Abstract
Chronic increases or decreases in neuronal activity initiate compensatory changes in synaptic strength that emerge slowly over a 12–24 hr period, but the mechanisms underlying this slow homeostatic response remain poorly understood. Here, we show an essential role for the ubiquitin proteasome system (UPS) in slow homeostatic plasticity induced by chronic changes in network activity. In cultured hippocampal neurons, UPS inhibitors drive a slow increase in miniature excitatory postsynaptic current (mEPSC) amplitude and synaptic AMPA receptor subunit GluA1 and GluA2 expression that both mirrors and occludes the changes produced by chronic suppression of network activity with tetrodotoxin (TTX). These non-additive effects were similarly observed under conditions of chronic hyperactivation of network activity with bicuculline – the increase in mEPSC amplitude and GluA1/2 expression with chronic UPS inhibition persists during network hyperactivation, which scales synaptic strength and AMPA receptor expression in the opposite direction when UPS activity is intact. Finally, cell-autonomous UPS inhibition (via expression of the ubiquitin chain elongation mutant, UbK48R) enhances mEPSC amplitude in a manner that mimics and occludes changes in network activity, demonstrating a postsynaptic role for the UPS in slow homeostatic plasticity. Taken together, our results suggest that the UPS acts as an integration point for translating sustained changes in network activity into appropriate incremental compensatory changes at synapses.
Keywords: AMPA receptor, GluA1, GluA2, synaptic scaling, protein degradation, miniature excitatory postsynaptic current (mEPSC)
1. Introduction
Homeostatic forms of synaptic plasticity are thought to buffer otherwise debilitating changes in neural circuit activity through compensatory synaptic adaptations that drive network activity back towards a stable range (Marder and Prinz, 2002; Davis, 2006; Rich and Wenner, 2007; Nelson and Turrigiano, 2008; Rabinowitch and Segev, 2008). At central synapses, this homeostatic control of synapse function has been best characterized through chronic perturbations of activity in cultured neuron networks, where compensatory synaptic modifications emerge in a characteristically slow manner over a period of roughly 24–72 hrs (Rao and Craig, 1997; Turrigiano et al., 1998; O’Brien et al., 1998; Murthy et al., 2001). Recent studies have also demonstrated that more rapid forms of homeostatic plasticity are expressed in the nervous system, where compensatory synaptic modifications are evident in minutes to hours following activity challenges (Sutton et al., 2006; Frank et al., 2006). Although the molecular mechanisms underlying slower forms of homeostatic control are still largely unknown, several findings suggest that these mechanisms may be qualitatively distinct from more rapid homeostatic mechanisms. For example, whereas blocking both action potential (AP)-driven and spontaneous neurotransmission induces synaptic insertion of GluA1 homomeric receptors that requires dendritic protein synthesis (Ju et al., 2004; Thiagarajan et al., 2005 Sutton et al., 2006; Aoto et al., 2008), AP blockade on its own drives a slower form of compensation associated with an increase in AMPAR half-life (O’Brien et al., 1998), enhanced expression of GluA2-containing AMPARs at synapses (Wierenga et al., 2005), as well as accumulation of GFP-tagged GluA2 subunits at synapses (Ibata et al., 2008; Gainey et al., 2009). These findings suggest that de novo synthesis and degradation of existing proteins may differentially contribute to temporally distinct forms of homeostatic plasticity.
Neurons use two major protein degradation pathways – 1) endocytic retrieval and degradation of integral membrane proteins by the lysosome and, 2) regulated degradation of soluble proteins by the ubiquitin proteasome system (UPS; for recent reviews, see Tai and Schuman, 2008; Hegde, 2010). Degradation by the UPS requires covalent attachment of polyubiquitin chains to the target substrate, catalyzed by the sequential action of E1–E3 ubiquitin-conjugating enzymes (Hershko, 2005). Ubiquitin contains 7 lysine residues that can contribute to the polyubiquitination of target substrates, but it is polyubiquitination of a target substrate via lysine 48 (K48) that serves as the cellular marker for targeted protein degradation by the proteasome (Varshavsky, 2005; Schwartz and Ciechanover, 2009). Hence, expression of a mutant ubiquitin, where lysine 48 is mutated to an arginine (K48R), induces premature K48 chain termination and disrupts proteasome-dependent degradation without blocking other ubiquitin-dependent pathways.
Previous studies have shown that proteasome function in neurons is bi-directionally regulated by activity (Djakovic et al., 2009), suggesting that the UPS is poised to play an active role in regulating protein dynamics at the synapse. Indeed, Ehlers (2003) demonstrated that AP blockade and disinhibition of cortical cultures resulted in bi-directional changes in the protein composition of the postsynaptic density (PSD) that emerge slowly (24–48 hrs) and are UPS-dependent. These observations suggest that the UPS may play a critical role in slow homeostatic plasticity, but it is currently unknown whether homeostatic changes in synapse function similarly require the UPS. Moreover, other studies have recently demonstrated that UPS activity is necessary for homeostatic silencing of presynaptic terminals in response to excessive depolarization (Jiang et al., 2010) and may play other homeostatic roles presynaptically (Willeumier et al., 2006; Rinetti and Schweizer, 2010). Furthermore, astrocytes can play an active role in homeostatic plasticity during chronic changes in activity (Stellwagen and Malenka, 2006). These latter results raise questions about whether the changes in PSD composition observed by Ehlers (2003) reflect a requirement for the UPS postsynaptically, or whether UPS inhibition alters PSD composition indirectly via changes in astrocyte-driven signaling or presynaptic function.
Here, we demonstrate that the UPS plays a critical role in the postsynaptic cell in driving slow homeostatic changes in synaptic function. We show that AP blockade and network hyperactivation induce opposite changes in postsynaptic UPS activity, with AP blockade suppressing and hyperactivity enhancing, proteasome function. Similar to AP blockade, proteasome inhibitors induce a slow scaling of mEPSC amplitude and increase surface expression of the AMPAR subunits GluA1 and GluA2 at synapses. Both the enhancement of postsynaptic function and changes in synaptic AMPAR content induced by UPS inhibition occlude further increases with AP blockade, and also persist in the face of chronic network hyperactivation. Targeted UPS inhibition to individual neurons similarly enhances mEPSC amplitude in a manner that is non-additive with either chronic AP suppression or chronic hyperactivation. Together, our data demonstrate a critical role for activity-dependent regulation of proteasome function during the induction of slow homeostatic adaptations at synapses.
2. Experimental Procedures
2.1 Cell Culture
Hippocampi from Sprague-Dawley rat pups (P1–P3) were dissected in cold dissociation media (DM; 82 mM Na2SO4, 30 mM K2SO4, 5.8 mM MgCl2-6H2O, 252 µM CaCl2-2H2O, 1 mM HEPES, 200 mM glucose, 0.001% w/v phenol red), and transferred to a 15 ml conical tube. The DM was gently removed (leaving ~500 µl of DM remaining to keep the tissue covered) and replaced with 5 ml of pre-warmed (37°C) cysteine-activated papain solution (3.2 mg L-cysteine (Sigma-Aldrich; Saint Louis, MO) with 500 µl papain (Sigma-Aldrich; Saint Louis, MO) in 10 ml DM, pH ~ 7.2), and incubated for 15 min at 37°C to allow for tissue digestion; halfway through the incubation, the tube was inverted ~2–3 times. Cells were then washed 2X in ice-cold DM containing 12.5 % v/v fetal bovine serum to inactivate the papain followed by 2 washes in DM alone. The cells were then washed 2X in chilled normal growth medium [NGM; Neurobasal A (Gibco; Grand Island, NY) supplemented with 2% v/v B27 (Invitrogen; Carlsbad, CA) and 1% v/v Glutamax (Invitrogen; Carlsbad, CA)], then titurated ~10–15 times in 5 ml NGM to obtain a single cell suspension and placed on ice for ~3–5 min. 4.5 ml of the cell suspension was removed from the middle of the cell solution to avoid contaminant material and the cells were placed in a new 15 ml tube and centrifuged at 67 × g (0.5 × 1000 rcf) at 4°C. 50–70K cells (in a volume of 150 µl) were plated onto poly-d-lysine-coated glass-bottom petri dishes (Mattek; Ashland, MA) and maintained at 5% CO2/37°C. 4 hrs after plating, 2 ml of NGM-GC (NGM supplemented with 15% v/v glial conditioned media and 10% v/v cortical conditioned media) was added to each dish. Cells were fed 24 hr later by replacing 50% of their media with fresh NGM-GC and every 4 days thereafter by replacing 25% of their media with fresh media. Cells were maintained for 14 days in NGM-GC, then fed every 4 days thereafter with NGM alone. All neurons used for experiments developed for ≥ 21 DIV, a time at which the majority of synaptogenesis is completed and network activity is stable. Experiments were repeated in at least three independent cultures.
2.2 Whole-Cell Patch Clamp Electrophysiology
Pharmacological agents were added to cultured hippocampal neurons (≥21 DIV) in conditioned media at times as indicated for each reagent. Prior to recording, cells were washed 1X and maintained in HEPES-buffered saline (HBS; 119 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 30 mM glucose, 10 mM HEPES; pH 7.4) containing 1µM TTX (Calbiochem; San Diego, CA) and 10 µM bicuculline (Tocris; Ellisville, MO). Whole cell patch-clamp recordings were performed using glass recording pipettes, with resistances of 4–6 MΩ when filled with internal solution (100 mM cesium gluconate, 0.2 mM EGTA, 5 mM MgCl2, 40 mM HEPES, 2 mM Mg-ATP, 0.3 mM Li-GTP, pH 7.2). Pyramidal-like neurons were identified for recording based on cell morphology (the presence of a large apical dendrite and a large pyramidal-like cell body). Membrane potential was clamped at −70 mV and miniature excitatory postsynaptic currents (mEPSCs) were recorded using an Axopatch 200B amplifier and Clampex 8.0 software (Molecular Devices). mEPSCs were analyzed off-line using MiniAnalysis (Synaptosoft).
2.3 Surface GluA1/2 Immunocytochemistry
Following the appropriate treatment, cells were live labeled with primary antibodies against surface epitopes of GluA1 (sGluA1, 1:10, Calbiochem; San Diego, CA) and GluA2 (sGluA2, 1:100, Chemicon; USA) for 15 min at 37°C. Following the 15 min incubation, the cells were washed 3X in phosphate buffered saline with Mg2+ and Ca2+ (PBS-MC; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, 1 mM MgCl2 and 0.1mM CaCl2) and immediately fixed in PBS-MC containing 2% paraformaldehyde and 2% sucrose for 20 min at room temperature (RT). The cells were blocked (2% BSA/PBS-MC for 30 min at RT) and incubated with Alexa555-conjugated secondary antibodies (goat anti-rabbit and goat anti-mouse for GluA1 and GluA2, respectively 1:500; Molecular Probes; Eugene, OR) for 60 min at RT in blocking solution. Cells were then permeabalized with PBS-MC containing 0.1% Triton X-100 for 5 min at RT, and stained for PSD95 as above [a mouse monoclonal anti-PSD95 antibody (1:200; Fisher) for GluA1 co-labeling and a rabbit polyclonal anti-PSD95 antibody for GluA2 co-labeling (1 µg/ml; AbCam; Cambridge, MA)] using Alexa488-conjugated secondary antibodies (goat anti-mouse and goat anti-rabbit for GluA1 and GluA2 co-labeling, respectively, 1:500; Molecular Probes; Eugene, OR).
Neurons stained for sGluA1/PSD95 or sGluA2/PSD95 were imaged on an Olympus FV1000 inverted confocal microscope using a Plan Apochromat 60×/1.4 NA objective and 2X digital zoom. Areas of interest were selected for imaging guided by epifluorescent visualization of the PSD95 channel, to ensure blind sampling of surface GluA1 and GluA2 expression. Acquisition settings were identical for all treatment groups and were determined to ensure: 1) optimization of the dynamic range of signal intensities to limit saturation, 2) the absence of detectable fluorescence in a no GluA1/2 antibody condition included in all experimental runs, and 3) no fluorescence bleed-through between channels. Image analysis was performed with NIH Image J on maximal intensity z-projections, as described (Sutton et al., 2006). Dendrites were linearized using the straighten plugin for Image J, and extracted from the full-frame image. For analysis, a “synaptic” GluA1/2 particle was defined as a particle that occupied greater than 10% of the area defined by a PSD95 particle, and the integrated fluorescence intensity of synaptic GluA1/2 particles was quantified. Integrated fluorescence intensity per particle was determined by multiplying each pixel in a particle by its intensity (1–255) and summing this product across all pixels occupying a given particle. Image analysis was performed using custom written analysis routines for Image J.
2.4 paGFPu Imaging and Data Analysis
GFPu (in pEGFP-C1 plasmid backbone; Clontech; Mountain View, CA), a fusion of the CL1 degron (degradation signal) on the C terminus of GFP, was kindly provided by Dr. Ron Kopito (Stanford University, Palo Alto, CA). GFPu is ubiquitinated and specifically degraded by the UPS (Bence et al., 2001, 2005). The AgeI–BsrGI fragment from photoactivatable (pa) GFP (a kind gift provided by Jennifer Lipponcott-Schwartz, National Institutes of Health, Bethesda, MD) was subcloned into the GFPu plasmid. paGFPu or paGFP was then subcloned into pSinRep5 (Invitrogen; Carlsbad, CA). For production of recombinant Sindbis virions, RNA was transcribed using the SP6 mMessage mMachine Kit (Ambion; Austin, TX) and electroporated into BHK cells using a BTX ECM 600 at 220 V, 129Ω, and 1050 µF. Virion was collected after 24h and stored at −80°C until use.
Neurons were infected for 14–16 hours with Sindbis viral vectors prior to imaging. Pyramidal-like neurons infected with paGFPu or paGFP (identified by co-expression of mCherry) were then photoactivated for 10s with a 100 W Hg2+ lamp and a D405/40× with 440 DCLP dichroic filter set (Chroma). Confocal images were acquired using a Leica DMI6000 inverted microscope outfitted with a Yokogawa spinning disk confocal head, an Orca ER high-resolution black-and-white cooled CCD camera (6.45 µm/pixel at 1×) (Hamamatsu), a Plan Apochromat 63×/1.4 NA objective, and a Melles Griot argon/krypton 100 mW air-cooled laser for 488/568/647 nm excitations. Exposure times were held constant during acquisition of all images for each experiment. For image analysis, maximum intensity z-projections were used. Dendrites from individual neurons were then straightened and total integrated density (normalized to dendritic length) of reporter fluorescence was quantified.
2.5 Data Analysis
Statistical differences between experimental conditions were determined by either unpaired t-tests (2 groups) or by analysis of variance (ANOVA) and post-hoc Fisher’s LSD test (> 2 experimental conditions). Differences were deemed significant if α < 0.05 (two-tailed).
3. Results
3.1 Chronic, but not acute, proteasome inhibition scales excitatory synaptic strength
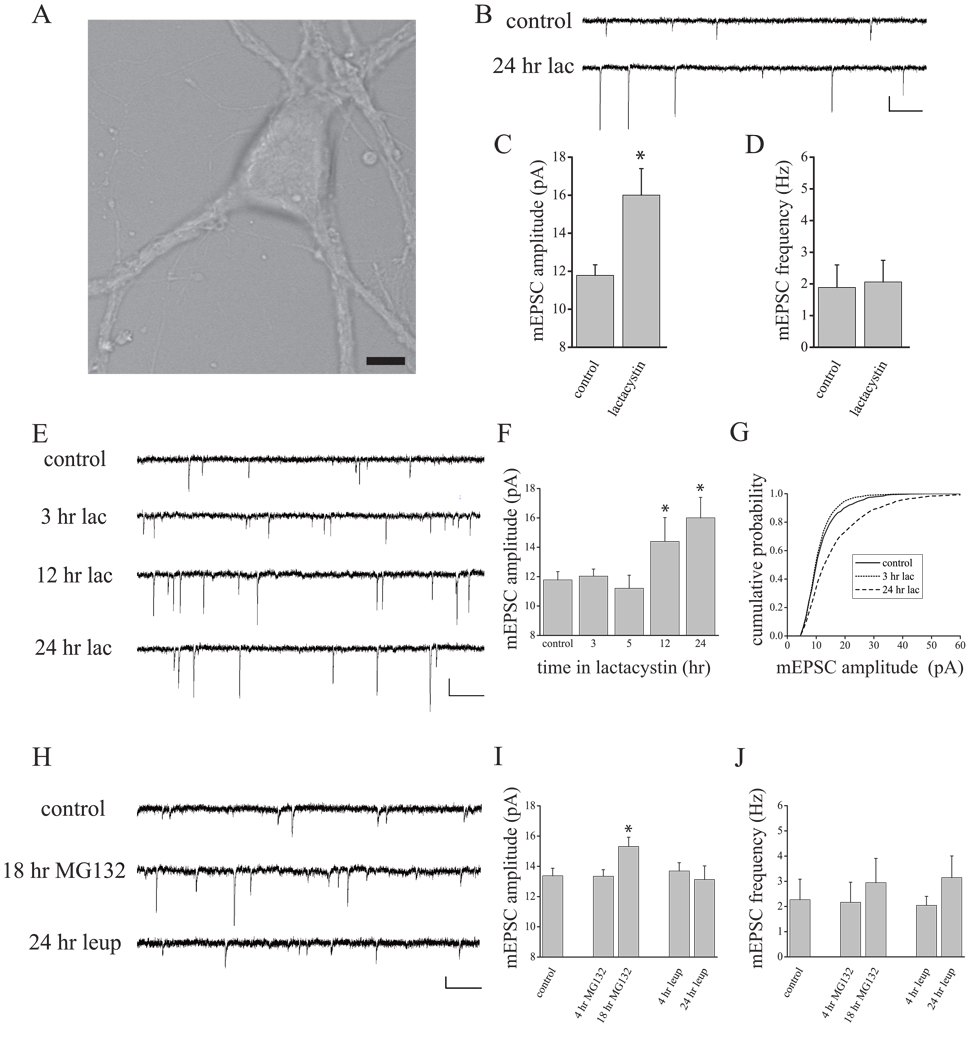
Homeostatic changes in synapse function can occur slowly over a period of 24–72 hrs (Rao and Craig, 1997; Turrigiano et al., 1998, O’Brien et al., 1998; Murthy et al., 2001), or more rapidly within minutes to hours (Sutton et al., 2006; Frank et al., 2006; Aoto et al., 2008). While mechanisms underlying rapid homeostatic changes are partially understood, very little is known regarding how activity drives slower homeostatic adaptations at synapses. Chronic changes in activity (> 24 hr) induce bi-directional changes in synaptic protein composition mediated by the ubiquitin proteasome system (UPS) (Ehlers, 2003), but a role for the UPS in homeostatic changes in synaptic function has not been assessed. To examine if chronic changes in proteasome function mimic aspects of homeostatic plasticity, we treated cultured hippocampal neurons with a selective proteasome inhibitor lactacystin (10µM), and recorded miniature excitatory postsynaptic currents (mEPSCs) 24 hours later in neurons with a pyramidal-like morphology (Fig. 1A–D). We found that this chronic inhibition of the UPS induced a robust increase in mEPSC amplitude relative to untreated control cells (Fig. 1B–C; t18 = 8.00, p < 0.05). In contrast, there was no difference in mEPSC frequency between cells treated with lactacystin and untreated control cells (Fig. 1D; t18 = 0.37, NS) suggesting a selective effect of chronic UPS inhibition on scaling of postsynaptic function in hippocampal neurons. To determine when functional synaptic changes first appear in the course of UPS inhibition, we treated neurons with lactacystin (10µM) and recorded mEPSCs 3, 5, 12, and 24 hr later. Similar to the time-course of slow homeostatic plasticity induced by changes in network activity in cortical (see Turrigiano et al., 1998) and hippocampal neurons (see Sutton et al., 2006), UPS inhibition produced a slow, time-dependent increase in mEPSC amplitude (F4,44 = 3.59, p < 0.05; Figure 1E–G) with no effect on mEPSC frequency (data not shown; F4,44 = 0.76, NS). The increase in synaptic function induced by chronic UPS inhibition was associated with a near uniform rightward shift of mEPSC amplitudes (Figure 1G), a profile highly similar to the multiplicative scaling of synaptic function observed following chronic TTX treatment (Turrigiano et al., 1998). These results suggest that UPS inhibition mimics slow homeostatic compensation induced by AP blockade.
Figure 1. Scaling of mEPSC amplitude accompanies chronic UPS inhibition.
(A) Representative pyramidal-like neuron used for experiments. Scale bar = 10 µm. Sample recordings (B) and mean (+SEM) mEPSC amplitude (C) and frequency (D) from control hippocampal neurons (n = 11) or neurons treated with lactacystin (10 µM) for 24 hrs (n = 8). Chronic lactacystin induced a significant (*p < 0.05) increase in mEPSC amplitude, but no change in mEPSC frequency. Scale bar = 10 pA, 250 ms in (B). (E–G) Time-course of mEPSC scaling during chronic lactacystin treatment. Representative recordings (E) and mean (+SEM) mEPSC amplitude (F) from control neurons (n = 10) or neurons treated with lactacystin (10 µM) for 3 (n = 9), 5 (n = 8), 12 (n = 9), or 24 hrs (n = 8). Lactacystin induced a significant (*p < 0.05) time-dependent increase in mEPSC amplitude that emerges slowly (12 hrs). Scale Bar = 10 pA, 250 ms in (E). (G) Cumulative probability distribution of mEPSC amplitudes for control, 3 hr and 24 hr lactacystin-treated neurons. Whereas the distribution of mEPSC amplitudes after 3 hr lactacystin is similar to controls, a near uniform rightward shift of mEPSC amplitudes is observed after 24 hr proteasome inhibition. (H–J) Representative traces (H) and mean (+SEM) mEPSC amplitude (I) and frequency (J) from control neurons (n = 10) or neurons treated with a different proteasome inhibitor MG132 (10 µM) or the lysosomal inhibitor leupeptin (10 µM). Similar to the effects of lactacystin, chronic (18 hr; n = 8 neurons), but not acute (4 hr; n = 9 neurons) treatment with MG132 produced a significant (*p < 0.05, relative to control) increase in mEPSC amplitude without altering mEPSC frequency. Neither chronic (24 hr; n = 9 neurons) nor acute (4 hr, n = 10 neurons) treatment with leupeptin altered mEPSCs.
To confirm that these changes in mEPSCs were due specifically to disruption of proteasome function, we compared the acute (4 hr) and chronic (18 hr) effects of another UPS inhibitor, MG132 (10µM) with acute and chronic (4 and 24 hr, respectively) treatment with leupeptin (10 µM), a potent inhibitor of cellular proteases found in the lysosome. As we observed with lactacystin, UPS inhibition with MG132 produced no change in mEPSC amplitude at the early (4 hr) time-point but induced a robust increase in mEPSC amplitude after chronic (18 hr) treatment relative to untreated control cells (F2,21 = 4.80, p < 0.05; 18 hr vs control, p < 0.05 Fisher’s LSD; Figure 1H–I). Additionally, like lactacystin treatment, MG132 treatment did not significantly alter mEPSC frequency between groups (F2,21 = 0.24, NS; Figure 1J). By contrast, neither brief (4 hr) nor prolonged (24 hr) lysosomal inhibition with leupeptin altered mEPSC amplitude (F2,24 = 0.19, NS; Figure 1I) or frequency (F2,24 = 0.77, NS; Figure 1J). These results thus demonstrate that proteasome inhibition, but not inhibition of lysosomal-mediated degradation, mimics slow homeostatic changes in synapse function induced by suppression of network activity.
3.2 AP blockade and UPS inhibition drive similar changes in synaptic AMPAR expression
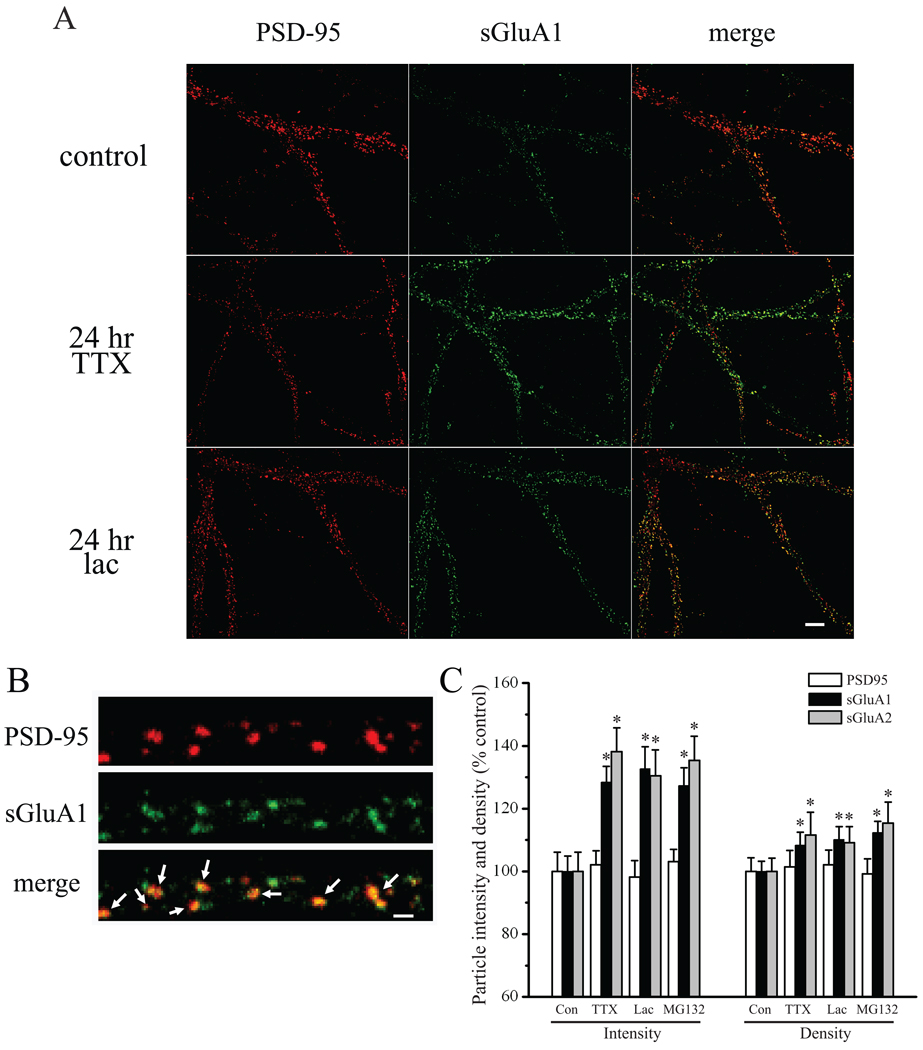
Rapid forms of homeostatic plasticity are associated with an increase in GluA1 homomeric receptor expression at synapses (Sutton et al., 2006; Aoto et al., 2008; see also, Ju et al., 2004; Thiagarajan et al., 2005), which is a mechanism that is likely distinct from that underlying slow homeostatic adaptations (e.g., see Sutton et al., 2006; Aoto et al., 2008). For example, in cortical neuron cultures, chronic AP blockade with TTX induces a coordinate increase in both GluA1 and GluA2 AMPAR subunit expression at synapses (Wierenga et al., 2005; see also, Ibata et al., 2008). Likewise, in hippocampal neurons, scaled mEPSCs following chronic TTX treatment are insensitive to polyamine toxins (Sutton et al., 2006), suggesting an increase in GluA2-containing AMPARs in these neurons during slow homeostatic compensation. To examine changes in surface expression of AMPAR subunits at synapses following changes in activity or UPS inhibition, we live-labeled neurons with antibodies recognizing extracellular epitopes of the GluA1 or GluA2 subunits, then fixed and stained for PSD95 to identify excitatory synapses. The integrated fluorescence intensity of GluA1/A2 particles that colocalized with PSD95 was used to measure relative changes in surface expression of these subunits at synaptic sites. We found that 24 hr TTX treatment induced a significant increase in surface expression of both GluA1 (F3,352 = 9.45, p < 0.05; TTX vs control, p < 0.05 Fisher’s LSD) and GluA2 (F3,262 = 7.33, p < 0.05; TTX vs control, p < 0.05 Fisher’s LSD) at PSD95-labeled excitatory synapses (Figure 2; see also, Figure S1). These changes in AMPAR subunits were largely accounted for by an increase in GluA1/2 content at synapses (as reflected in integrated fluorescence intensity per particle) and to a lesser extent, an increase in density of GluA1 (F3,352 = 3.03, p < 0.05; TTX vs control, p < 0.05 Fisher’s LSD) and GluA2 particles (F3,262 = 3.26, p < 0.05; TTX vs control, p < 0.05 Fisher’s LSD). It is likely that this latter measure reflects enhanced detection of particles owing to their increased intensity rather than insertion of receptors at previously AMPAR-silent synapses, since chronic TTX treatment selectively enhances mEPSC amplitude without changes in mEPSC frequency (Turrigiano et al., 1998; Sutton et al., 2006; see also, Figure 6B–C). In contrast to the changes in GluA1/2 expression, the density (F3,352 = 0.36, NS) and intensity (F3,352 = 0.29, NS) of PSD95 particles from the same dendrites was unchanged by chronic TTX treatment (Figure 2). These observations thus suggest that slow homeostatic increases in synaptic strength are associated with enhanced expression of GluA2-containing AMPARS at existing synapses.
Figure 2. Similar synaptic changes in AMPAR expression accompany AP and UPS blockade.
(A) Representative images of PSD95 (red) and surface GluA1 (sGluA1; green) immunostaining in neurons treated as indicated; merged images are shown in the right panel. Scale Bar = 10 µm. (B) High magnification images of PSD95 (red), sGluA1 (green), and PSD95 and sGluA1 merged; arrows indicate colocalized particles used for analysis of synaptic sGluA1/2 expression. Scale Bar = 1 µm. (C, left) Mean (+SEM) normalized (relative to the average control value) intensity of PSD95 particles, and synaptic sGluA1/A2 particles that colocalize with PSD95. (C, right) Mean (+SEM) normalized particle density of PSD95, sGluA1, and sGluA2 in groups, as indicated. AP blockade (2 µM TTX, 24 hr) induced a significant (*p < 0.05, Fisher’s LSD) increase in sGluA1 (n = 82 neurons) and sGluA2 (n = 61 neurons) intensity at synapses relative to untreated control neurons (n = 89 and 64 neurons, respectively), but did not alter PSD95 particle intensity or density. Similar changes in sGluA1 and sGluA2 expression at synapses were observed following chronic (24 hr) UPS inhibition with lactacystin (10 µM; n = 87 and 66 neurons, respectively) and MG132 (10 µM; n = 98 and 75 neurons, respectively). AP blockade and UPS inhibition also induced a modest, but significant (*p < 0.05, Fisher’s LSD) increase in sGluA1 and sGluA2 particle density.
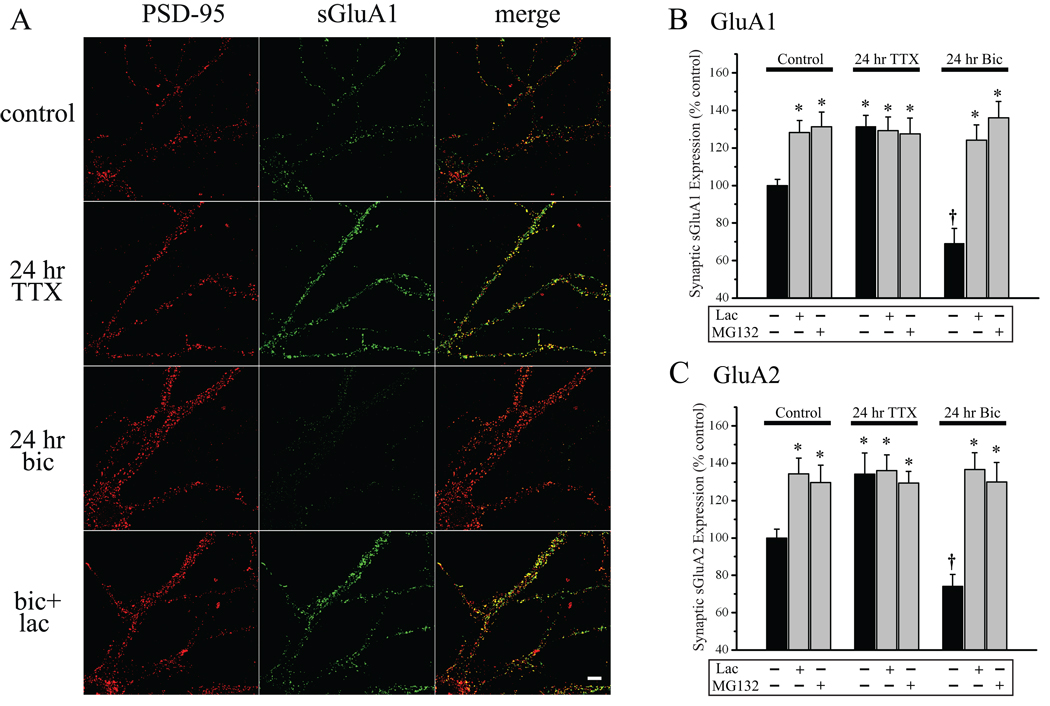
Figure 6. UPS inhibition drives enhancement of synaptic AMPAR expression irrespective of activity levels.
(A) Representative full-frame examples of PSD95 (left, red) and surface GluA1 (middle, green) staining from neurons treated, as indicated; merged PSD95/sGluR1 images are shown in the right panel; scale bar = 10 µm. (B–C) Mean (+SEM) normalized (relative to the average control value) intensity of sGluA1 (B) and sGluA2 (C) particles at synapses (i.e., that overlap with PSD95 particles). Chronic AP blockade (2 µM TTX, 24 hrs) signicantly enhances (*p < 0.05), whereas chronic network hyperactivity (50 µM Bic, 24 hrs) significantly diminishes († p < 0.05), sGluA1 and sGluA2 expression at synapses relative to control (black bars). UPS inhibition (10 µM lactacystin or 10 µM MG132, 24 hrs) significantly enhances synaptic sGluA1 and sGluA2 expression on its own; this effect is non-additive with chronic AP blockade when UPS inhibitors are applied with TTX and, UPS inhibition still drives increases in sGluA1/2 expression during hyperactivity when UPS inhibitors are applied with bicuculline. For the groups listed from left to right in (B), n’s = 64, 75, 62, 45, 46, 38, 44, 52, and 49 neurons. For the groups listed from left to right in (C), n’s = 44, 52, 49, 47, 45, 39, 44, 43, and 47 neurons.
We next examined expression of GluA1 and GluA2 after chronic UPS inhibition to determine if a similar or distinct profile in synaptic AMPAR expression was apparent. Similar to chronic TTX treatment, we found that UPS inhibition with both lactacystin and MG132 induced a coordinate increase in surface GluA1 and GluA2 expression at synapses (both p < 0.05 vs control, Fisher’s LSD). Also similar to chronic AP blockade, these changes in synaptic AMPAR content following UPS inhibition were not associated with changes in the density or intensity of PSD95 particles. Therefore, in addition to parallel effects on mEPSC amplitude, UPS inhibition and AP blockade induce similar changes in synaptic AMPAR expression at synapses.
3.3 Chronic changes in neuronal activity drive bi-directional changes in proteasome function
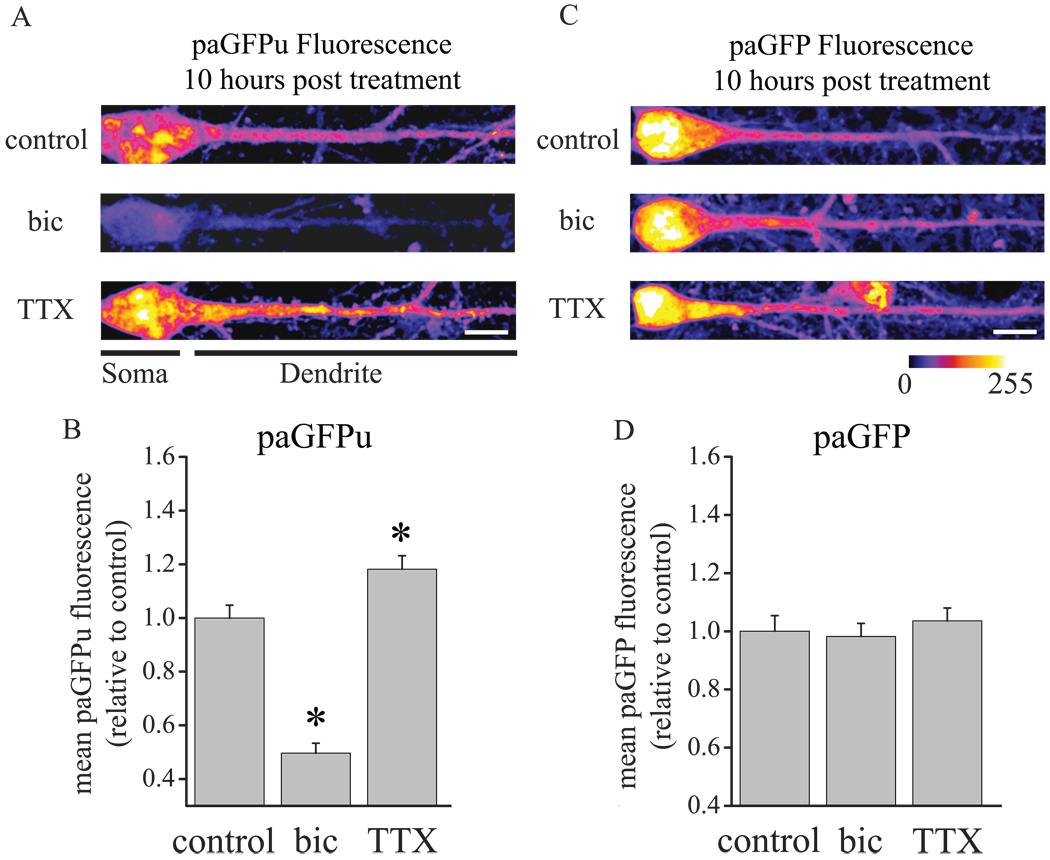
The observed proteasome-dependent changes in neuronal strength occur on the same slow timescale as those induced by chronic AP blockade, suggesting that slow activity-dependent homeostatic compensation may arise via sustained bi-directional changes in proteasome function. Indeed, a recent study found that AP blockade (with TTX) induced an acute decrease in neuronal UPS activity, whereas network hyperactivity (via disinhibition with bicuculline) induced acute enhancement of proteasome function (Djakovic et al., 2009). To test if these bi-directional changes in UPS activity are sustained during chronic changes in activity, we imaged a photoactivatable GFP fluorescent UPS reporter (paGFPu), in which paGFP (Patterson and Lippincott-Schwartz, 2002) is fused with a 16 amino acid degron (CL1) that is constitutively polyubiquitinated and degraded by the proteasome; it is well established that this reporter is selectively degraded by the proteasome (Bence et al., 2001, 2005, Djakovic et al., 2009). As a control for changes in paGFPu fluorescence independent of UPS activity, we also examined expression of paGFP lacking the proteasome-sensitive degron. Both the UPS reporter (paGFPu) and the UPS-insensitive control reporter (paGFP) were co-expressed with mCherry via an internal ribosomal entry site (IRES) and expressed in neurons using Sindbis viral vectors. We found that chronic activity suppression (2 µM TTX, 10 hrs) significantly diminished paGFPu degradation by the proteasome compared to untreated control neurons, whereas chronic network hyperactivation (40 µM bicuculline) significantly enhanced paGFPu degradation (Fig 3A–B; F2,258 = 56.25, p < 0.05; TTX and Bic vs Control; each p < 0.05 Fisher’s LSD). Relative to control neurons (mean ± SEM dendritic paGFPu expression, 1020.1 ± 90.5 a.u.), TTX-treated neurons exhibited an 18.2% increase in dendritic paGFPu expression (1287.4 ± 79.6 a.u.), whereas bicuculline-treated neurons exhibited a 50.3% decrease in dendritic paGFPu expression (522.3 ± 65.9 a.u.). By contrast, mCherry expression was similar among groups (data not shown), as was expression of paGFP lacking the UPS-sensitive degron (Figure 3C–D; mean ± SEM dendritic paGFP expression, Control 913.7 ± 95.6 a.u., TTX 909.1 ± 72.3 a.u., Bicuculline 918.1 ± 87.1 a.u.; F2,159 = 0.56, NS). These data demonstrate that sustained bi-directional changes in UPS activity accompany chronic changes in activity that induce opposing slow homeostatic synaptic adaptations.
Figure 3. Chronic changes in neuronal activity induce bidrectional changes in proteasome activity.
Neurons were infected with Sindbis viral vectors expressing the 26S proteasome reporter paGFPu-IRES-mCherry or the control (UPS-insensitive) reporter paGFP-IRES-mCherry, and were then treated with 2 µM TTX (n = 92 and 71 neurons, respectively), 50 µM bicuculline (n = 77 and 65 neurons, respectively) or were untreated (control, n = 92 and 70 neurons, respectively) prior to imaging 10 hrs later. . (A,C) Representative expression of paGFPu (A) and paGFP (C) in neurons treated, as indicated. Somatic and dendritic divisions are indicated below the images in (A); quantification was restricted to dendritic regions. Fluorescence intensity is indicated by color look-up table; scale bars = 10 µm. (B,D) Mean (+SEM) normalized (relative to the average control value) dendritic paGFPu (B) and paGFP fluorescence intensity in neurons, treated as indicated. Network hyperactivation (bic) induced a significant (*p < 0.05, Fisher’s LSD) increase in paGFPu degradation (indicated by loss of paGFPu expression), whereas suppression of network activity (TTX) significantly diminished paGFPu degradation relative to controls; fluorescence intensity of the UPS-insensitive paGFP reporter was similar among treatment groups.
3.4 Postsynaptic UPS activity drives cell autonomous scaling of mEPSC amplitude
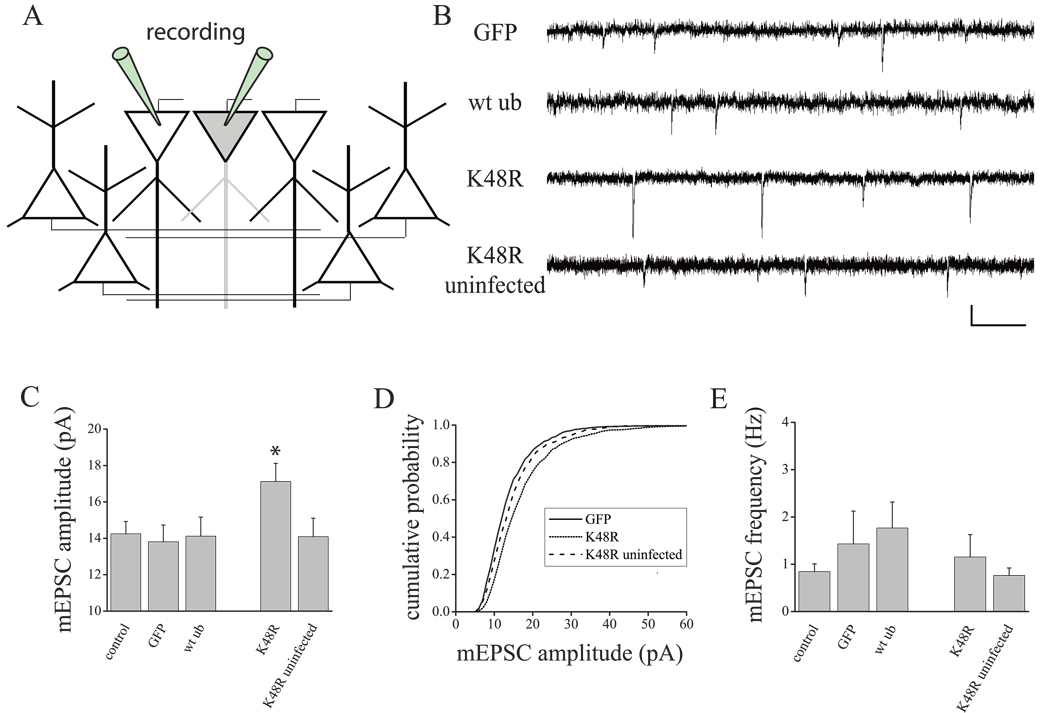
The results described above indicate that chronic UPS inhibition is sufficient to scale mEPSC amplitude and that postsynaptic UPS activity is regulated by changes in activity that normally drive slow homeostatic scaling of synaptic strength. We next examined whether manipulations of UPS function restricted to the postsynaptic cell are sufficient to drive slow homeostatic changes in synaptic strength. To examine this question, we used Sindbis viral vectors to express a dominant-negative ubiquitin chain elongation mutant (UbK48R) in which lysine 48 (K48) is mutated to an arganine. Ub48R retards the growth of polyubiquitination chains at lysine 48 necessary for proteasome-dependent degradation, without depleting the free ubiqutin pool. As controls, we examined the effects of expressing wild-type ubiquitin (wtUb) or GFP alone in sister cultures. Importantly, the low infection efficiency of Sindbis vectors allowed us to manipulate UPS function in a small (< 1%) proportion of neurons in the network (Figure 4A), allowing us to test the cell autonomous role of the UPS in a hippocampal network that is otherwise unperturbed. Consistent with the idea that the UPS functions postsynaptically to mediate slow homeostatic plasticity in a cell autonomous manner, we found that 24 hr expression of K48R-IRES-GFP in pyramidal-like hippocampal neurons selectively enhanced mEPSC amplitude (Figure 4B–C; F4,51 = 2.324, p < 0.05; p < 0.05 vs control, Fisher’s LSD) without altering mEPSC frequency (Figure 4E; F4,51 = 0.75, NS), a profile similar to both chronic AP blockade and pharmacological UPS inhibition. Moreover, the cumulative probability distribution of mEPSC amplitudes in UbK48R-expressing neurons exhibited a near uniform shift to the right, similar to the characteristic multiplicative scaling induced by AP blockade (Turrigiano et al., 1998). In contrast to the effects of the chain elongation ubiquitin mutant, expression of wtUb-IRES-GFP or GFP alone had no effect on mEPSCs (vs. control, Fisher’s LSD, NS). Moreover, mEPSCs recorded from un-infected cells in the same dish as those expressing Ub48R were also unchanged, indicating that postsynaptic UbK48R expression enhances mEPSC amplitude in a cell autonomous fashion.
Figure 4. Postsynaptic UPS inhibition scales excitatory synaptic function in a cell autonomous manner.
(A) Sindbis viral vectors were used to achieve sparse (< 1% of neurons) expression of the ubiquitin chain elongation mutant, UbK48R-IRES-GFP, wild-type ubiquitin (wtUb), or GFP alone. The effects of UbK48R expression were compared with uninfected neurons in the same culture and the expression of wtub-IRES-GFP and GFP alone in sister cultures. (B–E) Representative mEPSC recordings and summary data (C–E) from cells expressing GFP (n = 9), wt ub (n = 13), K48R (n = 14), and uninfected cells present in the K48R-infected dish (n = 8); untreated control neurons (n = 11) from sister cultures were also examined. (C–E) Mean (+ SEM) mEPSC amplitude (C) and frequency (E) from neurons, treated as indicated. UbK48R expression (24 hr) produced a significant (*p < 0.05, Fisher’s LSD) increase in mEPSC amplitude (but not mEPSC frequency) relative to both untreated control neurons and uninfected neurons present in the K48R dish; no changes in mEPSC amplitude were found in neurons expressing wtUb or GFP alone. (D) Similar to global AP suppression in cultured hippocampal neurons, restricted postsynaptic UbK48R expression induced a rightward shift in the mEPSC amplitude cumulative probability distribution, relative to control neurons (GFP expressing neurons in sister cultures and K48R uninfected neurons recorded from the same culture as K48R-expressing neurons).
3.5 UPS inhibition occludes slow homeostatic plasticity
If bi-directional slow homeostatic changes in synaptic strength require changes in synaptic composition mediated by the proteasome, then UPS inhibition should occlude homeostatic changes in synaptic strength induced by chronic AP blockade and prevent synaptic compensation driven by chronic hyperactivation. To test this idea, we treated neurons with lactacystin (10 µM) either alone, or 30 min prior to chronic treatment with TTX (2 µM), and assessed excitatory synaptic function 4 or 24 hr later. Consistent with previous results (Turrigiano et al., 1998; Sutton et al., 2006; Figure 1), we found that neither AP blockade nor UPS inhibition altered mEPSC amplitude or frequency at the early, 4 hr, time-point (Figure 5A–C; NS, Fisher’s LSD). Moreover, coincident treatment with TTX and lactacystin also failed to alter mEPSCs at this early time-point (NS, Fisher’s LSD), indicating that AP blockade and UPS inhibition does not accelerate the slow enhancement of synaptic strength that accompanies either treatment alone. By contrast, 24 hr AP blockade induced a robust scaling of mEPSC amplitude (F6,49 = 6.85, p < 0.05; 24 hr TTX vs control, p < 0.05 Fisher’s LSD) without changes in mEPSC frequency (F6,49 = 0.85, NS). As in earlier experiments, 24 hr lactacystin treatment induced a similar scaling of mEPSC amplitude (p < 0.05 vs control, Fisher’s LSD), and this effect was not additive with AP blockade when TTX and lactacystin were co-applied (Figure 5B; 24 hr lactacystin vs 24 hr TTX+lactacystin, NS, Fisher’s LSD). These results indicate that chronic UPS inhibition induces slow enhancement of synaptic strength that occludes slow homeostatic compensation induced by AP blockade.
Figure 5. UPS inhibition Occludes Slow Homeostatic Plasticity.
(A–C). Interaction between network activity suppression and chronic UPS inhibition. Representative mEPSC recordings (A) and mean (+ SEM) mEPSC amplitude (B) and frequency (C) from neurons, treated with TTX alone (2 µM), lactacystin alone (10 µM), or TTX + lactacystin for 4 or 24 hrs. For the groups shown left to right in (B) and (C), n = 16, 8, 10, 9, 7, 8, and 7 cells, respectively; scale bar in (A) = 10 pA and 250 ms. While neither AP blockade (TTX) nor UPS inhibition (lac) altered synapse function acutely (4 hr, NS), both significantly (* p < 0.05, Fisher’s LSD) enhanced mEPSC amplitude in response to chronic (24 hr) treatment. These effects on mEPSCs were non-additive, as combined TTX+lactacystin treatment for 24 hr, while significantly (* p < 0.05, Fisher’s LSD) enhancing mEPSC amplitude relative to controls, did not enhance mEPSCs over and above that observed with AP and UPS blockade in isolation (NS). In contrast to the effects on mEPSC amplitude, no significant changes in mEPSC frequency were evident following these treatments. (D–F). Interaction between network hyperactivation and chronic UPS inhibition. Representative mEPSC recordings (D) and mean (+ SEM) mEPSC amplitude (E) and frequency (F) from neurons, treated with bicuculline alone (50 µM), lactacystin alone (10 µM), or bicuculline + lactacystin for 4 or 24 hrs. For the groups shown left to right in (E) and (F), n = 26, 6, 10, 8, 6, 7, and 9 cells, respectively; scale bar in (D) = 10 pA and 250 ms. These experiments were done in parallel with those described in Figure 5A–C, and the data shown for 4 hr and 24 hr lactacystin alone conditions are replotted in (E) and (F) for comparison. Whereas chronic (24 hr) hyperactivity imposed by bicuculline alone induces a significant (* p < 0.05, Fisher’s LSD) decrease in mEPSC amplitude, mEPSC amplitude remains significantly (* p < 0.05, Fisher’s LSD) enhanced relative to control when hyperactivity is made coincident with UPS inhibition. Network hyperactivation is completely ineffective in reducing the enhanced mEPSCs driven by UPS inhibition (lactacystin vs bic+lactacystin, NS). No significant changes in mEPSC frequency accompany acute or chronic hyperactivity or UPS inhibition.
We next examined how UPS inhibition interacts with chronic network hyperactivation induced by bicuculline. Acutely (4 hr treatment), no changes in mEPSC frequency or amplitude accompanied either hyperactivity (bicuculline) or UPS inhibition alone; combined hyperactivity/UPS inhibition also failed to alter mEPSCs at the early 4 hr time-point (Figure 5E). Chronic (24 hr) hyperactivation, however, lead to a robust decrease in mEPSC amplitude (Figure 5D–E; F6,55 = 6.02, p < 0.05; p < 0.05 vs control, Fisher’s LSD) without altering mEPSC frequency (Figure 5F; F6,55 = 0.64, NS), similar to previous studies (Turrigiano et al., 1998; Leslie et al., 2001; Seeburg et al., 2008). Remarkably, despite the robust weakening of synaptic strength induced by chronic hyperactivity alone, mEPSCs scaled upward in strength with coincident lactacystin treatment (p < 0.05 vs control, Fisher’s LSD) to a degree similar to UPS inhibition alone (24 hr lactacystin vs 24 hr bic+lactacystin, NS, Fisher’s LSD), demonstrating that UPS inhibition completely prevents slow synaptic compensation driven by chronic increases in network activity. These results indicate that UPS inhibition occludes homeostatic strengthening and prevents homeostatic weakening of synaptic strength, and thus suggest a critical role for the proteasome in driving bi-directional synaptic adaptations underlying homeostatic scaling.
Since chronic AP blockade and UPS inhibition induce similar changes in AMPAR subunit expression at synapses, we next examined if these synaptic modifications themselves are bi-directionally regulated by chronic activity and whether changes in AMPARs induced by UPS inhibition occludes changes induced by alterations of network activity. We treated hippocampal neurons with TTX or bicuculline for 24 hr alone or in combination with either lactacystin (10 µM) or MG132 (10 µM), prior to live surface GluA1 or GluA2 labeling and subsequent immunocyctochemistry against PSD95 to label excitatory synapses. As before, we quantified synaptic GluA1/2 expression by analyzing the intensity of GluA1/2 particles that colocalized with PSD95. We found that, in the absence of coincident UPS inhibition, TTX and bicuculline induced symmetrically opposite changes in synaptic expression of both GluA1 (F8,466 = 11.13, p < 0.05) and GluA2 (F8,399 = 10.64, p < 0.05), with AP blockade significantly enhancing GluA1/GluA2 expression and hyperactivity significantly diminishing GluA1/GluA2 expression (Figure 6A–C, black bars; p < 0.05 vs control, Fisher’s LSD). These bi-directional changes in AMPAR subunit expression at synapses parallel slow homeostatic strengthening and weakening, respectively, with chronic activity suppression and elevation. As before (Figure 2), chronic UPS inhibition with either lactacystin or MG132 induced a significant increase in expression of both GluA1 and GluA2 at synapses under control levels of activity (both p < 0.05 vs control, Fisher’s LSD). As we observed for synaptic currents, the changes in synaptic AMPAR expression induced by UPS inhibition occluded the increase in GluA1 and GluA2 expression induced by chronic activity suppression (24 hr TTX; Fisher’s LSD vs TTX alone, NS) and also prevented the reduction of these subunits induced by chronic activity elevation (24 hr bic; Lac+bic and MG132+bic, each p < 0.05 vs control, Fisher’s LSD; Figure 6A–C; see also, Figure S2). These results thus suggest that UPS inhibition drives coordinate increases in GluA1 and GluA2 expression at synapses in a manner that both mimics and occludes slow homeostatic compensation induced by chronic changes in network activity.
3.6 Postsynaptic UPS activity determines the direction of synaptic compensation
We next examined whether manipulation of postsynaptic UPS function, specifically, is sufficient to drive slow homeostatic changes in synaptic strength that occlude that induced by chronic changes in network activity. To address this issue, we used Sindbis vectors to express UbK48R in a small (< 1%) percentage of neurons present in individual cultures, and examined the effects of network-wide activity suppression or elevation in both these neurons and un-infected neurons in the same culture. Neurons expressing Ub48R (24 hr post-infection) exhibited a significant increase in mEPSC amplitude relative to both non-expressing neurons in the same culture as well as GFP-expressing neurons in sister cultures (Figure 7A–B; F4,43 = 4.76, p < 0.05; p < 0.05 vs control, Fisher’s LSD); by contrast, no significant changes in mEPSC frequency were observed (Figure 7D; F4,43 = 0.73, NS). Moreover, the increase in mEPSC amplitude induced by UbK48R expression was not additive with suppressing network activity, as 24 hr TTX treatment effectively scaled mEPSCs in uninfected neurons (TTX vs control, p < 0.05, Fisher’s LSD) but not in UbK48R-expressing neurons (Figure 7A–B; K48R vs K48R+TTX, NS, Fisher’s LSD). In both UbK8R-expressing and non-expressing neurons following 24 hr TTX treatment, a similar near uniform rightward shift of mEPSC amplitudes was observed relative to untreated controls (Figure 7C), again demonstrating that postsynaptic UPS inhibition occludes slow homeostatic increases in synaptic strength induced by chronic suppression of network activity.
Figure 7. Postsynaptic UPS activity overrides changes in network activity during slow homeostatic plasticity.
Sindbis vectors encoding UbK48R-IRES-GFP (or GFP alone, as a control) were used to infect a small (< 1%) percentage of neurons and slow homeostatic plasticity in these neurons and their uninfected neighbors was compared. The indicated accompanying treatments were applied 30 min prior to infection. (A) Representative mEPSC recordings from untreated controls, K48R-expressing neurons treated with TTX (2µM, 24hr), and uninfected neurons in the same K48R-infected dish (TTX). (B–D) Mean (+ SEM) mEPSC amplitude (B) and frequency (D) from neurons, treated as indicated. For the groups shown left to right in (B) and (D) n = 9, 8, 14, 7 and 9 cells, respectively; the K48R uninfected and K48R groups in the absence of AP blockade are re-plotted from Figure 5 for comparison. Chronic AP blockade induced a significant (*p < 0.05, Fisher’s LSD) increase in mEPSC amplitude that was similar in Ub48R-expressing neurons and uninfected neurons from the same culture (NS), revealing a non-additive effect of postsynaptic UPS inhibition and AP blockade for scaling mEPSCs. (C) Cumulative probability distribution of mEPSC amplitudes from the conditions indicated. A similar rightward shift in mEPSC amplitude cumulative probability distributions is observed for neurons treated with TTX alone and those which express UbK48R and are treated with TTX. (E) Representative mEPSC recordings and summary data (F–H) for experiments where postsynaptic UPS inhibition was paired with chronic network hyperactivity (24 hr bic). Shown in (E) are controls and K48R-expressing and non-expressing neurons in the same dish treated with bicuculline (50 µM); scale bar = 10 pA, 250 ms. Mean (+ SEM) mEPSC amplitude (F) and frequency (H) in neurons, treated as indicated. For the groups shown left to right in (F) and (H), n = 9, 8, 14, 7 and 6 cells respectively. Whereas chronic network hyperactivity induced significant (*p < 0.05, Fisher’s LSD) compensatory decreases in mEPSC amplitude in uninfected neurons, this homeostatic weakening of synaptic strength was completely prevented by postsynaptic expression of UbK48R, as these neurons still exhibit significantly enhanced mEPSC amplitude relative to untreated controls. (G) Opposite shifts of the mEPSC cumulative probability distribution are found in UbK48R-expressing and non-expressing neurons in the same culture exposed to bicuculline for 24 hrs.
We next examined whether postsynaptic UPS inhibition prevents the changes in synaptic strength induced by chronic network hyperactivation. In uninfected neurons, 24 hr bicuculline treatment induced a significant decrease in mEPSC amplitude relative to untreated control neurons (Figure 7E–G; F4,39 = 4.38 p < 0.05; p < 0.05 vs control, Fisher’s LSD), but mEPSC frequency was not significantly altered (Figure 7H; F4,39 = 0.72, NS). In neurons expressing UbK8R, bicuculline treatment was completely ineffective in scaling mEPSCs, as these neurons still exhibited a significant increase in mEPSC amplitude relative to controls (p < 0.05, Fisher’s LSD) that was not significantly different from UbK8R-expressing neurons in the absence of bicuculline treatment (Figure 7E–H). Taken together, our results indicate that postsynaptic blockade of UPS-dependent degradation drives slow homeostatic increases in synaptic strength in these neurons irrespective of changes in network activity.
4. Discussion
Our results reveal a postsynaptic role for the ubiquitin proteasome system (UPS) in slow homeostatic adaptations driven by chronic changes in network activity of hippocampal neurons. Specifically, sustained inhibition of the UPS produces a slow increase in mEPSC amplitude that parallels the slow homeostatic plasticity induced by suppression of network activity with TTX (Turrigiano et al., 1998; Sutton et al., 2006). The increase in synaptic strength induced by UPS inhibition is associated with a coordinate increase in surface GluA1 and GluA2 expression at synapses, similar to that observed following chronic TTX treatment. These functional changes are consistent with the activity-dependent regulation of proteasome function observed during chronic activity changes – activity suppression induces a sustained decrease in UPS-dependent degradation, whereas network hyperactivation significantly enhances proteasome function (Figure 3, see also, Djakovic et al., 2009), suggesting that activity suppression may enhance synaptic function though its effect on the UPS. Additional experiments provide strong evidence for this possibility, as UPS inhibition enhances postsynaptic function and synaptic AMPAR expression in a manner that occludes increases induced by chronic activity suppression. More strikingly, UPS inhibition drives homeostatic increases in postsynaptic function even in the face of network hyperactivation, which normally drives homeostatic weakening of synaptic strength. Cell-restricted expression of the ubiquitin chain elongation mutant UbK48R further revealed that postsynaptic UPS inhibition is sufficient to drive slow homeostatic scaling of synaptic strength that occludes the effects of chronic activity suppression and prevents synaptic compensation driven by hyperactivation of network activity. Taken together, these results reveal a critical role for postsynaptic proteasome-mediated degradation in slow homeostatic plasticity.
4.1 Distinct compartment-specific roles for the proteasome in homeostatic synaptic plasticity
Our results, taken together with earlier observations, suggest that the UPS may play distinct roles in the presynaptic and postsynaptic compartments of the synapse during homeostatic plasticity. More generally, a role for the UPS in synaptic plasticity was first suggested by work in Aplysia, where UPS-mediated degradation of PKA regulatory subunits leads to long-lasting autonomous PKA activity necessary for long-lasting presynaptic facilitation of neurotransmission (Hegde et al., 1997; Chain et al., 1999). A more recent study (Zhao et al., 2003) demonstrated that proteasome inhibition in either presynaptic or postsynaptic neurons is sufficient to increase synaptic efficacy at Aplysia sensorimotor synapses. Similarly, in mammalian neurons, the UPS is necessary for forms of LTP (Fonseca et al., 2006; Karpova et al., 2006; Dong et al., 2008), and LTD (Patrick et al., 2003; Colledge et al., 2003; Hou et al., 2006) that are known to be induced and expressed postsynaptically. During homeostatic synaptic plasticity, there may be distinct pre- and postsynaptic roles for the UPS, as well. For example, Ehlers (2003) demonstrated large scale and bidirectional changes in protein composition of postsynaptic densities (PSDs) in response to chronic suppression (with TTX) or elevation (with bicuculline) of neuronal activity. Importantly, these changes in PSD composition emerged gradually over the course of chronic treatment, and were shown to arise via changes in UPS function. On the other hand, UPS activity is necessary for homeostatic silencing of presynaptic terminals in response to excessive depolarization (Jiang et al., 2010) and may play other homeostatic roles presynaptically (Willeumier et al., 2006; Rinetti and Schweizer, 2010). These latter results raise questions about whether the changes in PSD composition observed by Ehlers (2003) reflect a requirement for the UPS postsynaptically, or whether UPS inhibition alters PSD composition indirectly via presynaptic effects. Our results demonstrate that the UPS drives postsynaptic compensation in a cell autonomous fashion, thus supporting the notion that the changes in PSD composition revealed by Ehlers (2003) likely reflect cell intrinsic actions of the proteasome operating on the postsynaptic compartment. Interestingly, while acute UPS inhibition induces a transient increase in presynaptic function in hippocampal neurons (Rinetti and Schweizer, 2010), we did not observe persistent changes in presynaptic function following chronic UPS inhibition, suggesting that a distinct presynaptic role for the UPS may exist for more rapid forms of homeostatic plasticity.
4.2 Multiple homeostatic mechanisms operate in hippocampal neurons
It is becoming increasingly apparent that neurons express multiple homeostatic mechanisms that operate over distinct temporal/spatial domains and are responsive to unique facets of neural activity. For example, the synaptic recruitment of GluA2-lacking AMPARs during rapid homeostatic plasticity requires local dendritic synthesis, likely of GluA1 itself (Sutton et al., 2006; Poon and Chen, 2008) and is independent of gene transcription (Aoto et al., 2008), whereas compensation induced by AP blockade has been shown to require CREB-mediated transcriptional activation (Ibata et al., 2008). These mechanistic differences may also map onto whether the resulting compensation is implemented locally (Sutton et al., 2006; Branco et al., 2008) or globally (Turrigiano et al., 1998; Ibata et al., 2008). Although changes in synapse composition are likely the ultimate locus of expression of slow homeostatic plasticity (Ehlers, 2003), it is presently unclear whether the UPS functions on a global (cell-wide) or more local level to drive these synaptic adaptations. On the one hand, synaptic scaling can be induced by focal application of TTX to the cell body (Ibata et al., 2008), suggesting that global aspects of neuronal activity such as firing rate are sufficient to induce compensatory modifications at synapses. The fact that AP suppression inhibits UPS function, and UPS inhibition alone drives slow homeostatic adaptations that occlude further plasticity imposed by changes in network activity, suggests that the UPS may be responsive to global features of activity and promote synaptic changes in a cell-wide fashion. On the other hand, recent studies have demonstrated activity-dependent synaptic trafficking of proteasomes in dendrites (Bingol and Schuman, 2006; Bingol et al., 2010) raising the possibility that the UPS could also act locally to alter the composition of a more restricted set of synapses.
While a number of studies have all documented increases in AMPAR content at synapses accompanying homeostatic increases in synaptic function, the population of AMPARs that is regulated appears to differ depending on the mode of activity blockade. For example, Turrigiano et al. (1998) originally reported that mEPSC kinetics were unchanged in visual cortical cultures following chronic TTX treatment, suggesting that the increase in postsynaptic function was mediated by an increase in the number, but not type, of AMPARs. Similarly, a coordinate increase in synaptic expression of GluA1 and GluA2 has been observed in cortical neurons treated chronically with TTX (Wierenga et al.,2005; see also, Ibata et al., 2008; Gainey et al., 2009) as well as in spines of hippocampal neurons apposed to presynaptic terminals rendered silent by Kir2.1 expression (Hou et al., 2008). On the other hand, multiple laboratories have documented a recruitment of GluA2-lacking (and presumptive GluA1 homomeric) AMPARs to synapses (Ju et al., 2004; Thiagarajan et al., 2005; Sutton et al., 2006; Aoto et al., 2008), although it is notable that each of these studies employed receptor blockade either alone or in conjunction with AP blockade, to suppress activity. Our results suggest that the recruitment of GluA2-lacking AMPARs may be a mechanism unique to rapid forms of homeostatic plasticity, since we find that slow synaptic compensation in hippocampal neurons driven by AP blockade alone drives coordinate increases in GluA1 and GluA2 expression at synapses, similar to that observed in other studies. Conversely, we find that chronic network hyperactivation (with bicuculline treatment) induces a coordinate loss of GluA1 and GluA2 from synapses. These findings also explain why scaled mEPSCs in hippocampal neurons following blockade of miniature neurotransmission are effectively reversed by agents that block GluA2-lacking AMPARs, but scaled mEPSCs following chronic TTX treatment are insensitive to these agents (Sutton et al., 2006). Similarly, recent evidence indicates that blocking miniature transmission, but not AP blockade alone, drives retinoic acid synthesis in neurons, an essential intermediate for de novo GluA1 synthesis necessary for rapid homeostatic plasticity (Aoto et al., 2008; Poon and Chen, 2008). It is unlikely that the UPS plays a role in the relatively fast recruitment of GluA2-lacking AMPARs (within 1–3 hrs, Sutton et al., 2006) during rapid homeostatic plasticity, since the changes in postsynaptic function accompanying UPS inhibition are intrinsically slow to develop, similar to synaptic scaling induced by AP blockade (Turrigiano et al., 1998). Taken together, these observations suggest that changes in AMPAR subunit composition accompany some forms of compensatory plasticity, but not others, indicating that neurons express multiple forms of homeostatic plasticity that are mechanistically distinct.
4.3 Activity-dependent regulation of proteasome function links changes in network activity with alterations of synapse function
A major unresolved issue in activity-dependent homeostatic control is the nature of the activity sensor(s) that read out changes in activity above or below the normal range. Given the extended time-course over which synaptic modifications arise during AP blockade, it has been especially difficult to define an activity sensor for slow homeostatic plasticity. Although definitive evidence that any one protein serves as a bona fide homeostatic activity sensor is still lacking, several activity-responsive enzymes have emerged as attractive candidates, including CAMKII (Thiagarajan et al., 2002), eEF2 kinase (Sutton et al., 2007), and Ca2+-sensitive adenylate cyclases (Gong et al., 2007). Irrespective of the true nature of the slow homeostatic sensor, our results suggest that the UPS serves as an important integration point through which such a sensor can engage mechanisms that drive compensatory synaptic modifications. For example, previous work has shown that network activity bi-directionally regulates degradation of a 26S proteasome reporter in dendrites (Djakovic et al., 2009), effects that appear sustained during chronic activity changes (Figure 3). Moreover, we show that UPS inhibition dictates changes in synaptic function irrespective of network activity, suggesting that the UPS couples sustained changes in network activity with downstream compensatory synaptic modifications.
While our results indicate that network activity is integrated postsynaptically by the proteasome, the manner by which activity interacts with the proteasome on a molecular level is still unknown. One interesting possibility is that activity engages the UPS via posttranslational modification of the proteasome itself, perhaps through direct phosphorylation by activity-dependent protein kinases. For example, direct activity-dependent phosphorylation of the proteasome has been shown to gate proteasome function (Zhang et al., 2007; Djakovic et al., 2009) and trafficking into dendritic spines (Bingol et al., 2010). Specifically, CAMKII is capable of directly phosphorylating Rpt6 (Djakovic et al., 2009; Bingol et al., 2010) an AAA-ATPase subunit located in the 19S regulatory cap of the proteasome and whose phosphorylation is required for proteasome function (Zhang et al., 2007). In so far as CAMKII expression (Thiagarajan et al., 2002) and/or function serves an activity-sensing role, our data suggest that interactions with the proteasome provide an interface for linking the detection of activity levels outside the normal range with the appropriate compensatory response. Likewise, it has been shown that PKA can directly phosphorylate Rpt6 as well (Zhang et al., 2009) which provides a similar platform for linking the actions of a putative activity sensor with changes in proteasome function.
Our results demonstrate that the UPS plays a critical role in slow homeostatic adaptations at synapses in hippocampal neurons. It seems likely that this role reflects a combination of direct changes in proteasome function in response to long term changes in activity as well the targeted degradation of specific synaptic proteins ultimately responsible for regulating AMPAR expression and synaptic function. In recent years, many synaptic proteins have been shown to be regulated by the proteasome (e.g., Yi and Ehlers, 2007; Ding and Shen, 2008; Segref and Hoppe, 2009), suggesting numerous candidate UPS targets that could contribute to changes in synaptic strength, including Arc/Arg3.1 (Shepherd et al., 2006; Greer et al., 2010), PICK1 (Joch et al., 2007), ROMK1 (Lin et al., 2005), Shank (Gong et al., 2009), GRIP (Guo and Wang, 2007), and GKAP (Hung et al., 2010). Determining the effectors that operate downstream of proteasome function, and how they act coordinately to drive appropriate homeostatic synaptic adaptations, is an important challenge for future studies.
Acknowledgements
We thank Cindy Carruthers for preparing beautiful hippocampal neurons and other members of the Sutton lab for helpful discussions. This work was supported by RO1MH085798 from The National Institute of Mental Health (M.A.S.) and a grant from the Pew Biomedical Scholars Program (M.A.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
S.K.J. and M.A.S. conceived the experimental ideas, performed experiments and wrote the manuscript. R.M.N, S.N.D and G.N.P. performed experiments.
References
- Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic Signaling by All-Trans Retinoic Acid in Homeostatic Synaptic Plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- Bence NF, Bennett EJ, Kopito RR. Ubiquitin and Protein Degradation. Pt B. San Diego: Elsevier Academic Press Inc.; 2005. Application and analysis of the GFP(u) family of ubiquitin-proteasome system reporters; pp. 481–490. [DOI] [PubMed] [Google Scholar]
- Bingol B, Schuman EM. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature. 2006;441:1144–1148. doi: 10.1038/nature04769. [DOI] [PubMed] [Google Scholar]
- Bingol B, Wang CF, Arnott D, Cheng DM, Peng JM, Sheng M. Autophosphorylated CaMKII alpha Acts as a Scaffold to Recruit Proteasomes to Dendritic Spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Branco T, Staras K, Darcy KJ, Goda Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron. 2008;59:475–485. doi: 10.1016/j.neuron.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C-elegans. Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- Chain DG, Casadio A, Schacher S, Hegde AN, Valbrun M, Yamamoto N, Goldberg AL, Bartsch D, Kandel ER, Schwartz JH. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron. 1999;22:147–156. doi: 10.1016/s0896-6273(00)80686-8. [DOI] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Ann Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Ding M, Shen K. The role of the ubiquitin proteasome system in synapse remodeling and neurodegenerative diseases. Bioessays. 2008;30:1075–1083. doi: 10.1002/bies.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakovic SN, Schwarz LA, Barylko B, DeMartino GN, Patrick GN. Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2009;284:26655–26665. doi: 10.1074/jbc.M109.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Upadhya SC, Ding L, Smith TK, Hegde AN. Proteasome inhibition enhances the induction and impairs the maintenance of late-phase long-term potentiation. Learn Mem. 2008;15:335–347. doi: 10.1101/lm.984508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nägerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Gainey MA, Hurvitz-Wolf JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Wang H, Gu S, Heximer SP, Zhuo M. Genetic evidence for the requirement of adenylyl cyclase 1 in synaptic scaling of forebrain cortical neurons. Eur J Neurosci. 2007;26:275–288. doi: 10.1111/j.1460-9568.2007.05669.x. [DOI] [PubMed] [Google Scholar]
- Gong YS, Lippa CF, Zhu JH, Lin QS, Rosso AL. Disruption of glutamate receptors at Shank-postsynaptic platform in Alzheimer's disease. Brain Res. 2009;1292:191–198. doi: 10.1016/j.brainres.2009.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman Syndrome Protein Ube3A Regulates Synapse Development by Ubiquitinating Arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Wang Y. Glutamate stimulates glutamate receptor interacting protein 1 degradation by ubiquitin-proteasome system to regulate surface expression of GluR2. Neuroscience. 2007;145:100–109. doi: 10.1016/j.neuroscience.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hegde AN. The ubiquitin-proteasome pathway and synaptic plasticity. Learning and Memory. 2010;17:314–327. doi: 10.1101/lm.1504010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain DG, Martin KC, Kandel ER, Schwartz JH. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 1997;89:115–126. doi: 10.1016/s0092-8674(00)80188-9. [DOI] [PubMed] [Google Scholar]
- Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death and Differentiation. 2005;12:1191–1197. doi: 10.1038/sj.cdd.4401702. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci USA. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Sung CC, Brito IL, Sheng M. Degradation of postsynaptic scaffold GKAP and regulation of dendritic spine morphology by the TRIM3 ubiquitin ligase in rat hippocampal neurons. PLoS One. 2010;5:11. doi: 10.1371/journal.pone.0009842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Jiang XP, Litkowski PE, Taylor AA, Lin Y, Snider BJ, Moulder KL. A Role for the Ubiquitin-Proteasome System in Activity-Dependent Presynaptic Silencing. J Neurosci. 2010;30:1798–1809. doi: 10.1523/JNEUROSCI.4965-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joch M, Ase AR, Chen CXQ, MacDonald PA, Kontogiannea M, Corera AT, Brice A, Seguela P, Fon EA. Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels. Mol Biol Cell. 2007;18:3105–3118. doi: 10.1091/mbc.E05-11-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Karpova A, Mikhaylova M, Thomas U, Knöpfel T, Behnisch T. Involvement of protein synthesis and degradation in long-term potentiation of Schaffer collateral CA1 synapses. J. Neurosci. 2006;26:4949–4955. doi: 10.1523/JNEUROSCI.4573-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie KR, Nelson SB, Turrigiano GG. Postsynaptic depolarization scales quantal amplitude in cortical pyramidal neurons. J Neurosci. 2001;21:RC170. doi: 10.1523/JNEUROSCI.21-19-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DH, Sterling H, Wang ZJ, Babilonia E, Yang BF, Dong K, Hebert SC, Giebisch G, Wang WH. ROMK1 channel activity is regulated by monobuquitination. Proc Natl Acad Sci U S A. 2005;102:4306–4311. doi: 10.1073/pnas.0409767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Prinz AA. Modeling stability in neuron and network function: the role of activity in homeostasis. Bioessays. 2002;24:1145–1154. doi: 10.1002/bies.10185. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu YL. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Oliet SHR, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Park EC, Glodowski DR, Rongo C. The Ubiquitin Ligase RPM-1 and the p38 MAPK PMK-3 Regulate AMPA Receptor Trafficking. PLoS One. 2009;4:15. doi: 10.1371/journal.pone.0004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Bingol B, Weld HA, Schuman EM. Ubiquitin-mediated proteasome activity is required for agonist-induced endocytosis of GluRs. Curr Biol. 2003;13:2073–2081. doi: 10.1016/j.cub.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- Poon MM, Chen L. Retinoic acid-gated sequence-specific translational control by RAR alpha. Proc Natl Acad Sci U S A. 2008;105:20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Rich MM, Wenner P. Sensing and expressing homeostatic synaptic plasticity. Trends Neurosci. 2007;30:199–125. doi: 10.1016/j.tins.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Rinetti GV, Schweizer FE. Ubiquitination Acutely Regulates Presynaptic Neurotransmitter Release in Mammalian Neurons. J Neurosci. 2010;30:3157–3166. doi: 10.1523/JNEUROSCI.3712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raninowitch I, Segev I. Two opposing plasticity mechanisms pulling a single synapse. Trends Neurosci. 2008;31:377–383. doi: 10.1016/j.tins.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Ciechanover A. Targeting proteins for destruction by the ubiquitin system: Implications for human pathobiology. Ann Rev Pharmacol Toxicol. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DTS, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segref A, Hoppe T. Think locally: control of ubiquitin-dependent protein degradation in neurons. EMBO Rep. 2009;10:44–50. doi: 10.1038/embor.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci. 2008;9:826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and beta CaMKII: Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. Regulated protein degradation. Trends Biochem Sci. 2005;30:283–286. doi: 10.1016/j.tibs.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeumier K, Pulst SM, Schweizer FE. Proteasome inhibition triggers activity-dependent increase in the size of the recycling vesicle pool in cultured hippocampal neurons. J Neurosci. 2006;26:11333–11341. doi: 10.1523/JNEUROSCI.1684-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Takagi H, Konishi Y, Ageta H, Ikegami K, Yao I, Sato S, Hatanaka K, Inokuchi K, Seog DH, Setou M. Transmembrane and ubiquitin-like domain-containing protein 1 (Tmub1/HOPS) facilitates surface expression of GluR2-containing AMPA receptors. PLoS One. 2008;3:13. doi: 10.1371/journal.pone.0002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JJ, Ehlers MD. Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol Rev. 2007;59:14–39. doi: 10.1124/pr.59.1.4. [DOI] [PubMed] [Google Scholar]
- Zhang FX, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE. Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem. 2007;282:22460–22471. doi: 10.1074/jbc.M702439200. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hegde AN, Martin KC. The ubiquitin proteasome system functions as an inhibitory constraint on synaptic strengthening. Curr. Biol. 2003;13:887–898. doi: 10.1016/s0960-9822(03)00332-4. [DOI] [PubMed] [Google Scholar]