appropriate value, the electron mean free path can be minimized for a given sample surface so that the oxidation state and the composition of the surface layer with a thickness of ≈1 nm can be determined.
AP-XPS has been applied to studying the oxidation states of Rh nanoparticles with sizes ranging from 2 to 11 nm during the CO oxidation reaction (119). It was found that the thickness of the surface oxide on the Rh nanparticles increases with size reduction, which is correlated with an increase of the reaction turnover.
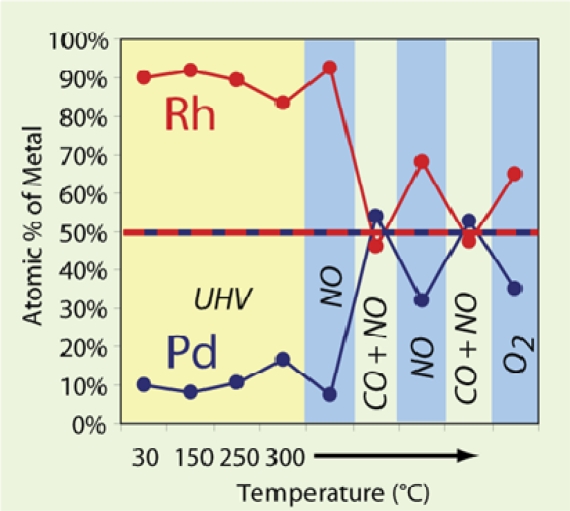
The active site on the bimetallic catalyst surfaces usually consists of an atomic arrangement with a specific composition. It is of great importance to determine the surface composition of bimetallic nanoparticle catalysts under reaction conditions. A recent AP-XPS study (120) of the surface composition of Rh/Pd and Pd/Pt bimetallic nanoparticles demonstrated that the surface composition of nanoparticles are extremely sensitive to the ambient chemical environment. For example, under oxidizing conditions of NO, the Rh atoms are pulled out to the surface of the Rh0.5Pd0.5 nanoparticles (Fig. 4). Under reducing conditions of a mixture of CO and NO, the Pd surface concentration increases. The surface composition variation of a given metal is up to 20% at 300 °C. These results indicate that the surface active sites on bimetallic nanoparticle catalysts may markedly change under varying reaction conditions.
Fig. 4.
Chemical environment effects on surface segregation of the Rh/Pd nanoparticles detected by AP-XPS. In the experiment, the partial pressure of NO or CO is ≈100 mtorr.
High Pressure Scanning Tunneling Microscopy (HP-STM).
Scanning tunneling microscopy operating under high pressure and high temperature conditions offers a promising way to monitor the structure of surfaces and adsorbates on the molecular level during surface reactions. Since the first system (121) was demonstrated in 1992, several HP-STM systems have been designed and applied to in situ catalytic reaction studies (122–125). A recently developed system (126) is shown in Fig. S2. By integrating a small, high pressure reactor into the UHV chamber, the new system is capable of imaging surfaces with atomic resolution under a wide range of pressures (from 10−13 bar to several bars) and temperatures (from 300 to 700 K).
The adsorbate mobility, an important factor in hydrocarbon conversion reactions, has been studied by HP-STM. During the hydrogenation/dehydrogenation of cyclohexene over the Pt(111) surface, no distinguishable feature of the surface can be resolved by STM (Fig. S3) (127). Given the limited scanning speed of the STM tip (typically, 10 nm/ms), the featureless STM image implies that the adsorbates move at a speed much higher than that of the STM tip. After poisoning the reaction by a small amount of CO, the reaction turnover stops. Meanwhile, the ordered structures emerge on the surface since the coadsorption of strongly bonded CO limits the mobility of hydrocarbon adsorbates. These results and similar observations during ethylene hydrogenation (128) suggest that, on the catalyst surfaces saturated by various hydrocarbon adsorbates under high pressures, adsorbate mobility is crucial for the active sites occupied by inactive species to be continuously released to allow the reactive species access to the active sites.
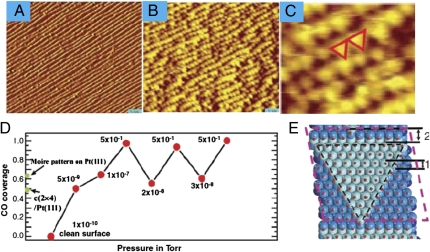
The HP-STM studies on catalyst surfaces unveiled many phenomena that were not seen under ultrahigh vacuum conditions. For example, HP-STM, AP-XPS, and DFT simulations were combined in a recent study of CO chemisorption-induced reconstruction of stepped Pt surfaces at elevated pressures. It was found that stepped Pt surfaces can undergo extensive and reversible restructuring when exposed to carbon monoxide (CO) at pressures >0.1 torr (Fig. 5 A–C). Scanning tunneling microscopy and photoelectron spectroscopy studies under gaseous environments near ambient pressure at room temperature revealed that as the CO surface coverage approaches 100% (Fig. 5D) under ≈0.5 torr of CO, the originally flat terraces of (557) oriented Pt crystals break up into nanometer-sized clusters and revert to the initial morphology after pumping out the CO gas. Density functional theory calculations provide a rationale of the observations whereby the creation of increased concentrations of low-coordination Pt edge sites in the formed nanoclusters relieves the strong CO–CO repulsion in the highly compressed adsorbate film (Fig. 5E). This example and many others highlight the indispensible role of in situ techniques in surface chemistry research.
Fig. 5.
STM images of Pt(557) in ultrahigh vacuum (A) and under 1 torr of CO (B). (Scale bars: 5 nm.) (C) An enlarged view of B showing the roughly triangular shape of the nanoclusters formed at 1 torr. (D) Coverage of CO on Pt(557) under different pressures as determined by AM-XPS measurements. (E) The relatively stable triangular-shaped nanoclusters predicated by DFT calculations, which provides an explanation to the formation of triangular nanoclusters roughly observed in the STM image shown in C. (Reproduced from ref. 142)
Sum-Frequency Generation (SFG) Vibrational Spectroscopy.
SFG vibrational spectroscopy (129) is a nonlinear spectroscopy technique in which two high-energy pulsed laser beams are overlapped spacially and temporally on an interface of interest. Because of the properties of the nonlinear susceptibility tensor, media with inversion symmetry such as isotropic gases or bulk metal crystals cannot generate a SFG signal. Thus, the entire signal is generated at the interface. The infrared visible SFG process (Fig. S4) can be thought of as an infrared excitation followed by an anti-Stokes Raman relaxation process, the result of which is emitted radiation at the sum of the two incoming frequencies. This technique has been applied in our laboratory to detect surface intermediates of many catalytic reactions over single crystal and nanoparticle model catalysts at high pressures (130, 131).
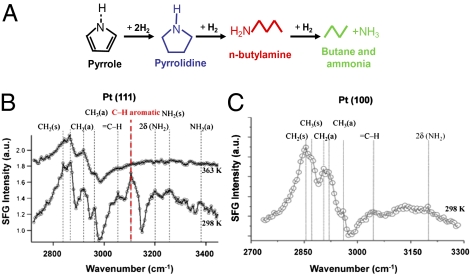
This technique helps us not only to determine the major reaction intermediates on the catalyst surfaces but also to understand the competition between intermediates for active sites. For example, Fig. 6 shows the SFG spectra of the Pt(111) and Pt(100) surfaces during pyrrole hydrogenation (132, 133). In these spectra, the C–Hx vibrational modes below ≈3,000 cm−1 can be attributed to the adsorbed n-butylamine. On the Pt(111) surface, the C–H aromatic stretching mode at 3,105 cm−1 indicates that an intact aromatic ring is adsorbed on the surface at 298 K. Thus, at 298 K, n-butylamine and certain aromatic species are coadsorbed on the Pt(111) surface. The disappearance of the aromatic stretching mode on the Pt(100) surface at 363 K indicates n-butylamine is bonded more strongly to the surface than the aromatic species, which blocks the sites for the adsorption of the aromatic species on the Pt(100) surface. These findings can be applied to understand the shape dependence of selectivity over the nanoparticle catalyst.
Fig. 6.
(A) Pyrrole hydrogenation. The major products are the aromatic molecule, pyrrolidine, and the ring-opening product, n-butylamine. (B) The SFG spectra of the Pt(111) surface during the pyrrole hydrogenation reaction at 298 K and 363 K. (C) The SFG spectrum of the Pt(100) surface during the pyrrole hydrogenation at 298 K. Pyrrole (3 torr) and 30 torr of hydrogen are used in these studies. Note that the C–H aromatic peak at 3,105 cm−1 (the red color dash line) shows up on the Pt(111) surface at 298 K, and that it is missing on the Pt(111) surface at high temperature (363 K) or on the Pt(100) surface at 298 K.
Summary and Outlook
The huge economic impact of surface chemistry is manifested by its pervasive applications in modern industries that produce chemicals, fuels, microelectronic and optical devices, and medical devices. The challenges associated with high-impact industrial applications provide a major thrust to the progressive advances in surface chemistry. At present, there are many technological challenges in surface chemistry applications. A few examples include the development of energy-efficient and environmentally benign processes for chemical and energy conversion, further size reduction of transistors down to sub-10 nm, the development of biocompatible implant materials with long-term stability in the human body, and the development of biosensors with fast detection time and multiple-analyte response capabilities.
Surfaces are involved in many important chemical and biological processes in nature and in industry largely because of their high chemical reactivity. Understanding surface processes at the molecular level, however, has proven to be challenging because spatial resolution down to a few nanometers is a prerequisite for any useful surface science technique. The advancement of various surface-specific techniques is the main theme in the development of modern surface chemistry. These techniques have been used to investigate surfaces at an unprecedented spatial, time, and chemical resolution.
A recent trend in surface chemistry is to study surface reactions at the molecular level under conditions close to those of the real applications by using experimental techniques combined with theoretical simulations (134). This type of research highlights the importance of developing new in situ techniques and is expected to directly aid in the rational design of surface properties for technological applications (135).
During the past decade, studying nanomaterials has become a thrust in surface chemistry. The high surface-volume ratio makes surface properties dominant in nanomaterials. Because of classical and quantum confinement effects, a nanomaterial may exhibit novel physical and chemical properties not seen in its bulk form. These properties can be tailored for advanced technological applications through contolling the size, shape, compositon, and assembly structure of nanomaterials. Surface science principles and techniques have been extensively applied in size- and shape-controlled nanoparticle synthesis, nanostructure assembly, and material property characterization (136–140). Advanced applications of nanomaterails in medicine, electronics, biomaterials, chemicals, and energy production are under intensive investigations. For example, the catalytic activity and selectivity of a transition metal nanocatalyst can be tuned by controlling the size and shape of the nanocatalyst (35–38). A solid lubricant made of hollow MoS2 nanoparticles exhibits superior frictional properties and chemical stability over the thin film MoS2 (88).
In many potential technological applications of nanomaterials, the development of large-scale yet well-controlled synthesis and assembly approaches is a challenging task. Nanomaterials are usually more susceptible to environmental changes than the bulk material. Thus, their thermal and chemical stabilities are major concerns in many applications (141).
Given the rapid molecular-level advances in surface chemistry and their intimate roles in the development of modern technologies, molecular surface chemistry has a bright future in the 21th century.
Supplementary Material
Acknowledgments
This work was supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract DE-AC02-05CH11231.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006669107/-/DCSupplemental.
References
- 1.Somorjai GA, Li Y. Introduction to Surface Chemistry and Catalysis. 2nd Ed. Hoboken, NJ: Wiley; 2010. [Google Scholar]
- 2.Ertl G. Reactions at Solid Surfaces. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 3.Adamson AW, Gast AP. Physical Chemistry of Surfaces. 6th Ed. New York: Wiley; 1997. [Google Scholar]
- 4.Hiemenz PC, Rajagopalan R. Principles of Colloid and Surface Chemistry. 3rd Ed. New York: Marcel Dekker; 1997. [Google Scholar]
- 5.Woodruff DP, Delchar TA. Modern Techniques of Surface Science. 2nd Ed. New York: Cambridge Univ Press; 1994. [Google Scholar]
- 6.Nilsson A, Pettersson LGM, Norskov JK, editors. Chemical Bonding at Surfaces and Interfaces. Amsterdam: Elsevier Science & Technology; 2008. [Google Scholar]
- 7.Sinfelt JH. Role of surface science in catalysis. Surf Sci. 2002;500:923–946. [Google Scholar]
- 8.Ekerdt JG, Sun YM, Szabo A, Szulczewski GJ, White JM. Role of surface chemistry in semiconductor thin film processing. Chem Rev. 1996;96:1499–1518. doi: 10.1021/cr950236z. [DOI] [PubMed] [Google Scholar]
- 9.D'Orazio P. Biosensors in clinical chemistry. Clin Chim Acta. 2003;334:41–69. doi: 10.1016/s0009-8981(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 10.Bartholomew CH, Farrauto RJ. Fundamentals of Industrial Catalytic Processes. 2nd Ed. Hoboken, NJ: Wiley; 2006. [Google Scholar]
- 11.Taylor KC. Automobile catalytic converters. In: Anderson JR, Boudart M, editors. Catalysis: Science and Technology. Berlin: Springer; 1984. pp. 120–170. [Google Scholar]
- 12.Bagot PAJ. Fundamental surface science studies of automobile exhaust catalysis. Mater Sci Technol. 2004;20:679–694. [Google Scholar]
- 13.Anastas PT, Kirchhoff MM, Williamson TC. Catalysis as a foundational pillar of green chemistry. Appl Catal A Gen. 2001;221:3–13. [Google Scholar]
- 14.Anastas PT, Kirchhoff MM. Origins, current status, and future challenges of green chemistry. Acc Chem Res. 2002;35:686–694. doi: 10.1021/ar010065m. [DOI] [PubMed] [Google Scholar]
- 15.Somorjai GA, Park JY. Molecular factors of catalytic selectivity. Angew Chem Int Ed Engl. 2008;47:9212–9228. doi: 10.1002/anie.200803181. [DOI] [PubMed] [Google Scholar]
- 16.Taniewski M. Sustainable chemical technologies in production of clean fuels from fossil fuels. Clean-Soil Air Water. 2008;36:393–398. [Google Scholar]
- 17.Taniewski M. Sustainable chemical technologies—Development trends and tools. Chem Eng Technol. 2006;29:1397–1403. [Google Scholar]
- 18.Tullo AH, Short PL. Propylene oxide routes take off. Chem Eng News. 2006;84:22. [Google Scholar]
- 19.Balzani V, Credi A, Venturi M. Photochemical conversion of solar energy. ChemSusChem. 2008;1:26–58. doi: 10.1002/cssc.200700087. [DOI] [PubMed] [Google Scholar]
- 20.Grätzel M. Photoelectrochemical cells. Nature. 2001;414:338–344. doi: 10.1038/35104607. [DOI] [PubMed] [Google Scholar]
- 21.Bak T, Nowotny J, Rekas M, Sorrell CC. Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int J Hydrogen Energ. 2002;27:991–1022. [Google Scholar]
- 22.Roy SC, Varghese OK, Paulose M, Grimes CA. Toward solar fuels: Photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano. 2010;4:1259–1278. doi: 10.1021/nn9015423. [DOI] [PubMed] [Google Scholar]
- 23.Centi G, Perathoner S. Towards solar fuels from water and CO2. ChemSusChem. 2010;3:195–208. doi: 10.1002/cssc.200900289. [DOI] [PubMed] [Google Scholar]
- 24.Steele BCH, Heinzel A. Materials for fuel-cell technologies. Nature. 2001;414:345–352. doi: 10.1038/35104620. [DOI] [PubMed] [Google Scholar]
- 25.Winter M, Brodd RJ. What are batteries, fuel cells, and supercapacitors? Chem Rev. 2004;104:4245–4269. doi: 10.1021/cr020730k. [DOI] [PubMed] [Google Scholar]
- 26.Carlini C, Patrono P, Galletti AMR, Sbrana G, Zima V. Selective oxidation of 5-hydroxymethyl-2-furaldehyde to furan-2,5-dicarboxaldehyde by catalytic systems based on vanadyl phosphate. Appl Catal A Gen. 2005;289:197–204. [Google Scholar]
- 27.Bianchi CL, Canton P, Dimitratos N, Porta F, Prati L. Selective oxidation of glycerol with oxygen using mono and bimetallic catalysts based on Au, Pd and Pt metals. Catal Today. 2005;102:203–212. [Google Scholar]
- 28.Crossley S, Faria J, Shen M, Resasco DE. Solid nanoparticles that catalyze biofuel upgrade reactions at the water/oil interface. Science. 2010;327:68–72. doi: 10.1126/science.1180769. [DOI] [PubMed] [Google Scholar]
- 29.Hara M. Biomass conversion by a solid acid catalyst. Energy Environ Sci. 2010;3:601–607. [Google Scholar]
- 30.Casanova O, Iborra S, Corma A. Biomass into chemicals: Aerobic oxidation of 5-hydroxymethyl-2-furfural into 2,5-furandicarboxylic acid with gold nanoparticle catalysts. ChemSusChem. 2009;2:1138–1144. doi: 10.1002/cssc.200900137. [DOI] [PubMed] [Google Scholar]
- 31.Casanova O, Iborra S, Corma A. Biomass into chemicals: One pot-base free oxidative esterification of 5-hydroxymethyl-2-furfural into 2,5-dimethylfuroate with gold on nanoparticulated ceria. J Catal. 2009;265:109–116. [Google Scholar]
- 32.Sheldon RA, Downing RS. Heterogeneous catalytic transformations for environmentally friendly production. Appl Catal A Gen. 1999;189:163–183. [Google Scholar]
- 33.Arakawa H, et al. Catalysis research of relevance to carbon management: Progress, challenges, and opportunities. Chem Rev. 2001;101:953–996. doi: 10.1021/cr000018s. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Somorjai GA. Nanoscale advances in catalysis and energy applications. Nano Lett. 2010;10:2289–2295. doi: 10.1021/nl101807g. [DOI] [PubMed] [Google Scholar]
- 35.Witham CA, et al. Converting homogeneous to heterogeneous in electrophilic catalysis using monodisperse metal nanoparticles. Nat Chem. 2010;2:36–41. doi: 10.1038/nchem.468. [DOI] [PubMed] [Google Scholar]
- 36.Somorjai GA, Frei H, Park JY. Advancing the frontiers in nanocatalysis, biointerfaces, and renewable energy conversion by innovations of surface techniques. J Am Chem Soc. 2009;131:16589–16605. doi: 10.1021/ja9061954. [DOI] [PubMed] [Google Scholar]
- 37.Burda C, Chen XB, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 38.Bond GC, Thompson DT. Catalysis by gold. Catal Rev Sci Eng. 1999;41:319–388. [Google Scholar]
- 39.Brattain WH. Surface properties of semiconductors. Science. 1957;126:151–153. doi: 10.1126/science.126.3265.151. [DOI] [PubMed] [Google Scholar]
- 40.Bardeen J. Nobel Lecture, Physics 1942-1962. Amsterdam: Elsevier; 1956. [Google Scholar]
- 41.Duke CB. The birth and evolution of surface science: Child of the union of science and technology. Proc Natl Acad Sci USA. 2003;100:3858–3864. doi: 10.1073/pnas.0730358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bent SF. Semiconductor surface chemistry. In: Nilsson A, Pettersson LGM, Norskov JK, editors. Chemical Bonding at Surfaces and Interfaces. Amsterdam: Elsevier Science & Technology; 2008. pp. 323–395. [Google Scholar]
- 43.Keyes RW. Moore's Law today. IEEE Circuits and Systems Magazine. 2008;8:53–54. [Google Scholar]
- 44.Gates SM. Surface chemistry in the chemical vapor deposition of electronic materials. Chem Rev. 1996;96:1519–1532. doi: 10.1021/cr950233m. [DOI] [PubMed] [Google Scholar]
- 45.Belau L, Park JY, Liang T, Seo H, Somorjai GA. Chemical effect of dry and wet cleaning of the Ru protective layer of the extreme ultraviolet lithography reflector. J Vac Sci Technol B. 2009;27:1919–1925. [Google Scholar]
- 46.Wilk GD, Wallace RM, Anthony JM. High-kappa gate dielectrics: Current status and materials properties considerations. J Appl Phys. 2001;89:5243–5275. [Google Scholar]
- 47.Mokkapati S, Jagadish C. III-V compound SC for optoelectronic devices. Mater Today. 2009;12:22–32. [Google Scholar]
- 48.Feng M, Shyh-Chiang S, Caruth DC, Huang JJ. Device technologies for RF front-end circuits in next-generation wireless communications. Proc IEEE. 2004;92:354–375. [Google Scholar]
- 49.Pearton SJ, Ren F, Zhang AP, Lee KP. Fabrication and performance of GaN electronic devices. Mater Sci Eng Rep. 2000;30:55–212. [Google Scholar]
- 50.Amano H, Sawaki N, Akasaki I, Toyoda Y. Metalorganic vapor-phase epitaxial-growth of a high-quality GaN film using an AlN buffer layer. Appl Phys Lett. 1986;48:353–355. [Google Scholar]
- 51.Castner DG, Ratner BD. Biomedical surface science: Foundations to frontiers. Surf Sci. 2002;500:28–60. [Google Scholar]
- 52.Baier RE, Meyer AE, Natiella JR, Natiella RR, Carter JM. Surface properties determine bioadhesive outcomes: Methods and results. J Biomed Mater Res. 1984;18:327–355. doi: 10.1002/jbm.820180404. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman AS. Principles governing biomolecule interactions at foreign interfaces. J Biomed Mater Res. 1974;8:77–83. doi: 10.1002/jbm.820080309. [DOI] [PubMed] [Google Scholar]
- 54.Vroman L, Adams AL. Findings with the recording ellipsometer suggesting rapid exchange of specific plasma proteins at liquid/solid interfaces. Surf Sci. 1969;16:438–446. [Google Scholar]
- 55.Vroman L. Effects of hydrophobic surfaces upon blood coagulation. Thromb Diath Haemorrh. 1964;10:455–493. [PubMed] [Google Scholar]
- 56.Tanaka M, et al. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)—relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials. 2000;21:1471–1481. doi: 10.1016/s0142-9612(00)00031-4. [DOI] [PubMed] [Google Scholar]
- 57.Chen H, Yuan L, Song W, Wu Z, Li D. Biocompatible polymer materials: Role of protein-surface interactions. Prog Polym Sci. 2008;33:1059–1087. [Google Scholar]
- 58.Wang YX, Robertson JL, Spillman WB, Jr, Claus RO. Effects of the chemical structure and the surface properties of polymeric biomaterials on their biocompatibility. Pharm Res. 2004;21:1362–1373. doi: 10.1023/b:pham.0000036909.41843.18. [DOI] [PubMed] [Google Scholar]
- 59.Luong JHT, Male KB, Glennon JD. Biosensor technology: Technology push versus market pull. Biotechnol Adv. 2008;26:492–500. doi: 10.1016/j.biotechadv.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Bosch ME, Sánchez AJR, Rojas FS, Ojeda CB. Optical chemical biosensors for high-throughput screening of drugs. Comb Chem High Throughput Screen. 2007;10:413–432. doi: 10.2174/138620707781996402. [DOI] [PubMed] [Google Scholar]
- 61.Grieshaber D, MacKenzie R, Voros J, Reimhult E. Electrochemical biosensors—Sensor principles and architectures. Sensors. 2008;8:1400–1458. doi: 10.3390/s80314000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Xu H, Zhang JM, Li G. Electrochemical sensors for clinic analysis. Sensors. 2008;8:2043–2081. doi: 10.3390/s8042043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J, Koffas TS, Lawrence CC, Somorjai GA. Surface structural characterization of protein- and polymer-modified polystyrene microspheres by infrared-visible sum frequency generation vibrational spectroscopy and scanning force microscopy. Langmuir. 2004;20:4640–4646. doi: 10.1021/la0498511. [DOI] [PubMed] [Google Scholar]
- 64.Koffas TS, Amitay-Sadovsky E, Kim J, Somorjai GA. Molecular composition and mechanical properties of biopolymer interfaces studied by sum frequency generation vibrational spectroscopy and atomic force microscopy. J Biomater Sci Polym Ed. 2004;15:475–509. doi: 10.1163/156856204323005325. [DOI] [PubMed] [Google Scholar]
- 65.Harder P, Grunze M, Dahint R, Whitesides GM, Laibinis PE. Molecular conformation in oligo(ethylene glycol)-terminated self-assembled monolayers on gold and silver surfaces determines their ability to resist protein adsorption. J Phys Chem B. 1998;102:426–436. [Google Scholar]
- 66.Zolk M, et al. Solvation of oligo(ethylene glycol)-terminated self-assembled monolayers studied by vibrational sum frequency spectroscopy. Langmuir. 2000;16:5849–5852. [Google Scholar]
- 67.Roberge PR. Corrosion Basics: An Introduction. 2nd Ed. Houston: NACE Intl; 2006. [Google Scholar]
- 68.Schmuki P. From Bacon to barriers: A review on the passivity of metals and alloys. J Solid State Electrochem. 2002;6:145–164. [Google Scholar]
- 69.Olsson COA, Landolt D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim Acta. 2003;48:1093–1104. [Google Scholar]
- 70.Yang JC, de Groh KK. Materials issues in the space environment. MRS Bull. 2010;35:12–16. [Google Scholar]
- 71.Williams DE, Newman RC, Song Q, Kelly RG. Passivity breakdown and pitting corrosion of binary alloys. Nature. 1991;350:216–219. [Google Scholar]
- 72.Kirchheim R, et al. The passivity of iron-chromium alloys. Corros Sci. 1989;29:899–917. [Google Scholar]
- 73.Payer JH, Scully JR. Research opportunities in corrosion science for long termprediciton of materials performance. J Corros Sci Eng. 2005;7 Preprint 15. [Google Scholar]
- 74.Reddy MR. Effect of low-earth orbit atomic oxygen on spacecraft materials. J Mater Sci. 1995;30:281–307. [Google Scholar]
- 75.Bhushan B. Tribology: Friction, Wear, and Lubrication. In: Dorf RC, editor. The Engineering Handbook. Boca Raton, FL: CRC; 2000. [Google Scholar]
- 76.Booser ER. American Society of Lubrication Engineers, Society of Tribologists and Lubrication Engineers . CRC Handbook of Lubrication: Theory and Practice of Tribology. Boca Raton, FL: CRC; 1983. [Google Scholar]
- 77.Campbell ME. Solid Lubricants: A Survey. Washington, DC: Natl Aeronaut Space Admin; 1972. [Google Scholar]
- 78.Freeman ME, Furey MJ, Love BJ, Hampton JM. Friction, wear, and lubrication of hydrogels as synthetic articular cartilage. Wear. 2000;241:129–135. [Google Scholar]
- 79.Gellman AJ. Lubricants and overcoats for magnetic storage media. Curr Opin Colloid Interface Sci. 1998;3:368–372. [Google Scholar]
- 80.Mate CM. Preface: Friction, lubrication, and adhesion in micro- and nano-scale devices. Tribol Lett. 2005;19:1. [Google Scholar]
- 81.Mori S. Present and future of lubricants for magnetic disks. J Japanese Soc Tribol. 1998;43:388–393. [Google Scholar]
- 82.Israelachvili JN, McGuiggan PM, Homola AM. Dynamic properties of molecularly thin liquid films. Science. 1988;240:189–191. doi: 10.1126/science.240.4849.189. [DOI] [PubMed] [Google Scholar]
- 83.Bowden FP, Tabor D. The friction and lubrication of solids. New York: Oxford Univ Press; 2001. [Google Scholar]
- 84.Allen CM, Drauglis E. Boundary layer lubrication: Monolayer or multilayer. Wear. 1969;14:363–384. [Google Scholar]
- 85.Gellman AJ, Spencer ND. Surface chemistry in tribology. Proc Inst Mech Eng, Part J. 2002;216:443–461. [Google Scholar]
- 86.Spikes HA. Direct observation of boundary layers. Langmuir. 1996;12:4567–4573. [Google Scholar]
- 87.Rapoport L, et al. Hollow nanoparticles of WS2 as potential solid-state lubricants. Nature. 1997;387:791–793. [Google Scholar]
- 88.Chhowalla M, Amaratunga GAJ. Thin films of fullerene-like MoS2 nanoparticles with ultra-low friction and wear. Nature. 2000;407:164–167. doi: 10.1038/35025020. [DOI] [PubMed] [Google Scholar]
- 89.Berrien LSJ, Furey MJ, Veit HP. Tribological study of joint pathology. Crit Rev Biomed Eng. 2000;28:103–108. doi: 10.1615/critrevbiomedeng.v28.i12.170. [DOI] [PubMed] [Google Scholar]
- 90.Somorjai GA. The development of molecular surface science and the surface science of catalysis: Berkeley contribution. J Phys Chem B. 2000;104:2969–2979. [Google Scholar]
- 91.Somorjai GA, Park JY. Concepts, instruments, and model systems that enabled the rapid evolution of surface science. Surf Sci. 2009;603:1293–1300. [Google Scholar]
- 92.Van Hove MA, et al. The surface reconstructions of the (100) crystal faces of iridium, platinum and gold. 1. Experimental-observations and possible structural models. Surf Sci. 1981;103:189–217. [Google Scholar]
- 93.Lang E, et al. Leed intensity analysis of the (1×5) reconstruction of Ir(100) Surf Sci. 1983;127:347–365. [Google Scholar]
- 94.Wander A, Somorjai GA, Somorjai GA. Van Hove MA Molecule-induced displacive reconstruction in a substrate surface: Ethylidyne adsorbed on Rh(111) studied by low-energy-electron diffraction. Phys Rev Lett. 1991;67:626–628. doi: 10.1103/PhysRevLett.67.626. [DOI] [PubMed] [Google Scholar]
- 95.Blakely DW, Kozak EI, Sexton BA, Somorjai GA. New instrumentation and techniques to monitor chemical surface-reactions on single-crystals over a wide pressure range (10−8-105 torr) in same apparatus. J Vac Sci Technol. 1976;13:1091–1096. [Google Scholar]
- 96.Strongin DR, Carrazza J, Bare SR, Somorjai GA. The importance of C7 sites and surface roughness in the ammonia-synthesis reaction over iron. J Catal. 1987;103:213–215. [Google Scholar]
- 97.Ertl G. Primary steps in catalytic synthesis of ammonia. J Vac Sci Technol A. 1983;1:1247–1253. [Google Scholar]
- 98.Strongin DR, Bare SR, Somorjai GA. The effects of aluminum-oxide in restructuring iron single-crystal surfaces for ammonia-synthesis. J Catal. 1987;103:289–301. [Google Scholar]
- 99.Strongin DR, Somorjai GA. The effects of potassium on ammonia-synthesis over iron single-crystal surfaces. J Catal. 1988;109:51–60. [Google Scholar]
- 100.Toyoshima I, Somorjai GA. Heats of chemisorption of O2, H2, CO, CO2 and N2 on polycrystalline and single-crystal transition-metal surfaces. Catal Rev Sci Eng. 1979;19:105–159. [Google Scholar]
- 101.Hammer B, Norskov JK. Theoretical surface science and catalysis - Calculations and concepts. Adv Catal. 2000;45:71–129. [Google Scholar]
- 102.Norskov JK. Chemisorption on metal surfaces. Rep Prog Phys. 1990;53:1253–1295. [Google Scholar]
- 103.Norskov JK, Scheffler M, Toulhoat H. Density functional theory in surface science and heterogeneous catalysis. MRS Bull. 2006;31:669–674. [Google Scholar]
- 104.Bligaard T, Norskov JK. Heterogeneous catalysis. In: Nilsson A, Pettersson LGM, Norskov JK, editors. Chemical Bonding at Surfaces and Interfaces. Amsterdam: Elsevier Science & Technology; 2008. pp. 255–321. [Google Scholar]
- 105.Hammer B, Norskov JK. Why gold is the noblest of all the metals. Nature. 1995;376:238–240. [Google Scholar]
- 106.Lopez N, Nørskov JK. Catalytic CO oxidation by a gold nanoparticle: A density functional study. J Am Chem Soc. 2002;124:11262–11263. doi: 10.1021/ja026998a. [DOI] [PubMed] [Google Scholar]
- 107.Nørskov JK, et al. The nature of the active site in heterogeneous metal catalysis. Chem Soc Rev. 2008;37:2163–2171. doi: 10.1039/b800260f. [DOI] [PubMed] [Google Scholar]
- 108.West LA, Kozak EI, Somorjai GA. Molecular beam scattering from single crystal surfaces under ultrahigh vacuum conditions. J Vac Sci Technol. 1971;8:430. [Google Scholar]
- 109.Engel T, Ertl G. Molecular-beam investigation of catalytic oxidation of CO on Pd(111) J Chem Phys. 1978;69:1267–1281. [Google Scholar]
- 110.Jones RH, Olander DR, Schwarz JA, Siekhaus WJ. Investigation of gas-solid reactions by modulated molecular-beam mass-spectrometry. J Vac Sci Technol. 1972;9:1429. [Google Scholar]
- 111.Salmeron M, Gale RJ, Somorjai GA. Molecular beam study of H2-D2 exchange reaction on stepped platinum crystal surfaces: Dependence on reactant angle of incidence. J Chem Phys. 1977;67:5324–5334. [Google Scholar]
- 112.Tully JC. Dynamics of chemical processes at surfaces. Acc Chem Res. 1981;14:188–194. [Google Scholar]
- 113.Binnig G, Rohrer H. Scanning tunneling microscopy - from birth to adolescence. Rev Mod Phys. 1987;59:615–625. [Google Scholar]
- 114.Besenbacher F, et al. Atomic-scale surface science phenomena studied by scanning tunneling microscopy. Surf Sci. 2009;603:1315–1327. [Google Scholar]
- 115.Briner BG, Doering M, Rust HP, Bradshaw AM. Microscopic molecular diffusion enhanced by adsorbate interactions. Science. 1997;278:257–260. [Google Scholar]
- 116.Wintterlin J, Volkening S, Janssens TVW, Zambelli T, Ertl G. Atomic and macroscopic reaction rates of a surface-catalyzed reaction. Science. 1997;278:1931–1934. doi: 10.1126/science.278.5345.1931. [DOI] [PubMed] [Google Scholar]
- 117.Salmeron M, Schlogl R. Ambient pressure photoelectron spectroscopy: A new tool for surface science and nanotechnology. Surf Sci Rep. 2008;63:169–199. [Google Scholar]
- 118.Bluhm H, et al. In situ x-ray photoelectron spectroscopy studies of gas-solid interfaces at near-ambient conditions. MRS Bull. 2007;32:1022–1030. [Google Scholar]
- 119.Grass ME, et al. A reactive oxide overlayer on rhodium nanoparticles during CO oxidation and its size dependence studied by in situ ambient-pressure X-ray photoelectron spectroscopy. Angew Chem Int Ed Engl. 2008;47:8893–8896. doi: 10.1002/anie.200803574. [DOI] [PubMed] [Google Scholar]
- 120.Tao F, et al. Reaction-driven restructuring of Rh-Pd and Pt-Pd core-shell nanoparticles. Science. 2008;322:932–934. doi: 10.1126/science.1164170. [DOI] [PubMed] [Google Scholar]
- 121.McIntyre BJ, Salmeron MB, Somorjai GA. A scanning tunneling microscope that operates at high pressures and high temperatures (430-K) and during catalytic reactions. Catal Lett. 1992;14:263–269. [Google Scholar]
- 122.Rasmussen PB, Hendriksen BLM, Zeijlemaker H, Ficke HG, Frenken JWM. The “reactor STM”: A scanning tunneling microscope for investigation of catalytic surfaces at semi-industrial reaction conditions. Rev Sci Instrum. 1998;69:3879–3884. [Google Scholar]
- 123.Petersen L, et al. A fast-scanning, low- and variable-temperature scanning tunneling microscope. Rev Sci Instrum. 2001;72:1438–1444. [Google Scholar]
- 124.Kolmakov A, Goodman DW. In situ scanning tunneling microscopy of individual supported metal clusters at reactive gas pressures from 10(-8) to 10(4) Pa. Rev Sci Instrum. 2003;74:2444–2450. [Google Scholar]
- 125.Rossler M, Geng P, Wintterlin J. A high-pressure scanning tunneling microscope for studying heterogeneous catalysis. Rev Sci Instrum. 2005;76:023705. [Google Scholar]
- 126.Tao F, Tang D, Salmeron M, Somorjai GA. A new scanning tunneling microscope reactor used for high-pressure and high-temperature catalysis studies. Rev Sci Instrum. 2008;79:084101. doi: 10.1063/1.2960569. [DOI] [PubMed] [Google Scholar]
- 127.Montano M, Salmeron M, Somorjai GA. STM studies of cyclohexene hydrogenation/dehydrogenation and its poisoning by carbon monoxide on Pt(111) Surf Sci. 2006;600:1809–1816. [Google Scholar]
- 128.Grunes J, Zhu J, Yang MC, Somorjai GA. CO poisoning of ethylene hydrogenation over Pt catalysts: A comparison of Pt(111) single crystal and Pt nanoparticle activities. Catal Lett. 2003;86:157–161. [Google Scholar]
- 129.Shen YR. Surface properties probed by second-harmonic and sum-frequency generation. Nature. 1989;337:519–525. [Google Scholar]
- 130.Cremer PS, Su XC, Somorjai GA, Shen YR. High pressure catalytic processes studied by infrared-visible sum frequency generation. J Mol Catal A Chem. 1998;131:225–241. [Google Scholar]
- 131.Somorjai GA, Kliewer CJ. Reaction selectivity in heterogeneous catalysis. React Kinet Catal Lett. 2009;96:191–208. [Google Scholar]
- 132.Tsung CK, et al. Sub-10 nm platinum nanocrystals with size and shape control: Catalytic study for ethylene and pyrrole hydrogenation. J Am Chem Soc. 2009;131:5816–5822. doi: 10.1021/ja809936n. [DOI] [PubMed] [Google Scholar]
- 133.Kliewer CJ, Bieri M, Somorjai GA. Pyrrole hydrogenation over Rh(111) and Pt(111) single-crystal surfaces and hydrogenation promotion mediated by 1-methylpyrrole: A kinetic and sum-frequency generation vibrational spectroscopy study. J Phys Chem C. 2008;112:11373–11378. [Google Scholar]
- 134.Somorjai GA, Li YM. Major successes of theory-and-experiment-combined studies in surface chemistry and heterogeneous catalysis. Top Catal. 2010;53:311–325. [Google Scholar]
- 135.Norskov JK, Bligaard T, Rossmeisl J, Christensen CH. Towards the computational design of solid catalysts. Nat Chem. 2009;1:37–46. doi: 10.1038/nchem.121. [DOI] [PubMed] [Google Scholar]
- 136.Yin Y, Alivisatos AP. Colloidal nanocrystal synthesis and the organic-inorganic interface. Nature. 2005;437:664–670. doi: 10.1038/nature04165. [DOI] [PubMed] [Google Scholar]
- 137.Somorjai GA, Park JY. Colloid science of metal nanoparticle catalysts in 2D and 3D structures. Challenges of nucleation, growth, composition, particle shape, size control and their influence on activity and selectivity. Top Catal. 2008;49:126–135. [Google Scholar]
- 138.Tao AR, Habas S, Yang P. Shape control of colloidal metal nanocrystals. Small. 2008;4:310–325. [Google Scholar]
- 139.Zhao DY, Yang PD, Huo QS, Chmelka BF, Stucky GD. Topological construction of mesoporous materials. Curr Opin Solid State Mater Sci. 1998;3:111–121. [Google Scholar]
- 140.Murray CB, Kagan CR, Bawendi MG. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu Rev Mater Sci. 2000;30:545–610. [Google Scholar]
- 141.Campbell CT, Parker SC, Starr DE. The effect of size-dependent nanoparticle energetics on catalyst sintering. Science. 2002;298:811–814. doi: 10.1126/science.1075094. [DOI] [PubMed] [Google Scholar]
- 142.Tao F, et al. Break-up of stepped platinum catalyst surfaces of high CO coverage. Science. 2010;327:850–853. doi: 10.1126/science.1182122. [DOI] [PubMed] [Google Scholar]
- 143.Bylinsky G. Fortune. May 27, 1985. The magic of designer catalysts; pp. 82–88. [Google Scholar]
- 144.Deutschmann O, Knozinger H Kochloefel, Turek T. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag, Weinheim: Wiley; 2009. Heterogeneous catalysis and solid catalysts. Electronic Release, 7th Ed. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.