Abstract
Background
Decreased red blood cell (RBC) deformability and activation of neutrophils (polymorphonuclear leukocytes [PMN]) after trauma-hemorrhagic shock (T/HS) have been implicated in the development of multiple organ dysfunction. Experimentally, female animals seemed to be protected from the effects of T/HS, at least in part, because of elevated estrogen levels. Thus, we examined the relative role of estrogen receptor (ER)-α and -β in this protective response.
Methods
To accomplish this goal, RBC deformability and neutrophil respiratory burst activity were measured in several groups of hormonally intact or ovariectomized (OVX) female rats subjected to T/HS (laparotomy plus hemorrhage to an MAP of 30 mm Hg to 35 mm Hg for 90 minutes) or trauma-sham shock (T/SS) and 3 hours of reperfusion. These groups included rats receiving vehicle, estradiol, or either an ER-α agonist or an ER-β agonist administered at the end of the shock period just before volume resuscitation.
Results
RBC deformability and neutrophil activation were similar among all the T/SS groups and were not different from that observed in the non-OVX female rats subjected to T/HS. In contrast, RBC deformability was reduced and neutrophil activation was increased in the OVX, T/HS female rats as compared with the T/SS groups or the non-OVX, T/HS rats. The administration of estrogen to the T/HS, OVX rats returned RBC and neutrophil function to normal. Both the ER-α and -β agonist partially, but not completely, protected the OVX rats from T/HS-induced loss of RBC deformability, whereas only the ER-β agonist prevented the increase in neutrophil activation.
Conclusions
The protective effects of estrogen on T/HS-induced RBC deformability are mediated, at least in part, via activation of both ER-α and -β, whereas ER-β activation is involved in limiting T/HS-induced neutrophil activation.
Keywords: Gender, Hemorrhagic shock, Estrogen receptors
Organ failure is a common cause of morbidity and mortality in patients sustaining major trauma, and hence, the pathophysiology of trauma-induced organ injury and dysfunction has been an important line of research.1 Based on these trauma studies, it is clear that shock-induced changes and trauma-induced changes in neutrophil activation are involved in the pathogenesis of the adult respiratory distress syndrome and contribute to the development of the multiple organ dysfunction syndrome.1 Likewise, recent work suggests that impaired red blood cell (RBC) deformability, which occurs in sepsis, after trauma, and during shock states,2–4 contributes to the development of multiple organ dysfunction syndrome by impairing tissue microvascular blood flow.5 Consequently, understanding the factors that modulate the magnitude and extent of neutrophil activation and RBC deformability might provide important insights into ways to limit post-trauma organ dysfunction.1 Because gender and sex hormones have been shown to modulate the development of organ injury as well as the immune system in preclinical models of trauma-hemorrhagic shock (T/HS)6–10 and in some but not all clinical studies,11 we have been interested in investigating the effects of gender and sex hormones on T/HS-induced changes in neutrophil and RBC function.
Our earlier studies documented that neutrophil activation12 and RBC rigidification13 were reduced in female as opposed to male rats subjected to T/HS and that estrogen played a protective role in these studies. Because the beneficial effects of estrogen (17β-estradiol) seem to occur through high-affinity estrogen receptors (ER), the major goal of this study was to investigate the relative role that each of the two principal ERs (ER-α and -β) played in protecting against trauma-shock-induced RBC dysfunction, as measured by RBC deformability and neutrophil activation. To accomplish this goal, we administered either the ER-α agonist propyl pyrazole triol (PPT) or the ER-β agonist diarylpropiolnitrile (DPN) to ovariectomized (OVX) rats. These agents were chosen as PPT has a 410-fold higher binding affinity preference for ER-α over ER-β,14 whereas DPN exhibits a 70-fold higher binding affinity to ER-β than ER-α.15
MATERIALS AND METHODS
Experimental Design
The goal of this study was to examine the relative role played by individual ER subtypes in protecting female rats from T/HS-induced RBC injury and neutrophil activation. This was accomplished by examining the differential protective effects of estrogen versus ER-α or -β agonists in OVX rats subjected to trauma plus hemorrhagic or sham shock. The control groups consisted of hormonally intact female rats in the proestrus stage of the cycle subjected to T/HS or trauma-sham shock (T/SS) as well as OVX rats receiving vehicle.
Thus, in addition to proestrus female rats, four groups of OVX rats were subjected to either laparotomy and controlled hemorrhagic shock (T/HS; 30–35 mm Hg for 90 minutes) or laparotomy plus sham-shock (T/SS). The OVX animals were randomized into four groups: group 1 received vehicle and acted as the negative control; group 2 received the ER-α agonist, PPT (5 μg/kg BW); group 3 received the ERβ agonist, DPN (5 μg/kg BW); and group 4 received 17β-estradiol (50 μg/kg BW). The dosing for all the drugs was obtained from previously published articles.16,17 All the drugs were dissolved in dimethylsulfoxide (Sigma) and were administered at the end of the 90-minute shock period and immediately before volume resuscitation, via intraperitoneal (i.p.) injection. At 3 hours after the end of the shock or sham-shock period, the animals were killed, and blood was collected for the evaluation of neutrophil priming as well as measurements of RBC deformability, as our previous studies documented that these parameters had become abnormal by this time point.12,13
Animals
OVX and non-OVX female Sprague-Dawley rats, weighing 300 g to 375 g, were purchased from Charles River, Wilmington, MA, and were used after a minimum acclimatization period of 5 days. OVX animals were allowed at least 14 days after ovarectomy before use. All animals were housed under barrier-sustained conditions and kept at 25°C with 12-hour light/dark cycles. The rats had free access to water and chow (Teklan 22/5 Rodent Diet W-8640, Harlan Teklad, Madison, WI). All rats were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals. The New Jersey Medical School Animal Care Committee approved all animal protocols.
Surgical Procedure
Rats were anesthetized with i.p. sodium pentobarbital (50 mg/kg). Using aseptic techniques, we isolated the femoral artery and internal jugular vein, and cannulated it with polyethylene (PE-50) tubing and 50-gauge silicone catheter containing 0.1 mL heparinized saline (10 units/mL), respectively. Both the catheters remained in place for the duration of the experiment. Next, a 3-cm midline laparotomy (trauma) was performed with exposure of the intestine for 15 minutes, followed by closure with a one-layer running 4-0 silk suture.
Continuous blood pressure monitoring was achieved via the femoral artery catheter (BP-2 Digital Blood Pressure Monitor, Columbus Instruments, Columbus, OH). Blood was then withdrawn from the internal jugular vein catheter. The mean arterial pressure was reduced to 30 mm Hg to 35 mm Hg and was maintained at this level for 90 minutes by withdrawing or reinfusing shed blood as needed. The animals’ temperature was maintained during the shock period at approximately 37°C by using an electric heating pad under the surgical platform as needed. At the end of the shock period, the animals were resuscitated by reinfusing all of the shed blood, which returned the blood pressure to preshock levels. All the treatment drugs were administered via i.p. injection at the end of the shock period and just before volume resuscitation with shed blood. The T/SS rats were anesthetized, their vessels cannulated, and they underwent a laparotomy, but no blood was withdrawn or infused. All the animals remained anesthetized throughout the duration of the experiment.
Determination of Menstrual Stage
The non-OVX female rats were periodically screened via vaginal swabbing to determine the stage of the estrus cycle, as described by Baker et al.18 Only proestrus female rats were used in this experiment.
Determination of RBC Deformability
At 3 hours after resuscitation (end of the T/HS period), whole blood was collected through an intravenous catheter, and RBC deformability was determined with a laser-assisted ekta-cytometer (LORCA, RR Mechatronics, The Netherlands), as previously described.19 Briefly, an aliquot of RBCs (15 μL) containing approximately 30 million cells was suspended in 1 mL of 5% polyvinylpyrrolidone (molecular weight 360,000; Sigma, St. Louis, MO) in phosphate-buffered saline at a final viscosity of 30 mPa. After gently mixing for 15 minutes at room temperature to assure complete oxygenation of the hemoglobin, cell deformability was determined at 37°C. Cell deformability was assessed by calculating the elongation index (EI) at shear stresses ranging from 0.3 Pa to 30 Pa, as described previously.19 From the shear-stress elongation curve created previously, the data were analyzed using the Lineweaver-Burke analysis to determine the stress required for the erythrocytes to reach 50% of their maximal elongation (KEI).19 Thus, an elevation in KEI reflects a decrease in RBC deformability.
Neutrophil Respiratory Burst Activity
A blood sample was obtained in a heparinized syringe from each animal at 3 hours, after the end of T/SS or T/HS for the measurement of polymorphonuclear leukocytes (PMN) respiratory burst activity, as previously described.12 The samples were treated with RBC lysis buffer (1% PharM Lyse; BD Pharmingen, San Diego, CA) and incubated for 15 minutes at room temperature to remove the RBCs. They were then centrifuged at 25°C and washed twice with Hank’s balanced salt solution, and the supernatant was then discarded. The resulting pellet was suspended in Hank’s balanced salt solution at a concentration of 4 × 106cells. Dihydrorhodamine (15 ng/mL) was added to 100 μL of the PMN sample and warmed to 37°C. Phorbol myristic acid (0.4 μmol/L, 15 minutes, 37°C) was then added to stimulate the cells. The PMN respiratory burst was measured by flow cytometry, where the neutrophils were identified by forward and side scatter analysis. The data are expressed as the mean fluorescence index.12
ER-α and -β Determination on RBC and PMN Membranes
Fresh-heparinized blood was centrifuged at 900 g for 10 minutes and the plasma and buffy coat was removed. To ensure removal of leukocytes and platelets, the packed RBCs were washed 3 times with GASP (9 mmol/L Na2HPO4, 1.3 mmol/L NaH2PO4, 140 mmol/L NaCl, 5.5 mmol/L glucose, and 0.08% bovine serum albumin). For preparation of RBC ghosts, the washed, packed RBCs were lysed with ice cold 5 mmol/L sodium phosphate buffer, pH 8.0, containing 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L sodium orthovanadate, and 1 mmol/L phenylmethylsulfonyl fluoride (lysis buffer) and incubated on ice for 10 minutes. The RBC ghosts were isolated by centrifugation at 20,000g for 20 minutes at 4°C. The isolated ghosts were washed three times with the same lysis buffer and centrifuged (20,000g) for 10 minutes at 4°C.
Rat PMN were isolated from naïve rat whole blood collected in heparinized syringes, and the blood was centrifuged at 150 g for 10 minutes, and the buffy coat was isolated and layered onto Polymorphoprep (Robbins Scientific Corp, Sunnyvale, CA) and centrifuged at 300 g for 30 minutes at room temperature. The PMN-enriched fraction was collected and washed with RPMI medium, and the PMN pellet (10–50 million cells) was suspended in 50 μL to 100 μL of 1× RIPA Lysis buffer (Upstate Cell Signaling, Temecula, CA) containing 1× protease inhibitor cocktail (Sigma St Louis, MO) and 1× phosphatase inhibitor cocktails 1 and 2 (Sigma). The cell suspension, while on ice, was sonicated two to three times for 5 seconds to 10 seconds and kept on ice for 30 minutes. The sonicated cell suspension was centrifuged at 9,000 g for 5 minutes at 4°C. The supernatants were collected and stored at −80°C. This preparation includes cytosolic, membrane, and nuclear proteins.
The protein concentration of the RBC ghosts and PMN samples were determined by using the microbicinchoninic acid assay. Equal amounts of the RBC protein (10 μg) or PMN (40 μg) protein were loaded per lane of their respective gels. Electrophoresis was performed, according to the method of Fairbanks et al.,20 using 7% Tris-Acetate gels (Invitrogen, CA). The controls for each gel included an ovarian extract. The electrophoresed samples were transferred to nitrocellulose according to the manufacturer specifications (Invitrogen, CA). The nonspecific binding sites were blocked by incubating the nitrocellulose membrane in SuperBlock T-20 (BioRad, Hercules, CA) for 1 hour at room temperature. The samples were then incubated with either anti-ER-α or anti-ER-β polyclonal anti-rabbit antibody (Abcam Inc., Cambridge, MA) diluted to a final concentration of 1 μg/mL and an antiactin antibody diluted 1:5,000 in SuperBlock T-20, overnight at 4°C. The membranes were washed three times with Tris-buffered saline containing 0.1% Tween 20 and then incubated with the appropriate horseradish peroxide-conjugated secondary antibody diluted in SuperBlock T-20. Finally, the membranes were incubated with West Pico chemiluminescence reagent (Pierce, Rockford, IL) and exposed to an X-ray film.
Statistical Analysis
All the data were analyzed using analysis of variance with the Tukey-Kramer multiple comparison test. The data are expressed as mean ± SD. A p value <0.05 was considered significant.
RESULTS
RBC Deformability Measurements
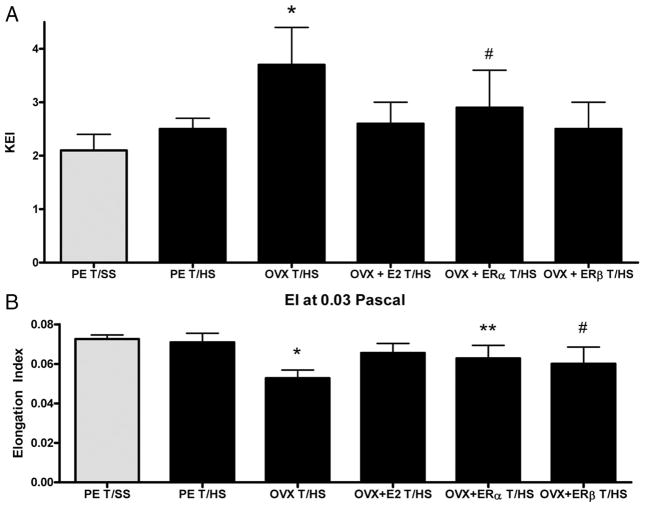
RBC deformability was similar between the T/SS groups, whether measured as the amount of stress required to half-maximally elongate the RBCs (KEI) or as the length of RBC deformability observed when the RBC is subjected to a low shear stress (0.3 Pa) (EI) (Table 1). Both the KEI as well as the EI were measured, because the KEI reflects the overall deformability of the RBC in various vascular beds, while the EI reflects the deformability observed at shear stress levels observed in the microvasculature. As shown in Figure 1A, the KEI or the amount of stress required to half-maximally deform the RBCs did not increase in the proestrus females subjected to T/HS but was significantly increased in the OVX, T/HS females. The administration of estradiol to the OVX females subjected to T/HS fully protected against T/HS-induced loss of RBC deformability as did the ER-β (DPN) agonist (Fig. 1). However, although the ER-α (PPT) agonist was protective, its protective effect was less than that observed with estradiol or the ER-β agonist (Fig. 1).
TABLE 1.
RBC and PNM Data From All Sham Groups
| Groups | RBC Data |
PMN Respiratory Burst | |
|---|---|---|---|
| KEI | EI at 0.3 Pa | ||
| PE T/SS | 2.1 ± 0.3 | 0.073 ± 0.002 | 209 ± 9 |
| OVX T/SS | 2.2 ± 0.3 | 0.071 ± 0.002 | 214 ± 12 |
| E2 T/SS | 2.2 ± 0.2 | 0.073 ± 0.002 | 207 ± 24 |
| ER-α T/SS | 2.3 ± 0.2 | 0.073 ± 0.002 | 211 ± 11 |
| ER-β T/SS | 2.1 ± 0.2 | 0.074 ± 0.002 | 217 ± 19 |
Data expressed as mean ± SD with seven to eight animals per group.
Figure 1.
(A) Effect of estrogenic hormonal manipulation on KEI showing that ovariectomy impairs RBC deformability after T/HS and that the effect of ovariectomy is abrogated by estrogen and the ER-β agonist to a greater degree than the ER-α agonist. N = 7–8 rats per group with data expressed as mean ± SD. *p < 0.05 vs. all other groups and #p < 0.05 vs. T/SS group. (B) Effect of estrogenic hormonal manipulation on T/HS-induced RBC elongation (EI) at 0.03 Pa showing that ovariectomy reduced the ability of RBC to elongate normally and that this defect in deformability was fully reversed by estradiol but only partially reversed by the ER-α or -β agonists. N = 7–8 rats per group with data expressed as mean ± SD. *p < 0.05 vs. all other groups and **p < 0.05 vs. T/SS group, #p < 0.05 vs. T/SS and T/HS groups.
The EI, which reflects the degree of RBC deformation at 0.03 Pa, did not increase in the proestrus rats subjected to T/HS (Fig. 1B). However, the EI of RBC from the OVX females subjected to T/HS was significantly decreased, reflecting a decreased ability of these RBC to elongate under a fixed 0.03-Pa shear stress (Fig. 1B). The administration of estradiol fully prevented the decrease in RBC deformability observed in the T/HS, OVX females, whereas the two ER agonists were partially protective (Fig. 1B).
PMN Activation–Respiratory burst
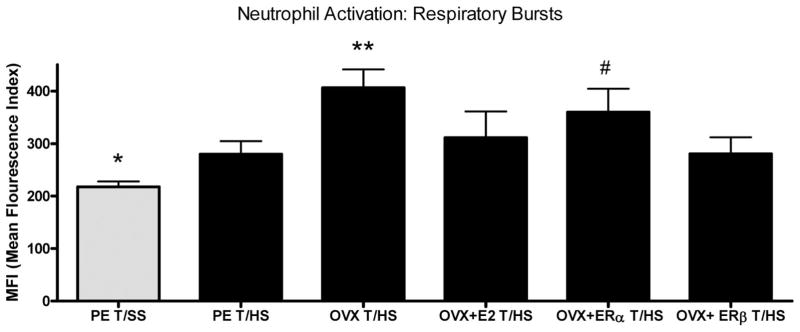
Because activation of PMNs is associated with lung and other organ injuries after shock, we measured the effects of estrogen manipulation on PMN activation in rats subjected to T/HS or T/SS. PMN activation was similar between all the T/SS groups, indicating that OVX itself does not prime for an increased PMN respiratory burst (Table 1). In contrast to the measurements of RBC deformability, the PMN respiratory burst was increased in the proestrus T/HS females as compared with the T/SS controls (Fig. 2). However, ovariectomy was associated with a further in increase in the T/HS-induced PMN respiratory burst, which was returned to the proestrus level by the administration of estradiol or the ER-β agonist (Fig. 2). However, the OVX rats subjected to T/HS were similar to and not ameliorated by the administration of the ER-α agonist, indicating that the protective effect of estradiol was not being mediated by the ER-α (Fig. 2). Thus, these results suggest that estradiol, through the ER-β, limits the increase in T/HS-induced PMN activation, as reflected in respiratory burst activity.
Figure 2.
Neutrophil activation is increased in all groups after T/HS with the greatest increases in the ovariectomized rats and the rats receiving the ER-α agonist. N = 7–8 rats per group with data expressed as mean ± SD. *p < 0.05 vs. all other groups; **p < 0.01 vs. the PE T/HS, OVX + E2, and OVX+ ER-β groups; and #p < 0.05 vs T/HS and the Ovx+ ER-β groups.
Determination of ER-α and -β on RBC and PMN
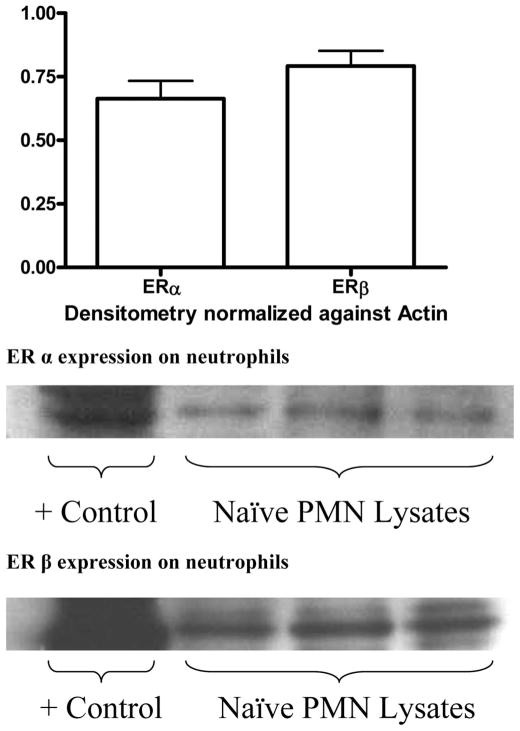
Because estrogenic hormonal manipulations affected both RBC and PMN functions, we tested whether naïve RBC or PMN expressed ER-α and/or ER-β, using protein separation via gel electrophoresis and subsequent western blot staining of the transfered nitrocellulose membrane. Using this approach, we failed to demonstrate any evidence of either ER present within the RBC in any of the samples tested. This was true of both naïve animals and rats subjected to T/HS or T/SS (data not shown). The activity of the antibodies themselves was confirmed by pairing the samples with a whole cell extract sample of a naïve female rat ovary. Staining for both ER-α and ER-β on PMN cell lysates indicates the presence of both ER subtypes within the rat neutrophil, with both receptors being approximately equally expressed (Fig. 3). Additionally, the levels of the ERs were not upregulated on rats subjected to T/HS (data not shown). This would indicate that estrogen could directly act on the neutrophil to decrease its activation, in addition to inducing changes within the external milieu of the cells.
Figure 3.
Both ER-α and -β are expressed on neutrophils with comparable density.
DISCUSSION
The effects of hormonal modulation of estrogen and its receptors were studied because estrogen seems to be protective during shock and septic states.8,11 Furthermore, in the context of shock and sepsis, excessive PMN activation as well as impaired RBC deformability has been implicated in organ injury and reduced microvascular perfusion.1–5 In this context, one key new observation of the current study is that the ER-β receptor seems to be more important than the ER-α receptor in transducing the ability of estradiol to limit T/HS-induced RBC rigidity and neutrophil activation. A second important observation is that estradiol, as well as the ER agonists, was effective when used in a post-treatment regimen and administered at the end of the shock period at the time of fluid resuscitation.
The biology of estrogen has been extensively studied for decades because of its important reproductive, cardiovascular, and other effects. Initially, estrogen was thought to work through a genomic mechanism where it induces gene transcription and subsequent protein synthesis. However, although the classic genomic effects of estrogen, where estrogens bind directly to nuclear ERs, are well recognized and important, it now seems that estrogens can also exert their physiologic effects via nongenomic mechanisms.21 The nongenomic effects of estrogens are more rapid than the genomic effects and occur when estrogen, as well as estrogenic and antiestrogenic molecules, bind to ERs and activate signaling systems within the cytoplasm. Thus, once activated by the binding of estrogens, ERs can function as hormone-regulated transcription factors (genomic effects) whose effects are observed in hours and/or through non-genomic signaling systems in the cytoplasm, whose effects are apparent within minutes and, thereby, can rapidly mediate the effects of estrogens on different cells and tissues.21 Our observation that the beneficial effects of estradiol administration or the administration of the ER agonists on neutrophil and RBC function were consistent with previous studies on the immune system7 and organ function,22,23 suggesting that the beneficial effects of ER agonists are operating primarily via a nongenomic pathway.
Because ER-α and -β not only have some overlapping but also different tissue distributions, the differences in ligand interactions with these two major ERs could translate into important differences in their biological actions at the tissue or cellular levels.24 For example, work from the laboratory of Chaudry has shown that tissues with higher densities of ER-β than ER-α, such as the lung, are better protected from T/HS-induced injury by ER-β than ER-α agonists.23 Likewise, this same group has documented that ER-α is more important than ER-β in mediating the immunoprotective effects of estrogen on T-cells and splenic macrophages as well as liver injury in rats subjected to trauma hemorrhage.7,24
Our current results indicating that an ER-β agonist was more effective than an ER-α agonist in limiting neutrophil activation and RBC deformability extend our previous work on the protective effect of female sex hormones on these cell populations after T/HS12,13 and are of potential clinical importance as both the neutrophils and RBC seem to be involved in T/HS-induced organ dysfunction.1–6 Because we could not find any studies defining ER expression on rat neutrophils or RBCs and the presence or relative cellular density of the ER-α and -β receptors on neutrophils and RBCs could help explain why ER-β was more protective than ER-α, we measured their levels in naïve proestrus rats. Our observation that the basal amount of the two ERs was similar in rat neutrophils does not explain why we found that the ER-β agonist limited T/HS-induced PMN activation better than the ER-α agonist. However, our finding that rat neutrophil contained approximately equal amounts of both ERs is consistent with a recent human study.25 Furthermore, work by Yu et al.,26 in which male rats subjected to trauma hemorrhage received either an ER-β or an ER-α agonist, found that the ability of the agonists to limit tissue neutrophil sequestration seemed to be related to the dominant ER expressed by the tissue studied. That is, tissues such as the lung that are ER-β dominant had less neutrophil sequestration when the animals received the ER-β agonist, whereas the liver, which is an ER-α-dominant organ, had less neutrophil sequestration when they received the ER-α agonist.26 Furthermore, our observation that one of the ERs seems more important in limiting neutrophil activation than the other, although both are expressed, also seems to be seen in lymphocytes, as lymphocytes seem to express both ER-α and ER-β27; yet, the ER-α agonist, but not the ER-β agonist, limited trauma hemorrhage-induced immune alterations.7
Nonetheless, these current experiments investigating the effects of ovarectomy, estrogen replacement, and individual ERs on neutrophil activation after T/HS do provide potentially important insights. First, as expected, neutrophil activation was greater in the OVX group than the non-OVX female rats subjected to T/HS, supporting the hypothesis that the removal of estrogenic hormones potentiates neutrophil activation. However, as ovarectomy also causes significant reductions in progesterone, prolactin, and dehydroepiandrosterone,28 estrogen is not the only sex-related hormone altered by ovariectomy. Thus, our results, showing that the administration of estradiol to the OVX rats was sufficient to suppress neutrophil activation, indicate that estrogen is the key sex hormone responsible for limiting neutrophil activation after T/HS in female rats. In addition, we demonstrated for the first time that administration of the ER-β agonist was more effective in limiting T/HS-induced neutrophil activation than an ER-α agonist. Because neutrophils express ERs, it seems reasonable to posit that at least part of the effect of estrogen on neutrophils could be related to its direct effects on the neutrophil, which are mediated by ERs. However, estrogen may also have limited neutrophil activation after T/HS, by indirect as well as direct mechanisms. This notion that estrogen could have limited neutrophil activation via an indirect mechanism is based on our earlier work12 showing that factors carried in mesenteric lymph are responsible for T/HS-induced neutrophil activation and that estrogen limits gut injury and the generation of gut-derived neutrophil activating factors. In addition to indicating that estrogen can limit neutrophil activation by limiting gut injury, this work also supported a direct modulatory effect of estrogen on neutrophil activation after T/HS, because naïve female neutrophils were found to be more resistant to activation by T/HS lymph or plasma than male neutrophils and that this cellular resistance to activation varied over the estrus cycle.12 Thus, estrogen is likely to have limited T/HS-induced neutrophil activation via both direct and indirect mechanisms.
In contrast to neutrophils, we did not find ERs to be expressed on the RBCs and also, somewhat in contrast to our neutrophil results, both the ER-α and -β agonists were partially protective, with the ER-β agonist showing slightly more protection. Our failure to identify RBC ERs is consistent with mapping studies of the RBC proteome, because in several studies neither ER subtype was identified among the more than 500 RBC proteins isolated.29–31 The lack of ERs on RBCs suggests that the protective effects of estrogen on the RBC cytoskeleton are indirectly mediated. One indirect mechanism by which estrogen and the ER agonists could have limited T/HS-induced loss of RBC deformability is by limiting gut injury and the production of biologically active mesenteric lymph. This notion is supported by our previous work showing that estrogen limits gut injury and the production of biologically active mesenteric lymph,7,12 and that T/HS-induced RBC deformability is abrogated in female rats and that mesenteric lymph duct ligation protects against T/HS-induced loss of RBC deformability in male rats.32 A second possible indirect pathway by which T/HS-induced RBC injury could have been limited is via limitation of neutrophil activation. This notion is supported by our unpublished work showing that exposure of whole blood but not isolated RBC from naïve rats to T/HS mesenteric lymph results in decreased RBC deformability. This work suggests that T/HS mesenteric lymph cannot damage normal RBC in vitro in the absence of white blood cells. However, until further studies are completed, the cellular mechanisms by which estrogen and the ER agonists limit the loss of RBC deformability must remain speculative.
Although current and other preclinical studies have generally documented that estrogen exerts a protective effect on various organs and cellular systems in shock trauma models, clinical studies are not clear in showing clinical benefit for females, especially when mortality is used as the outcome measure.11,33,34 There are several potential explanations for this fact. As recently discussed in an editorial by Proctor,34 some of these differences might be related to the limitations of the preclinical trauma shock models used, including our own. These include a controlled hemorrhage model, the use of small doses of heparin, and the fact that all the animals used are perfectly healthy and have no preexisting illnesses or confounding social issues such as alcohol ingestion. Additionally, the immunoinflammatory response of rodents differ to some degree from those of humans.35 Additionally, the levels of sex hormones rapidly change over time in trauma and other patient groups36,37 and these patients frequently develop complications, such as pneumonia and often require multiple operations (i.e., second hits) during their hospital course. Nonetheless, the current study supports the theory that females are protected from acute shock-induced RBC injury and neutrophil activation, and that this protection occurs though an estrogen-dependent pathway with at least part of the protective effects of estrogen being mediated via ERs. The exact clinical significance of these observations will require patient-based studies and raise the hope that selective ER agonists can be used as potential therapeutic agents to modulate specific cellular and tissue targets.
Acknowledgments
Supported by NIH grants 1P50GM069790 (to E.A.D. and R.F.), R01 GM059179 (to C.J.H.), and T32-GM069330 (to D.D.).
References
- 1.Deitch EA. Multiple organ failure: pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hurd T, Dasmahapatra K, Rush BF, Jr, Machiedo GW. Red cell deformability in human and experimental sepsis. Arch Surg. 1988;123:217–20. doi: 10.1001/archsurg.1988.01400260101012. [DOI] [PubMed] [Google Scholar]
- 3.Baskurt O, Temiz A, Meiselman H. Red blood cell aggregation in experimental sepsis. J Lab Clin Med. 1997;130:183–190. doi: 10.1016/s0022-2143(97)90094-9. [DOI] [PubMed] [Google Scholar]
- 4.Macheido GW, Powell RJ, Rush BF, Jr, Swislocki NI, Dikdan G. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple system organ failure. Arch Surg. 1989;124:1386–1389. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 5.Machiedo GW, Zaets SB, Berezina TL, et al. Trauma hemorrhagic shock-induced red blood cell damage leads to decreased microcirculatory blood flow. Crit Care Med. 2009;37:1000–1010. doi: 10.1097/CCM.0b013e3181962d39. [DOI] [PubMed] [Google Scholar]
- 6.Ananthakrishnan P, Cohen DB, Xu DZ, Lu Q, Feketeova E, Deitch EA. Sex hormones modulated distant organ injury in both trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137:56–65. doi: 10.1016/j.surg.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 7.Raju R, Bland KI, Chaudry IH. Estrogen: a novel therapeutic adjunct for the treatment of trauma-hemorrhage-induced immunological alterations. Mol Med. 2008;14:213–221. doi: 10.2119/2008-00001.Raju. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. The female reproductive system is an important variable in the response to trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2000;279:H1015–H1021. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- 9.Knöferl MW, Angele MK, Diadato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105–112. doi: 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remmers DE, Cioffi WG, Bland KI, Wang P, Angele MK, Chaudry IH. Testosterone: the crucial hormone responsible for depressing myocardial function in males after trauma-hemorrhage. Ann Surg. 1998;227:790–799. doi: 10.1097/00000658-199806000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anathakrishnan P, Deitch EA. Gut orgin sepsis and MODS: the role of sex hormones in modulating intestinal and distant organ injury. XX vs XY. 2003;1:108–117. [Google Scholar]
- 12.Deitch EA, Ananthakrishnan P, Cohen DB, Xu da Z, Feketeova E, Hauser CJ. Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am J Physiol Heart Circ Physiol. 2006;291:H1456–H1465. doi: 10.1152/ajpheart.00694.2005. [DOI] [PubMed] [Google Scholar]
- 13.Macheido GW, Zeats S, Berezina T, Xu DZ, Spolarics Z, Deitch EA. Red blood cell damage after trauma-hemorrhage is modulated by gender. J Trauma. 2004;56:837–844. doi: 10.1097/01.ta.0000058128.04007.90. [DOI] [PubMed] [Google Scholar]
- 14.Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 15.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure–activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Shimizu T, Yu HP, et al. Tissue compartment-specific role of estrogen receptor subtypes in immune cell cytokine production following trauma-hemorrhage. J Appl Physiol. 2007;102:163–168. doi: 10.1152/japplphysiol.00964.2006. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrand F, Hubbard WJ, Choudhry MA, Thobe BM, Pape HC, Chaudry IH. Are the protective effects of 17beta-estradiol on splenic macrophages and splenocytes are after trauma-hemorrhage mediated via estrogen receptor (ER)-alpha or ER-beta? J Leukoc Biol. 2006;79:1173–1180. doi: 10.1189/jlb.0106029. [DOI] [PubMed] [Google Scholar]
- 18.Baker HJ, Lindsey JR, Weisbroth SH. Biology and Diseases. Vol. 1. New York, NY: Academic Press; 1979. The Laboratory Rat; pp. 54–156. [Google Scholar]
- 19.Condon MR, Kim JE, Deitch EA, Machiedo GW, Spolarics Z. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am J Physiol Heart Circ Physiol. 2003;284:H2177–H2184. doi: 10.1152/ajpheart.01069.2002. [DOI] [PubMed] [Google Scholar]
- 20.Fairbanks G, Steck TL, Wallach DF. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- 21.Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target cells. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 22.Yu HP, Shimazu T, Choudhry MA, et al. Mechanism of cardioprotection following trauma-hemorrhagic-shock by selective estrogen receptor-β agonist: up-regulation of cardiac heat shock factor-1 and heat shock proteins. J Mol Cell Cardiol. 2006;40:185–194. doi: 10.1016/j.yjmcc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Yu HP, Hsieh YC, Suzuki T, et al. Salutary effects of estrogen receptor-β agonist on lung injury following trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1004–L1009. doi: 10.1152/ajplung.00504.2005. [DOI] [PubMed] [Google Scholar]
- 24.Kuiper GGJM, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 25.Molero L, Garcia-Duran M, Diaz-Recasens J, Rico L, Casado S, López-Farré A. Expression of estrogen receptor subtypes and neuronal nitric oxide synthase in neutrophils from women and men: regulation by estrogen. Cardiovasc Res. 2002;56:43–51. doi: 10.1016/s0008-6363(02)00505-9. [DOI] [PubMed] [Google Scholar]
- 26.Yu HP, Shimizu T, Hsieh YC, et al. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol. 2006;79:963–970. doi: 10.1189/jlb.1005596. [DOI] [PubMed] [Google Scholar]
- 27.Scariano JK, Emery-Cohen AJ, Pickett GG, Morgan M, Simons PC, Alba F. Estrogen receptors alpha (ESR1) and beta (ESR2) are expressed in circulating human lymphocytes. J Recept Signal Transduct Res. 2008;28:285–293. doi: 10.1080/10799890802084614. [DOI] [PubMed] [Google Scholar]
- 28.Jarrar D, Wang P, Knoferl MW, et al. Insight into the mechanisms by which estradiol improves organ function after trauma-hemorrhage. Surgery. 2000;128:246–252. doi: 10.1067/msy.2000.107376. [DOI] [PubMed] [Google Scholar]
- 29.Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood. 2006;108:791–801. doi: 10.1182/blood-2005-11-007799. [DOI] [PubMed] [Google Scholar]
- 30.Kakhniashvili DG, Griko NB, Bulla LA, Jr, Goodman SR. The proteomics of sickle cell disease: profiling of erythrocyte membrane proteins by 2D-DIGE and tandem mass spectrometry. Exp Biol Med. 2005;230:787–792. doi: 10.1177/153537020523001102. [DOI] [PubMed] [Google Scholar]
- 31.Low TY, Seow TK, Chung MCM. Separation of human erythrocyte membrane associate proteins with one dimentional and two dimentional gel electrophoresis followed by identification with matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Proteomics. 2002;2:1229–1239. doi: 10.1002/1615-9861(200209)2:9<1229::AID-PROT1229>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Zaets SB, Berezina TL, Caruso J, Xu DZ, Deitch EA, Machiedo GW. Mesenteric lymph duct ligation prevents shock-induced RBC deformability and shape changes. J Surg Res. 2003;109:51–56. doi: 10.1016/s0022-4804(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 33.Magnotti LJ, Fischer PE, Zarzaur BL, Fabian TC, Croce MA. Impact of gender on outcomes after blunt injury: a definitive analysis of more than 36,000 trauma patients. J Am Coll Surg. 2008;206:984–991. doi: 10.1016/j.jamcollsurg.2007.12.038. discussion: 991–992. [DOI] [PubMed] [Google Scholar]
- 34.Proctor KG. Gender differences in trauma theory vs practice: comments on “Mechanism of estrogen-mediated intestinal protection following trauma-hemorrhage: p38 MAPK-dependent upregulation of HO-1” by Hsu JT et al. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1822–R1824. doi: 10.1152/ajpregu.90301.2008. [DOI] [PubMed] [Google Scholar]
- 35.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Dossett LA, Swenson BR, Evans HL, Bonatti H, Sawyer RG, May AK. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg Infect (Larchmt) 2008;9:41–48. doi: 10.1089/sur.2007.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheingraber S, Dobbert D, Schmiedel P, Seliger E, Dralle H. Gender-specific differences in sex hormones and cytokines in patients undergoing major abdominal surgery. Surg Today. 2005;35:846–854. doi: 10.1007/s00595-005-3044-1. [DOI] [PubMed] [Google Scholar]