Abstract
Several lines of evidence suggest the existence of multiple progestin receptors that may account for rapid and delayed effects of progesterone in the central nervous system. The delayed effects have been long attributed to activation of the classical progestin receptor (Pgr). Recent studies have discovered novel progestin signaling molecules that may be responsible for rapid effects. These include, and progesterone receptor membrane component 1 (Pgrmc1), Pgrmc2, progestin and adipoQ receptor 7 (Paqr7) and Paqr8. The functions of these molecules have been investigated extensively in non-neural, but not in neural tissues, partly because it is unclear which are expressed in the brain and where they are expressed. To address these issues, we compared the distributions of mRNAs encoding Pgr, Pgrmc1, Pgrmc2, Paqr7 and Paqr8 using in situ hybridization with radiolabeled oligodeoxynucleotidyl probes in forebrain tissues of estradiol-treated female rats. We also examined the distribution of serpine mRNA binding protein 1 (Serbp1), a putative binding partner of Pgrmc1. Analysis of adjacent brain sections showed that the highest expression of mRNAs encoding Pgr, Pgrmc1, Pgrmc2 and Serbp1 was detected in several hypothalamic nuclei important for female reproduction. In contrast, expression patterns of Paqr7 and Paqr8 were low and homogeneous in the hypothalamus, and more abundant in thalamic nuclei. The neuroanatomical distributions of these putative progestin signaling molecules suggest that Pgrmc1 and Pgrmc2 may play a role in neuroendocrine functions while Paqr7 and Paqr8 are more likely to regulate sensory and cognitive functions.
Keywords: neuroendocrine, steroid, mPR, progestin, mapping
Introduction
Progesterone (P4) is widely recognized for its ability to regulate neural functions related to reproduction, but it also affects diverse processes such as cognition and neurogenesis (Berman et al., 1997, Giachino et al., 2003). The traditional tenet of P4 action is that it binds the cognate progestin receptor (Pgr), and functions as a ligand-activated transcription factor to regulate gene expression. However, rapid non-genomic effects have also been reported (Meyerson, 1972, Parsons et al., 1980, Mani et al., 1994), and P4 can act in the absence of Pgr (Frye et al., 2006). These data support the emerging concept that P4 actions in the brain may be through the classical Pgr and also through non-classical mechanisms.
Current research suggests that there are several possible candidates for mediating the non-classical effects of P4. One such protein is progesterone receptor membrane component 1 (Pgrmc1), but it does not appear to function as a traditional receptor because it requires a binding partner known as serpine mRNA binding protein 1 (Serbp1) (Peluso et al., 2005, Peluso et al., 2006). Moreover, the structure of Pgrmc1 does not share homology with either classical steroid receptors or G-coupled protein receptors (Mifsud and Bateman, 2002). Nonetheless, Pgrmc1 mediates several important Pgr-independent effects. For example, P4 acts through Pgrmc1 to activate phosphoinositide-dependent protein kinase 1 and phosphorylate Akt (Hand and Craven, 2003). In the ovary, Pgrmc1 and Serbp1 form a receptor complex required for the antiapoptotic effects of P4 in granulosa cells (Peluso et al., 2006, Zhang et al., 2008). Pgrmc2 is a closely related isoform of Pgrmc1, differing mainly in its N-terminus, but there is virtually no information regarding Pgrmc2 function (Falkenstein et al., 1999, Peluso et al., 2005). Pgrmc1 has been localized to several brain regions (Krebs et al., 2000, Sakamoto et al., 2004, Meffre et al., 2005), but no studies have systematically mapped its distribution and the role of this protein in the brain remains unknown. Likewise, no studies have mapped neural expression of Serbp1 or Pgrmc2. Despite these limitations, several lines of evidence indicate that Pgrmc1/Serbp1, and possibly Pgrmc2, may be important for non-classical P4 actions in the brain.
Two other candidates for mediating the non-genomic effects of P4 are progestin and adipoQ receptor 7 (Paqr7) and Paqr8. These are G-protein coupled receptors first discovered in spotted sea trout, and subsequently in mammalian tissues (Zhu et al., 2003a, Zhu et al., 2003b). Although activation of these receptors by P4 regulates cAMP levels and MAPK activity in fish (Hanna et al., 2006), there is some debate about whether they function as bona fide P4 receptors in mammals (Fernandes et al., 2008). Recent reports detected Paqr7 and Paqr8 mRNAs in hypothalamic tissue of mice, but the exact anatomical localization is unknown (Sleiter et al., 2009). Collectively, these findings raise the possibility that Paqr7 and Paqr8 also mediate P4 neural actions.
Although there is abundant evidence that these signaling molecules participate in P4 signaling, it is unclear which are important in the nervous system. Moreover, while many neural functions are modulated by P4, there is little information about which functions require Pgr, non-nuclear receptors or both. One obstacle to resolving this question is that neither the classical Pgr nor any of the non-classical P4 receptor candidates have been systematically mapped in the brain. To address this issue, we used in situ hybridization histochemistry (ISHH) to map the expression of mRNAs encoding Pgr, Pgrmc1, Pgrmc2, Serbp1, Paqr7 and Paqr8. In these studies, we used female rats because of the important role P4 plays in regulation of female-specific physiological functions.
Materials and Methods
Animals and tissue preparation
All protocols were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts and all animals were housed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Six adult female Sprague-Dawley rats (225–250 g; approximately 95 days of age; Harlan Sprague-Dawley, Madison WI) were individually housed in the Animal Care Facility on a 14:10 light:dark cycle with food and water provided ad libitum. To achieve a similar hormonal milieu among rats, we ovariectomized them and implanted two Silastic capsules containing E2 (150 μg/ml 17β-estradiol in sesame oil) a week later as described previously (Petersen and LaFlamme, 1997). Twenty-four hours later, we collected brains and rapidly froze and stored them at −80 °C until they were cryosectioned (Leica CM3000, Nussloch, Germany).
For three animals, 14-μm coronal forebrain sections were obtained and thaw-mounted onto gelatin-coated slides and stored at −80 °C until ISHH was performed. The remaining three animals were used for RNA isolation in validation studies described below.
Oligodeoxynucleotidyl probe preparation
In these studies, we used oligodeoxynucleotidyl probes of the same length and specific activity. Antisense oligodeoxynucleotide sequences used for end-tailing are provided in Table 1. Both sense and antisense sequences were produced by an automated DNA synthesizer and purified by reverse-phase HPLC by Integrated DNA Technologies (Coralville, IA). Oligodeoxynucleotides were 3'-end labeled with [α33P]-dATP (PerkinElmer, Waltham, MA) using terminal deoxynucleotidyl transferase (Roche, Indianapolis, IN) as described previously (Petersen et al., 1989). Incubation was halted by addition of TE (10mM Tris-HCL; pH 8.0, 1 mM EDTA), and the probe was purified by phenol-chloroform extraction and ethanol precipitation. The resulting pellet was washed with 70% ethanol and resuspended in 25 μl TE.
Table 1.
Sequences for Oligodeoxynucleotidyl Probes Used in ISHH Studies
| NCBI Gene Name and Accession # | Oligodeoxynucleotide Sequences 5'- 3' | Antisense to bases: |
|---|---|---|
| Pgrmc1 NM_021766 | TGTAGTTCCAACCCAATTACCAGGTGTGTGAGAGTTACTGTGTGTGGG | 1354-1305 |
| Pgrmc2 NM_001008374 | CAAAGTTCAGTCCTGTTTACTGTGATCCTTGGTGTCCTCCTCGTCTGT | 667-619 |
| Serbp1 NM_145086 | AAGGAACCAGTGTTGTATTATGGCATCCAGTTAGGCCAGAGCGGGGAA | 1274-1227 |
| Paqr7 NM_001034081 | ATAGTCCAGCGTCACAGCTTCTAGCTGGGCTAAAGTGCACAGCACC | 888-843 |
| Paqr8 NM_001014099 | GCCAGCCCCGCTGGTACCACTTGACAGATCTTCCGCATAACTGGATAA | 1112-1065 |
| PgR NM_022847 | CACATGGTAAGGCACAGCGAGTAGAATGACAACTCCTTCATCCTCTGC | 2375-2328 |
ISHH
The distribution pattern for each mRNA was determined in separate ISHH runs, and tissue sections were prehybridized as previously described (Ottem et al., 2004). Radioisotopic probes (0.5 × 106 cpm) were applied directly to brain tissue in 20 μl hybridization buffer. This buffer contained 4XSSC (1XSSC = 0.15 M NaCl/0.015 M sodium citrate, pH 7.2), 50% (v/v) formamide, 10% (w/v) dextran sulfate, 250 μg/ml yeast tRNA, 1X Denhardt's solution, 500 μg/ml heparin sodium salt, 0.1% sodium pyrophosphate and 0.05 M dithiothreitol added freshly before use. Sections were covered with glass coverslips and hybridized overnight at 37 °C in humidified plastic boxes. Slides were removed from 37 °C and allowed to cool, and coverslips were floated off in 1XSSC. They were washed four times for 15 minutes each in 2XSSC-50% formamide solution at 40 °C, followed by four washes, 15 minutes each, in 1XSSC. Finally, slides were rinsed in water and briefly dehydrated in 70% ethanol. The slides were air-dried and apposed to Kodak BioMax MR film (Rochester, NY) for signal detection. In order to acquire optimum signal, autoradiograms were developed at 1, 3 and 6 weeks by an X-ray film processor and images were acquired using BioQuant Imaging Software (Bio-Quant Inc, Nashville, TN,) and a CCD videocamera (QImaging QICAM FAST color).
Validation of probe specificity
To determine the specificity of the hybridization signal, sense strand probes to each target of interest were hybridized to representative sections. To verify specificity of each antisense probe, subsets of adjacent slides were treated with RNAse A solution (100 μg/ml RNAse A in 0.5 M NaCl, 0.05 M EDTA and 0.01 M Tris-HCl) for one hour at 37 °C following prehybridization. An additional set of slides was used for Nissl staining in order to provide reference material for identification of specific brain regions.
Regardless of exposure time, Paqr7 and Paqr8 antisense probes produced diffuse and homogeneous signal, therefore multiple probes (Table 2) were used for each gene to verify signal specificity. To ensure specificity in regions that displayed low ISHH signal for Paqr7, quantitative polymerase chain reaction (QPCR) was performed using cDNA derived from RNA of the diagonal band of Broca and striatum, regions with two different signal intensities. RNA was isolated from tissue punches using the RNeasy Lipid Tissue Kit (Qiagen, Valencia, CA) and reverse transcribed into cDNA using Quantitect Reverse Transcription Kit (Qiagen) and manufacturer's protocol. Reactions were performed in a Stratagene Mx3000P instrument programmed as follows: 95 °C, 10 min, and 40 cycles of 95 °C for 15 sec and 60 °C for 60 sec. Reactions contained reagents from QuantiTect SYBR Green Kit and manufacturer's protocols were used (Roche Diagnostics, Indianapolis, IN). Specific primer sets were obtained from Integrated DNA Technologies, and the forward and reverse primers used to detect Paqr7 mRNA were TGCACCGCATCATAGTGTC and TGATAGTCCAGCGTCACAGC. Resulting cycle thresholds were normalized using forward and reverse primers for beta-actin: GGGAAATCGTGCGTGACATT and GCGGCAGTGGCCATCTC. Samples with no cDNA were used as negative controls. Products were resolved using 2% agarose gel electrophoresis.
Table 2.
Sequences for Additional Oligodeoxynucleotidyl Probes Used in ISHH Validation Studies
| NCBI Gene Name and Accession # | Oligodeoxynucleotide sequences 5'- 3' | Antisense to bases: |
|---|---|---|
| PAQR7 NM_001034081 | GGTACACGGCCACACCCACATAGTCCAAGAAGAAGAAGCTGTAATGCC | 445-398 |
| GGGGCCAACGGGCGTGCAGAGGCTTATAGATGGCTCCCCGAGCCTGAT | 934-887 | |
| CAGGCTGGACAGCAGGTGGCTGAACTTCTGGGCTACTGCCATCGCCAT | 48-1 | |
| PAQR8 NM_001014099 | TTCTGGGACGGTGCATGGCATCTTGGGAAGCCCTTCTTCCAGAATCTT | 570-523 |
| GAGCCAGGCCGGTGGTGAGTTTGAGGCCAGCCTGCTCTATAGAGTGAG | 238-191 | |
| GTGAGCCAAGGCGCTGCCATACTGGTAGACACTGACCCCGACGTAGTC | 486-439 |
Data Analysis
Neuroanatomic mapping of Pgrmc1, Pgrmc2, Paqr7, Paqr8, Serbp1 and Pgr was performed with the aid of a rat brain atlas (Swanson, 1998) and Nissl-stained adjacent sections. Relative levels of mRNA were determined by optical densitometric measurements of the autoradiographic signals across different brain regions. Results of this semiquantitative ISHH were obtained by digitizing all autoradiographic images and four ranges of density of labeling were used to determine relative intensities across brain regions and across probes. Signal strength intensity was determined using arbitrary optical density units and denoted by – (background; 0–51), + (low; 52–102), ++ (moderate; 103–153), +++ (154–204), and ++++ (highest signal intensity; 205–255). Digitized images were imported into Adobe Photoshop 8.0 CS (Adobe Systems Inc., San Jose CA) and all figures were cropped to the same size for display.
For QPCR studies, relative levels of Paqr7 mRNA were analyzed using the 2-ΔΔCt method (Livak and Schmittgen, 2001).
Results
Probe specificity
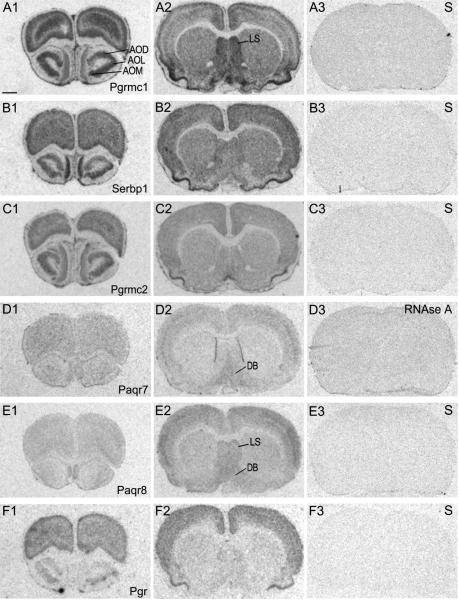
We verified the specificity of the probes used in these studies in several ways. All four oligodeoxynucleotidyl probes for each target produced the same labeling pattern. In addition, neither sense strand controls nor RNAse A-treated sections showed specific signal with the exception of the sense probe for Paqr7. Although Paqr7 sense strand control probes showed signal above background, the signal for antisense probes was abolished by tissue pretreatment with RNAse A (Fig. 1, D3). Moreover, QPCR detected a single band of expected size using primers to Paqr7. Consistent with our ISHH findings, QPCR detected higher mRNA levels in the diagonal band of Broca than in the striatum (data not shown).
Figure 1.
Photomicrographs of film autoradiograms of hybridized rat brain sections to 33P-labeled oligodeoxynucleotidyl probes for A) Pgrmc1 B) Serbp1 C) Pgrmc2 D) Paqr7 E) Paqr8 and F) Pgr. For Pgrmc1, Serbp1, Pgrmc2, Paqr8 and Pgr, the sense (S) strand probe is shown in the third image of each series. For Paqr7, the section was hybridized to the antisense probe and then treated with RNAse A. Scale bar = 200 μm
Distribution of Pgrmc1, Serbp1, Pgrmc2, Paqr7, Paqr8 and Pgr mRNAs
The neuroanatomical distributions of mRNAs encoding Pgrmc1, Serbp1, Pgrmc2, Paqr7, Paqr8 and Pgr are presented in Table 3. The distributions of mRNAs encoding Pgrmc1, Pgrmc2 and Serbp1 overlapped extensively throughout the brain, and some of these regions displayed high levels of the classical Pgr, most notably in ventral structures. Paqr7 and Paqr8 mRNAs were not highly expressed in these structures, but showed expression patterns similar to one another. Unlike signals for probes to Pgrmc1, Pgrmc2, Serbp1 and Pgr, signals for Paqr7 and Paqr8 probes were characterized by diffuse labeling found predominantly in the thalamus.
Table 3.
Localization and abundance of mRNA encoding Pgr, Pgrmcl, Serbp1, Pgrmc2, Paqr7 and Paqr8 in the female rat forebraina
| Anatomical region | Pgr | Pgrmc1 | Serbp1 | Pgrmc2 | Paqr7 | Paqr8 |
|---|---|---|---|---|---|---|
| Telencephalon | ||||||
| Olfactory cortex | ||||||
| Anterior olfactory nucleus external part | + | ++ | + | ++ | – | – |
| Anterior olfactory nucleus lateral part | + | +++ | +++ | ++ | – | – |
| Anterior olfactory nucleus medial part | + | +++ | +++ | +++ | + | – |
| Anterior olfactory nucleus dorsal part | – | +++ | ++ | ++ | – | – |
| Olfactory tubercle | + | + | + | + | + | + |
| Piriform cortex | ++ | ++++ | ++++ | +++ | + | + |
| Hippocampal formation | ||||||
| Taenia tecta | + | + | ++ | + | + | + |
| Dentate gyrus | + | ++ | ++++ | ++ | – | + |
| CA1 pyramidal layer | ++ | + | +++ | + | – | + |
| CA2 pyramidal layer | ++ | ++ | +++ | ++ | – | + |
| CA3 pyramidal layer | +++ | ++ | +++ | ++ | – | + |
| Amygdala | ||||||
| Medial nucleus of the amygdala | +++ | +++ | +++ | + | – | – |
| Posterolateral cortical amygdaloid nucleus | ++ | + | + | + | + | + |
| Central nucleus of the amygdala | + | ++ | + | + | – | – |
| Basolateral nucleus of the amygdala | ++ | + | + | + | – | – |
| Basomedial nucleus of the amygdala | ++ | + | + | + | – | – |
| Septal region | ||||||
| Triangular septal nucleus | – | ++ | + | + | – | – |
| Lateral septal nucleus | – | +++ | ++ | + | – | ++ |
| Medial septal nucleus | – | ++ | + | + | – | ++ |
| Horizontal limb of the diagonal band of Broca | + | ++ | + | + | + | + |
| Vertical limb of the diagonal band of Broca | + | ++ | + | + | + | + |
| Bed nucleus of the stria terminalis, anterior | + | +++ | ++ | + | – | – |
| Bed nucleus of the stria terminalis, posterior | + | ++ | + | + | + | + |
| Accumbens nucleus | + | +++ | ++ | + | + | + |
| Diencephalon | ||||||
| Thalamus | ||||||
| Medial habenular nucleus | + | +++ | +++ | ++ | – | + |
| Lateral habenular nucleus | + | ++ | + | – | – | + |
| Paraventricular nucleus | ++ | ++ | ++ | + | – | ++ |
| Zona incerta | +++ | +++ | ++ | ++ | + | + |
| Medial geniculate nucleus | + | ++ | ++ | – | + | + |
| Dorsal lateral geniculate nucleus | + | + | + | – | + | + |
| Anterodorsal thalamic nucleus | + | ++ | ++ | + | ++ | ++ |
| Anteroventral thalamic nucleus | + | ++ | + | – | ++ | ++ |
| Anteromedial thalamic nucleus | + | + | ++ | ++ | + | ++ |
| Ventral posteromedial thalamic nucleus | + | + | ++ | ++ | + | + |
| Lateralis | + | + | ++ | ++ | – | + |
| Lateralis posterior | + | + | ++ | ++ | + | + |
| Ventralis | + | + | ++ | – | + | + |
| Paracentral thalamic nucleus | + | + | + | + | + | ++ |
| Central medial thalamic nucleus | ++ | + | + | + | + | + |
| Reuniens thalamic nucleus | ++ | ++ | ++ | ++ | + | + |
| Hypothalamus/preoptic region | ||||||
| Anteroventral periventricular nucleus | ++++ | ++++ | +++ | +++ | + | + |
| Suprachiasmatic preoptic nucleus | + | ++++ | +++ | ++ | – | + |
| Supraoptic nucleus | + | ++++ | +++ | ++ | + | + |
| Paraventricular nucleus | ++ | ++++ | +++ | ++ | + | + |
| Rostral arcuate nucleus | ++ | ++++ | +++ | ++ | + | – |
| Caudal arcuate nucleus | +++ | +++ | +++ | ++ | + | + |
| Medial preoptic area | +++ | +++ | +++ | ++ | + | + |
| Median preoptic nucleus | ++++ | +++ | +++ | ++ | + | + |
| Anterior hypothalamic area | +++ | ++ | ++ | + | + | + |
| Ventromedial nucleus | +++ | +++ | +++ | ++ | + | + |
| Dorsal hypothalamic area | ++ | +++ | +++ | + | + | + |
| Premamillary nucleus, dorsal | ++ | +++ | +++ | + | + | + |
| Premamillary nucleus ventral | ++ | ++ | ++ | + | + | + |
| Medial mammillary nucleus | ++ | +++ | +++ | + | + | – |
| Dorsomedial nucleus | ++ | +++ | ++ | + | + | – |
| Lateral preoptic area | + | ++ | ++ | + | + | + |
| Lateral hypothalamic area | + | + | ++ | + | + | ++ |
| Mesencephalon | ||||||
| Interpeduncular nucleus | +++ | +++ | +++ | + | ++ | + |
| Substantia nigra | ++ | ++ | ++ | + | + | + |
| Superior colliculus | + | ++ | ++ | + | + | + |
| Periaqueductal gray | +++ | +++ | +++ | + | + | + |
| Ventral tegmental nucleus | + | ++ | ++ | + | + | + |
| Nucleus of the oculomotor cranial nerve | +++ | +++ | +++ | + | + | ++++ |
| Pretectum | + | ++ | +++ | + | + | ++++ |
| Red nucleus | ++ | ++ | ++ | ++ | ++ | ++ |
The ratings reflect the relative signal strength for each probe.
Diencephalon
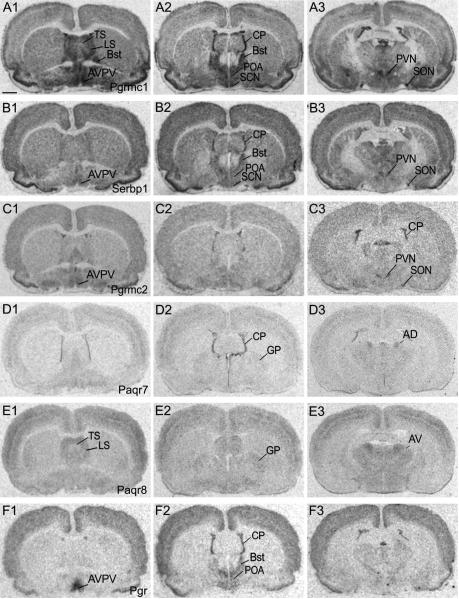
In the rostral diencephalon, high signal intensities for Pgrmc1 mRNA were detected in several olfactory nuclei as indicated in Fig. 1, A1. We found moderate signal for mRNAs encoding Pgrmc1, Paqr7 and Paqr8 in the diagonal band of Broca (Fig. 1; A2, D2 and D4, respectively). In the preoptic area (POA), the AVPV nucleus displayed very high levels of mRNAs encoding Pgrmc1 and Pgr (Fig. 2, A1 and F1). Serbp1 and Pgrmc2 also showed elevated signal intensities in this nucleus (Fig. 2, B1 and C1). Although Paqr7 and Paqr8 mRNAs were found in the POA, clearly defined signal within the AVPV was not apparent (Fig. 2, D1 and E3). The bed nucleus of the stria terminalis contained dense labeling for Pgrmc1, Serbp1 and Pgr (Fig. 2, A2, B2 and F2). Moderate levels of mRNAs encoding Pgrmc1 and Paqr8 were found in the rostral portion of the lateral septal nucleus (Fig. 2, A1 and E1).
Figure 2.
Rostral to caudal arrangement of photomicrographs showing film autoradiograms of hybridized rat forebrain sections to 33P-labeled oligodeoxynucleotidyl probes for A) Pgrmc1, B) Serbp1, C) Pgrmc2, D) Paqr7, E) Paqr8, and F) Pgr. Scale bar = 200 μm
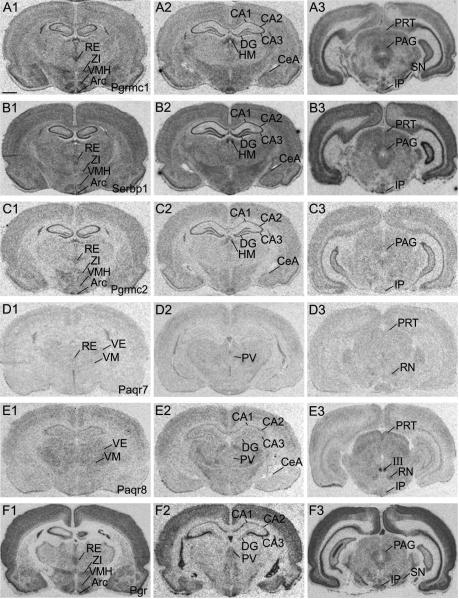
The paraventricular nucleus (PVN) and supraoptic nucleus (SON) showed abundant labeling for Pgrmc1, Serbp1, and Pgrmc2 (Fig. 2, A3, B3 and C3). High signal for probes encoding these mRNAs was also detected in the zona incerta, ventromedial nucleus (VMH) and arcuate nucleus (Arc), a finding consistent with the distribution of Pgr (Fig. 3, column 1). The reunions nucleus of the thalamus showed moderate levels of mRNAs encoding Pgrmc1, Serbp1, Pgrmc2 and Pgr mRNAs (Fig. 3, A1, B1, C1 and F1). In contrast, probes for these mRNAs showed low levels of signal intensity in the ventromedial thalamic nuclei while those encoding Paqr7 and Paqr8 were somewhat prominent (Fig. 3, column 1). The anterodorsal and anteroventral thalamic nuclei displayed similarly elevated levels of Serbp1 and Paqr8 gene expression (Fig. 3, B1 and E1).
Figure 3.
Rostral to caudal arrangement of photomicrographs showing film autoradiograms of hybridized rat forebrain sections to 33P-labeled oligodeoxynucleotidyl probes for A) Pgrmc1, B) Serbp1, C) Pgrmc2, D) Paqr7, E) Paqr8, and F) Pgr. Scale bar = 200 μm
Telencephalon
The hippocampus showed intense hybridization signals for Pgrmc1, Serbp1, Pgrmc2 and Pgr probes as shown in Fig. 3, columns 1 and 2. Within this region, signal intensities were highest in the CA2 and CA3 for all four mRNAs. In the dentate gyrus, stronger signal was detected for mRNAs encoding Pgrmc1, Serbp1 and Pgrmc2 (Fig. 3, A2, B2 and C2) than for those encoding Pgr (Fig. 3, F2). Paqr8 displayed low to moderate signal intensity within the hippocampus (Fig. 3, E2). In contrast, mRNAs encoding Paqr7 were undetectable in CA1, CA2, CA3 and dentate gyrus (Fig. 3, D1 and D2).
In the cortex, moderate levels of mRNAs were observed for Pgrmc1, Serbp1, Pgrmc2, Paqr8 and Pgr as shown in column 2 of Fig. 1. High signal intensity was detected in cortical regions for Pgrmc1, Serbp1 and Pgr mRNA (Fig. 3, row A, B and F). The medial amygdala showed moderate expression of Pgrmc1, Serbp1, Pgrmc2 and Pgr (Fig. 3, columns 1 and 2). Alternatively, Paqr7 and Paqr8 signals in the amygdala were low overall (Fig. 3, D2 and E2)
The highest levels of mRNAs encoding Pgrmc1, Serbp1 and Pgr in the caudal forebrain were detected in the periaqueductal gray (PAG) (Fig. 3, A3, B3 and F3). Pgrmc2 mRNA levels were moderately high within this region (Fig. 3, C3), but Paqr7 and Paqr8 mRNAs were very low (Fig. 3, D3 and E3). Interestingly, Paqr8 mRNA levels were highest in the nucleus of the oculomotor tract (Fig. 3, E3). Finally, within the substantia nigra and interpeduncular nucleus, we detected moderately high levels of mRNAs encoding Pgrmc1, Serbp1 and Pgr (Fig. 3, A3, B3 and F3).
Discussion
Collectively, these neuroanatomical mapping data provide important clues as to functions of putative progestin signaling molecules. The high levels of Pgrmc1 and Pgrmc2 mRNAs within virtually all hypothalamic nuclei suggest that these molecules are likely candidates for mediating rapid neuroendocrine effects. Moreover, signals for Pgrmc1 and Pgrmc2 mRNAs were strongest in regions that also contained Pgr and regulate reproductive functions, consistent with the idea that these functions require both rapid and delayed actions of P4. Pgrmc1 and Serbp1 mRNAs displayed strongly overlapping distribution patterns supporting the idea that these molecules interact to form a functional receptor (Peluso et al., 2004). Our data indicate that Paqr7 and Paqr8 may be important in sensory relay systems and other less well understood P4 functions regulated by thalamic and cortical brain regions. Our findings suggest that P4 may act through a number of signaling molecules to influence a much broader array of physiological effects than previously recognized.
The specific neural mechanisms underlying the role of Pgrmc1 have not yet been delineated, but the distinctive and overlapping patterns of Pgrmc1 and Serbp1 mRNAs suggest that these molecules may interact in neurons as they do in other cells. In granulosa cells, P4 activation of the Pgrmc1/Serbp1 complex stimulates phosphorylation cascades through activation of protein kinase G (Peluso et al., 2007). Protein kinase G can activate additional kinases such as phosphoinositide 3-kinase, resulting in phosphorylation of Akt (Kandel and Hay, 1999, Brazil and Hemmings, 2001). The rapid phosphorylation of Akt in response to P4 has been observed in cells from cortex and hippocampus (Singh, 2001, Hwang et al., 2009), and our study shows that both brain regions contain Pgrmc1 and Serbp1. Taken together, these observations suggest that phosphorylation events initiated by P4 may involve the Pgrmc1/Serbp1 complex.
Brain regions rich in Pgrmc1 and Serbp1, as well as Pgrmc2 and Pgr, include the AVPV, medial preoptic (MPN), Arc and suprachiasmatic nuclei (SCN). These findings are in agreement with previous immunocytochemical and ISHH studies of Pgr and Pgrmc1 (Parsons et al., 1982, Krebs et al., 2000, Curran-Rauhut and Petersen, 2002, Meffre et al., 2005). Each of these nuclei contribute to the timing and magnitude of the preovulatory luteinizing hormone (LH) surge, a process in which P4 exerts rapid and delayed effects. For example, estrogen induction of the LH surge requires Pgr expression in the AVPV/MPN (Chappell and Levine, 2000), and P4 rapidly advances and augments the LH surge in estradiol-primed female rats (Krey et al., 1973, DePaolo and Barraclough, 1979, Levine and Ramirez, 1980). Our findings that the AVPV/MPN displayed high levels of Pgrmc1, Serbp1 and Pgrmc2 support the idea that these signaling molecules mediate rapid P4 effects in the LH surge mechanism. P4 also rapidly represses basal LH levels, and this action may involve the Arc (Banks and Freeman, 1978, Goodman et al., 1981, Richter et al., 2005), where Pgrmc1, Pgrmc2 and Serbp1 mRNA levels were also high. Finally, levels of these mRNAs were elevated in the SCN, and while not directly linked to reproduction, this nucleus sends projections to the AVPV and is critical for the temporal regulation of the LH surge (Paxinos and Watson, 1982, Watts and Swanson, 1987, de la Iglesia et al., 1995). Although the rapid effects of P4 on LH surge release may be mediated by membrane-associated Pgr (Hammes and Levin, 2007), the current observations suggest that Pgrmc1, Pgrmc2 and Serbp1 may also play a role.
We found high levels of mRNAs encoding Pgrmc1, Pgrmc2, Serbp1 and Pgr in the VMH and in PAG, brain regions important for P4-dependent female sex behaviors (Pfaff and Sakuma, 1979, Rubin and Barfield, 1983). This confirms a previous report of Pgrmc1 in the VMH (Krebs et al., 2000). Rat copulatory behavior requires activation of the Pgr within the VMH (Pollio et al., 1993, Ogawa et al., 1994), a nucleus that sends projections to many brain regions including the PAG (Saper et al., 1976). In the PAG, P4 rapidly elicits female sexual receptivity (Gorski, 1974), an effect originally attributed to Pgr expression in this region. However, work in Pgr-null mice shows that sexual receptivity can be rapidly induced by midbrain injection of P4, indicating a role for other signaling mechanisms (Beyer et al., 1988, Frye and Vongher, 1999). One such mechanism involves P4 metabolism to allopregnanolone, a molecule that induces feminine sex behaviors when injected into the midbrain (Beyer et al., 1988, Pfaff et al., 1994). The present work suggests another possibility, namely that Pgrmc1 contributes to elicitation of sexual receptivity by mediating rapid P4 signaling through phosphorylation events as described above. Alternatively, Pgrmc1 may influence sex behavior through actions on neurosteroid production in the midbrain, a process that may be required for feminine sex behavior (Akesson et al., 1988, Frye et al., 2007). Consistent with this idea, Pgrmc1 interacts with key regulators of cholesterol homeostasis (Suchanek et al., 2005) and enhances steroidogenesis in diverse cell types (Laird et al., 1988, Min et al., 2004).
The present study identified high levels of Pgrmc1, Pgrmc2 and Serbp1 mRNAs in regions not directly linked to reproduction. For example, we confirmed previous findings that Pgrmc1 is expressed in the PVN and SON (Krebs et al., 2000, Meffre et al., 2005), and extend these findings to show that Pgrmc2 and Serbp1 mRNAs were also found in these nuclei. The physiological significance of these findings are unclear, but Meffre et al. found that Pgrmc1 protein was expressed specifically within vasopressinergic neurons of the PVN and SON, suggesting that Pgrmc1 may play a role in regulation of water and ion homeostasis (Meffre et al., 2005). We also found Pgrmc1, Pgrmc2 and Serbp1 mRNAs in CA1, CA2, CA3 and dentate gyrus. These findings may be relevant to the observation that P4 enhances hippocampal-dependent cognitive performance (Hoshina et al., 1994, Sandstrom and Williams, 2001). Our data also compliment previous findings of Pgrmc1 and Pgrmc2 mRNAs in neuroprogenitor cells derived from adult hippocampus (Liu et al., 2009). In these cells, the authors showed that P4 induces neurogenesis through Pgrmc1-dependent phosphorylation cascades that do not require Pgr. These findings suggest that non-genomic signaling events involving Pgrmc1, and possibly Pgrmc2 and Serbp1, underlie P4 actions in diverse neural functions.
Neither Paqr7 nor Paqr8 genes were strongly expressed in neural regions that control gonadotropin release and contain abundant Pgrmc1 mRNA. This was somewhat surprising because these genes are colocalized in other parts of the reproductive system. For example, Paqr7, Paqr8 and Pgrmc1 have been detected in cells of the ovary, uterus, and placenta in rats, sheep, and humans (Zhu et al., 2003a, Cai and Stocco, 2005, Peluso et al., 2006, Zhang et al., 2008, Ashley et al., 2009). Moreover, in the rat corpus luteum, the expression of each of these genes changes during pregnancy, and the patterns of change for Paqr7 and Pgrmc1 expression are strikingly similar (Cai and Stocco, 2005). In contrast to Pgrmc1, neither Paqr7 nor Paqr8 mRNA levels are found at levels much above background in AVPV/MPN. However, the AVPV provides afferent projections to GnRH neurons and results of recent studies show that Paqr7 and Paqr8 are functional in GT1-7 immortalized GnRH neurons (Sleiter et al., 2009). Thus, it is possible that Paqr7 and Paqr8 impact the neural control of reproduction, but through GnRH neurons scattered in the rostral preoptic area that also receive signals from Pgrmc1-containing neurons of the AVPV.
Though we did not find strong evidence for Paqr7 or Paqr8 gene expression in neuroendocrine nuclei, their expression was high in thalamic and cortical brain regions suggesting that these may participate in less well-studied actions of P4. The thalamus is known primarily as a sensory relay system but few studies have examined P4 actions within this brain region. However, P4 binding occurs in the lateral septal nucleus of the thalamus (Shughrue et al., 1992) where we found high levels of mRNA encoding Paqr8. This is important because present and previous studies have found that the Pgr is absent in this brain region (Parsons et al., 1982). P4 binding within the lateral septum decreases anxiety and depression-like behaviors (Pesold and Treit, 1992, Estrada-Camarena et al., 2002), and these effects have been presumed to be mediated by P4 metabolites acting on GABAA receptors (Majewska et al., 1986). Similarly, GABAA receptors are implicated in the neuroprotective effects of P4 in the cortex, a region in which we observed high levels of Paqr7, Paqr8 and Pgr mRNAs. In light of the present findings, it is also possible that Paqr8 mediates P4 actions in the lateral septum and cortex. This idea is supported by findings that in the cortex, maximal neuroprotective effects of P4 require activation of MAPK and phosphoinositide 3-kinase pathways (Kaur et al., 2007), suggesting a role for membrane-initiated P4 signaling, possibly involving Paqr7 and Paqr8.
In summary, we found that genes encoding Pgrmc1, Serbp1, Pgrmc2 and Pgr, but not Paqr7 and Paqr8, were highly expressed in many preoptic and hypothalamic nuclei and their projection sites. In contrast, Paqr7 and Paqr8 mRNA levels were most robust in thalamic nuclei and cortex. In view of these neuroanatomical findings, it is tempting to speculate that Pgrmc1 and Pgrmc2 control neuroendocrine functions, while Paqr7 and Paqr8 regulate affect and cognition. Additionally, the extensive overlap of Pgrmc1, Pgrmc2 and the classical Pgr mRNAs raise the intriguing possibility that these molecules interact within the same cells to regulate rapid and delayed P4 effects in the brain. However, there were some regions in which Pgrmc1 and Pgrmc2 may exert effects independently of the Pgr. Together, these data provide anatomical information that will be important for determining the functional roles of these novel P4 signaling molecules.
Acknowledgements
The authors thank Sarah Fadden for technical assistance with tissue preparation and in situ hybridizations. Funding for this work was provided by National Institutes of Health grants ES013885 and HD027305 to SLP and T32 MH020051 fellowship support to KAI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akesson TR, Simerly RB, Micevych PE. Estrogen-concentrating hypothalamic and limbic neurons project to the medial preoptic nucleus. Brain Res. 1988;451:381–385. doi: 10.1016/0006-8993(88)90789-5. [DOI] [PubMed] [Google Scholar]
- Ashley RL, Arreguin-Arevalo JA, Nett TM. Binding characteristics of the ovine membrane progesterone receptor alpha and expression of the receptor during the estrous cycle. Reprod Biol Endocrinol. 2009;7:42. doi: 10.1186/1477-7827-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JA, Freeman ME. The temporal requirement of progesterone on proestrus for extinction of the estrogen-induced daily signal controlling luteinizing hormone release in the rat. Endocrinology. 1978;102:426–432. doi: 10.1210/endo-102-2-426. [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–8841. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer C, Gonzalez-Mariscal G, Eguibar JR, Gomora P. Lordosis facilitation in estrogen primed rats by intrabrain injection of pregnanes. Pharmacol Biochem Behav. 1988;31:919–926. doi: 10.1016/0091-3057(88)90405-4. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146:5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Levine JE. Stimulation of gonadotropin-releasing hormone surges by estrogen. I. Role of hypothalamic progesterone receptors. Endocrinology. 2000;141:1477–1485. doi: 10.1210/endo.141.4.7428. [DOI] [PubMed] [Google Scholar]
- Curran-Rauhut MA, Petersen SL. The distribution of progestin receptor mRNA in rat brainstem. Brain Res Gene Expr Patterns. 2002;1:151–157. doi: 10.1016/s1567-133x(02)00011-x. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport. 1995;6:1715–1722. doi: 10.1097/00001756-199509000-00004. [DOI] [PubMed] [Google Scholar]
- DePaolo LV, Barraclough CA. Dose dependent effects of progesterone on the facilitation and inhibition of spontaneous gonadotropin surges in estrogen treated ovariectomized rats. Biol Reprod. 1979;21:1015–1023. doi: 10.1095/biolreprod21.4.1015. [DOI] [PubMed] [Google Scholar]
- Estrada-Camarena E, Contreras CM, Saavedra M, Luna-Baltazar I, Lopez-Rubalcava C. Participation of the lateral septal nuclei (LSN) in the antidepressant-like actions of progesterone in the forced swimming test (FST) Behav Brain Res. 2002;134:175–183. doi: 10.1016/s0166-4328(02)00023-2. [DOI] [PubMed] [Google Scholar]
- Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology. 1999;140:5999–6002. doi: 10.1210/endo.140.12.7304. [DOI] [PubMed] [Google Scholar]
- Fernandes MS, Brosens JJ, Gellersen B. Honey, we need to talk about the membrane progestin receptors. Steroids. 2008;73:942–952. doi: 10.1016/j.steroids.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Engaging in paced mating, but neither exploratory, anti-anxiety, nor social behavior, increases 5alpha-reduced progestin concentrations in midbrain, hippocampus, striatum, and cortex. Reproduction. 2007;133:663–674. doi: 10.1530/rep.1.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Sumida K, Lydon JP, O'Malley BW, Pfaff DW. Mid-aged and aged wild-type and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3alpha,5alpha-THP-facilitated lordosis. Psychopharmacology (Berl) 2006;185:423–432. doi: 10.1007/s00213-005-0300-4. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Progesterone has rapid and membrane effects in the facilitation of female mouse sexual behavior. Brain Res. 1999;815:259–269. doi: 10.1016/s0006-8993(98)01132-9. [DOI] [PubMed] [Google Scholar]
- Giachino C, Galbiati M, Fasolo A, Peretto P, Melcangi R. Neurogenesis in the subependymal layer of the adult rat: a role for neuroactive derivatives of progesterone. Ann N Y Acad Sci. 2003;1007:335–339. doi: 10.1196/annals.1286.032. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Bittman EL, Foster DL, Karsch FJ. The endocrine basis of the synergistic suppression of luteinizing hormone by estradiol and progesterone. Endocrinology. 1981;109:1414–1417. doi: 10.1210/endo-109-5-1414. [DOI] [PubMed] [Google Scholar]
- Gorski RA. The neuroendocrine regulation of sexual behavior. Adv Psychobiol. 1974;2:1–58. [PubMed] [Google Scholar]
- Hand RA, Craven RJ. Hpr6.6 protein mediates cell death from oxidative damage in MCF-7 human breast cancer cells. J Cell Biochem. 2003;90:534–547. doi: 10.1002/jcb.10648. [DOI] [PubMed] [Google Scholar]
- Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- Hoshina Y, Takeo T, Nakano K, Sato T, Sakuma Y. Axon-sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behav Brain Res. 1994;61:197–204. doi: 10.1016/0166-4328(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Hwang JY, Duncan RS, Madry C, Singh M, Koulen P. Progesterone potentiates calcium release through IP3 receptors by an Akt-mediated mechanism in hippocampal neurons. Cell Calcium. 2009;45:233–242. doi: 10.1016/j.ceca.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase-and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci U S A. 2000;97:12816–12821. doi: 10.1073/pnas.97.23.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey LC, Tyrey L, Everett JW. The estrogen-induced advance in the cyclic LH surge in the rat: dependency on ovarian progesterone secretion. Endocrinology. 1973;93:385–390. doi: 10.1210/endo-93-2-385. [DOI] [PubMed] [Google Scholar]
- Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology. 2009;150(7):3186–3196. doi: 10.1210/en.2008-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird SM, Vinson GP, Whitehouse BJ. Monoclonal antibodies against rat adrenocortical cell antigens. Acta Endocrinol (Copenh) 1988;119:420–426. doi: 10.1530/acta.0.1190420. [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD. In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology. 1980;107:1782–1790. doi: 10.1210/endo-107-6-1782. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, Allen JM, Law SW, O'Malley BW, Clark JH. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- Meffre D, Delespierre B, Gouezou M, Leclerc P, Vinson GP, Schumacher M, Stein DG, Guennoun R. The membrane-associated progesterone-binding protein 25-Dx is expressed in brain regions involved in water homeostasis and is up-regulated after traumatic brain injury. J Neurochem. 2005;93:1314–1326. doi: 10.1111/j.1471-4159.2005.03127.x. [DOI] [PubMed] [Google Scholar]
- Meyerson B. Latency between intravenous injection of progestins and the appearance of estrous behavior in estrogen-treated ovariectomized rats. Horm Behav. 1972;3:1–9. doi: 10.1016/0018-506x(72)90001-3. [DOI] [PubMed] [Google Scholar]
- Mifsud W, Bateman A. Membrane-bound progesterone receptors contain a cytochrome b5-like ligand-binding domain. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-research0068. RESEARCH0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Takemori H, Nonaka Y, Katoh Y, Doi J, Horike N, Osamu H, Raza FS, Vinson GP, Okamoto M. Characterization of the adrenal-specific antigen IZA (inner zone antigen) and its role in the steroidogenesis. Mol Cell Endocrinol. 2004;215:143–148. doi: 10.1016/j.mce.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Olazabal UE, Parhar IS, Pfaff DW. Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity in female rat. J Neurosci. 1994;14:1766–1774. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons B, MacLusky NJ, Krey L, Pfaff DW, McEwen BS. The temporal relationship between estrogen-inducible progestin receptors in the female rat brain and the time course of estrogen activation of mating behavior. Endocrinology. 1980;107:774–779. doi: 10.1210/endo-107-3-774. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: 1982. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab. 2009;94:2644–2649. doi: 10.1210/jc.2009-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso JJ, Liu X, Romak J. Progesterone maintains basal intracellular adenosine triphosphate levels and viability of spontaneously immortalized granulosa cells by promoting an interaction between 14-3-3sigma and ATP synthase beta/precursor through a protein kinase G-dependent mechanism. Endocrinology. 2007;148:2037–2044. doi: 10.1210/en.2006-1603. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Fernandez G, Wu CA. Involvement of an unnamed protein, RDA288, in the mechanism through which progesterone mediates its antiapoptotic action in spontaneously immortalized granulosa cells. Endocrinology. 2004;145:3014–3022. doi: 10.1210/en.2004-0067. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M. Expression and function of PAIRBP1 within gonadotropin-primed immature rat ovaries: PAIRBP1 regulation of granulosa and luteal cell viability. Biol Reprod. 2005;73:261–270. doi: 10.1095/biolreprod.105.041061. [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action. Endocrinology. 2006;147:3133–3140. doi: 10.1210/en.2006-0114. [DOI] [PubMed] [Google Scholar]
- Pesold C, Treit D. Excitotoxic lesions of the septum produce anxiolytic effects in the elevated plus-maze and the shock-probe burying tests. Physiol Behav. 1992;52:37–47. doi: 10.1016/0031-9384(92)90431-z. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Cheuk C, Hartman RD, Barraclough CA. Medial preoptic microimplants of the antiestrogen, keoxifene, affect luteinizing hormone-releasing hormone mRNA levels, median eminence luteinizing hormone-releasing hormone concentrations and luteinizing hormone release in ovariectomized, estrogen-treated rats. J Neuroendocrinol. 1989;1:279–283. doi: 10.1111/j.1365-2826.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Petersen SL, LaFlamme KD. Progesterone increases levels of mu-opioid receptor mRNA in the preoptic area and arcuate nucleus of ovariectomized, estradiol-treated female rats. Brain Res Mol Brain Res. 1997;52:32–37. doi: 10.1016/s0169-328x(97)00194-0. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neil E, editors. The physiology of reproduction. 1994. pp. 107–220. [Google Scholar]
- Pollio G, Xue P, Zanisi M, Nicolin A, Maggi A. Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Brain Res Mol Brain Res. 1993;19:135–139. doi: 10.1016/0169-328x(93)90158-l. [DOI] [PubMed] [Google Scholar]
- Richter TA, Robinson JE, Lozano JM, Evans NP. Progesterone can block the preovulatory gonadotropin-releasing hormone/luteinising hormone surge in the ewe by a direct inhibitory action on oestradiol-responsive cells within the hypothalamus. J Neuroendocrinol. 2005;17:161–169. doi: 10.1111/j.1365-2826.2005.01287.x. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogen-primed rats. Endocrinology. 1983;113:797–804. doi: 10.1210/endo-113-2-797. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Ukena K, Takemori H, Okamoto M, Kawata M, Tsutsui K. Expression and localization of 25-Dx, a membrane-associated putative progesterone-binding protein, in the developing Purkinje cell. Neuroscience. 2004;126:325–334. doi: 10.1016/j.neuroscience.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J Comp Neurol. 1976;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131:381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat Methods. 2005;2:261–267. doi: 10.1038/nmeth752. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain: a laboratory guide with printed and electronic templates for data, models, and schematics. Second edition Elsevier; 1998. [Google Scholar]
- Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kanda Y, Roberts DJ, Ecker JL, Losel R, Wehling M, Peluso JJ, Pru JK. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol Cell Endocrinol. 2008;287:81–89. doi: 10.1016/j.mce.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003a;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003b;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]