Abstract
Our previous studies have demonstrated that the natural chaperone complexes of full-length tumor protein antigens (e.g., gp100) and large stress proteins (e.g., hsp110 and grp170) with exceptional antigen holding capabilities augment potent tumor protective immunity. Here we assess the peptide-interacting property of these large chaperones and, for the first time, compare the immunogenicity of the recombinant chaperone vaccines targeting two forms of antigens (protein vs peptide). Both hsp110 and grp170 readily formed complexes with antigenic peptides under physiological conditions, and the peptide association could be further stimulated by heat shock. The large chaperones displayed similar, but distinct peptide-binding features compared to hsp70 and grp94/gp96. Immunization with hsp110- or grp170-tyrosinase-related protein 2 (TRP2175–192) peptide complexes effectively primed CD8+ T-cells reactive with TRP2-derived, MHC I-restricted epitope. However, the tumor protective effect elicited by the TRP2175–192 peptide vaccine was much weaker than that achieved by full-length TRP2 protein antigen chaperoned by grp170. Furthermore, immunization with combined chaperone vaccines directed against two melanoma protein antigens (i.e., gp100 and TRP2) significantly improved overall antitumor efficacy when compared to either of the single antigen vaccine. Lastly, treatment of tumor-bearing mice with these dual antigen-targeted chaperone complexes resulted in an immune activation involving epitope spreading, which was associated with a strong growth inhibition of the established tumors. Our results suggest that high molecular weight chaperones are superior to conventional chaperones as a vaccine platform to deliver large protein antigens, and provide a rationale for translating this recombinant chaperoning-based vaccine to future clinical investigation.
Keywords: large stress protein, chaperone, grp170, hsp110, antigen, immuogenicity
Introduction
It is well known that stress/heat shock proteins (HSPs) act as molecular chaperones that are actively involved in almost every aspect of protein homeostasis (e.g., protein synthesis, folding/unfolding, oligomeric assembly, transportation and degradation) (1). Stress proteins can be grouped into different families of protein based on their molecular size, which include hsp25/27, hsp70/grp78, hsp90/grp94 and hsp110/grp170 (2, 3). These molecules are constitutively expressed in mammalian cells, but can be strongly induced under stress conditions. As the most abundant and ubiquitous intracellular proteins, stress proteins play essential roles in the survival of cells due to their diverse housekeeping functions (4).
The immunological feature of stress protein that has been the greatest focus of attention thus far is their ability to effectively direct associated antigens into the endogenous antigen presentation pathway of professional antigen-presenting cells (APCs) (5). It has been well-documented that stress protein-peptide complexes purified from tumor, such as hsp70, hsp90 and grp94/gp96, are able to initiate efficient tumor-specific cytotoxic T-lymphocyte (CTL) response and protective immunity (6, 7). Recent studies have implicated that specific stress protein receptors on APCs are critical for the cross-presentation of stress protein-associated antigens (8–11).
High molecular weight stress proteins, including hsp110 and grp170, belong to hsp70 super-family, and represent large and highly “diverged” relatives of the hsp70 (3, 12). Chaperoning studies have shown that these large stress proteins are highly efficient peptide-chain binders compared to other classical chaperones (13, 14). Our earlier studies have demonstrated that hsp110 and grp170 purified from tumors suppress the same tumor growthin mice and induce tumor-specific CTLs (15, 16). In order to overcome the technical difficulties associated with conventional HSP vaccine approach (e.g., tumor tissue requirement, time-consuming preparation), we took advantage of the strong protein holding capability of these large chaperones and developed a recombinant chaperoning vaccine approach by complexing a full length protein antigen to hsp110 or grp170. The in vitro generated complexes of the large stress protein and tumor protein antigens resemble the natural chaperone-client/partner protein complexes that are formed while the molecular chaperones execute their intracellular functions. We have demonstrated that the hsp110- and grp170-protein antigen complexes exhibit potent antitumor activities by stimulating antigen-specific immune responses in both prophylactic and therapeutic settings (17–19). Thus, these large chaperone molecules represent an excellent choice for development of synthetic and non-toxic vaccines for cancer immunotherapy.
Although the large stress proteins display highly efficient protein substrate/antigen chaperoning capability, the interaction of these molecules and peptide antigens have not been investigated. In the present study, we have characterized the ability of large chaperones to interact with antigenic peptides in vitro under various conditions. Competition studies revealed that peptide binding to the large chaperones was specific. The reconstituted grp170-peptide complexes remain highly stable under high salt conditions, but are sensitive to low pH and high concentrations of reducing or oxidizing agents. For the first time we have compared the vaccine potency of large chaperones complexed with peptide antigens versus full length protein antigens. Interestingly, although the reconstituted peptide antigen chaperone complex was able to effectively elicit MHC I-restricted, antigen-specific CD8+ T-cell activation, the resultant antitumor response was significantly weaker than that generated by the full length protein antigen chaperone complex. Moreover, we have demonstrated that these large stress proteins are superior to conventional chaperones in holding tumor protein antigens. The use of full length protein antigens in chaperone complex with these large stress proteins can be exploited as a platform for formulating recombinant chaperone vaccines that target one or more protein antigens.
Materials and Methods
Mice and Cell lines
C57BL/6 mice purchased from the National Institutes of Health animal facilities were housed under pathogen-free conditions. Melanoma cell line B16-gp100 was maintained in DMEM, supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies, Grand Island, NY), 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. All of the experimental procedures were conducted according to the protocols approved by the Institutional Animal Care and Use Committee.
Peptides and proteins
Recombinant proteins including hsp110, grp170, hsp70, gp100 and TRP2 were expressed in a BacPAK™ baculovirous expression system (BD Biosciences Clontech, Palo Alto, CA) and prepared using a nickel nitriloacetic acid (Ni-NTA)-agarose (Qiagen, Valencia, CA) column as previously described (14, 17). Tumor-derived grp170 was purified from culture media of B16 tumor cells that have been engineered to secret grp170 (20). Endotoxin levels in recombinant proteins (<30 EU/mg protein) were measured using a Limulus Amebocyte lysate kit (Biowhittaker, Walkersville, MD). The following peptides (underlined sequences represent the precise MHC class I-binding epitope): AH1423–431(9mer, SPSYVYHQF), AH1429–436(18 mer, VTYHSPSYVYHQFERRAK), gp10025–32 (KVPRNQDWL), TRP2180–188 (SVYDFFVWL), TRP2175–192 (QIANCSVYDFFVWLHYYA) were synthesized and purified by HPLC to >90% homogeneity. Peptides were labeled in the N-terminal α-amino group using NHS-LC-Biotin (#21336, Pierce, Rockford, IL) as previously described (21). Non-reacted biotin reagent and byproducts were removed by HPLC.
Peptide chaperone complex formation and characterization
Grp170 or hsp110 (2 μg) was incubated with biotinylated peptides at 1:50 molar ratio in PBS under different conditions as indicated for 30 min, followed by incubation at room temperature for additional 60 min. The complexes were purified using a Centricon-10 filter to remove unbound peptides and resolved on non-reducing 10% SDS-PAGE, followed by immune blotting analysis using streptavidin-HRP. The complexes were visualized using chemiluminescence on X-ray film. For ATP/ADP binding study, the formed complexes were applied to an ADP-agarose column (Sigma), washed and eluted with buffer (20 mm Tris acetate, 20 mm NaCl, 3 mm MgCl2, pH7.5) containing either 5 mm ADP or ATP.
Protein thermal aggregation assay
150 nM protein antigen alone or in the presence of 1:1 molar ratio of hsp70, hsp110 or grp170, were equilibrated to room temperature in 25 mM Hepes, pH 7.4, 5 mM magnesium acetate, 50 mM KCl, 5 mM β-mercaptoethanol followed by incubation at the indicated temperatures in a thermostated cuvette. In some cases, excess protein substrate was added to the reaction solution. Light scattering by protein aggregation was determined by measuring the increase of optical density at 320 nm with a spectrophotometer.
Immunization, ELISPOT and CTL assays
For preparation of protein antigen chaperone complexes, recombinant gp100 or TRP2 protein and stress proteins (1:1 molar ratio) were under heat shock conditions for 30 min, followed by incubation at 37°C for 30 min (17). For preparation of peptide antigen chaperone complexes, peptides and stress proteins (50:1 molar ratio) were incubated at 50°C for 30 min, followed by incubation at 37°C for 30 min. Mice were immunized intradermally twice at two-week intervals with 30 μg of large stress proteins complexed with protein or peptide antigens. Two weeks after immunizations, splenocytes (5×105/well) were stimulated with protein antigen (20 μg/ml) or peptide antigen (1 μg/ml), or irradiated tumor cells (2.5×104/well, splenocyte: tumor cell= 20:1) at 37°C for 24 h. IFN-γ production was assessed by an ELISPOT assay (17). For in vitro CTL assays, cells from spleen or tumor-draining lymph nodes were stimulated with TRP2180–188 or mitomycin C-treated tumor cells and then co-cultured with 51Cr-labeled B16 cells at varying E: T ratios as described previously (17).
Tumor studies
For tumor challenge study, mice were inoculated intradermally with 1×105 B16-gp100 cells on the contralateral site after immunizations. Tumor growth was monitored every two days by measuring perpendicular tumor diameters using an electronic digital caliper. The tumor volume is calculated using the formula V=(The shortest diameter2× the longest diameter)/2. For tumor treatment, mice were established with tumors by injection of 2×105 B16 cells on day 0, followed by 4 treatments with protein antigen chaperone complexes or tumor-derived grp170 on days 3, 5, 7 and 9.
Isolation of tumor-infiltrating immune cell and intracellular IFN-γ staining
Freshly excised tumor tissues were gently minced into small pieces using a razor blade, and incubated in the digestion solution (400 U/ml of collagenase D, 25ug/mL Hyaluronidase, 10ug/mL DNase I) for 30 min at 37 °C. Single cell suspensions were prepared using a mesh filter in combination with Lymphocyte@-M centrifugation procedure (CEDARLANE Laboratories, Burlington, NC). Cells were stimulated with CTL peptide epitopes from gp100/TRP2 overnight in the presence of IL-2. Cells were then treated with BD GolgiPlug protein transport inhibitor containing brefeldin A and Cytofix/CytoPerm solution (BD Bioscience, San Jose, CA) before intracellular staining with antibodies for CD8 and IFN-γ was performed and analyzed on BD FACScaliburr.
Statistical analysis
Comparisons between two groups were performed using Student’s t test. Comparisons between multiple groups were carried out using ANOVA test. A value of p < 0.05 is considered to be statistically significant.
Results
Large stress proteins are capable of forming complexes with antigenic peptides in vitro
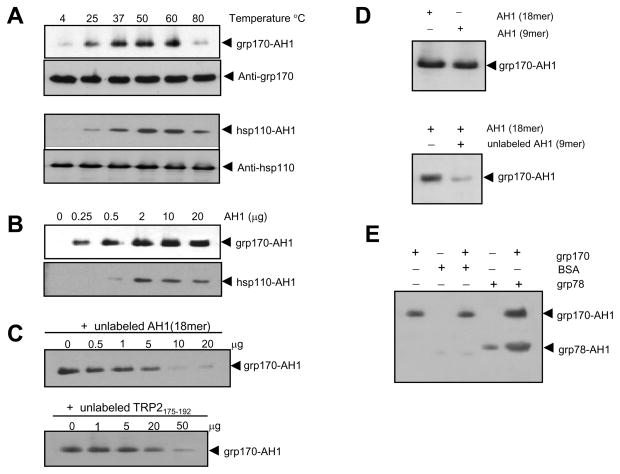
We first investigated the ability of grp170 and hsp110 to bind peptides by incubating purified recombinant proteins with a molar excess of labeled peptides (protein:peptide molar ratio = 1:50). The presence of biotinylated AH1 peptide associated with grp170 or hsp110 proteins was traced using gel electrophoresis and immunoblot with streptavidin-HRP. Biotinylated AH1(18 mer) peptide (VTYHSPSYVYHQFERRAK) containing an Ld-restricted epitope of a murine leukemia virus envelop protein gp70 (22) was used as a tracer. While the exogenously added AH1 peptides associated with grp170 in a temperature-dependent manner, high temperature (i.e., 80°C) clearly interfered with the formation of grp170-AH1 peptide complex (Fig. 1A, top). The temperature-dependent increase in peptide-binding was also observed when hsp110, another hsp70 super-family member, was used in the assay (Fig. 1A, bottom). Although both chaperones displayed the greatest peptide binding at 50°C, a strong peptide interaction was readily detected under physiological condition (i.e., 37°C). Similar observations have been made when large stress proteins were incubated with several other antigenic peptides, including HPV E749–57 and melanoma antigen gp10025–32 (data not shown).
Figure 1. Large stress proteins form complexes with antigenic peptides in vitro.
A. Temperature effect on the complex formation. Grp170 or hsp110 was incubated with biotinylated AH1(18mer, 1:50 molar ratio) peptides in PBS at different temperatures for 30 min, followed by incubation at room temperature for additional 60 min. The purified complexes were loaded on to 10% SDS-PAGE, followed by immune blotting analysis using streptavidin-HRP. Membranes were stripped and re-probed with antibodies against grp170 or hsp110, which serves as loading controls. B. Effect of peptide concentrations on the complex formation. Grp170 or hsp110 was incubated with increased concentrations of biotinylated AH1 peptides as indicated at 50°C for 30 min followed by immunoblot analysis. C. Competition effect of unlabeled peptides on the complex formation. Grp170 was incubated with labeled AH1 peptides in the presence of excess unlabeled AH1 or TRP2 peptides. D. Effect of the peptide length on the complex formation. Grp170 was incubated with labeled AH1 (18 mer) or AH1 (9 mer) peptides (Top). Or grp170 was incubated with labeled AH1 (18 mer) in the presence of excess unlabeled AH1 (9 mer) peptide (Bottom), followed by immunoblot analysis. E. Competition effect of proteins on the complex formation. Grp170 was incubated with labeled AH1 (9 mer) in the presence of BSA or chaperone grp78/BiP.
Next we assessed the effect of peptide concentrations on the complex formation at 50°C, a heat shock condition that stimulated the highest peptide binding. Grp170 (Fig. 1B, top) or hsp110 (Fig. 1B, bottom) was incubated with different concentrations of biotinylated AH1 peptides. Increased peptide concentrations resulted in a dose dependent increase in the complex formation. It was estimated that the binding reached the highest levels when grp170 or hsp110 was incubated with AH1 peptide at a molar ratio of approximately 25:1.
We also examined the grp170-AH1 peptide complex formation in the presence of unlabeled AH1 peptides. Addition of excess, unlabeled AH1 peptide effectively competed off the labeled AH1 peptides bound to grp170 in a dose-dependent manner (Fig. 1C, top). Furthermore, the presence of unrelated TRP2175–192 peptides, which contains an immunodominant CTL epitope (SVYDFFVWL) derived from melanoma antigen tyrosinase-related protein 2, also markedly inhibited AH1 peptide binding (Fig. 1C, bottom). In addition, grp170 was seen to bind to AH1 (18 mer) peptides and AH1 (9 mer) peptides in a comparable manner (Fig. 1D, top). Addition of unlabeled short AH1 (9 mer) peptides interfered with the complex formation between grp170 and the long AH1 (18 mer) peptides (Fig. 1D, bottom), suggesting that the size of peptides does not appear to significantly affect the peptide binding affinity of grp170. These studies suggest that grp170 is able to specifically bind a diverse array of peptides and displays no recognition preference for the peptide sequences under the conditions used here.
We next examined the competition effect of proteins on the grp170 interactions with AH1 peptides. As expected, the non-chaperone molecule BSA only showed marginal peptide binding whereas chaperones grp170 and grp78/BiP bound labeled AH1 peptides efficiently. The presence of excess BSA had no effect on the interaction between grp170 and AH1 peptides. Intriguing, addition of grp78/BiP to the solution of grp170 and AH1 peptides enhanced peptide bound to not only grp170, but also grp78/BiP (Fig. 1E).
Large stress protein grp170 is more efficient in binding peptides than other chaperones
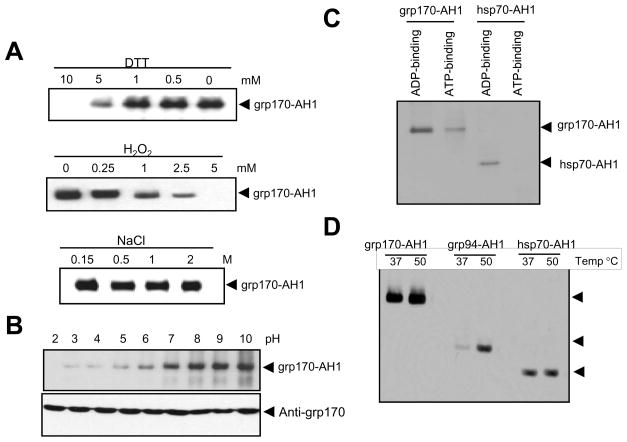
We next characterized the non-covalent interaction between grp170 and antigenic peptides in vitro under redox conditions. Grp170-peptide complex formation remained intact under reducing conditions (i.e., 1 mM DTT). However, a 5 mM concentration of DTT started to diminish the peptide complexing (Fig. 2A, top). Additionally, the grp170 binding of peptides became weak in the presence of increased concentrations of hydrogen peroxide (i.e., H2O2). 5 mM H2O2 completely dissociated the grp170-AH1 complex (Fig. 2A, Middle). Surprisingly, high salt concentrations up to 2M had a negligible effect on the grp170 interaction with AH1 peptides (Fig. 2A, Bottom). The peptide-binding activity of grp170 was also evaluated under different pH conditions. It is evident that the complex of grp170 and AH1 peptides was highly susceptible to acidic conditions. Even a pH of 6 resulted in reduction in peptide association with grp170 (Fig. 2B). Another peptide TRP2 was also tested and showed an identical binding pattern (data not shown).
Figure 2. Large stress protein grp170 is more efficient in binding peptides than other chaperones.
A. Effect of reducing, oxidative agents and salt concentrations on the complex formation. Grp170 was incubated with biotinylated AH1 peptides in the presence of DTT, H2O2 or NaCl at different concentrations as indicated. B. pH effect on the complex formation. Grp170 was incubated with biotinylated AH1 peptides in PBS with different pH, and analyzed by immunoblot. Membranes were stripped and re-probed with antibodies against grp170. C. Effect of ATP/ADP on grp170-AH1 peptide complex. Grp170- and hsp70-AH1 peptide complexes were generated by incubation of chaperones with biotinylated AH1(18mer) peptide at 37°C. The AH1 complexes were loaded onto ADP-agarose (lane 1 and 3) or ATP-agarose (lane 2 and 4) column, followed by washing with 500 mM NaCl containing PBS. Grp170 or hsp70 was eluted from column with PBS containing 5 mM ADP (lane 1 and 3) or ATP (lane 2 and 4). Eluted fraction was examined by immunoblot using streptavidin-HRP. D. Comparison of peptide binding ability of different stress proteins. Grp170, gp96/grp94 or hsp70 was incubated with biotinylated AH1 peptide in PBS at 37°C or 50 °C for 30 min, followed by incubation at room temperature for additional 1h. Representative data from three independent experiments are shown.
It has been demonstrated that a conformational change associated with ATP binding stimulates release of bound peptides from hsp70 (23). Therefore, we examined the effect of ATP/ADP on peptides bound to grp170. Stress protein-AH1 peptide complexes were incubated with ADP- or ATP-agarose beads and eluted with ADP or ATP, respectively. Analyses of eluted samples showed that grp170 bound ATP as well as ADP efficiently, and excessive ATP resulted in partial dissociation of peptides from grp170 (Fig. 2C). In contrast, the presence of ATP completely removed the AH1 peptides from hsp70, which agrees with the earlier report of the presence of peptides bound to Hsp70 after ADP purification and their absence after ATP purification (24). Given the highly efficient protein holding property of large chaperones, we compared the peptide-binding abilities of grp170 and two other conventional stress proteins, i.e., grp94/gp96 and hsp70. While grp94/gp96 was a weak peptide-binder at 37°C, the grp170-peptide complexes readily formed at the physiological condition (Fig. 2D). Similar results were obtained when different peptides were tested (data not shown). Although heat shock at 50°C modestly stimulated peptide association with grp170 and hsp70, it markedly increased the amount of peptide bound to grp94/gp96, which is consistent with the previous finding that an elevated temperature elicited an irreversible tertiary conformational change that is required for its enhanced peptide-binding activity (25, 26). Quantification of the peptides associated with grp170 by liquid chromatography-mass spectrometry indicates that grp170-AH1 complexes contained approximately 8.2 ng peptides per 1 μg of grp170 (data not shown), which is 5-fold increase of the reported peptides bound to grp94/gp96 (27).
Grp170 enhances the immunogenicity of associated antigenic peptides in vivo
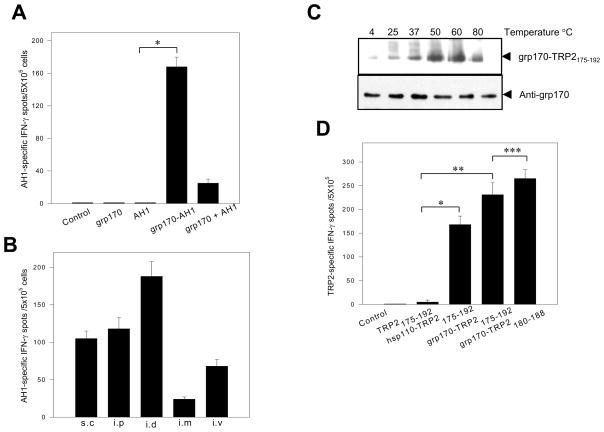
Given that large stress protein-peptide complexes can be reconstituted in vitro, we next examined the ability of grp170-AH1 peptide complexes to elicit an antigen-specific immune response in vivo. Neither AH1 peptide alone nor grp170 alone was able to generate a measurable immune response against AH1. However, immunization of mice with the grp170-AH1 peptide complex promoted a potent AH1-specific T-cell activation, as indicated by an increase in the frequency of IFN-γ producing cells compared to other groups. Mice receiving grp170 mixed with AH1 also developed an AH1-specific immune response, probably due to the peptide-binding at room temperature (Fig. 3A). We also immunized mice with the grp170-AH1 peptide complexes via different injection routes. While intramuscular (i.m.) vaccination produced the weakest AH1-specific response, subcutaneous (s.c.) and intraperitoneal (i.p.) injections both generated a modest AH1-specific T-cell activation. Intradermal (i.d.) delivery of the grp170-AH1 peptide complex appeared to be the most effective for induction of T-cell response against associated AH1 peptides (Fig. 3B).
Figure 3. Large stress proteins enhance the immunogenicity of associated peptides in vivo.
A. Increased immunogenicity of grp170-associated viral peptide. BALB/c mice (n=3) were immunized i.d. with 30 μg of grp170 alone, AH1 peptide alone, grp170-AH1 (18 mer) peptide complexes, or grp170+AH1 mixture. Splenocytes were stimulated with AH1 (9mer) in the presence of IL2 and assayed for IFN-γ production by ELISPOT (*, p< 0.001). B. Effect of injection routes on the grp170-AH1 induced immune response. Mice were immunized with reconstituted grp170-AH1 complexes via different routes as indicated. Splenocytes were subjected to ELISPOT assays upon stimulation with AH1 peptide. C. Temperature dependence of TRP2175–192 bound to grp170. Grp170 was incubated with biotinylated TRP2175–192 under different temperature conditions. The complex formation was examined by immunoblot as described. D. Enhanced T-cell activation recognizing melanoma antigen-derived CTL epitope. Mice (n=3) were immunized with TRP2175–192 peptide alone, hsp110-TRP2175–192 complexes, grp170-TRP2175–192 complex or grp170-TRP2180–188 complex. Splenocytes were assayed for IFN-γ production using an ELISPOT (*, p< 0.001; **, p< 0.001; ***, p> 0.05). Data represent one of three independent experiments.
In a similar manner, melanoma antigen TRP2-derived precursor peptide TRP2175–192, which contains a Kb restricted epitope SVYDFFVWL(180–188), was evaluated for its interaction with grp170. The complexes between grp170 and TRP2175–192 were readily formed, and increasing temperatures stimulated the amount of peptide bound to the grp170 (Fig. 3C). The same binding pattern was also seen with hsp110 (data not shown). The in vitro generated hsp110- or grp170-TRP2175–192 complexes were used to immunize C57BL/6 mice. ELISPOT assays showed that both TRP2 chaperone peptide complexes induced a more effective TRP2180–188-specific CTL response than did the TRP2175–192 peptide alone (Fig. 3D). Mice were also immunized with grp170 complexed with precisely processed MHC I-restricted peptide TRP2180–188. These mice only developed a slightly higher T-cell response against the CTL epitope than those immunized with grp170 complexed with TRP2175–192 peptide complex.
Protein antigen chaperone complex generates a more potent antitumor response than peptide antigen chaperone complexes in vivo
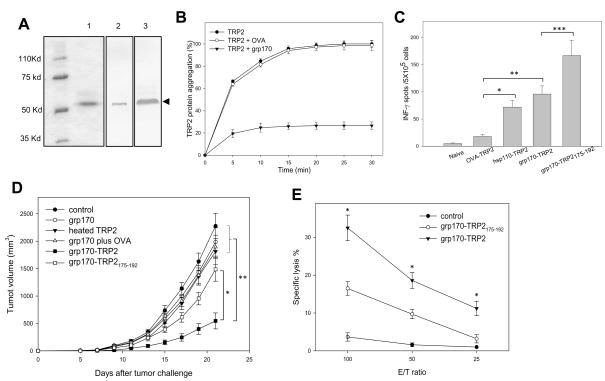
TRP2 is highly expressed in human primary as well as metastatic melanomas and has also been defined as a potential tumor-rejectionantigen in murine B16 melanoma (28). In light of the efficient priming of antigen-specific T-cells by both protein and peptide chaperone complexes, we sought to compare the antitumor activities generated by these two forms of antigen chaperone complexes. We first prepared recombinant human TRP2 protein using a BacPAK™ Baculovirus expression system, which not only offers a high yield of recombinant protein with proper tertiary structure and post-translational modifications, but also greatly minimizes potential endotoxin contamination in protein preparation. Protein purity was assessed by SDS-PAGE stained with Coomassie blue and immunoblotting using anti-TRP2 antibodies as well as anti-His-tag antibodies (Fig. 4A). Since it is the partially denatured peptide chain of a protein that is recognized by chaperone molecules and stimulates their chaperoning-binding function, we determined the thermal sensitivity of TRP2 protein antigen using an in vitro aggregation assay (17). This study indicated that the ‘melting temperature’ of TRP2 protein is approximately 45°C (data not shown), which was then used for subsequent complexing experiments. Protein aggregation assay showed that the large chaperone hsp110, but not ovalbumin (OVA), effectively blocked heat-induced TRP2 protein aggregation (Fig. 4B), which is consistent with our previous observations with another melanoma antigen gp100 (17). The ability of the TRP2 protein chaperone complex to generate antigen-specific CTL response was examined using an ELISPOT assay. Following vaccination, splenocytes from animals immunized with the hsp110- or grp170-TRP2 protein complex vaccines displayed high levels of IFN-γ production compared to those from untreated or OVA-TRP2 protein immunized mice (Fig. 4C). However, mice immunized with the grp170-TRP2175–192 peptide complex developed a stronger T-cell activity recognizing the CTL epitope TRP2180–188 than did the TRP2 protein complex (Fig. 4C).
Figure 4. Grp170-TRP2 protein complexes exhibit more potent antitumor activities than grp170-TRP2 peptide complexes in vivo.
A. Characterization of purified recombinant TRP2 protein by SDS-PAGE followed by Coomassie blue staining (lane 1) and immunoblot using antibodies for TRP2 (lane 2) or His-tag (lane 3). B. Protection of heat shock-induced TRP2 protein aggregation by hsp110. Hsp110 and TRP2 protein were incubated at 45°C. TRP2 aggregation was determined by optical density changes at 320 nm. C. Induction of a TRP2-specific immune response by immunization with an hsp110-TRP2 protein chaperone complex. Mice (n=5) were immunized i.d. with or without the TRP2 protein alone, hsp110-TRP2 complex, grp170-TRP2 complex, or grp170-TRP2175–192 peptide complex. Splenocytes were stimulated with TRP2180–188 and assayed for IFN-γ (*, p< 0.001; **, p< 0.001; ***, p< 0.01). D. Generation of a stronger antitumor response by grp170-TRP2 protein complex compared to grp170-TRP2175–192 peptide complex. Mice (n=5) were immunized with different vaccine regimens as indicated or left untreated. Two immunizations were administrated at weekly intervals, followed by tumor challenge with B16 tumor cells (*, p <0.005; **, p<0.001). Representative data from three independent experiments are shown. E. Induction of a robust CTL response by the grp170-TRP2 protein complex. After stimulation of splenocytes with TRP2180–188, CD8+ T-cells were magnetically isolated as effector cells for 51Cr release assays using B16 cells as targets.
Since the TRP2180–188 has been previously characterized as the most immunogenic epitope in the TRP2 amino acid sequence and this conserved non-mutated epitope can be recognized by anti-B16 melanoma CTLs as well as human HLA-A2.1 restricted anti-melanoma CTLs (28, 29), we carried out tumor prevention studies to compare the immunogenicity of TRP2175–192 peptide and full-length TRP2 protein when complexed to grp170. Although grp170-TRP2175–192 peptide vaccine induced a considerably higher T-cell response against CTL epitope TRP2180–188, it only provided a modest tumor inhibitory effect. In contrast, the grp170-TRP2 protein chaperone complex augmented a robust antitumor response (Fig. 4D). We also showed that vaccination with grp170 protein alone, heated TRP2 protein or grp170 mixed with irrelevant antigen OVA failed to induce tumor-protective effects, indicating that chaperone complex formation is required for the observed antitumor activity. This result is consistent with our previously published data on gp100-targeted chaperone vaccines (17, 19).
We next performed the chromium release assays to measure the cytolytic activity of T effector cells from immunized mice. It was shown that CD8+ T-cells from the grp170-TRP2 protein complex immunized mice exhibited significantly higher cytotoxic activity compared to those from the grp170-TRP2175–192 peptide complex treated mice (Fig. 4E), suggesting that protein antigen-targeted chaperone vaccines efficiently promote functional activation of CTLs.
Large stress proteins exhibit a superior antigen-holding capability
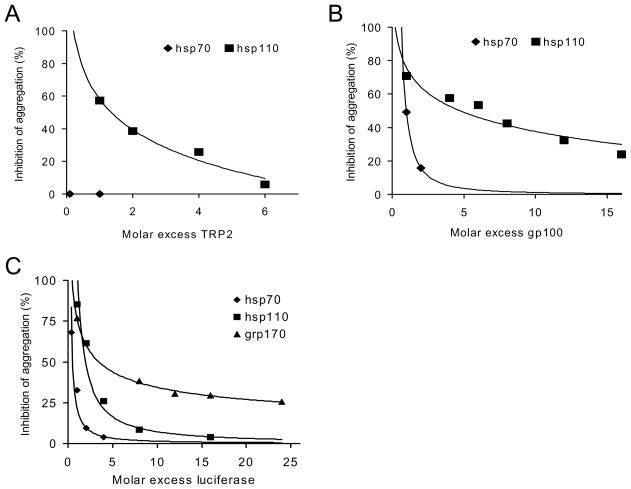
We have shown previously that hsp110 and grp170 are highly efficient at preventing aggregation of reporter protein luciferase and tumor-associated protein antigens such as gp100 upon heat shock at a 1:1 molar ratio compared to hsp70 and grp94/gp96 in vitro (17, 20, 30). We next compared the antigen-holding capabilities of hsp110 and hsp70 by performing the similar protein aggregation assays with the addition of excess substrate (i.e., antigen) under heat shock conditions. While the chaperoning activity of hsp70 was negligible at less than 4-fold excess of protein substrate, hsp110 was able to protect gp100 from heat-induced protein aggregation even with more than 10 fold excess gp100 (Fig. 5A). The same observations were made with TRP2 protein antigen, although hsp110 prevented aggregation of TRP-2 less efficiently than gp100 (Fig. 5B). In addition, we showed that grp170 has more capacity for preventing heat shock-induced aggregation of excess model antigen luciferase compared to hsp70 and hsp110 in vitro (Fig. 5C). These results indicate that large stress proteins chaperone or bind to antigen substrate in a quantitatively greater degree than conventional hsp70.
Figure 5. Large stress proteins display highly efficient antigen-holding capability.
A and B. Effective prevention of tumor protein antigen aggregation by hsp110 compared to hsp70. Increasing concentrations of (A) gp100, and (B) TRP2 was incubated with 150 nM of hsp70, or hsp110 under heat shock conditions. Protein denaturation was monitored by measuring optical density at 320nm. The optical density of the substrate heated alone at 30 min was set to 100%. Each point represents inhibition of aggregation at 30 min time point with increasing molar ratio of substrate:chaperone. C. A greater holding capacity of grp170 compared to other chaperones. Increasing concentrations of luciferase was added to each chaperone, heated at 43°C and assessed for aggregation as described. Data shown are representative of two independent experiments.
Large stress protein-based chaperone vaccines targeting dual protein antigens enhance antigen-specific tumor immunity
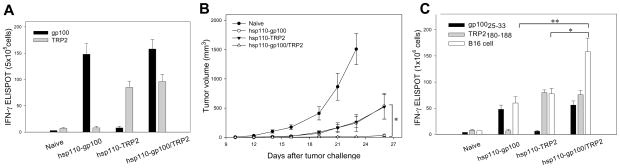
Given their unique and highly efficient antigen-holding capabilities, these large chaperones may be used as a platform to deliver multiple antigens that could enhance immunotherpeutic efficacy against tumor. We examined whether the vaccine efficacy might be further improved by simultaneous immunization with hsp110-based chaperone vaccines directed against two melanoma antigens instead of one. Hsp110-gp100 protein complex and hsp110-TRP2 protein complex were prepared separately under heat shock conditions. Mice were immunized with either single protein antigen complexed with hsp110, or the combination of gp100 and TRP2 chaperone vaccines. The frequency of gp100 protein-specific IFN-γ-producing splenocytes was significantly higher in mice immunized with the gp100 chaperone vaccine and the ‘polyvalent’ vaccines than in mice untreated or immunized with TRP2 chaperone vaccine. Similarly, a TRP2-specific immune response was also specifically provoked in hsp110-TRP2 or the combined hsp110-gp100/TRP2 complex vaccine immunized mice, not in hsp110-gp100 immunized animals (Fig. 6A). These results suggest that immunization with individual melanoma antigens complexed with hsp110 does not augment immune reactivity against each other, and the combined vaccinations do not appear to interfere with the activation of antigen-specific immune responses induced by the individual chaperone vaccine.
Figure 6. Chaperone vaccines targeting gp100 and TRP2 result in robust tumor protective immunity.
A. Mice (n=3) were immunized weekly with the hsp110-gp100 vaccine, hsp110-TRP2 vaccine, or combination of gp100 and TRP2 chaperone complexes for three times. Splenocytes were harvested and incubated with gp100 or TRP2 protein, and the frequency of IFN-γ producing T-cells was measured using an ELISPOT assay. B. Improved antitumor response by the combined chaperone vaccines. Following immunizations as described above, mice (n=5) were challenged with 1×105 B16 cells. Tumor growth was monitored by measuring tumor size (*, p< 0.001) C. Enhanced immune recognition following the administration of combined chaperone vaccines. One week after the last immunization, splenocytes were stimulated with gp10025–33, TRP2180–188 peptides or irradiated B16 cells overnight, and subjected to an ELISPOT assay (*, p< 0.001; **, p< 0.001).
To investigate the feasibility and antitumor efficacy of the polyvalent protein chaperone vaccines targeting both gp100 and TRP2, mice were immunized with different vaccine regimens, followed by B16 tumor challenge. The ‘polyvalent’ vaccines resulted in an effective protection against subsequent tumor challenge, which was more potent than that achieved with either of the single antigen-chaperone complexes alone (Fig. 6B), suggesting that this ‘polyvalent’ vaccines simultaneously targeting dual antigens promotes the overall immune response against B16 tumors.
Since the B16 tumor protective immunity was induced by administration of chaperone-based protein vaccines, we next sought to determine whether the combined vaccines elicited gp100 and TRP2-specific CD8+ T-cell responses. Splenocytes from immunized or naïve animals were stimulated with MHC I-restricted CTL epitopes, gp10025–33 or TRP2180–188 peptides, and assayed for IFN-γ production. In contrast to naïve or mice immunized with TRP2 protein chaperone vaccine alone, those immunized with the gp100 protein vaccine or combined gp100/TRP2 protein chaperone vaccines displayed greater activation of gp10025–33-specific T-cells. Similarly, TRP2180–188-specific T-cells were also primed in mice immunized with vaccine formulations containing TRP2 protein antigen. In addition, irradiated B16 tumor cells were used to stimulate splenocytes harvested from treated mice. Compared to individual protein antigen chaperone vaccine (i.e., gp100 or TRP2), administration of the dual chaperone vaccines markedly enhanced the B16 tumor-specific IFN-γ production, suggesting that the combined vaccinations resulted in an improved immune recognition of the B16 tumor cell target (Fig. 6C).
Combined grp170 chaperone vaccines targeting gp100 and TRP2 protein antigens result in a greater antitumor immunity compared to tumor-derived grp170
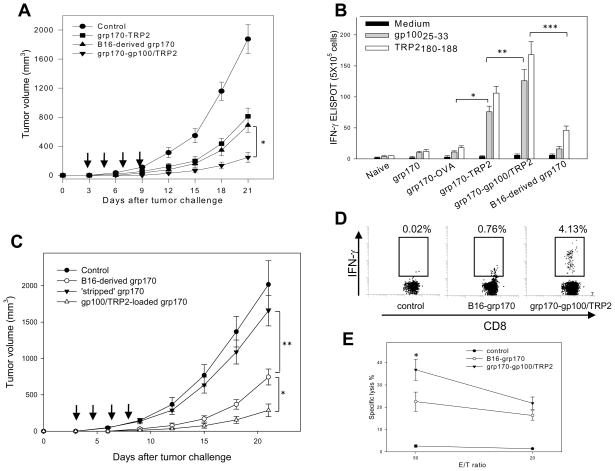
We compared the antitumor activities of recombinant grp170-gp100/TRP2 protein antigen complex and tumor-derived/purified grp170 in a therapeutic setting. Tumor-bearing mice were treated with grp170-TRP2 complex, grp170-gp100/TRP2 complexes or grp170-derived/purified from B16 tumor cells. It was observed that TRP2 protein antigen carried by grp170 exhibited comparable antitumor activities to tumor-derived grp170. However, treatment with combined protein chaperone vaccines targeting both gp100 and TRP2 led to a significantly more effective inhibition of tumor growth than single antigen chaperone vaccine or tumor-derived grp170 (Fig. 7A). Splenocytes were also collected from treated mice and stimulated with CD8+ T-cell epitopes gp10025–33 or TRP2180–188. A greater activation of gp100 and TRP2-specific T-cell responses was observed in mice immunized with the combined chaperone vaccines than those immunized with tumor-derived grp170 or grp170-TRP2 protein complex. Interestingly, cells from the grp170-TRP2 protein chaperone vaccine treated mice showed increased production of IFN-γ upon stimulation with gp10025–33 peptides, suggesting that the TRP2 chaperone vaccine administrated in this treatment setting also triggered the activation of gp10025–33-specific T-cells (Fig. 7B). Vaccination with grp170 alone, grp170 plus OVA or B16-grp170 failed to induce gp100-reactive T-cells, suggesting that antigen specificity as well as antigen dose both play a role.
Figure 7. Chaperone vaccines targeting dual antigens induce potent therapeutic antitumor immunity.
A. Superior therapeutic efficacy generated by the combined chaperone vaccines. Mice (n=5) were established with B16 tumors 3 days prior to vaccine treatment. Grp170-TRP2 complex, tumor-derived grp170 or grp170-gp100/TRP2 complexes were administrated every three days for a total 4 treatments (*, p< 0.01). B. Priming of T-cells reactive with gp10025–33 following TRP2-chaperone vaccine treatment. Splenocytes from treated mice were stimulated with gp10025–33 or TRP2180–188 and subjected to an ELISPOT assay (*, p< 0.001; **, p< 0.01; ***, p < 0.001). Data shown are from two independent experiments. C. Potent antitumor immunity augmented by endogenous grp170 complexed with gp100/TRP2 proteins. B16 tumor-bearing mice were treated with tumor-derived grp170, antigen ‘stripped’ grp170, or ‘stripped’ grp170 complexed with gp100/TRP2 protein antigens. Tumor growth was followed as described (* and **, p< 0.01). D. High frequency of gp100/TRP2-specific TILs elicited by the chaperone vaccines. TILs were isolated from pooled tumor tissues after treatment and stimulated with gp10025–33/TRP2180–188 peptides, followed by intracellular staining for IFN-γ. Cells were analyzed using FACS by gating on CD8+ cells. E. Robust tumor-killing activity of TDLN cells from chaperone vaccine-treated mice. Single cell suspensions were prepared using TDLNs pooled from treated mice. Cells were stimulated with mitomycin and IFN-γ-treated B16 cells for 5 days. T-cells were used as effector cells in cytotoxic assays against B16 cells as targets (*, p< 0.005). Data shown are from two independent experiments.
We next examined whether the enhanced antitumor effect by recombinant grp170-gp100/TRP2 complexes resulted from increased molar ratio of tumor-associated antigens in the chaperone vaccine formulation. To this end, we removed endogenous antigens associated with B16 tumor-derived grp170 using ATP-agarose column as previously described by Peng et al., (24), and subsequently complexed recombinant gp100/TRP2 proteins to the ‘stripped’ grp170. Tumor-bearing mice were treated with B16-grp170, the ‘stripped’ grp170 or gp100/TRP2-reloaded grp170. As expected, the ‘stripped’ grp170 lost its immunogenicity against B16 tumors. However, the enhanced therapeutic effect was observed in mice treated with endogenous grp170-loaded with gp100/TRP2 compared to those treated with B16-grp170 (Fig. 7C).
We subsequently investigated antigen-specific tumor-infiltrating lymphocytes (TILs) following the therapeutic vaccinations. Intracellular IFN-γ staining of antigen-stimulated TILs showed that recombinant chaperone vaccine resulted in a significant increase in the frequency of gp100/TRP2 reactive CD8+ T-cells in tumor site compared to control or B16-grp170 (Fig. 7D). It is not surprising since the antigens in the chaperone vaccine formulation are highly concentrated whereas only small percentage of antigens carried by B16-grp170 is likely to be tumor-specific. In addition, cytotoxic T-cell assays were performed to assess the effector function of T-cells from tumor-draining lymph nodes (TDLNs). TDLN-derived CD8+ T-cells from the grp170-gp100/TRP2 chaperone vaccine treated mice displayed a higher cytolytic activity than their counterparts from B16-grp170 treated mice (Fig. 7E). Collectively, these results demonstrate the superior capability of the recombinant chaperone vaccines to augment robust antitumor immunity targeting carried protein antigens.
Discussion
Several lines of evidence have shown that stress proteins, including hsp70, hsp90 and grp94/gp96, are peptide-binding chaperones and stress protein-peptide complexes released into the extracellular milieu as a consequence of pathological cell death may play an important role in antigen cross-presentation. Given the strong vaccine potency exhibited by high molecular weight chaperones, we have investigated here the peptide-binding ability of large stress proteins as well as the immunogenicity of in vitro reconstituted hsp110- and grp170-peptide complexes. We have demonstrated for the first time that full-length protein antigen chaperone complex is superior to peptide chaperone complex in augmenting an antitumor immune response in both prophylactic and therapeutic settings, strongly supporting the idea of using large chaperone-protein antigen complexes as a new clinical approach to the treatment of cancers or other diseases.
Our results show that hsp110 and grp170 readily form complexes in vitro with a variety of antigenic peptides derived from viral or tumor antigens. Competition studies reveal that the peptide binding can be efficiently blocked in the presence of excess unlabeled peptides or peptides of different sequences or length, suggesting that these large chaperones may bind peptides in a promiscuous manner. The in vitro formed complexes are highly stable as indicated by their resistance to high salt concentrations. However, grp170 association with antigenic peptides is susceptible to acidic pH, which may have implications for grp170-mediated peptide loading/release process in vivo, since MHC I molecules were also found to be able to exchange peptides under acidic conditions (31). Indeed, earlier studies have shown that grp170 interacts with transporter associated with antigen processing (TAP) translocated peptides and is involved in peptide trafficking in the antigen presentation pathway (32, 33).
Hsp70 interactions with peptides in vitro have been shown to be promoted by oxidative conditions (34). However, no significant stimulation of peptide association with grp170 or hsp110 (data not shown) is observed under oxidative or reducing conditions, even though they represent highly diverged and distant family members of hsp70, suggesting that different chaperones may bind to peptides through distinct mechanisms. Compared to the hsp70 as shown here and grp94/gp94 with a reported adenine nucleotide independent peptide binding activity (25, 26), ATP only partially dissociates antigenic peptides from grp170, suggesting that ATP might play a regulatory role in the grp170-involved peptide-binding and release processes in vivo. One interesting finding was that the presence of grp78/BiP significantly stimulates peptide association with both grp170 and grp78, indicating the existence of the functional interactions between these two chaperones (35). In fact, Lhs1p, the grp170 homologue in yeast, can serve as an alternative nucleotide exchange factor, and it is believed that the coordination of both grp78/BiP (Kar2p) and grp170 (Lhs1p) promotes productive folding of unfolded polypeptide substrate (36). In a similar scenario, either sequentially or simultaneously association with the substrate has been proposed for the hsp110-hsp70 complex (37). However, how exactly grp78/BiP works together with grp170 to promote peptide association under conditions used here remains unclear.
High temperature appears to stimulate grp170 association with peptides, but not as dramatically as it does to grp94/gp96 (4-fold increase) (26). It has been reported that the transient heat shock results in the formation of higher order oligomers of stress protein, which, however, is not responsible for the increase in peptide-binding activity of grp94. Indeed, the activation of peptide interaction directly correlates with the exposure of a hydrophobic pocket(s) (26). It should be noted that the binding of peptides to large stress proteins is probably not optimum under our experimental conditions, likely due to the non-physiological parameters of the experiment, and the lack of co-chaperones. Nevertheless, the strong grp170 association with peptides at 37°C implies that grp170 may be more efficient in interacting with and actively participate in shaping the antigenic peptide repertoire in vivo compared to other chaperones, which may contribute to the highly potent vaccine activity of this molecule (15). In contrast to the reported weak CTL responses elicited by hsp70-peptide complex (38), the grp170-peptide complex effectively induces T-cell activation against associated antigenic peptides, which are in line with several reports suggesting that the peptide-binding affinity is important for the generation of a CTL response by chaperone-peptide complexes in vivo (39–41).
Coincident with the observations of highly efficient peptide-binding capability, the protein aggregation assays in the present study have demonstrated that large stress proteins display superior capability to stabilize and hold full length client proteins/substrates compared to hsp70 and grp94/gp96. These results are consistent with our previous observations that both hsp110 and grp170 can efficiently stabilize thermally denatured luciferase in a folding-competent state (13, 14, 20). Studies of grp170 deletion mutants show that grp170 contains two essential substrate-binding regions i.e., the β-sheet domain and the C-terminal helix domain (14). Therefore, it is evident that these large stress proteins, grp170 in particular, represent a unique vaccine vehicle for shuttling protein antigens. Strikingly, we found that the TRP2 protein antigen chaperone complex augments a more potent tumor protective response against B16 melanoma than the grp170-TRP2175–192 peptide complex, even though T-cell activity specific for a major CTL epitope (i.e, TRP2180–188) appears to be higher in mice immunized with the TRP2 peptide complex. We reason that full-length protein antigen contains CD4 T-helper epitopes and other unknown CD8 epitopes that may have contributed to the observed robust antitumor effect. The result agrees with our earlier studies showing that CD4+ cells are involved in the protein chaperone complex-induced antitumor immunity (16).
Using an experimental murine model of melanoma, we have demonstrated that immunization with large chaperone vaccines targeting two different melanoma antigens (i.e., gp100 and TRP2) dramatically enhances overall antitumor efficacy compared to either single antigen-targeted vaccine. Furthermore, treatment of B16 tumor-bearing mice with the ‘polyvalent’ grp170 chaperone complex vaccines result in a more potent therapeutic efficacy against established tumors when compared to tumor-derived grp170. This finding has two implications for the use of chaperoning-based vaccine approach. First, these large stress proteins can be utilized as a platform or a building block method to formulate multivalent vaccines targeting multiple protein antigens. Secondly, although it is not clear whether the improved antitumor activity is due to additive or synergistic effect derived from the combined vaccines, such a multivalent vaccine design is expected to significantly reduce the possibility of tumor escape as a result of antigen loss during antigen-targeted immunotherapy. Interestingly, we also show in the present study that re-loading of the ‘stripped’ endogenous grp170 with gp100/TRP2 protein antigens recapitulated the enhanced therapeutic effect of the recombinant chaperone vaccines-targeting gp100/TRP2, suggesting that the high concentrations of tumor antigens may contribute to the improved antitumor activities elicited by the chaperone vaccines. However, it should be noted that, given the nature of the antigen targets in these two different forms of vaccine regimens (protein vs peptide; well-defined antigens vs undefined and presumably polyvalent), it is possible that the immunostimulatory actions of these two vaccine approaches may involve distinct mechanisms.
Of particular interest, we found that administration of a TRP2-targeted chaperone vaccine in the therapeutic setting augmented an immune activation against another melanoma antigen gp100. It is likely that the observed epitope spreading was caused by tumor destruction and subsequent cross-presentation of tumor cell-associated antigens following the chaperone vaccine therapies. In addition, the TRP2-specific T-cell activities induced by the combined chaperone vaccines are significantly higher than that generated by the grp170-TRP2 complex in this melanoma treatment model, suggesting that administration of dual antigen chaperone vaccines may be able to amplify the immune responses specific for targeted antigens after the initial tumor killing event. It is not clear whether T-cells specific for melanoma antigens other than gp100 and TRP2 have been stimulated in this setting. B16 melanoma is a more relevant model for evaluating antitumor immunity, since it expresses multiple melanoma associated antigens and the sequences of these murine melanoma antigens including gp100, TRP2 and tyrosinase are all highly homologous to their human counterparts (42, 43). Therefore, immunization by combining different tumor protein antigens using the same chaperoning formulations should be clinically beneficial relative to the use of tumor-derived stress proteins.
Recent clinical trial using patient-specific autologous grp94/gp96 showed that overall survival in the treatment arm is statistically indistinguishable from that in the control arm. However, retrospective analyses show that patients in certain sub-stages receiving a larger number of grp94/gp96 immunizations survived longer than those receiving fewer such treatments (44). Unfortunately, the yield of tumor-derived stress proteins was a limiting factor and many patients were unable to participate or complete the vaccine trial. Clearly the synthetic production of the recombinant chaperone complexes will provide several advantages over the preparation of autologous or tumor derived HSP vaccines, including no requirement for tumor specimen, unlimited quantities of vaccine with significant uniformity from batch to batch, broad applicability to all patients with antigen(s)-positive tumors, increased chance of stimulating polyepitope directed cellular and humoral responses as well as post-treatment immunomonitoring by using well-defined whole protein antigens. As a tumor specimen is not required for vaccine production, patients with no measurable disease or inaccessible tumor can still be treated using this approach. This also makes it an ideal approach for eventual adjuvant therapy in high risk patients with completely resected disease. Collectively, the recombinant large chaperone-based vaccines targeting dual or multiple melanoma antigens presented here warrant further development and evaluations in the clinic for treatment of human melanoma. Additionally, this synthetic “building block” approach of complexing antigen with immuno-stimulatory large stress proteins can serve as a model to evaluate other different antigens, either alone or in combination vaccines.
Footnotes
This work was supported by National Cancer Institute Grants CA129111, CA099362 and CA557501, American Cancer Society Grant RSG-08-187-01-LIB, Harrison Endowed Scholarship and NCI Cancer Center Support Grant to Massey Cancer Center.
References
- 1.Welch WJ. Heat shock proteins functioning as molecular chaperones: their roles in normal and stressed cells. Philos Trans R Soc Lond B Biol Sci. 1993;339:327–333. doi: 10.1098/rstb.1993.0031. [DOI] [PubMed] [Google Scholar]
- 2.Subjeck JR, Shyy TT. Stress protein systems of mammalian cells. Am J Physiol. 1986;250:C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- 3.Easton DP, Kaneko Y, Subjeck JR. The hsp110 and Grp1 70 stress proteins: newly recognized relatives of the Hsp70s. Cell Stress Chaperones. 2000;5:276–290. doi: 10.1379/1466-1268(2000)005<0276:thagsp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 5.Wang XY, Facciponte JG, Subjeck JR. Molecular chaperones and cancer immunotherapy. Handb Exp Pharmacol. 2006;172:305–329. doi: 10.1007/3-540-29717-0_13. [DOI] [PubMed] [Google Scholar]
- 6.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 7.Graner M, Raymond A, Romney D, He L, Whitesell L, Katsanis E. Immunoprotective activities of multiple chaperone proteins isolated from murine B-cell leukemia/lymphoma. Clin Cancer Res. 2000;6:909–915. [PubMed] [Google Scholar]
- 8.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 9.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, Jeannin P. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 10.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. Embo J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J Biol Chem. 2004;279:51250–51257. doi: 10.1074/jbc.M406202200. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Easton D, Oh HJ, Lee-Yoon DS, Liu X, Subjeck J. The 170 kDa glucose regulated stress protein is a large HSP70-, HSP110-like protein of the endoplasmic reticulum. FEBS Lett. 1996;380:68–72. doi: 10.1016/0014-5793(96)00011-7. [DOI] [PubMed] [Google Scholar]
- 13.Oh HJ, Easton D, Murawski M, Kaneko Y, Subjeck JR. The chaperoning activity of hsp110. Identification of functional domains by use of targeted deletions. J Biol Chem. 1999;274:15712–15718. doi: 10.1074/jbc.274.22.15712. [DOI] [PubMed] [Google Scholar]
- 14.Park J, Easton DP, Chen X, MacDonald IJ, Wang XY, Subjeck JR. The chaperoning properties of mouse grp170, a member of the third family of hsp70 related proteins. Biochemistry. 2003;42:14893–14902. doi: 10.1021/bi030122e. [DOI] [PubMed] [Google Scholar]
- 15.Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166:490–497. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 16.Wang XY, Kazim L, Repasky EA, Subjeck JR. Immunization with tumor-derived ER chaperone grp170 elicits tumor-specific CD8+ T-cell responses and reduces pulmonary metastatic disease. Int J Cancer. 2003;105:226–231. doi: 10.1002/ijc.11058. [DOI] [PubMed] [Google Scholar]
- 17.Wang XY, Chen X, Manjili MH, Repasky E, Henderson R, Subjeck JR. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res. 2003;63:2553–2560. [PubMed] [Google Scholar]
- 18.Manjili MH, Wang XY, Chen X, Martin T, Repasky EA, Henderson R, Subjeck JR. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol. 2003;171:4054–4061. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 19.Park J, Facciponte JG, Chen X, MacDonald IJ, Repasky E, Manjili MH, Wang XY, Subjeck JR. Chaperoning Function of Stress Protein grp170, a Member of the hsp70 Superfamily, Is Responsible for its Immunoadjuvant Activity. Cancer Res. 2006;66:1161–1168. doi: 10.1158/0008-5472.CAN-05-2609. [DOI] [PubMed] [Google Scholar]
- 20.Wang XY, Arnouk H, Chen X, Kazim L, Repasky EA, Subjeck JR. Extracellular targeting of endoplasmic reticulum chaperone glucose-regulated protein 170 enhances tumor immunity to a poorly immunogenic melanoma. J Immunol. 2006;177:1543–1551. doi: 10.4049/jimmunol.177.3.1543. [DOI] [PubMed] [Google Scholar]
- 21.Selo I, Negroni L, Creminon C, Grassi J, Wal JM. Preferential labeling of alpha-amino N-terminal groups in peptides by biotin: application to the detection of specific anti-peptide antibodies by enzyme immunoassays. J Immunol Methods. 1996;199:127–138. doi: 10.1016/s0022-1759(96)00173-1. [DOI] [PubMed] [Google Scholar]
- 22.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, Pardoll DM, Jaffee EM. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci U S A. 1996;93:9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palleros DR, Reid KL, Shi L, Welch WJ, Fink AL. ATP-induced protein-Hsp70 complex dissociation requires K+ but not ATP hydrolysis. Nature. 1993;365:664–666. doi: 10.1038/365664a0. [DOI] [PubMed] [Google Scholar]
- 24.Peng P, Menoret A, Srivastava PK. Purification of immunogenic heat shock protein 70-peptide complexes by ADP-affinity chromatography. J Immunol Methods. 1997;204:13–21. doi: 10.1016/s0022-1759(97)00017-3. [DOI] [PubMed] [Google Scholar]
- 25.Wearsch PA, Nicchitta CV. Interaction of endoplasmic reticulum chaperone GRP94 with peptide substrates is adenine nucleotide-independent. J Biol Chem. 1997;272:5152–5156. doi: 10.1074/jbc.272.8.5152. [DOI] [PubMed] [Google Scholar]
- 26.Wearsch PA, Voglino L, Nicchitta CV. Structural transitions accompanying the activation of peptide binding to the endoplasmic reticulum Hsp90 chaperone GRP94. Biochemistry. 1998;37:5709–5719. doi: 10.1021/bi9801006. [DOI] [PubMed] [Google Scholar]
- 27.Staib F, Distler M, Bethke K, Schmitt U, Galle PR, Heike M. Cross-presentation of human melanoma peptide antigen MART-1 to CTLs from in vitro reconstituted gp96/MART-1 complexes. Cancer Immun. 2004;4:3. [PubMed] [Google Scholar]
- 28.Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, Yang JC. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997;185:453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh HJ, Chen X, Subjeck JR. Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J Biol Chem. 1997;272:31636–31640. doi: 10.1074/jbc.272.50.31636. [DOI] [PubMed] [Google Scholar]
- 31.Gromme M, Neefjes J. Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways. Mol Immunol. 2002;39:181–202. doi: 10.1016/s0161-5890(02)00101-3. [DOI] [PubMed] [Google Scholar]
- 32.Dierks T, Volkmer J, Schlenstedt G, Jung C, Sandholzer U, Zachmann K, Schlotterhose P, Neifer K, Schmidt B, Zimmermann R. A microsomal ATP-binding protein involved in efficient protein transport into the mammalian endoplasmic reticulum. Embo J. 1996;15:6931–6942. [PMC free article] [PubMed] [Google Scholar]
- 33.Spee P, Subjeck J, Neefjes J. Identification of novel peptide binding proteins in the endoplasmic reticulum: ERp72, calnexin, and grp170. Biochemistry. 1999;38:10559–10566. doi: 10.1021/bi990321r. [DOI] [PubMed] [Google Scholar]
- 34.Callahan MK, Chaillot D, Jacquin C, Clark PR, Ménoret A. Differential Acquisition of Antigenic Peptides by Hsp70 and Hsc70 under Oxidative Conditions. Journal of Biological Chemistry. 2002;277:33604–33609. doi: 10.1074/jbc.M202890200. [DOI] [PubMed] [Google Scholar]
- 35.Lin HY, Masso-Welch P, Di YP, Cai JW, Shen JW, Subjeck JR. The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol Biol Cell. 1993;4:1109–1119. doi: 10.1091/mbc.4.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steel GJ, Fullerton DM, Tyson JR, Stirling CJ. Coordinated activation of Hsp70 chaperones. Science. 2004;303:98–101. doi: 10.1126/science.1092287. [DOI] [PubMed] [Google Scholar]
- 37.Polier S, Dragovic Z, Hartl FU, Bracher A. Structural Basis for the Cooperation of Hsp70 and Hsp110 Chaperones in Protein Folding. Cell. 2008;133:1068–1079. doi: 10.1016/j.cell.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Kurotaki T, Tamura Y, Ueda G, Oura J, Kutomi G, Hirohashi Y, Sahara H, Torigoe T, Hiratsuka H, Sunakawa H, Hirata K, Sato N. Efficient Cross-Presentation by Heat Shock Protein 90-Peptide Complex-Loaded Dendritic Cells via an Endosomal Pathway. J Immunol. 2007;179:1803–1813. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 39.Moroi Y, Mayhew M, Trcka J, Hoe MH, Takechi Y, Hartl FU, Rothman JE, Houghton AN. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci U S A. 2000;97:3485–3490. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacAry PA, Javid B, Floto RA, Smith KG, Oehlmann W, Singh M, Lehner PJ. HSP70 peptide binding mutants separate antigen delivery from dendritic cell stimulation. Immunity. 2004;20:95–106. doi: 10.1016/s1074-7613(03)00357-1. [DOI] [PubMed] [Google Scholar]
- 41.Tobian AA, Canaday DH, Harding CV. Bacterial heat shock proteins enhance class II MHC antigen processing and presentation of chaperoned peptides to CD4+ T cells. J Immunol. 2004;173:5130–5137. doi: 10.4049/jimmunol.173.8.5130. [DOI] [PubMed] [Google Scholar]
- 42.Zhai Y, Yang JC, Spiess P, Nishimura MI, Overwijk WW, Roberts B, Restifo NP, Rosenberg SA. Cloning and characterization of the genes encoding the murine homologues of the human melanoma antigens MART1 and gp100. J Immunother. 1997;20:15–25. doi: 10.1097/00002371-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkhurst MR, Fitzgerald EB, Southwood S, Sette A, Rosenberg SA, Kawakami Y. Identification of a shared HLA-A*0201-restricted T-cell epitope from the melanoma antigen tyrosinase-related protein 2 (TRP2) Cancer Res. 1998;58:4895–4901. [PubMed] [Google Scholar]
- 44.Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, Yuh L, Gupta R, Srivastava PK. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. 2008;26:955–962. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]