Abstract
Plasma factor XIII (FXIII) is responsible for stabilization of fibrin clot at the final stage of blood coagulation. Because FXIII has also been shown to modulate inflammation and endothelial permeability, we hypothesized that FXIII diminishes multiple organ dysfunction caused by gut I/R injury. A model of superior mesenteric artery occlusion (SMAO) was used to induce gut I/R injury. Rats were subjected to 45-min SMAO or sham SMAO and treated with recombinant human FXIII A2 subunit (rFXIII) or placebo at the beginning of the reperfusion period. Lung permeability, lung and gut myeloperoxidase activity, gut histology, neutrophil respiratory burst, and microvascular blood flow in the liver and muscles were measured after a 3-h reperfusion period. The effect of activated rFXIII on transendothelial resistance of human umbilical vein endothelial cells was tested in vitro. Superior mesenteric artery occlusion–induced lung permeability as well as lung and gut myeloperoxidase activity was significantly lower in rFXIII-treated versus untreated animals. Similarly, rFXIII-treated rats had lower neutrophil respiratory burst activity and ileal mucosal injury. Rats treated with rFXIII also had higher liver microvascular blood flow compared with the placebo group. Superior mesenteric artery occlusion did not cause FXIII consumption during the study period. In vitro, activated rFXIII caused a dose-dependent increase in human umbilical vein endothelial cell monolayer resistance to thrombin-induced injury. Thus, administration of rFXIII diminishes SMAO-induced multiple organ dysfunction in rats, presumably by preservation of endothelial barrier function and the limitation of polymorphonuclear leukocyte activation.
Keywords: Fibrin-stabilizing factor, superior mesenteric artery occlusion, oxidative stress, lung injury, microcirculatory disorders
INTRODUCTION
Gut ischemia and intestinal permeability disorders caused by acute pathological conditions, such as trauma-hemorrhagic shock, burn, and sepsis, play an important role in the development of multiple organ dysfunction (MODS) (1–3). I/R injury of the gut can be a dangerous complication of other pathological states or interventions, for example, intestinal transplantation, abdominal aortic aneurysm surgery, and cardiopulmonary bypass (4). Gut-derived factors contained in intestinal lymph coming from the ischemic gut have been shown to be key factors in the pathogenesis of acute lung injury, bone marrow dysfunction, red blood cell alterations, endothelial cell injury, upregulation of endothelial cell adhesion molecule expression, and neutrophil activation (5–9). In addition, in severe inflammatory states, various blood and tissue cells, including polymorphonuclear granulocytes, release lysosomal proteinases that, together with other oxidizing agents produced during phagocytosis, enhance the inflammatory response by degrading connective tissue structures, membranes, and soluble proteins. Thus, agents that could reduce the severity of gut damage and inflammatory response after gut I/R injury are useful for the prevention and treatment of subsequent MODS.
Factor XIII (FXIII) or fibrin-stabilizing factor is a trans-glutaminase involved in the final stage of blood coagulation. It consists of two catalytic A subunits and two noncatalytic B subunits. Factor XIII circulates in the plasma as an inactive precursor and is activated by thrombin. Activated FXIII stabilizes fibrin clots by cross-linking fibrin monomers with covalent bonds, which increase the mechanical strength of the clot, retard fibrinolysis, and enhance platelet adhesion to the injured tissue (10).
The role of FXIII is not limited to the area of hemostasis. Activated FXIII has been shown to stabilize endothelial barrier function by reducing endothelial permeability (11, 12). In vitro studies revealed that FXIII promotes intestinal healing (13). In vivo animal experiments confirmed that FXIII is effective in the treatment of trinitrobenzenesulfonic acid-induced colitis (14). In addition, limited clinical experience with FXIII has suggested its efficacy in the treatment of ulcerative colitis and chronic inflammatory bowel diseases (15, 16). Finally, there is evidence that FXIII modulates the inflammatory response by retardation of macrophage migration (17).
Because FXIII has been suggested to improve endothelial function and modulate the inflammatory response, we hypothesized that treatment with FXIII could protect from the development of MODS after gut I/R.
MATERIALS AND METHODS
Study design
Male Sprague-Dawley rats weighing between 250 and 300 g received standard rat chow and water ad libitum and were allowed an acclimatization period of at least 1 week before the experiment. Animals were subjected to a cycle of 12-h light/12-h dark, controlled humidity, and room temperature between 18°C and 22°C. Animal study protocols were approved by Novo Nordisk Ethical Review Committee and the University of Medicine and Dentistry–New Jersey Medical School Animal Care and Use Committee. Experiments were performed in adherence to the guidelines of the Danish Animal Experiments Council, Danish Ministry of Justice, and in concordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals.
Rats subjected to superior mesenteric artery occlusion (SMAO) or sham SMAO were treated in blinded fashion with placebo or recombinant human FXIII A2 subunit (rFXIII; Novo Nordisk A/S, Maaloev, Denmark). Animals were randomly divided into four groups (eight animals each): group 1: SMAO + plus vehicle treatment; group 2: SMAO + rFXIII treatment; group 3: sham SMAO + vehicle treatment, and group 4: sham SMAO + rFXIII treatment. The vehicle represented a buffer consisting of 40 mM histidine, 8.5% sucrose, and 0.02% Tween 20 at pH 8.0. Lyophilized rFXIII was resuspended in the same buffer to achieve a final concentration of 1 mg/mL. The vehicle (1.0 mL/kg) or rFXIII (1.0 mg/kg) was given intravenously after 45 min of ischemia (in SMAO groups) immediately after mesenteric blood circulation was restored or after 45 min of sham SMAO (in sham groups). The chosen dose of rFXIII was in alignment with available literature data (14).
The majority of the end-point parameters (lung permeability, lung and gut myeloperoxidase [MPO] activity, neutrophil respiratory burst, gut histology, and microvascular blood flow in the muscle and liver) were assessed after 3 h of reperfusion. Factor XIII activity in rat plasma was measured before SMAO/sham SMAO and at the end of reperfusion period.
SMAO protocol
Rats were anesthetized with pentobarbital (50 mg/kg, i.p.). Using aseptic technique, the femoral artery and internal jugular vein were dissected out and cannulated with PE-50 tubing containing trisodium citrate (0.13 M). The jugular vein and femoral artery lines were used for drug administration and blood withdrawal.
Through a 5-cm midline laparotomy, the superior mesenteric artery was isolated and temporarily occluded by placing a 2-0 suture around the artery at its origin from the aorta. The abdomen was then covered with a sterile moist gauze pad. After 45 min of intestinal ischemia, the ligature was removed from around the artery, and after return of the blood supply to the gut was verified, the laparotomy incision was closed.
Rats subjected to sham SMAO were anesthetized, had a laparotomy, and had their superior mesenteric artery looped with 2-0 suture, but the vessel was not occluded. After 45 min of sham SMAO, the suture was removed, and the laparotomy incision was closed.
Rats were killed 3 h after SMAO or sham SMAO using i.v. pentobarbital injection.
Lung permeability assay
Lung permeability was measured using the Evans blue dye (EBD) technique. Rats were injected with 10 mg of EBD through the internal jugular catheter at 2 h 40 min after SMAO/sham SMAO. After 5 min, to allow for complete circulation of the dye, a blood sample (1.0 mL) was withdrawn from the femoral artery catheter and centrifuged at 1,500 revolutions per minute (rpm) at 4°C for 20 min. The plasma was used to determine the plasma EBD concentration. Twenty minutes after injection of the dye, the rats were killed, and the tracheobronchial tree and lungs were harvested as a unit. Bronchoalveolar lavage was performed by lavaging the lungs three times with 5-mL aliquots of normal saline. The recovered bronchoalveolar lavage fluid (BALF) was then centrifuged at 1,500 rpm at 4°C for 20 min to remove any cells. The supernatant fluid was then assayed spectrophotometrically at 620 nm to measure the concentration of the EBD in the BALF. The concentration of EBD in the BALF was then expressed as the percentage of that present in the plasma.
Tissue perfusion and oxygenation measurements
Tissue perfusion and oxygenation were investigated using combination of OxyFlo and OxyLite monitors (Oxford Optotronix Ltd, Oxford, UK). OxyFlo is a multichannel system for measuring tissue blood flow (perfusion), which combines laser Doppler technology and digital signal processing. OxyLite operates according to the principle of oxygen quenching of fluorescence and uses a small optical sensor featuring zero oxygen consumption for monitoring rapid temporal oxygen changes in a given tissue microregion. Precalibrated probes (sensor) are able to provide both spatial and continuous real-time measurement of dissolved oxygen (tissue Po2) and temperature.
Blood flow and Po2 in muscles of the medial surface of the thigh were measured using NP/O/E/4 needle-encased sensor inserted percutaneously via an 18-gauge guiding cannula. Liver blood flow was measured by reusable MSP300XP (Oxford Optotronix Ltd., Oxford, UK) miniature surface sensor. Blood flows are expressed in conditional units. The Win Daq software package (Dataq Instruments Inc, Akron, Ohio) was used to record and display tissue blood flow, Po2, and temperature in real time on a computer screen as well to save these data for further analysis.
MPO assay
Myeloperoxidase activity was measured in the ileum and lung. Harvested tissue samples were homogenized for 30 s in 4 mL of 20 mmol/L potassium phosphate buffer (pH 7.4) and centrifuged (40,000g) at 4°C. The pellet was resuspended in 4 mL of 50 mmol/L potassium phosphate buffer (pH 6) containing 0.5 g/dL hexadecyltrimethylammonium bromide. Samples were sonicated for 90 s, incubated in a 60°C water bath for 2 h, and centrifuged. The supernatant in the amount of 0.1 mL was added to 2.9 mL of 50 mmol/mL potassium phosphate buffer (pH 6) containing 0.167 mg/mL o-dianisidine and 0.0005% hydrogen peroxide. Absorbance at 460 nm of visible light (A460) was measured for 3 min. Myeloperoxidase activity was calculated using the following formula: MPO activity [in units per gram of tissue] = (δ A460 × 13.5)/weight [in grams], where δ A460 equals the rate of change in absorbance at 460 nm between 1 and 3 min. The coefficient 13.5 was empirically determined such that one-unit MPO activity is the amount of enzyme that will reduce 1 μmol peroxide per minute (18).
Neutrophil respiratory burst assay
Flow cytometry was used to assess neutrophil respiratory burst. Heparinized whole-blood samples (100 μL) were placed into 5-mL polystyrene round-bottom tubes containing an equal volume of Dulbecco modified Eagle medium, and the red blood cells were lysed by means of 1% Pharm Lyse solution (BD Biosciences, Franklin Lakes, NJ). The tubes were spun at 1,135 rpm for 5 min at 25°C. The supernatants were discarded, and the cells were washed twice with Hanks balanced salt solution. After the white blood cell pellets were resuspended in 400 μL of Hanks balanced salt solution, 15 ng/mL of dihydrorhodamine was added to the tubes. Five minutes after dihydrorhodamine was added, polymorphonuclear neutrophils were stimulated with phorbol myristyl acetate. After 15-min incubation at 37°C, the polymorphonuclear neutrophil respiratory burst was measured by flow cytometry.
Histological examination
After the rats were killed, a segment of the terminal ileum was excised and fixed in 10% buffered formalin. After processing, semithin (2–4 μm) sections were cut and stained with 1% hematoxylineosin. Five random fields with 100 to 250 villi were analyzed in a blinded fashion by means of light microscopy at 100× magnification. The overall percentage of ileal villous damage was determined by dividing the number of injured villi by the total number of villi examined. Ileal mucosal damage score was calculated as described by Chiu et al. (19).
FXIII activity assay
The photometric FXIII activity assay (Berichrom FXIII; Dade Behring, Newark, Del) was performed on rat plasma samples according to the manufacturer’s instructions but adapted to a microtiter plate format read on a SpectraMax (Molecular Devices, Sunnyvale, Calif) at 340 nm in the kinetic mode. Plasma samples were diluted 1:3 in 20 mM HEPES and 150 mM NaCl, pH 7.4, and rFXIII standards were diluted in FXIII-deficient plasma (George King Biomedical, Overland Park, Kans).
Measurement of endothelial cell electrical resistance
Endothelial cell electrical resistance was measured on human umbilical vein endothelial cells (HUVECs). They were grown in endothelial growth medium (EGM-2) (Lonza Inc, Allendale, NJ) containing epidermal growth factor (EGF), hydrocortisone, GA-1000, 2% fetal bovine serum (FBS), vascular endothelial growth factor (VEGF), human fibroblast growth factor, recombinant insulin-like growth factor (R-IGF-1), heparin, and ascorbic acid.
The barrier function of confluent endothelial cell layers was measured using the Electrical Cell-substrate Impedance Sensing (ECIS) system, 1600R (Applied Biophysics, Troy, NY). The ECIS arrays consisted of eight-well chamber slides containing gold film surface microelectrodes through which current was flowing using culture medium as the electrolyte. As it has been shown by Keese et al. (20), the attachment of cells to the electrodes causes changes in resistance that are measured by the ECIS system. Human umbilical vein endothelial cells were grown to confluent monolayers on gelatin-coated ECIS chamber slides, as confirmed by morphology and impedance measurement, and then treated with various combinations of thrombin (0.05–0.2 nM), activated rFXIII (rFXIIIa) (0.5–100 nM), or albumin, which served as a control. The response to the treatment was measured for 20 h. Human α-thrombin was obtained from Haematological Technologies Inc (Essex Junction, Vt). Recombinant human FXIII A2 subunit was activated by incubating with thrombin-agarose beads (Sigma, St. Louis, Mo) for 4 h at room temperature with shaking, which provided more than 80% activation of the purified FXIII as determined by a fluorometric FXIIIa assay (N-zyme Biotech GmbH, Darmstadt, Germany), as described by Oertel et al. (21) Human albumin (Sigma) received similar thrombin-agarose treatment before adding to control wells.
Resistance values for each microelectrode were normalized as the ratio of measured resistance to baseline resistance and plotted as a function of time. Concentration-response relationships were established from at least three experiments/concentration of FXIIIa and thrombin.
Statistics
Data were analyzed using SPSS 9.0 for Windows (SPSS, Chicago, Ill) and presented as the mean ± SD. Mean values were compared using one-way ANOVA followed by Tukey test. The level of statistical significance was set at P < 0.05.
RESULTS
In vivo experiments
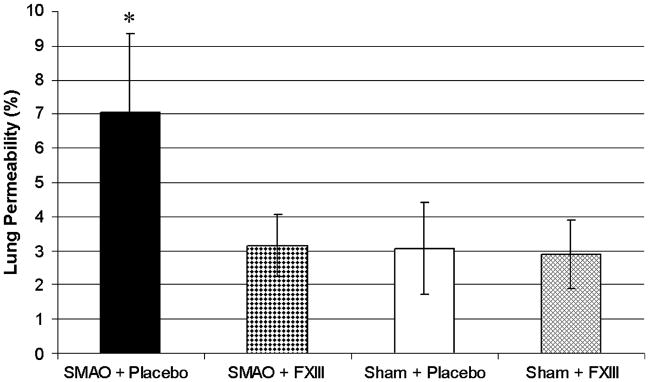
Lung permeability in rats subjected to SMAO and treated with rFXIII was significantly lower than in placebo-treated animals and did not differ from sham values (Fig. 1).
Fig. 1. rFXIII diminishes lung permeability alterations after gut I/R injury.
Lung permeability is evaluated by measuring concentration of EBD in BALF and expressed as the percentage of that present in the plasma. Data are expressed as means ± SD (n = 8 in each group). *P < 0.05 vs. all other groups.
Treatment with rFXIII improved regional microvascular blood flow and oxygenation after SMAO. Muscle Po2 as well as muscle and liver blood flows after SMAO were significantly higher in rFXIII-treated rats; however, these values were lower than in sham animals (Table 1).
Table 1.
rFXIII improves tissue oxygenation and perfusion after gut I/R injury
| Groups of animals | Muscle Po2 (mmHg) | Muscle blood flow (conditional units) | Liver blood flow (conditional units) |
|---|---|---|---|
| SMAO + placebo | 32.0 ± 7.2* | 163.5 ± 82.1* | 509.6 ± 138.8* |
| SMAO + FXIII | 56.2 ± 4.1† | 265.9 ± 54.2† | 841.3 ± 137.4† |
| Sham + placebo | 66.2 ± 7.3 | 483.9 ± 238.7 | 1,211.7 ± 272.4 |
| Sham + FXIII | 68.2 ± 13.2 | 464.8 ± 385.3 | 1,159.3 ± 250.7 |
P < 0.05 vs. all other groups.
P < 0.05 vs. shams.
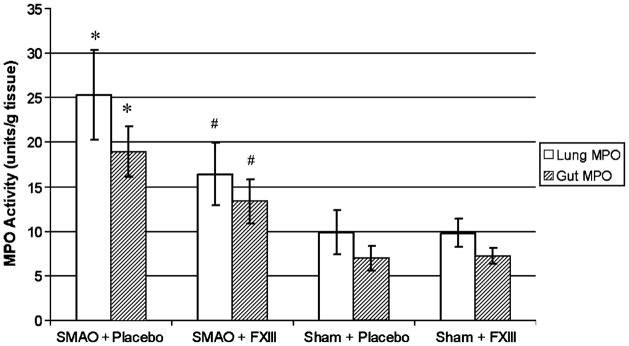
Lung and gut MPO activity after SMAO was also significantly lower in rFXIII-treated rats; however, these values were higher than in sham animals (Fig. 2).
Fig. 2. rFXIII reduces neutrophil sequestration in lung and gut after gut I/R injury.
The level of neutrophil sequestration is evaluated by measuring MPO activity. Data are expressed as means ± SD (n = 8 in each group). *P < 0.05 vs. all other groups; #P < 0.05 vs. shams.
Neutrophil respiratory burst in rats subjected to SMAO and treated with rFXIII was significantly lower than in placebo-treated animals and did not differ from sham values (Fig. 3).
Fig. 3. rFXIII decreases the level of oxidative stress after gut I/R injury.
The level of oxidative stress is assessed by neutrophil respiratory burst activity. Data are expressed as means ± SD (n = 8 in each group). *P < 0.05 vs. all other groups.
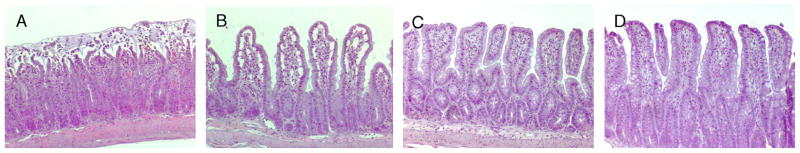
The number of injured ileal villi after SMAO did not differ in placebo- or rFXIII-treated rats and was significantly higher than in sham animals (Table 2). However, ileal mucosal injury score after SMAO was significantly less with rFXIII treatment. Rats subjected to SMAO and placebo treatment demonstrated severe disruption of villi tips (Fig. 4A). Ileal mucosal injury was less profound after SMAO and rFXIII treatment. However, an extension of the subepithelial space as well as villi edema was recorded (Fig. 4B). Ileal damage was minimal in both sham groups (Fig. 4, C and D).
Table 2.
rFXIII reduces the severity of ileal mucosal damage after gut I/R injury
| Groups of animals | Villi examined | Injured villi (%) | Injury score |
|---|---|---|---|
| SMAO + placebo | 240 ± 13 | 56.7 ± 14.0* | 2.88 ± 0.35† |
| SMAO + FXIII treatment | 256 ± 19 | 58.3 ± 14.5* | 2.25 ± 0.46† |
| Sham + placebo | 257 ± 23 | 11.5 ± 4.7 | 1.0 ± 0 |
| Sham + FXIII treatment | 247 ± 18 | 9.8 ± 3.6 | 1.12 ± 0.35 |
P < 0.05 vs. shams.
P < 0.05 vs. all other groups.
Fig. 4. rFXIII reduces the severity of ileal mucosal damage after gut I/R injury.
Hematoxylineosin staining. Original magnification ×100. A, Rat is subjected to SMAO and placebo treatment. Villi tips are disrupted. Red blood cell congestion is seen. Gut injury level is classified as grade 3 mucosal damage score. B, Rat is subjected to SMAO and rFXIII treatment. Extension of the subepithelial space as well as villi edema is seen. Gut injury level is classified as grade 2 mucosal damage score. C, Rat is subjected to sham SMAO and placebo treatment. Almost normal villi are seen (grade 0–1 mucosal damage score). D, Rat is subjected to sham SMAO and rFXIII treatment. Almost normal villi are seen (grade 0–1 mucosal damage score).
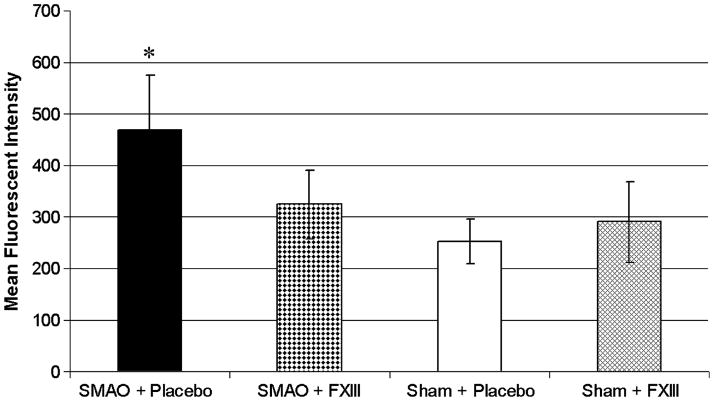
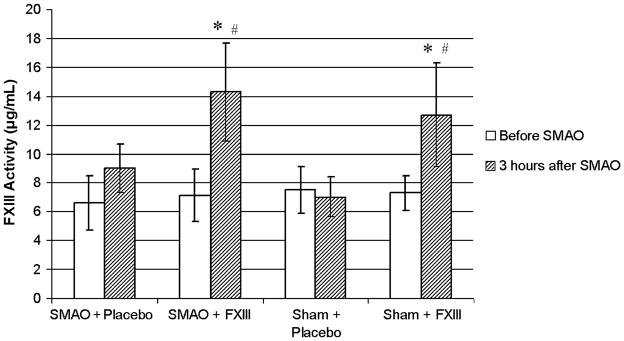
Superior mesenteric artery occlusion did not cause a drop in FXIII activity. Factor XIII activity in rats subjected to SMAO and treated with placebo did not differ from sham placebo-treated animals (Fig. 5). Similarly, FXIII activity increased equally in SMAO and sham animals if they were treated with rFXIII.
Fig. 5. FXIII activity does not change after gut I/R injury.
Factor XIII activity in rat plasma is determined using Berichrom assay before and 3 h after SMAO or sham SMAO. Data are expressed as means ± SD (n = 8 in each group). *P < 0.05 vs. before SMAO; #P < 0.05 vs. sham + placebo and SMAO + placebo.
In vitro experiments
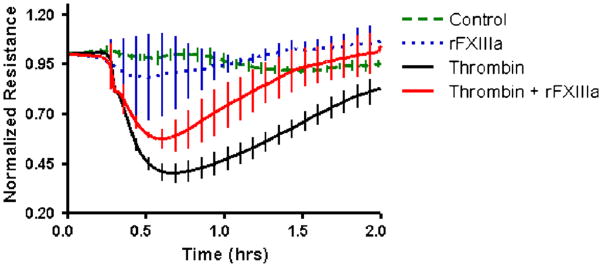
Thrombin-induced barrier disruption of HUVECs was attenuated by rFXIIIa (Fig. 6). Treatment with unactivated rFXIII did not affect endothelial permeability (not shown). In the absence of thrombin, neither rFXIII alone nor albumin caused detectable alterations in endothelial cell electrical resistance.
Fig. 6. rFXIIIa protects from thrombin-induced HUVEC resistance alterations.
Transendothelial electrical resistance of HUVECs after the following types of treatment is shown: 0.1 nM thrombin, 50 nM rFXIIIa, combination of thrombin and rFXIIIa, or albumin (control). Data are expressed as means ± SE (n = 8 in each group). Human umbilical vein endothelial cell resistance after thrombin administration (starting from 0.5 h after treatment) is lower than in all other groups (P < 0.05). Human umbilical vein endothelial cell resistance after thrombin + rFXIIIa administration (starting from 0.5 h and up to 1.2 h after treatment) is higher than after sole thrombin administration alone but lower than after sole rFXIIIa treatment or albumin administration (P < 0.05).
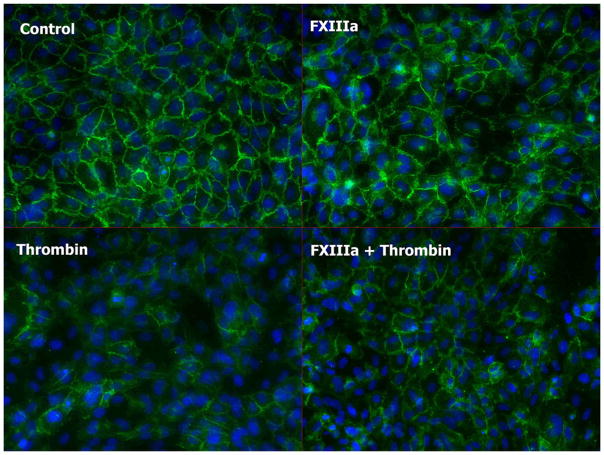
Immunostaining of HUVEC monolayers demonstrated that thrombin disrupted adherens junctions as evidenced by the decreased vascular endothelial (VE)–cadherin signal starting at 5 min after treatment (not shown) and continues to be lower at 60 min (Fig. 7). Addition of rFXIIIa caused less disruption of VE-cadherin along cell-cell contact points than thrombin alone, with almost complete recovery by 60 min.
Fig. 7. Immunofluorescent staining of HUVECs treated with the following agents: thrombin, combination of thrombin and rFXIIIa, rFXIII, or albumin (control).
Cells are fixed in 4% paraformaldehyde and stained with anti–VE-cadherin antibody (Cell Signaling, Beverly, Mass), Alexa-Fluor488–conjugated secondary antibody (Invitrogen, Carlsbad, Calif), and DAPI (blue) at 60 min after treatment. Disruption of adherens junctions by thrombin is seen by the decrease in green signal intensity. Addition of rFXIIIa reduces HUVEC alterations.
DISCUSSION
Intestinal ischemia followed by reperfusion injury causes severe alterations in gut permeability and the loss of its barrier function as well as the initiation of an inflammatory cascade that can lead to MODS. A significant increase in intestinal permeability has been well documented using the experimental model of SMAO (22–25). Gut I/R activates circulating neutrophils, which promote elastase-mediated injury in distant organs, especially in the lungs (26). Similarly, SMAO has been shown to amplify neutrophil accumulation in the lungs and to increase pulmonary permeability (27, 28). Different treatment strategies have been proposed to ameliorate gut I/R-induced lung permeability alterations and to modulate the inflammatory response following this injurious event. These therapies include the use of nutrition agents (29), elastase inhibitors (26), antioxidants (25), complement blockers (27), inhibitors of nuclear factor κB activation and proinflammatory cytokine production (23), and NO synthase inhibitors (28). In our study, we tested the hypothesis that rFXIII would decrease SMAO-induced endothelial permeability alterations in the lung and modulate the inflammatory response. The main rationale for this hypothesis is based on the ability of activated FXIII to stabilize endothelial barrier function by reducing endothelial permeability, as shown in monolayers of cultured porcine aortic endothelial cells (11, 12). In these studies, the flux of albumin across the endothelial monolayer served as a measure of paracellular permeability. Treatment with activated FXIII led to a 30% reduction in permeability. The authors demonstrated that FXIII not only lowered basal permeability of the endothelial monolayer but also prevented the increase in permeability provoked by an inhibition of endothelial energy production. In addition, the promotion of intestinal epithelial wound healing by enhancement of epithelial cell restitution has been achieved by the in vitro use of FXIII (13). The mechanisms by which FXIII might stimulate tissue repair and remodeling include stimulation of endothelial cell proliferation and migration, inhibition of apoptosis (30), and mediation of platelet adhesion to endothelial cells through αvβ3 and glycoprotein IIb/IIIa integrins (31).
Data from our current study indicate that rFXIII partially protects distant organs after gut I/R injury. Specifically, lung permeability at 3 h after SMAO was significantly lower in the rFXIII-treated animals compared with placebo-treated ones. Similarly, rFXIII treatment favorably influenced microvascular blood flow and tissue oxygenation in both liver and muscle tissue after gut I/R injury. Less severe gut and lung injury can be explained by lower levels of inflammatory response and oxidative stress achieved by rFXIII treatment because placebo-treated animals had significantly higher levels of gut and lung MPO activity and neutrophil respiratory burst.
The concentration of FXIII A subunit in human plasma is reported to be 10 to 15 μg/mL (32). Our data show that endogenous levels of FXIII A subunit in rat plasma are slightly lower and range from 5 to 12 μg/mL (mean, 7 μg/mL). In our series of in vivo experiments, we used the dose of recombinant human FXIII A subunit that is equal to 28 μg/mL. It means that supraphysiological levels of FXIII are needed to achieve a protective effect after gut I/R injury. It should be mentioned that the half-life of rFXIII when used in presumably pharmacological doses is 270 to 320 h (33).
We suggest that one of the key elements of rFXIII-induced protection of distant organs after gut I/R injury is a reduction of SMAO-induced intestinal damage. The severity of ileal mucosal injury was lower in rFXIII-treated rats, compared with placebo-treated ones.
In vitro experiments with HUVEC were performed to reveal the direct influence of rFXIIIa on injured endothelium. Thrombin was applied to cause barrier disruption of HUVECs, which was confirmed by the lowering of their electrical resistance. Activated rFXIII attenuated the thrombin-induced endothelial barrier dysfunction. These data are in alignment with findings of Dardik et al. (30, 31), who revealed the effect of rFXIII on endothelial cell proliferation and inhibition of apoptosis. However, the authors used higher concentrations of rFXIII (50–70 μg/mL).
Factor XIII deficiency has been recorded after trauma, burn, and sepsis (34–36). However, we did not reveal any drop in FXIII activity 3 h after gut I/R injury: rats subjected to SMAO or sham SMAO demonstrated an equal increase in FXIII activity if rFXIII was given. The increased FXIII levels were sufficient to show a protective effect in rats that were subjected to SMAO.
We conclude that rFXIII ameliorates MODS caused by gut I/R injury in this rat model. The mechanism of the protective effect of rFXIII might be explained by preservation of the gut endothelial barrier function, amelioration of permeability alterations in distant organs, and by modulation of the inflammatory response. Future studies are necessary to clarify the mechanisms of rFXIII action.
Footnotes
The abstract was presented at the 6th Congress of the International Federation of Shock Societies and the 31st Annual Conference on Shock in Cologne, Germany on June 28 to July 2, 2008.
References
- 1.Deitch EA, Sambol JT. Sepsis and Multiple Organ Dysfunction: A Multidisciplinary Approach. Philadelphia, PA: WB Saunders Co; 2002. The gut origin hypothesis of MODS; pp. 105–116. [Google Scholar]
- 2.De-Souza DA, Greene LJ. Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med. 2005;33(5):1125–1135. doi: 10.1097/01.ccm.0000162680.52397.97. [DOI] [PubMed] [Google Scholar]
- 3.Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. J Burn Care Rehabil. 2005;26(1):85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- 4.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA, Forsythe R, Anjaria D, Livingston DH, Lu Q, Xu DZ, Redl H. The role of lymph factors in lung injury, bone marrow suppression and endothelial cell dysfunction in a primate model of trauma-hemorrhagic shock. Shock. 2004;22(3):221–228. doi: 10.1097/01.shk.0000133592.55400.83. [DOI] [PubMed] [Google Scholar]
- 6.Zallen G, Moore EE, Johnson JL, Tamura DY, Ciesla DJ, Silliman CC. Posthemorrhagic shock mesenteric lymph primes circulating neutrophils and provokes lung injury. J Surg Res. 1999;83(2):83–88. doi: 10.1006/jsre.1999.5569. [DOI] [PubMed] [Google Scholar]
- 7.Zaets SB, Berezina TL, Caruso J, Xu DZ, Deitch EA, Machiedo GW. Mesenteric lymph duct ligation prevents shock-induced red blood cell deformability and shape changes. J Surg Res. 2003;109(1):51–56. doi: 10.1016/s0022-4804(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 8.Deitch EA, Shi HP, Lu Q, Feketova E, Skumick J, Xu DZ. Mesenteric lymph from burned rats induces endothelial cell injury and activates neutrophils. Crit Care Med. 2004;32(2):533–538. doi: 10.1097/01.CCM.0000109773.00644.F4. [DOI] [PubMed] [Google Scholar]
- 9.Xu DZ, Lu Q, Adams CA, Issekutz AC, Deitch EA. Trauma-associated shock-induced upregulation of endothelial cell adhesion molecules is blunted by mesenteric lymph duct ligation. Crit Care Med. 2004;32(3):760–765. doi: 10.1097/01.ccm.0000114815.88622.9d. [DOI] [PubMed] [Google Scholar]
- 10.Muszbek L, Adany R, Mikkola H. Novel aspects of blood coagulation factor XIII. Structure, distribution, activation, and function. Crit Rev Lab Sci. 1996;33(5):357–421. doi: 10.3109/10408369609084691. [DOI] [PubMed] [Google Scholar]
- 11.Noll T, Wozniak G, McCarson K, Hajimohammad A, Metzner HJ, Inserte J, Kummer W, Hehrlein FW, Piper HM. Effect of factor XIII on endothelial barrier function. J Exp Med. 1999;189(9):1373–1382. doi: 10.1084/jem.189.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wozniak G, Noll T, Brunner U, Hehrlein FW. Topical treatment of venous ulcer with fibrin stabilizing factor: experimental investigation of effects on vascular permeability. Vasa. 1999;28(3):160–163. doi: 10.1024/0301-1526.28.3.160. [DOI] [PubMed] [Google Scholar]
- 13.Cario E, Goebell H, Dignass AU. Factor XIII modulates intestinal epithelial wound healing in vitro. Scand J Gastroenterol. 1999;34(5):485–490. doi: 10.1080/003655299750026218. [DOI] [PubMed] [Google Scholar]
- 14.D’Argenio G, Grossman A, Cosenza V, Valle ND, Nazzacca G, Bishop PD. Recombinant factor XIII improves established experimental colitis in rats. Dig Dis Sci. 2000;45(5):987–997. doi: 10.1023/a:1005541512152. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz R, Born P, Classen M. Substitution of factor XIII concentrate in treatment refractory ulcerative colitis. A prospective pilot study. Med Klin. 1994;89(10):534–537. [PubMed] [Google Scholar]
- 16.Lorenz R, Olbert P, Born P. Factor XIII in chronic inflammatory bowel diseases. Semin Thromb Hemost. 1996;22(5):451–455. doi: 10.1055/s-2007-999045. [DOI] [PubMed] [Google Scholar]
- 17.Lanir N, Ciano PS, Van de Water L, McDonagh J, Dvorak AM, Dvorac HF. Macrophage migration in fibrin gel matrices. II. Effects of clotting factor XIII, fibronectin, and glycosaminoglycan content on cell migration. J Immunol. 1988;140(7):2340–2349. [PubMed] [Google Scholar]
- 18.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23(3):179–186. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- 19.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101(4):478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 20.Keese CR, Bhawe K, Wegener J, Giaever I. Real-time impedance assay to follow the invasive activities of metastatic cells in culture. Biotechniques. 2002;33(4):842–850. doi: 10.2144/02334rr01. [DOI] [PubMed] [Google Scholar]
- 21.Oertel K, Hunfeld A, Specker E, Reiff C, Seitz R, Pasternack R, Dodt J. A highly sensitive fluorometric assay for determination of human coagulation factor XIII in plasma. Anal Biochem. 2007;367(2):152–158. doi: 10.1016/j.ab.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Wattanasirichaigoon S, Menconi MJ, Delude RL, Fink MP. Effect of mesenteric ischemia and reperfusion or hemorrhagic shock on intestinal mucosal permeability and ATP content in rats. Shock. 1999;12(2):127–133. doi: 10.1097/00024382-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama T, Delude RL, Fink MP. Dose-dependent effects of ethyl pyruvate in mice subjected to mesenteric ischemia and reperfusion. Intensive Care Med. 2003;29(11):2050–2058. doi: 10.1007/s00134-003-1966-x. [DOI] [PubMed] [Google Scholar]
- 24.Saito T, Unno N, Yamamoto N, Inuzuka K, Sagara D, Suzuki M, Konno H. Intraperitoneal administration of hyperbarically oxygenated perfluorochemical enhances preservation of intestinal mucosa against ischemia/reperfusion injury. Shock. 2006;26(6):620–624. doi: 10.1097/01.shk.0000230297.93762.a8. [DOI] [PubMed] [Google Scholar]
- 25.Nosalova V, Navarova J, Mihalova D, Sotnikova R. Mesenteric ischemia/reperfusion-induced intestinal and vascular damage: effect of stobadine. Methods Find Exp Clin Pharmacol. 2007;29(11):39–45. doi: 10.1358/mf.2007.29.1.1063495. [DOI] [PubMed] [Google Scholar]
- 26.Carden D, Xiao F, Moak C, Willis BH, Robinson-Jackson S, Alexandr S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am J Physiol. 1998;275(2 Pt 2):385–392. doi: 10.1152/ajpheart.1998.275.2.H385. [DOI] [PubMed] [Google Scholar]
- 27.Xiao F, Eppihimer MJ, Willis BH, Carden DL. Complement-mediated lung injury and neutrophil retention after intestinal ischemia-reperfusion. J Appl Physiol. 1997;82(5):1459–1465. doi: 10.1152/jappl.1997.82.5.1459. [DOI] [PubMed] [Google Scholar]
- 28.Uchida K, Mishima S, Ohta S, Yukioka T. Inhibition of inducible nitric oxide synthase ameliorates lung injury in rats after gut ischemia-reperfusion. J Trauma. 2007;63(3):603–607. doi: 10.1097/TA.0b013e3181271b0b. [DOI] [PubMed] [Google Scholar]
- 29.Kozar RA, Schultz SG, Bick RJ, Poindexter BJ, DeSoignie R, Moore FA. Enteral glutamine but not alanine maintains small bowel barrier function after ischemia/reperfusion injury in rats. Shock. 2004;21(5):433–437. doi: 10.1097/00024382-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Dardik R, Solomon A, Loscalzo J, Eskaraev R, Bialik A, Goldberg I, Schiby G, Inbal A. Novel proangiogenic effect of factor XIII associated with suppression of thrombospondin 1 expression. Arterioscler Thromb Vasc Biol. 2003;23(8):1472–1477. doi: 10.1161/01.ATV.0000081636.25235.C6. [DOI] [PubMed] [Google Scholar]
- 31.Dardik R, Shenkman B, Tamarin I, Eskaraev R, Harsfalvi J, Varon D, Inbal A. Factor XIII mediates adhesion of platelets to endothelial cells through αvβ3 and glycoprotein IIb/IIIa integrins. Thromb Res. 2002;105(4):317–323. doi: 10.1016/s0049-3848(02)00014-2. [DOI] [PubMed] [Google Scholar]
- 32.Cheung PP, Kunapuhi SP, Scott CF, Wachfogel YT, Colman RW. Genetic basis of total kininogen deficiency in Williams’ trait. J Biol Chem. 1993;268(31):23361–23365. [PubMed] [Google Scholar]
- 33.Reynolds TC, Butine JE, Visich JE, Gunewardena KA, Machahon M, Pederson S, Bishop PD, Morton KM. Safety, pharmcokinetics, and immunogenicity of single-dose rFXIII administration to healthy volunteers. J Thromb Haemost. 2005;3(5):922–928. doi: 10.1111/j.1538-7836.2005.01224.x. [DOI] [PubMed] [Google Scholar]
- 34.Seekamp A, Barthels M, Sturm JA. Factor XIIIA2/XIIIB2 ratio in severely traumatized patients with soft tissue trauma. Thromb Res. 1992;65(6):809–814. doi: 10.1016/0049-3848(92)90119-u. [DOI] [PubMed] [Google Scholar]
- 35.Burkhardt H, Zellner PR, Moller I. Factor XIII deficiency in burns. Chirurg. 1977;48(8):520–523. [PubMed] [Google Scholar]
- 36.Zeerleder S, Schroeder V, Lammle B, Wuillemin WA, Hack CE, Kohler HP. Factor XIII in severe sepsis and septic shock. Thrombosis Res. 2007;119(3):311–318. doi: 10.1016/j.thromres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Noll T, Wozniak G, McCarson K, Hajimohammad A, Metzner HJ, Inserte J, Kummer W, Hehlein FW, Piper HM. Effect of factor XIII on endothelial barrier function. J Exp Med. 1999;189(9):1373–1382. doi: 10.1084/jem.189.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wozniak G, Noll T, Brunner U, Hehrlein FW. Topical treatment of venous ulcer with fibrin stabilizing factor: experimental investigation of effects on vascular permeability. Vasa. 1999;28(3):160–163. doi: 10.1024/0301-1526.28.3.160. [DOI] [PubMed] [Google Scholar]