Abstract
Selenoproteins, in particular thioredoxin reductase, have been implicated in countering oxidative damage occurring during aging but the molecular functions of these proteins have not been extensively investigated in different animal models. Here we demonstrate that TRXR-1 thioredoxin reductase, the sole selenoprotein in Caenorhabditis elegans, does not protect against acute oxidative stress but functions instead together with GSR-1 glutathione reductase to promote the removal of old cuticle during molting. We show that the oxidation state of disulfide groups in the cuticle is tightly regulated during the molting cycle, and that when trxr-1 and gsr-1 function is reduced, disulfide groups in the cuticle remain oxidized. A selenocysteine-to-cysteine TRXR-1 mutant fails to rescue molting defects. Furthermore, worms lacking SELB-1, the C. elegans homolog of Escherichia coli SelB or mammalian EFsec, a translation elongation factor known to be specific for selenocysteine in E. coli, fail to incorporate selenocysteine, and display the same phenotype as those lacking trxr-1. Thus, TRXR-1 function in the reduction of old cuticle is strictly selenocysteine dependent in the nematode. Exogenously supplied reduced glutathione reduces disulfide groups in the cuticle and induces apolysis, the separation of old and new cuticle, strongly suggesting that molting involves the regulated reduction of cuticle components driven by TRXR-1 and GSR-1. Using dauer larvae, we demonstrate that aged worms have a decreased capacity to molt, and decreased expression of GSR-1. Together, our results establish a function for the selenoprotein TRXR-1 and GSR-1 in the removal of old cuticle from the surface of epidermal cells.
The life cycles of nematodes, arthropods, tardigrades, onychophoruns, nematomorphs, kinorhynchs, and priapulids all involve distinct stages separated by molts. However, despite the fact that together these phyla contain the most abundant and diverse group of animals presently living, much remains to be learned about the molecular mechanisms by which molting occurs and is regulated. In insects, molting and metamorphosis is triggered by ecdysone, whose synthesis is modulated in response to environmental, developmental, and physiological cues (1, 2). However, precisely how ecdysone leads to the shedding of the old cuticle is not yet understood. In Caenorhabditis elegans, molting occurs at the end of each of the four larval stages of the reproductive life cycle. It also occurs upon entry into and exit from the dauer developmental diapause. Both cholesterol (3) and nuclear hormone receptors (4) are required for molting in C. elegans, suggesting that, although ecdysone itself is not produced, molting may be regulated by a steroid-derived hormone. A number of different tissues have been implicated in the control of molting, suggesting that, as it is in insects, the process is likely to be under complex regulation (5). Genome-wide screens for molting mutants have led to the identification of genes encoding proteins involved in a variety of different processes, and established a hierarchy for their function (5).
Thioredoxin reductases are NADPH-dependent oxidoreductases that, together with thioredoxins, form the thioredoxin system (6, 7). Mammalian thioredoxin reductases are homodimeric selenoproteins: The single selenocysteine residue is in a C-terminal Gly-Cys-Sec-Gly motif (8), the cysteine and selenocysteine residues of which constitute one of two redox active sites in the enzyme (9, 10). Selenocysteine has been shown to be required for the ability of rat thioredoxin reductase 1 (TrxR1) to reduce hydrogen peroxide in vitro (11). However, the significance of selenocysteine for activity in vivo is presently unclear. TrxR1 and TrxR2 are essential for mouse embryogenesis (12, 13). Mouse embryos lacking TrxR2 have defects in cell growth, programmed cell death, and heart function (13). However, the function of selenium-containing thioredoxin reductases in other animal models has not been reported. Thioredoxin reductase activity is induced in epidermal cells in mammals in response to exposure to UV light (14, 15), but the function of the enzyme in epidermal cells is not well understood.
Results
Disulfide Groups in the Cuticle Are Reduced During Molting.
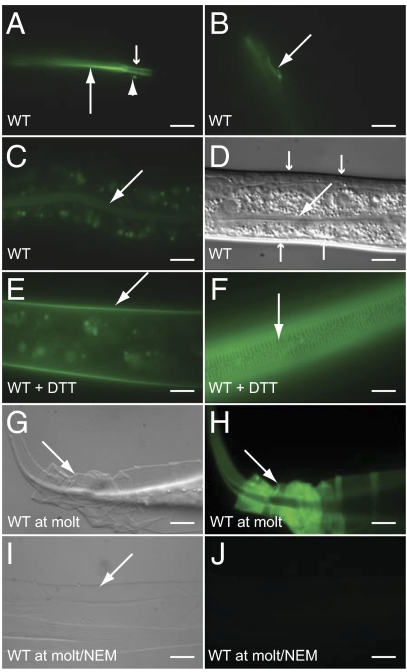
The cuticle in C. elegans and other nematodes is a highly ordered matrix composed of collagens, cuticulins, glycoproteins, and lipids (16). Collagens, which make up a substantial fraction of the cuticle, are highly cross-linked, in part through nonreducible di- and trityrosine cross-links and in part through disulfide bonds that link cysteine residues in different collagen molecules (16). Treatment with reducing agents leads to permeabilization of the cuticle and the release of soluble collagens (17). To investigate whether cross-linking through disulfides changes during molting, we stained worms with Alexa Fluor 488 C5-maleimide, a fluorescently-labeled maleimide derivative that reacts in vivo with accessible (extracellular) thiol (–SH) groups but not with oxidized sulfur atoms within disulfides (18). Worms at intermolt stages of the cycle (i.e., those that were not molting) showed staining of the pharyngeal lumen and the buccal cavity (and weakly of the intestinal lumen) but not of the cuticle (Fig. 1 A–D). Pretreatment of intermolt worms with the reducing agent DTT allowed high levels of cuticle staining, demonstrating that reduction of cuticle disulfides causes increased staining (Fig. 1 E and F). Exposure of worms at ecdysis to the label without pretreatment with DTT led to strong staining of the cuticle (Fig. 1 G and H). Cuticles shed at the L1, L2, L3, and L4 molts were all stained (Fig. S1 A–D). Treatment of detached cuticle with N-ethylmaleimide, which reacts specifically with thiols in biological systems, completely blocked staining (Fig. 1 I and J). Thus, disulfide groups in the cuticle are actively reduced during molting.
Fig. 1.
Disulfide groups in the cuticle are reduced during molting in C. elegans. Micrographs of worms stained with Alexa Fluor 488 C5-maleimide viewed with DIC (D, G, and I) or fluorescence (A–C, E, F, and H) optics. The large and small arrows in A indicate the lumen of the pharynx and buccal cavity, respectively. The arrowhead in A and the arrow in B indicate one of a pair of cell or neuronal exensions lying adjacent to the buccal cavity stained by the dye. The large arrows in C and D indicate the lumen of the intestine. The small arrows in D indicate the cuticle, which is not appreciably stained by the dye in nonmolting worms under normal conditions. (E and F) Nonmolting worms that had been incubated with 5 mM DTT for 30 min before staining with the dye. (G and H) Worms at molt that had not been incubated with DTT. The arrows indicate cuticle. (I and J) Molted cuticle that had been incubated with N-ethylmaleimide before staining. (Scale bars, 10 μm.)
Diamide is a small diffusible molecule that rapidly and specifically oxidizes thiol groups in vivo and in vitro (19). We found that, when L3 larvae that had recently completed the L2 molt were exposed to 18 mM diamide, they were able to continue growing. The divisions of six 6 Pn.p cells that form the vulval equivalence group (P3.p-P8.p), for example, were normal (Fig. S1 E and F). However, such worms arrested at the L3 molt with defects in apolysis, the separation of old and new cuticle (n = 10; Fig. S1G). When late L2 larvae were exposed to diamide, they arrested at the L2 molt (n = 10), and P3.p-P8.p failed to divide (Fig. S1H). That diamide can cause a block in molting in C. elegans without preventing growth strongly suggests that reduction of either cuticle components or a regulator of molting is necessary for molting to occur.
Thioredoxin Reductase 1 and Glutathione Reductase Are Essential for Molting.
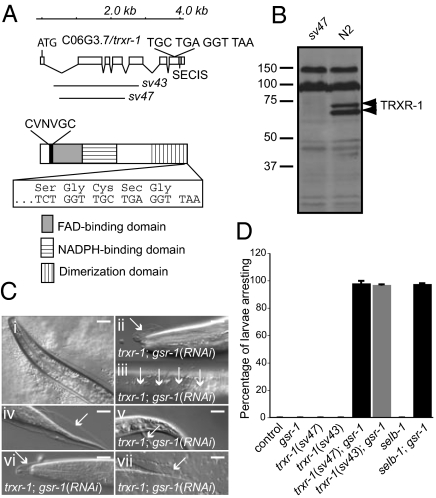
Thioredoxin reductases are proposed to be required for the reduction of oxidized disulfides in metazoans. C. elegans contains two thioredoxin reductase homologs, TRXR-1 and TRXR-2, but only TRXR-1 is a selenoprotein (20–22). We generated a trxr-1 deletion allele, sv47 (Fig. 2A) that is very likely null for trxr-1 function: no TRXR-1 protein was detected on Western blots of extracts from sv47 mutants (Fig. 2B). Worms homozygous for sv47 were not obviously different from the wild-type strain, N2, when grown under standard laboratory conditions: They grew normally and had normal life spans and brood sizes (Fig. S2A and Table S1). They did not show increased sensitivity to oxidative stress (Fig. S2 B–L) or defects in molting. trxr-1(sv47) worms that were also mutant for a deletion of trxr-2, tm2047, were also viable and fertile and did not arrest during molting. Thus, in an otherwise wild-type genetic background, thioredoxin reductases are dispensable for growth, development, and molting in C. elegans.
Fig. 2.
C. elegans TRXR-1 is required together with GSR-1 glutathione reductase for molting. (A Upper) The exon intron structure of the trxr-1 gene. Boxes represent exons, and lines represent introns. The sequence of the last four codons is shown, as is the position of the selenocysteine insertion sequence (SECIS) element in the 3′ untranslated region. The lines underneath denote the regions deleted in the sv43 and sv47 mutant alleles. (A Lower) Domain structure of the TRXR-1 protein. The sequence of the N-terminal redox active site is shown above, and the sequence of the C-terminal site is shown boxed below. (B) Western blot of C. elegans protein extracts probed with an antibody raised against TRXR-1. N2 indicates wild-type control. The upper bands result from nonspecific cross-reactivity of the antibody. (C) Micrographs of molting worms viewed with DIC optics. Old cuticle (indicated by arrows) associated with the buccal cavity (ii), midbody (iii), and rectum (v) is shown. The other panels show cuticle associated with either the head or tail regions. (i and iv) Wild-type control worm at molt. The complete genotype of the trxr-1 worms was trxr-1(sv47); rrf-3(pk1426); gsr-1(RNAi). (Scale bars, 10 μm.) (D) Graph showing percentage of larvae that arrest growth before becoming adults. All worms were homozygous for rrf-3(pk1426).
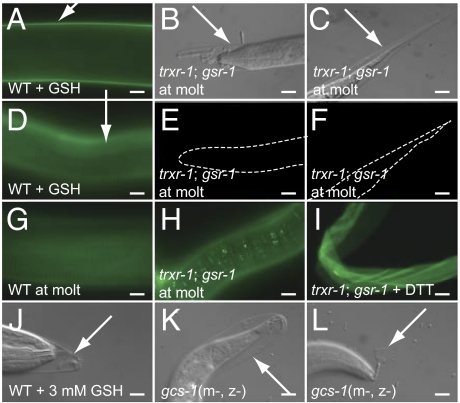
Work in yeast (23) and Drosophila (24) has indicated that enzymes with redox functions can have overlapping functions in vivo. Therefore, to test whether TRXR-1 functioned together with other proteins to regulate molting, we performed RNAi in a trxr-1(sv47) background against a panel of genes encoding proteins with known or proposed redox functions (Table S2). RNAi of the single glutathione reductase gene in the worm, gsr-1, was found to cause a highly penetrant growth arrest specifically in the absence of trxr-1 function (Fig. 2D). No synthetic effects were observed with any of the other genes tested. Inspection of the arrested trxr-1; gsr-1(RNAi) larvae under high magnification revealed that they had invariably arrested during molting (Fig. 2C). The arrest did not occur specifically at one molt, but occurred at any one of the four larval molts: 71% of trxr-1(sv47); gsr-1(RNAi) animals arrest at the L1 molt; 18% arrest at the L2 molt, 9% arrest at the L3 molt, and 2% arrest at the L4 molt. The majority of the trxr-1; gsr-1(RNAi) animals that arrested were surrounded by partially detached old cuticle, suggesting that apolysis and ecdysis were defective. When arrested trxr-1; gsr-1(RNAi) worms were stained with C5-maleimide, they were either not appreciably labeled or were labeled only at low levels (Fig. 3 B, C, E, F, and H). Furthermore, in worms in which low levels of labeling could be seen, the pattern was abnormal: It was markedly punctate (Fig. 3H) rather than smooth (Fig. 3G). However, when cuticle from trxr-1; gsr-1(RNAi) worms was first treated with DTT, it was labeled by C5-maleimide (Fig. 3I), implying that cuticle in these worms is not lacking thiol groups. We conclude that worms with decreased trxr-1 and gsr-1 function are defective in their ability to reduce cuticle components during molting.
Fig. 3.
TRXR-1 and GSR-1 are required for the reduction of disulfide groups in the cuticle during molting, which is promoted by GSH. (A–I) Micrographs of worms stained with Alexa Fluor 488 C5-maleimide viewed with DIC (B and C) or fluorescence (A and D–I) optics. The exposure time for the image in H was 1 s; for all other fluorescence images, exposure time was 100 ms. (A and D) Intermolt worms that had been incubated with 5 mM GSH for 30 min at room temperature before staining with the dye. (B, C, and E–H) Worms at molt that had not been incubated with GSH. The arrows indicate cuticle. (H) Note the punctate staining seen in some trxr-1; gsr-1(RNAi) worms at longer exposure times. (I) Cuticle from trxr-1; gsr-1(RNAi) worms that had been incubated with DTT before staining. (J–L) Partially detached cuticle in worms exposed to either 3 mM GSH (J) or that lacked maternal (m−) and zygotic (z−) activity of gcs-1 (K and L). (Scale bars, 10 μm.)
Reduced Glutathione Induces Apolysis.
In yeast, oxidative refolding of proteins containing disulfide bonds requires protein disulfide isomerase (25), which contains a thioredoxin domain. Furthermore, mutants in C. elegans pdi-2, which encodes a protein disulfide isomerase, have been shown to have defects in cuticle synthesis and molting (26). We found that PDI-2 and a second protein disulfide isomerase in C. elegans, PDI-3, appear to be required for proper protein folding because RNAi of the pdi-2 or pdi-3 genes resulted in induction of the unfolded protein response (UPR; Fig. S3 E and F). However, the UPR was not induced in trxr-1(sv47); gsr-1(RNAi) animals (Fig. S3 G and H). Furthermore, whereas cuticle synthesis is severely disrupted in pdi-2 mutants (26), this was not the case for trxr-1(sv47); gsr-1(RNAi) animals. First, formation of both the annuli and the alae on the L1 cuticle appear normal in animals lacking trxr-1 and gsr-1 activity (Fig. S3 I–L). In animals with aberrant cuticle secretion, L1 alae are absent or flattened and the annuli are disrupted (26). Analysis of the cuticle by electron microscopy failed to reveal any defects in trxr-1; gsr-1(RNAi) larvae (Fig. S3 M and N). Furthermore, the expression of GFP reporters for both QUA-1, a cuticle protein required for molting (27), and for LON-3, a cuticle collagen, was normal (Fig. S3 O–R). These observations argue against a model in which the molting defect seen in trxr-1; gsr-1 animals is the result of a defect in the proper folding of cuticle proteins within the secretory pathway.
In both Saccharomyces cerevisiae and Drosophila, the thioredoxin system has been shown to promote the reduction of glutathione (23, 24). Treatment of worms with GSH at a concentration of 3 mM, the concentration within eukaryotic cells, led to apolysis, the separation of old and new cuticle (Fig. 3J). All of the worms (n = 20) were affected but, at this concentration, the separation observed was confined to the cuticle in the head region; cuticle posterior of pharynx was not obviously detached. Treatment of worms with 10 mM GSH, however, led to detachment of the cuticle along the entire length of the worm (n = 20; Fig. S4 A–C). Such worms were very fragile and frequently burst during mounting for microscopy, an observation that suggests that GSH can induce apolysis even before the new cuticle is properly formed. Even at low concentrations, GSH treatment permitted strong staining by C5-maleimide (Fig. 3 A and D). Thus, extracellularly administered GSH can promote the reduction of disulfide groups in the cuticle, and reduction of cuticle components leads to detachment of the old cuticle. Consistent with the possibility that trxr-1; gsr-1(RNAi) animals arrest because they have reduced levels of GSH, exogenously supplied GSH promoted the separation and removal of cuticle from arrested trxr-1; gsr-1(RNAi) larvae (n = 10; Fig. S4 D–G). Furthermore, a defect in glutathione synthesis blocked molting: Animals with reduced zygotic and maternal activity of gcs-1, which encodes the enzyme that catalyses the first step in the synthesis of glutathione, arrested at the molt (n = 40; Fig. 3 K and L).
TRXR-1 Requires Selenocysteine for Function in Vitro and in Vivo.
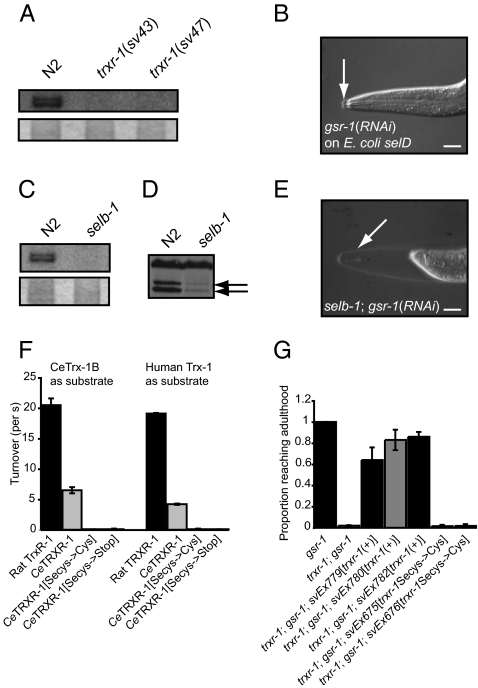
Previous work has suggested that trxr-1 is the only gene to encode selenoprotein in C. elegans (20–22). Consistent with this suggestion, when trxr-1(sv47) homozygous mutant worms [or those homozygous for a second allele, trxr-1(sv43) (Fig. 2A)] were grown on Escherichia coli bacteria radioactively labeled with 75Se, no label was detected in protein extracted from the mutants (Fig. 4A and Fig. S5A). Because the trxr-1 mutants lacked the closely spaced doublet of bands seen in wild type, trxr-1 encodes two proteins, both of which contain selenocysteine.
Fig. 4.
TRXR-1 is a selenoprotein; incorporation of selenium requires selb-1, and is necessary for function. (A Upper) Autoradiogram of a gel containing protein extracts of worms raised on 75Se-labeled E. coli. (A Lower) The Coomassie-stained gel. (B) Nomarski micrograph of an arrested worm grown on selD mutant E. coli that expressed gsr-1 dsRNA. (Scale bars, 10 μm.) (C Upper) Autoradiogram of a gel containing protein extracts of worms raised on 75Se-labeled E. coli. (C Lower) The Coomassie-stained gel. (D) Western blot of C. elegans protein extracts probed with an antibody raised against TRXR-1. (E) Micrograph of a selb-1 mutant worm grown on E. coli, wild type with respect to selD, that expressed gsr-1 dsRNA. Arrows indicate old cuticle. (F) Graph showing the activities of wild-type and mutant TRXR-1 proteins in catalyzing thioredoxin-mediated reduction of insulin in vitro (Materials and Methods). (G) Graph indicating the ability of wild-type or mutant trxr-1 transgenes to rescue the growth arrest defect displayed by trxr-1(sv47); gsr-1(RNAi) larvae. All strains were homozygous for rrf-3(pk1426).
In prokaryotes, archaea, and eukaryotes, selenocysteine is incorporated into selenoproteins by the alternate decoding of UGA stop codons by a selenocysteine tRNA, tRNA[Ser]Sec (28). Among other factors, incorporation also requires a cis-acting sequence in the mRNA, selenocysteine insertion element, and a dedicated translation elongation factor, SelB or EFsec (28). In rats, in the absence of selenium the UGA codon can code for cysteine in place of selenocysteine (29). To test the importance of selenocysteine for TRXR-1 function in C. elegans, we generated an E. coli strain lacking SelD selenophosphate synthetase, an enzyme that activates selenium for specific incorporation into bacterial selenoproteins and selenium-containing tRNA species, and fed worms these bacteria. Genotypically trxr-1(+) worms were able to develop in to fertile adults on the E. coli selD deletion strain. However, when gsr-1 was knocked down by RNAi, worms raised on this strain arrested growth during molting (Fig. 4B). To study further the requirement for selenocysteine for thioredoxin reductase function in vivo, we generated a deletion mutant of the single SelB/EFsec homolog in C. elegans, selb-1 (ref. 30; Fig. S5B). Consistent with a requirement for SELB-1 for selenocysteine incorporation, no radiolabeled proteins were detected in protein extracts from selb-1(sv36) mutant worms grown on 75Se-labeled E. coli wild type with respect to selD (Fig. 4C and Fig. S5C). The sv36 mutants showed no obvious defects under standard laboratory growth conditions (Fig. S2A and Table S1). However, when subjected to gsr-1(RNAi), they arrested growth during molting displaying phenotypes identical to those of trxr-1; gsr-1(RNAi) animals (Figs. 2D and 4E).
The TRXR-1 protein present in selb-1 mutants is predicted to lack the last two amino acids, Sec and Gly (Fig. 2A). The truncation apparently affects TRXR-1 stability or synthesis because levels of the protein are strongly reduced in selb-1 mutants (Fig. 4D). In the context of the full-length protein, replacement of selenocysteine with cysteine dramatically reduced the ability to complement defects in vivo (Fig. 4G) without affecting protein stability (Fig. S5D). Replacement of selenocysteine with cysteine also dramatically reduced the ability of C. elegans TRXR-1 to reduce the C. elegans or human thioredoxin 1 in vitro (Fig. 4F and Fig. S5F). We conclude that selenocysteine incorporation is required for TRXR-1 activity in vivo and in vitro.
trxr-1 and gsr-1 Are Expressed and Required in Cells That Secrete Cuticle.
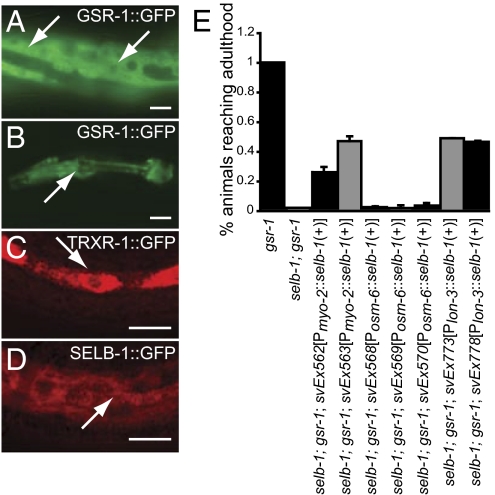
Expression of trxr-1::gfp reporter was seen in the hypodermis, in the pharynx and within the nervous system (Fig. 5C and Fig. S6 A, B, and E). A gsr-1::gfp reporter was strongly expressed in the hypodermis and the pharynx (Fig. 5 A and B) but not in the nervous system. Expression of a selb-1 cDNA under the control of either pharyngeal- or hypodermal-specific promoters (but not of a neuronal-specific promoter) partially rescued the selb-1(sv36); gsr-1(RNAi) arrest phenotype (Fig. 5E), implying that selb-1 (and consequently trxr-1) activity in the pharynx and hypodermis promotes molting.
Fig. 5.
Expression patterns of gsr-1::gfp, trxr-1::gfp and selb-1::gfp transgenes and focus for selb-1 activity. (A–D) Micrographs of worms with the indicated transgenes viewed with fluorescence (A and B) or confocal fluorescence (C and D) optics. The arrows in A, C, and D indicate GFP fluorescence in the hypodermis; arrows in B indicate GFP fluorescence in the pharynx. The worms in C and D were fixed and stained with an anti-GFP antibody. (Scale bars, 10 μm.) (E) Graph indicating the ability of seven-transgene arrays to rescue the growth arrest defect displayed by selb-1; gsr-1(RNAi) larvae. The arrays contain a selb-1 cDNA under the control of different tissue-specific promoters. The myo-2 promoter is active in the pharynx. The osm-6 and lon-3 promoters are active in ciliated sensory neurons and the hypodermis, respectively. All strains in E were homozygous for rrf-3(pk1426).
Molting Efficiency Is Reduced in Old Worms.
The redox activity of selenoproteins, in particular of thioredoxin reductases, has led to the suggestion that these proteins inhibit the changes associated with aging and promote longevity (31). However, the oxidative damage theory of aging remains controversial (32). To examine whether molting is less efficient in older C. elegans worms, we investigated whether aged dauer larvae have a reduced capacity to undergo the postdauer molt. When 1-wk-old dauer larvae were stimulated to exit dauer by being placed on food, they invariably began molting within 13 h (n = 20) and took, at most, only 15 min to complete ecdysis. In contrast, of 20 3-mo-old dauer larvae placed on food, none completed the molt within 24 h. Furthermore, 5 of the 20 larvae arrested during the molt (Fig. S6 G and H) and subsequently died without maturing into adults. Thus, molting efficiency dramatically decreases with age in wild-type dauer larvae. The reduced ability to undergo the molt correlated with a decrease in the amount of reduced thiols in the cuticle: Unlike wild-type intermolt animals, in which the cuticle is not strongly stained by C5-maleimide, appreciable fluorescence is seen when young dauer larvae are stained with the dye (Fig. S6 I and J). The level of fluorescence, however, is dramatically reduced in old dauer larvae (Fig. S6 K and L). Consistent with earlier global studies in which a reduction in gsr-1 expression was seen with age in dauer larvae (33), we found that young dauer larvae showed robust expression of a gsr-1::gfp reporter in hypodermal cells, but fluorescence of the reporter was almost not detectable in aged larvae (Fig. S6 M and N). trxr-1 and gsr-1 are together required for the postdauer molt: Whereas knockdown of gsr-1 by RNAi in trxr-1(+) animals did not prevent 3-d-old dauer larvae from developing into normal fertile adults (n = 66), gsr-1(RNAi) in a trxr-1 mutant background caused a 100% penetrant growth arrest during the postdauer molt (n = 128; Fig. S6 O–Q). Similar effects were seen with selb-1: 99% (n = 145) of 3-d-old selb-1(sv36); gsr-1(RNAi) dauer larvae arrested at the molt when stimulated to exit dauer (Fig. S6R).
Discussion
Selenoproteins are found in organisms in all three categories of life: archaea, prokaryotes, and eukaryotes. Although extensive work has been done on the functions on eukaryotic selenoproteins in vitro, few reports exist on the functions of selenoproteins in general or of thioredoxin reductases in particular in animal models. C. elegans represents an attractive system with which to address the importance of selenocysteine and thioredoxin reductase function in vivo.
Collagens and other components of the cuticle are known to be cross-linked via disulfide groups both in C. elegans and larger metazoans, including mammals. However, that disulfide groups are reduced during molting has not previously been demonstrated. Our results indicate that the thioredoxin and glutathione systems are required for the reduction of disulfide groups in the cuticle during molting. Earlier work identified BLI-3 (a dual oxidase), MLT-7 (a heme peroxidase), and PDI-2 (a putative protein disulfide isomerase) as important for molting (26, 34). BLI-3 and MLT-7 act by promoting the oxidative cross-linking of collagens via dityrosine groups rather than the reduction of disulfides. PDI-2, on the other hand, has two separate functions in cuticle formation: Alone, it acts as a dithiol oxidase to catalyze the formation of disulfides in cuticle collagens; in complex with DPY-18/PHY-1 or PHY-2, it acts as a prolyl hydroxylase to catalyze the hydroxylation of proline residues in collagens, a modification important for the stability of the triple helical fold. It is noteworthy that although bli-3, mlt-7 or pdi-2 mutants display molting defects, they also show severe defects in cuticle synthesis; many arrest as embryos, and the larvae that escape embryonic arrest are severely Dpy or otherwise malformed (26, 34). The observations that the UPR is not activated in trxr-1; gsr-1 larvae, and that the cuticle in such larvae is not severely defective, argue against a model in which the molting defect in trxr-1; gsr-1 larvae is an indirect result of defects in cuticle synthesis or secretion.
Our analysis, together with earlier studies (20–22), strongly suggests that trxr-1 is the only gene in C. elegans encoding selenoprotein. Thus, the six or more dedicated proteins and RNAs thought to be required for the insertion of selenocysteine in metazoans appear to exist in C. elegans for the sole purpose of decoding a single codon. Caenorhabditis briggsae TRXR-1 is also predicted to be a selenoprotein (22). Because the common ancestor of C. elegans and C. briggsae is thought to have lived between 40 and 100 million years ago, there appears to be considerable evolutionary pressure to maintain selenocysteine in TRXR-1. One possibility is that, in the wild, neither TRXR-1 nor GSR-1 alone provides sufficient reducing power during molting and, therefore, that both proteins are necessary under conditions found in natural habitats. Notably, the two tissues in which thioredoxin reductase functions to promote molting (the hypodermis and the pharynx) are exposed to molecular oxygen. Molting might require extra reducing power to overcome the oxidizing environment of the atmosphere.
That trxr-1 and gsr-1 have overlapping functions during molting is consistent with the possibility that the thioredoxin and glutathione systems have a common target or targets. Although glutathione and thioredoxin reductases are related to one another in sequence, extensive biochemical analyses have revealed that glutathione reductases are unable to reduce thioredoxins in vitro. It is noteworthy, however, that the Drosophila thioredoxin system can catalyze the reduction of GSSG by NADPH in a two-step, thioredoxin-dependent reaction (24). S. cerevisiae strains lacking both the thioredoxins present in this organism, Trx1 and Trx2, have increased levels of GSSG (23). Thus, the genetic redundancy between trxr-1 and gsr-1, and the observation that GSH can reduce disulfides in the cuticle, suggests that TRXR-1 and GSR-1 might act in molting by increasing GSH concentrations at the expense of GSSG. GSH can be exported from cells by certain proteins in the ATP-binding cassette (ABC) family, including ABCG2 (35), and thus could potentially exert its molting-promoting functions extracellularly (e.g., together with secreted glutaredoxins). However, other models are also consistent with our results. Although isomerization of disulfides has not, to date, been shown to be important for molting, TRXR-1 and GSR-1 could potentially act to promote the isomerization of disulfides by facilitating the reductive step in isomerization.
Thioredoxin reductases are widely thought to be required for cell proliferation (possibly by supporting ribonucleotide reduction) and to provide protection against oxidative stress. However, worms lacking trxr-1 display no obvious developmental defects under laboratory growth conditions and are not hypersensitive to acute oxidative stress. It is possible that other unrelated proteins are able to reduce thioredoxins in C. elegans. Alternatively, thioredoxins may play no or only minor roles in protecting against acute oxidative stress or supporting cell proliferation in this organism.
Although still controversial (32), the oxidative damage theory of aging remains one of the theories most often cited in modern aging studies. Through their ability to affect redox status, thioredoxin reductases have been suggested to be important for the ability of cells and organisms to inhibit processes that occur during aging. However, experimental evidence supporting a role for thioredoxin reductases in inhibiting such processes is presently lacking. One of the most visible signs of aging in many animals is a change in quality of the skin. The observation that the efficiency of molting is reduced in aged dauer worms, and that TRXR-1 (together with GSR-1) promotes molting, highlights a function for TRXR-1 in epidermal cells in C. elegans. Thioredoxin reductase activity is induced in mammalian epidermal cells by agents (such as UV light) that cause age-associated changes to skin (14, 15). Thus, a function for thioredoxin and glutathione reductases in the removal of old and damaged cuticle may have been conserved in evolution.
Materials and Methods
Isolation of C. elegans Deletion Mutants.
selb-1(sv36), trxr-1(sv43), and trxr-1(sv47) were isolated by screening a deletion library of wild-type N2 worms mutagenized with ethyl methanesulfonate. The sequences of the primers used for identifying and backcrossing the mutants are described in SI Materials and Methods.
75Se Labeling of C. elegans Worms.
Worms were labeled with 75Se by feeding them E. coli bacteria that had been grown in the presence of 75Se. A 1.3-mL culture of E. coli strain OP50 was grown overnight in LB medium supplemented with cysteine to a final concentration of 100 μg/mL and 20 μCi of 75Se. Two hundred microliters of the overnight culture was spread onto an NGM agar C. elegans culture plate and allowed to grow for 24 h at room temperature (∼21 °C). After this time, the plate was seeded with five L4 hermaphrodite worms of the appropriate strain. The worms were harvested shortly before they had consumed all of the bacteria.
Staining of Worms with Alexa Fluor 488 C5-Maleimide.
Worms were washed individually in 50 μL of M9 buffer in a depression slide and then left in the buffer to cool on ice for 15 min. One microliter of dye stock solution was added to give a final concentration of 5 μM. The worms were left for 15 min on ice, washed in ice-cold M9, and then mounted for microscopy.
Isolation of the E. coli HT115(DE3) Lacking selD.
The plasmids and the sequences of the primers used create the selD deletion mutation in the background of E. coli HT115(DH3) are given in SI Materials and Methods.
Generation and Use of anti-CeTRXR-1 Antibodies.
An anti-peptide antibody was raised in rabbits against a peptide with the sequence NH2–CTLEKKEGDEEKQAS–CONH2, corresponding to the residues 650–663 of C. elegans TRXR-1 protein. Immunizations were performed by Agrisera, Vännäs, Sweden. Western blots were probed with affinity purified anti-TRXR-1 antibody at a 1:1,000 dilution.
Measurements of Enzymatic Activities in Vitro.
The Trx-insulin and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) assays were used to determine the activities of purified wild-type and mutant TRXR-1 proteins in vitro. The assays were performed as described by Arnér et al. (36).
Supplementary Material
Acknowledgments
We thank E. Jonsson and A. Rönnlund for excellent technical assistance and K. Forsgren for help with electron microscopy. We are grateful to the Caenorhabditis Genetics Center (which is funded by the National Institute for Health) for strains, K. Blackwell and A. Fire for plasmids, and Y. Kohara for cDNAs. J. Yochem, M. Wikström, R. Gaur, and J. Näsvall are gratefully acknowledged for helpful discussions, and G. Kao, J. Yochem, P. Askjaer, and M. Muñoz for comments on the manuscript. The work was supported by grants from Cancerfonden (09 0507) and Vetenskapsrådet (K2009-67X-20441-03-3) (to S.T.), grants from The Swedish Foundation for Strategic Research, Vetenskapsrådet, and the NordForsk Nordic C. elegans Network (to P.S.), a grant from The Carl Trygger Foundation and Vetenskapsrådet (B650-19981122/2000) (to O.P.P.), grants from the Instituto de Salud Carlos III (Projects PI050065 and PI080557, cofinanced with the Fondo Social Europedeo) and Junta de Andalucía (Projects P07-CVI-02697 and P08-CVI-03629) (to A.M.-V.), and a predoctoral fellowship from CONACYT, Mexico (to B.C.-V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006328108/-/DCSupplemental.
References
- 1.Kozlova T, Thummel CS. Steroid regulation of postembryonic development and reproduction in Drosophila. Trends Endocrinol Metab. 2000;11:276–280. doi: 10.1016/s1043-2760(00)00282-4. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- 3.Yochem J, Tuck S, Greenwald I, Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development. 1999;126:597–606. doi: 10.1242/dev.126.3.597. [DOI] [PubMed] [Google Scholar]
- 4.Kostrouchova M, Krause M, Kostrouch Z, Rall JE. CHR3: a Caenorhabditis elegans orphan nuclear hormone receptor required for proper epidermal development and molting. Development. 1998;125:1617–1626. doi: 10.1242/dev.125.9.1617. [DOI] [PubMed] [Google Scholar]
- 5.Frand AR, Russel S, Ruvkun G. Functional genomic analysis of C. elegans molting. PLoS Biol. 2005;3:e312. doi: 10.1371/journal.pbio.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gromer S, Urig S, Becker K. The thioredoxin system—from science to clinic. Med Res Rev. 2004;24:40–89. doi: 10.1002/med.10051. [DOI] [PubMed] [Google Scholar]
- 7.Arnér ES. Focus on mammalian thioredoxin reductases—important selenoproteins with versatile functions. Biochim Biophys Acta. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Gladyshev VN, Jeang KT, Stadtman TC. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc Natl Acad Sci USA. 1996;93:6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong L, Arnér ES, Holmgren A. Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc Natl Acad Sci USA. 2000;97:5854–5859. doi: 10.1073/pnas.100114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SR, et al. Mammalian thioredoxin reductase: oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci USA. 2000;97:2521–2526. doi: 10.1073/pnas.050579797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong L, Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J Biol Chem. 2000;275:18121–18128. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]
- 12.Jakupoglu C, et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrad M, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Holmgren A. Induction of thioredoxin, thioredoxin reductase and glutaredoxin activity in mouse skin by TPA, a calcium ionophore and other tumor promoters. Carcinogenesis. 1999;20:1761–1767. doi: 10.1093/carcin/20.9.1761. [DOI] [PubMed] [Google Scholar]
- 15.Schallreuter KU, Wood JM. Thioredoxin reductase - its role in epidermal redox status. J Photochem Photobiol B. 2001;64:179–184. doi: 10.1016/s1011-1344(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 16.Page AP, Johnstone IL. The cuticle. WormBook. 2007. The C. elegans Research Community, WormBook doi/10.1895/wormbook.1.138.1, http://www.wormbook.org. Accessed March 19, 2007. [DOI] [PMC free article] [PubMed]
- 17.Cox GN, Kusch M, Edgar RS. Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J Cell Biol. 1981;90:7–17. doi: 10.1083/jcb.90.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahaf B, Heydari K, Herzenberg LA, Herzenberg LA. Lymphocyte surface thiol levels. Proc Natl Acad Sci USA. 2003;100:4001–4005. doi: 10.1073/pnas.2628032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosower NS, Kosower EM. Diamide: an oxidant probe for thiols. Methods Enzymol. 1995;251:123–133. doi: 10.1016/0076-6879(95)51116-4. [DOI] [PubMed] [Google Scholar]
- 20.Buettner C, Harney JW, Berry MJ. The Caenorhabditis elegans homologue of thioredoxin reductase contains a selenocysteine insertion sequence (SECIS) element that differs from mammalian SECIS elements but directs selenocysteine incorporation. J Biol Chem. 1999;274:21598–21602. doi: 10.1074/jbc.274.31.21598. [DOI] [PubMed] [Google Scholar]
- 21.Gladyshev VN, et al. Selenocysteine-containing thioredoxin reductase in C. elegans. Biochem Biophys Res Commun. 1999;259:244–249. doi: 10.1006/bbrc.1999.0765. [DOI] [PubMed] [Google Scholar]
- 22.Taskov K, et al. Nematode selenoproteome: the use of the selenocysteine insertion system to decode one codon in an animal genome? Nucleic Acids Res. 2005;33:2227–2238. doi: 10.1093/nar/gki507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller EG. A glutathione reductase mutant of yeast accumulates high levels of oxidized glutathione and requires thioredoxin for growth. Mol Biol Cell. 1996;7:1805–1813. doi: 10.1091/mbc.7.11.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanzok SM, et al. Substitution of the thioredoxin system for glutathione reductase in Drosophila melanogaster. Science. 2001;291:643–646. doi: 10.1126/science.291.5504.643. [DOI] [PubMed] [Google Scholar]
- 25.Frand AR, Cuozzo JW, Kaiser CA. Pathways for protein disulphide bond formation. Trends Cell Biol. 2000;10:203–210. doi: 10.1016/s0962-8924(00)01745-1. [DOI] [PubMed] [Google Scholar]
- 26.Winter AD, McCormack G, Page AP. Protein disulfide isomerase activity is essential for viability and extracellular matrix formation in the nematode Caenorhabditis elegans. Dev Biol. 2007;308:449–461. doi: 10.1016/j.ydbio.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Hao L, et al. The hedgehog-related gene qua-1 is required for molting in Caenorhabditis elegans. Dev Dyn. 2006;235:1469–1481. doi: 10.1002/dvdy.20721. [DOI] [PubMed] [Google Scholar]
- 28.Berry MJ. Knowing when not to stop. Nat Struct Mol Biol. 2005;12:389–390. doi: 10.1038/nsmb0505-389. [DOI] [PubMed] [Google Scholar]
- 29.Lu J, et al. Penultimate selenocysteine residue replaced by cysteine in thioredoxin reductase from selenium-deficient rat liver. FASEB J. 2009;23:2394–2402. doi: 10.1096/fj.08-127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagegaltier D, et al. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 2000;19:4796–4805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 32.Gems D, Doonan R. Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1681–1687. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- 33.Jones SJ, et al. Changes in gene expression associated with developmental arrest and longevity in Caenorhabditis elegans. Genome Res. 2001;11:1346–1352. doi: 10.1101/gr.184401. [DOI] [PubMed] [Google Scholar]
- 34.Thein MC, et al. Combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the dual oxidase BLI-3 is critical for post-embryonic viability in Caenorhabditis elegans. J Biol Chem. 2009;284:17549–17563. doi: 10.1074/jbc.M900831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brechbuhl HM, et al. Glutathione transport is a unique function of the ATP-binding cassette protein ABCG2. J Biol Chem. 2010;285:16582–16587. doi: 10.1074/jbc.M109.090506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arnér ES, Zhong L, Holmgren A. Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol. 1999;300:226–239. doi: 10.1016/s0076-6879(99)00129-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.