Abstract
Background
We tested the hypothesis that females are more resistant to trauma-hemorrhagic shock (T/HS)-induced gut injury than males, and this is related to better preservation of their intestinal mucus layer, which is influenced in turn by the estrus cycle stage at the time of injury.
Methods
Male, proestrus and diestrus female rats underwent a laparotomy (trauma) and 90 minutes of shock (~35 mm Hg). At 3 hours after reperfusion, terminal ileum was harvested and stained with Carnoy’s Alcian Blue for mucus assessment, hematoxylin and eosin, and periodic acid schiff for villous and goblet cell morphology and injury. Ileal permeability was measured in separate intestinal segments using the ex vivo everted gut sac technique.
Results
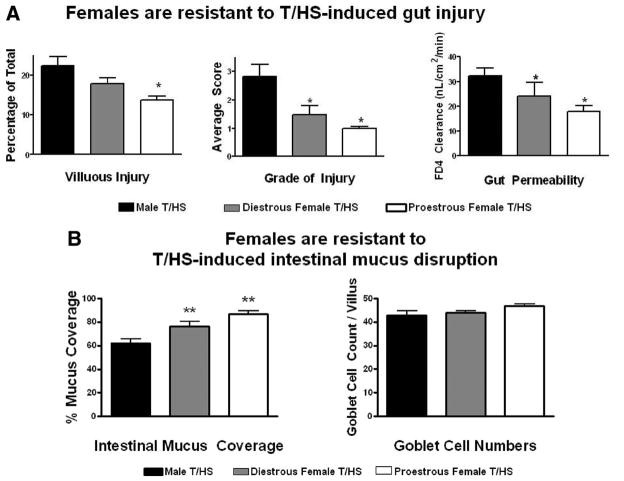
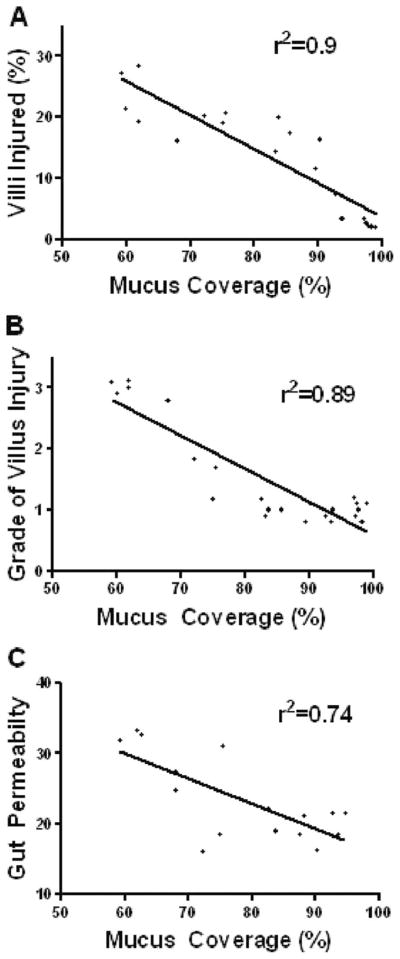
When compared with males, proestrus female rats were significantly more resistant to T/HS-induced morphologic gut injury, as reflected in both a lower incidence of villous injury (14% vs. 22%; p < 0.05) and a lesser grade of injury (1.0 vs. 2.8; p < 0.05) as well as preservation of gut barrier function (17.9 vs. 32.2; p < 0.05). This resistance to gut injury was associated with significant preservation of the mucus layer (87% vs. 62%; p < 0.05) and was influenced by the estrus cycle stage of the female rats. There was a significant inverse correlation between mucus layer coverage and the incidence (r2 = 0.9; p < 0.0001) and magnitude (r2 = 0.89; p < 0.0001) of villous injury and gut permeability (r2 = 0.74; p < 0.001).
Conclusions
The resistance of female rats to T/HS-induced intestinal injury and dysfunction was associated with better preservation of the intestinal mucus barrier and was to some extent estrus cycle-dependent. Preservation of the mucus barrier may protect against shock-induced gut injury and subsequent distant organ injury by limiting the ability of luminal contents such as bacteria and digestive enzymes from coming into direct contact with the epithelium.
Keywords: Trauma, Hemorrhagic Shock, Intestinal Mucus Layer, Gender, Gut Injury
Multiple organ dysfunction syndrome (MODS) is a significant but poorly understood cause of intensive care unit mortality after trauma-hemorrhagic shock (T/HS). Over the years, studies have suggested that the ischemic gut plays an important role in the pathophysiology of postshock systemic inflammatory response syndrome, adult respiratory distress syndrome, and MODS, in at least some patient subgroups, including the trauma patient.1,2 These preclinical and clinical studies have led to the gut hypothesis of MODS.1 Because the first step in the paradigm of gut origin sepsis and MODS involves gut injury, understanding the exact mechanisms by which trauma-shock leads to gut injury is of potential clinical significance. Traditionally, studies investigating the pathogenesis of gut injury have focused on the splanchnic circulation and systemic host factors, because shock or major trauma leads to shunting of blood away from the gut resulting in an intestinal ischemia-reperfusion (I/R) injury and an I/R-induced intestinal inflammatory response.3 However, much of the barrier function of the intestine is because of the unstirred mucus layer, which protects the underlying intestinal villi and enterocytes from potentially damaging substances such as digestive enzymes and bacteria that are contained within the intestinal lumen in high concentrations.3,4 Recently, we reported that disruption of the small intestinal mucus layer was associated with trauma- and shock-induced loss of gut barrier function in male rats.5 However, based on preclinical and some clinical studies demonstrating a protective effect of the female gender in outcomes after trauma and shock,6–9 we began investigating whether female rats were more resistant to gut injury and gut-induced distant organ dysfunction than male rats. These studies documented that the female intestine was more resistant to T/HS and I/R-induced gut injury than male rats.10–12 Furthermore, this resistance to gut injury was estrus cycle-specific with protection being greatest during the proestrus phase of the cycle (high estrogen) and lowest during the diestrus phase of the cycle when estrogen levels are greatly reduced.10 In addition, protection against T/HS-induced gut injury was largely lost after ovariectomy.11 Consequently, in this study, we began testing the hypothesis that females are more resistant to T/HS-induced gut injury than males due to better preservation of their intestinal mucus layer and that this resistance to T/HS-induced loss of the mucus barrier was estrus cycle-dependent.
MATERIALS AND METHODS
Animals
Sprague-Dawley (SD) rats weighing 300 g to 450 g were housed under barrier-sustained conditions kept at a temperature of 25°C with 12-hour light/dark cycle. The rats had free access to water and chow (Teklan 22/5 Rodent Diet W-8640; Harlan Tecklad, Madison, WI) for at least 1 week before the study. All animals were maintained in accordance with the recommendation of the Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the New Jersey Medical School Animal Care and Use Committee. The estrus cycle stages of the female rats were determined by histologic examination of vaginal scrapings.13
Experimental Design
The major goal of this study was to test the hypothesis that the degree of loss of the gut mucus layer after T/HS would correlate directly with the magnitude of gut injury and that both T/HS-induced mucus loss and gut injury would be less in proestrus female than male rats. To test this hypothesis, we performed several experiments on male and female (proestrus and diestrus) rats that underwent T/HS or trauma sham shock (T/SS). In the T/HS group, animals underwent a laparotomy followed by 90 minutes of hemorrhagic shock. After shock, animals were resuscitated with their own shed blood and sacrificed after a 3-hour period. In the T/SS group, animals underwent vessel cannulation and laparotomy but were not subjected to hemorrhagic shock. Three hours after resuscitation, the animals were killed. In experiment 1, morphologic markers of injury (villous injury, mucus coverage, and goblet cell number) were assessed. In experiment 2, the terminal ileum was collected and assessed functionally for permeability using the everted gut sac technique.
Hemorrhagic Shock Model
As described previously,14 rats were fasted overnight to eliminate feces, weighed, and anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg). Using aseptic techniques, the internal jugular vein and the femoral artery were isolated and cannulated with polyethylene (PE-50) tubing and 50-guage silicon catheter, respectively, both containing 0.1 mL heparinized saline solution (concentration, 10 units/mL). Both catheters remained in situ for the duration of the experiment. Next, a 3-cm midline laparotomy (trauma) was performed. After closure of the abdomen, the femoral artery catheter was attached in line to a blood pressure monitor (BP-2 digital blood pressure monitor; Columbus Instruments, Columbus, OH) for continuous blood pressure monitoring. In T/HS group, blood was then withdrawn from the internal jugular vein, and the mean arterial pressure was maintained at ~35 mm Hg for 90 minutes by withdrawing or infusing the shed blood at a rate of 1 mL/min. The T/HS animals were resuscitated by the reinfusing the shed blood which had been maintained at 37°C. Using heating pads, body temperature was maintained at 37°C until the animals were killed at 3 hours after the end of the 90 minutes T/SS or T/HS period.
Tissue Fixing and Staining Technique
The stool-free segments of terminal ileum were opened along the mesenteric border without rinsing. Specimens were laid flat, mucosal surface up, and pinned to cardboard. Alcian Blue (3%; Sigma, St. Louis, MD) solution in distilled water was sprayed on the mucosal surface to stain the mucus layer, followed by immediate immersion in Carnoy’s solution (60% ethanol, 30% acetic acid, and 10% chloroform) for 2 hours at 4°C. The samples were then placed in 100% ethanol, cleared in xylene, and embedded in paraffin.15 The tissue sections were cut longitudinally into 4-μm sections for histologic analysis.
Histologic Analysis
Ileal Villous Injury and Mucus Coverage
To assess the mucus layer, cut sections of tissue were restained with hematoxylin and eosin to evaluate ileal villous injury and mucus coverage simultaneously. Five random fields with 150 to 250 villi from each animal were analyzed in a blinded fashion using light microscopy at 100× magnification. The overall incidence of villous damage was expressed as a percentage of injured villi to total villi counted. Injury was graded using the method described by Chiu et al.16 Briefly, grade 0 corresponds to normal mucosal villi, grade 1 is development of subepithelial space, grade 2 is an extension of this subepithelial space with moderate lifting of epithelial layer from the lamina propria, grade 3 is massive epithelial lifting, grade 4 has denuded villi, and grade 5 involves digestion and disintegration of lamina propria, hemorrhage, and ulceration. The percentage of mucus coverage of the ileal surface was measured in microns (μm), with an objective micrometer in a blinded fashion as described by Rupani et al.5
Goblet Cell Count
The Carnoy’s fixed cut sections were restained with periodic acid schiff to reveal goblet cells. The goblet cells were identified within the ileal mucosa by their classic goblet-like shape and deep-blue stain. Total goblet cell counts were determined along the combined crypt-villus axis of 20 well-oriented crypt-villus units from the crypt base to the villus tip and expressed as number per villus.17 All studies were performed in a blinded fashion.
Measurement of Ex Vivo Intestinal Permeability
Intestinal permeability was determined using the everted gut sac method with fluorescein isothiocyanate dextran (molecular weight 4,000 Da; FD4; Sigma) tracer as described by Rupani et al.5
Statistical Analysis
Results were expressed as mean ± standard deviation (SD). Continuous data were analyzed by unpaired t test and one-way analysis of variance using the post hoc Tukey-Kramer or Newman-Keuls multiple comparison tests. Statistical significance was considered to be a p value <0.05.
RESULTS
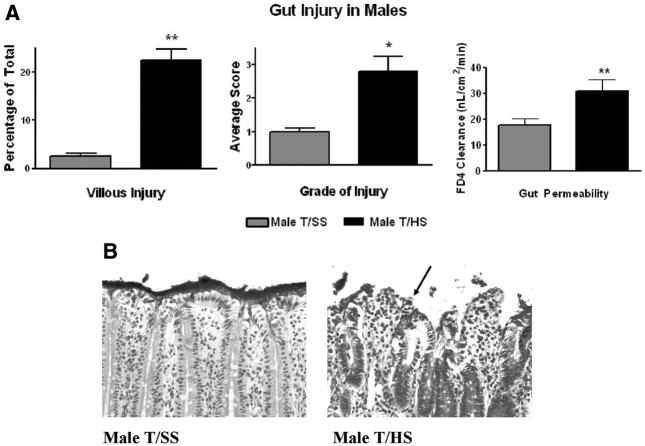
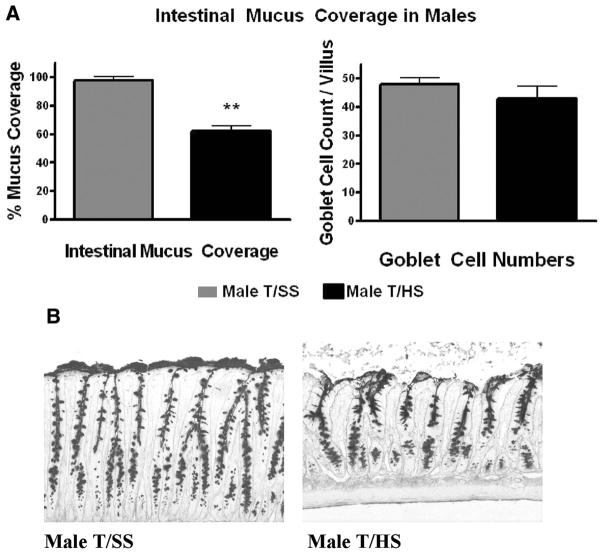
Male rats subjected to T/HS, but not T/SS, had morphologic evidence of significant villous damage and this was manifest as a 22% incidence of villous injury as well as a high grade of injury (Fig. 1). This morphologic evidence of T/HS-induced villous injury was associated with loss of gut barrier function and a twofold increase in gut permeability (Fig. 1, A). T/HS also caused over a 40% decrease in the intestinal mucus barrier layer, which was morphologically manifest as loss of the continuous mucus layer separating the underlying enterocytes and villi from the intraluminal contents of the gut (Fig. 2). This T/HS-induced disruption and decrease in the mucus layer was not associated with a significant reduction in goblet cell numbers (Fig. 2).
Figure 1.
(A) In male rats, T/HS increased the number of intestinal villi injured plus the magnitude of villous injury as well as intestinal permeability. **p < 0.01 vs. male T/SS group. *p < 0.05 vs. male T/SS group. Data are expressed as mean ± SD (n = 4–6 rats/group). (B) Representative photomicrographs of terminal ileum from T/SS or T/HS rats obtained 3 hours after reperfusion (200×) showing high-grade villous injury after T/HS manifest as sloughing of villous tips and a severely disrupted mucus layer (arrow).
Figure 2.
(A) Males rats subjected to T/HS had a significant reduction in their intestinal mucus layer coverage but no change in goblet cell numbers. **p < 0.01 versus male T/SS groups. Data are expressed as mean ± SD (n = 4–6 rats/group). (B) Representative photomicrographs of terminal ileum 3 hours after reperfusion (100×) showing loss of the mucus layer in the T/HS group.
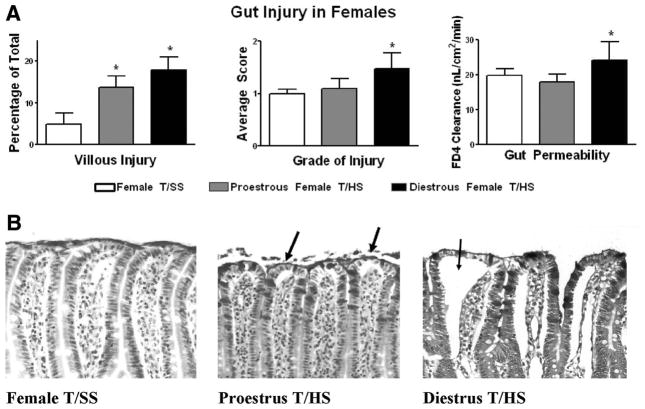
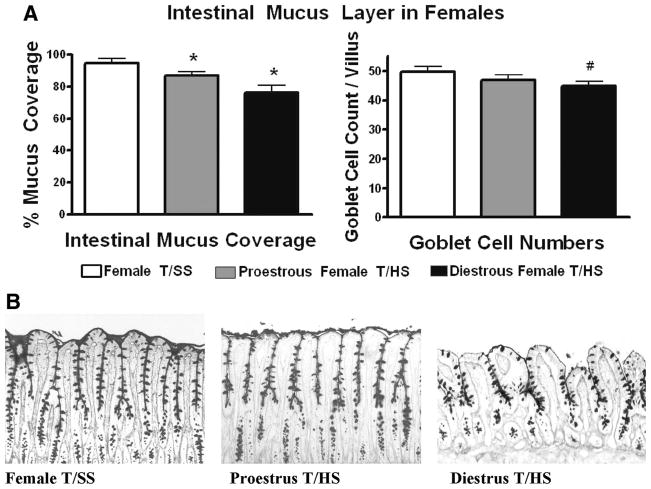
Proestrus female rats seemed to be significantly resistant to T/HS-induced gut injury when compared with T/SS because the incidence of villous injury in the proestrus female rats subjected to T/HS was only modestly increased, while the magnitude of gut injury and gut permeability were similar between the T/HS and the T/SS groups (Fig. 3). This resistance to T/HS-induced gut injury seemed to be estrus cycle-dependent, because female rats subjected to T/HS during the diestrus stage of the estrus cycle, when estrogen levels are reduced, had a higher incidence and magnitude of villous injury (p < 0.05) as well as increased gut permeability (p < 0.05) when compared with the proestrus T/HS and the T/SS control groups (Fig. 3, A). Similar to what was observed in terms of villous injury, the proestrus female rats subjected to T/HS had a small, but significant decrease in the mucus layer while the amount of the mucus layer lost was greater in the diestrus rats subjected to T/HS and only the diestrus T/HS rats had a decrease in goblet cell numbers (Fig. 4).
Figure 3.
(A) The incidence of T/HS-induced villous injury was increased to a greater degree in the diestrus than the proestrus rats subjected to T/HS as was gut permeability. *p < 0.05 versus all other groups. Data are expressed as mean ± SD (n = 4–6 rats/group). (B) Representative photomicrographs of terminal ileum 3 hours after reperfusion (200×) showing low-grade villous injury after T/HS in proestrus females manifest as subepithelial spaces at the tips (arrows). The diestrus group shows higher degree of gut injury with subepithelial spaces extending all the way to the crypt (arrow).
Figure 4.
(A) After T/HS, the magnitude of loss of the mucus layer was greater in the diestrus than the proestrus females when compared with the T/SS group. *p < 0.05 versus all other groups #p < 0.05 versus T/SS group. Data are expressed as mean ± SD (n = 4–6 rats/group). (B) Representative photomicrographs of terminal ileum 3 hours after reperfusion (100×) showing better preservation of the mucus layer and goblet cells in proestrus compared with diestrus rats subjected to T/HS.
There was no difference in any of the gut parameters measured between the male and female T/SS groups. However, the incidence and severity of intestinal villous injury, the degree of gut permeability, and the magnitude of loss of the mucus layer were greater in the male than the proestrus females subjected to T/HS (Fig. 5, A). Furthermore, the extent of gut injury and loss of the mucus layer in the diestrus females subjected to T/HS was significantly less than that observed in the male rats subjected to T/HS but significantly greater than in the proestrus T/HS females (Fig. 5, B). This observation that the susceptibility of the diestrus females to T/HS-induced gut changes was intermediate between the males and proestrus females suggests that while females are more resistant to gut injury, the degree of that resistance varies over the estrus cycle as the concentration of sex hormones change.
Figure 5.
Both proestrus and diestrus female rats subjected to T/HS were more resistant to gut injury (A), loss of gut barrier function (A) or loss of the mucus layer than male rats (B). *p < 0.05 versus all other groups. **p < 0.01 versus all other groups. Data are expressed as mean ± SD (n = 5–6 rats/group).
The relative resistance to gut injury in the female as opposed to the male T/HS groups was associated with significant preservation of the mucus layer, because there was a significant inverse correlation between the mucus layer coverage and the incidence (r2 = 0.9; p < 0.0001) and magnitude (r2 = 0.89; p < 0.0001) of villous injury as shown in Figure 6. Similarly, mucus layer coverage also correlated inversely with the loss of gut barrier function as measured by increased gut permeability (r2 = 0.74; p < 0.001).
Figure 6.
There is significant inverse correlation between that the degree of mucus layer coverage with the incidence (r2 = 0.9; p < 0.0001; A) and grade (r2 = 0.89; p < 0.0001; B) of villous injury and gut permeability (r2 = 0.74; p < 0.001; C). All T/HS and T/SS animals were used.
DISCUSSION
The concept that loss of the gut barrier can lead to adverse clinical events has resulted in a large body of work culminating in the gut hypothesis of MODS, the first and critical step of which is gut injury and loss of barrier function. This notion is not surprising because the lumen of the small and large intestine is a hostile environment that contains sufficient bacteria and bacterial products to kill the host thousands of times over if they were to reach the systemic circulation.1 Furthermore, if the luminal digestive enzymes, especially the pancreatic proteases, were to come into direct contact with enterocytes lining the intestinal villous, these host cells would be digested in a fashion similar to that of ingested proteins and other food stuffs. Thus, if the gut barrier were lost, the host would be in danger of being injured not only by its own gut bacteria but also of being digested by its own luminal digestive enzymes. Thus, a complex set of host defenses has evolved to maintain gut barrier function, the failure of which can have dire consequences. Consequently, significant efforts have been made to investigate the mechanisms of gut injury and loss of barrier function under stress situations associated with splanchnic hypoperfusion and gut I/R states, such as those that occur after major trauma and/or during shock states. Based on the exact experimental model tested, a number of factors have been implicated in the pathogenesis of gut injury including reactive oxygen species, reactive nitrogen intermediates, proinflammatory molecules, and inflammatory cells such as neutrophils18–20 and intraluminal digestive enzymes.21,22 Although significant attention has been focused on the intestinal villous and the enterocyte response to injury and repair, the effects of gut I/R on the unstirred mucus layer have been relatively ignored. However, under normal conditions, the gut is largely protected from direct contact with intestinal bacteria or harmful luminal contents by a layer of mucus that is being continuously secreted by goblet cells.23,24 One potential reason for the limited attention being focused on the mucus layer under conditions of gut ischemia or stress is that the mucus layer is dissolved and lost during ordinary histologic fixation procedures, and what is not seen is often ignored and not studied. Consequently, because of the potential importance of the unstirred mucus layer in preserving intestinal barrier function, we have recently begun studying this component of the intestinal barrier. To accomplish this, we have used a modification of the Carnoy’s fixation technique, which allows the mucus layer to be preserved during fixation, while allowing standard morphologic analysis of the intestinal villous. With this technique, direct correlations between the mucus layer and gut injury can be made.
In this context, the current studies show that T/HS leads to a significant loss of the intestinal mucus layer in male rats and that this loss of the mucus layer is tightly associated with an increase in villous injury and gut permeability. In contrast, in proestrus female rats where the mucus layer is largely preserved, very limited T/HS-induced gut injury and dysfunction was observed. Furthermore, in diestrus female rats, where T/HS led to an intermediate degree of mucus loss, the extent of gut injury and the increase in gut permeability was also intermediate. Thus, these studies support the idea that T/HS-induced loss of the unstirred mucus layer is directly associated with gut injury and dysfunction. This notion that loss of the mucus layer is involved in T/HS-induced gut injury is supported by studies showing that the mucus layer is an important protective barrier in the stomach where stress-or ischemia-related loss of the gastric mucus layer contributes to gastric mucosal injury caused by intraluminal acid.25,26 The current results are also consistent with earlier studies using frozen sections of the gut to assess the mucus layer, where the magnitude of T/HS-induced increases in gut permeability correlated better with the degree of mucus loss than the extent of gut injury based on morphologic studies.5 Others have reported that qualitative and quantitative changes in the intestinal mucus gel may disrupt the functional mucus barrier leading to increased bacterial translocation,27–29 and a recent report showed that the enteral administration of high molecular weight polyethylene glycol, as a mucus surrogate, prevented lethal gut origin sepsis.30 In addition, using a whole gut transport model, it was found that the decisive barrier to the transport of a compound across the gut wall was related to the mucus layer and not the lipid membrane of the underlying enterocytes.31 Finally, in proof of principle studies, we and others32,33 documented that the intraluminal administration of the mucolytic agent N-acetyl cysteine into the normal intestine resulted in loss of the mucus layer and an increase in intestinal permeability that correlated in a dose-dependent fashion.32
Having shown that T/HS leads to loss of the mucus barrier and that loss of the mucus layer correlates with increased gut permeability, the question arises as to the potential mechanisms by which this occurs. Because it is the polymerization of mucin glycoproteins to form a viscous gel that is responsible for the hydrophobic and barrier natures of mucus,34,35 destruction of polymerized mucin could contribute to T/HS-induced loss of the mucus layer and increased gut permeability. Because it has been reported that reactive oxygen and nitrogen species damage and degrade mucin molecules leading to a loss of viscosity and a decrease in hydrophobicity,36 it seems likely that under conditions of gut I/R, the presence of damaging reactive oxygen and nitrogen free radicals could result in mucin molecules loosing their viscoelastic properties and thereby compromise the barrier function of the mucus layer. Furthermore, the ability of pancreatic enzymes to cleave and degrade mucins in vivo is significantly augmented when the mucins have been chemically modified as may occur under conditions of oxidant stress.37,38 This potential relationship between oxidant-modified mucin molecules and subsequent mucin degradation by luminal pancreatic enzyme resulting in increased gut injury and loss of barrier function is supported by studies showing that intraluminal pancreatic enzyme neutralization or pancreatic duct ligation largely prevents T/HS-induced gut injury, increased intestinal permeability, and loss of the mucus layer.22,39,40 These studies22,39,40 documented that not only the gut mucosa and the mucus layer were preserved, but also pancreatic proteases were necessary for T/HS-induced lung injury, neutrophil activation, and the production of biologically active mesenteric lymph.
The reasons for why the female rodent gut seems to be resistant to T/HS-induced gut injury seem to be multifactorial. For example, studies have shown both cardiac output and regional blood flow to the organs, including the intestine, are better preserved during as well as after an episode of T/HS in female than male rats.41,42 Furthermore, even when the intestine of male and female rats are subjected to an equivalent gut I/R insult, where the reduction in intestinal blood flow is equivalent between the groups, the proestrus female rat gut is more resistant to injury than the male gut.12 Thus, more than a difference in intestinal blood flow after T/HS seems to account for the increased resistance of female versus male intestine. The fact that estrogen is involved appears likely from our earlier study,10 as well as the current study, showing that the susceptibility to T/HS-induced gut injury is highest when estrogen levels are lowest as during the diestrus stage of the estrus cycle. In this light, it is known that female sex hormones increase both the quantity and quality of mucus secretion in the genital and nasal epithelia43,44 and, in the same way, female sex hormones may augment the mucus barrier in the gut and thereby help in protecting the gut from injury. In addition, the female gut produces less of a nitric oxide response10 and is more resistant to T/HS-induced gut inflammation and manifests a blunted cytokine response45–47 after a gut I/R insult. Thus, whether the increased mucus layer observed in the proestrus female rats after T/HS represents a direct effect of female sex hormones on the mucus layer or an indirect effect due to the limiting effects of estradiol on gut inflammation, or even a combination of the two, will require further study.
In summary, this study provides further evidence to suggest that nonbacterial factors on the luminal side of the gut may be important modulators of gut injury during stress states, with two of these luminal factors being the unstirred mucus layer and pancreatic proteases. These results also suggest that there are two distinct processes occurring simultaneous, contributing to gut injury during shock states, one systemically and the other at the luminal surface of the gut. Therefore, studies limited to assessing tight junction function between enterocytes or even villous injury are unlikely to provide a complete picture of the mechanisms involved in T/HS as well as other gut I/R insults that lead to gut injury and loss of barrier function. Based on this information, our working hypothesis for the mechanisms by which T/HS causes gut injury and increases gut permeability is as follows. T/HS induces a gut I/R injury, which in turn results in mild enterocyte injury and an oxidant-mediated injury to the mucus layer, which of itself is not sufficient to completely disrupt the mucus layer. However, the chemically modified mucus loses enough of its hydrophobic and other barrier properties to allow further mucin degradation by intraluminal, water soluble pancreatic proteases. These pancreatic proteases, as well as other lumen factors, including bacteria and their products, now reach and cross the compromised enterocyte barrier, where they initiate a response that results in the generation of biologically active lymph, as well as potentiate gut and systemic inflammation and injury. If this notion is true, then luminal therapies directed at supporting the mucus layer and limiting the actions of digestive enzymes may join systemic therapies directed at limiting the magnitude or consequences of gut injury and hence gut-origin sepsis and MODS.
Acknowledgments
Supported by NIH grants GM 59841 and T32 069330.
Footnotes
Presented at the 68th Annual Meeting of the American Association for the Surgery of Trauma, October 1–3, 2009, Pittsburgh, Pennsylvania.
References
- 1.Deitch EA, Xu DZ, Lu Q. Gut lymph hypothesis of early shock and trauma-induced multiple organ dysfunction syndrome: a new look at gut origin sepsis. J Organ Dysfunct. 2006;2:70–79. [Google Scholar]
- 2.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–393. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharpe SM, Doucet DR, Qin X, Deitch EA. Role of intestinal mucus and pancreatic proteases in the pathogenesis of trauma-hemorrhagic shock induced gut barrier failure and multiple organ dysfunction syndrome. J Organ Dysfunct. 2008;4:168–176. [Google Scholar]
- 4.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol. 1991;260:C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
- 5.Rupani B, Caputo F, Watkins A, et al. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2004;141:481–489. doi: 10.1016/j.surg.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Sperry JL, Minei JP. Gender dimorphism following injury: making the connection from bench to bedside. J Leukoc Biol. 2008;83:499–506. doi: 10.1189/jlb.0607360. [DOI] [PubMed] [Google Scholar]
- 7.Haider AH, Crompton JG, Oyetunji T, et al. Females have fewer complications and lower mortality following trauma than similarly injured males: a risk adjusted analysis of adults in the National Trauma Data Bank. Surgery. 2009;146:308–315. doi: 10.1016/j.surg.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Ananthakrishnan P, Deitch EA. Gut origin sepsis and MODS: the role of sex hormones in modulating intestinal and distant organ injury. XX vs XY. 2003;1:108–117. [Google Scholar]
- 9.Deitch EA, Livingston DH, Lavery RF, Monaghan SF, Bongu A, Machiedo GW. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surg. 2007;246:447–453. doi: 10.1097/SLA.0b013e318148566. discussion 453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caruso JM, Deitch EA, Xu DZ, Lu Q, Dayal SD. Gut injury and gut-induced lung injury after trauma hemorrhagic shock is gender and estrus cycle specific. J Trauma. 2003;55:531–539. doi: 10.1097/01.TA.0000025584.46870.95. [DOI] [PubMed] [Google Scholar]
- 11.Ananthakrishnan P, Cohen DB, Xu DZ, et al. Sex hormones modulate distant organ injury in a trauma/hemorrhagic shock model and a burn model. Surgery. 2005;137:56–65. doi: 10.1016/j.surg.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 12.Deitch EA, Feketeova E, Lu Q, Lu Q, Feketeova E, Deitch EA. Resistance of the female, as opposed to the male, intestine to I/R-mediated injury is associated with increased resistance to gut-induced distant organ injury. Shock. 2008;29:78–83. doi: 10.1097/shk.0b013e318063e98a. [DOI] [PubMed] [Google Scholar]
- 13.Baker HJ, Lindsey JR, Weisbroth SH. The Laboratory Rat, Volume 1, Biology and Diseases. New York: Academic Press; 1979. pp. 54–156. [Google Scholar]
- 14.Magnotti LJ, Upperman JS, Xu DZ, Lu Q, Deitch EA. Gut derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann Surg. 1998;228:518–527. doi: 10.1097/00000658-199810000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo K, Ota H, Akamatsu T, Sugiyama A, Katsuyama T. Histochemistry of the surface mucus gel layer of the human colon. Gut. 1997;40:782–789. doi: 10.1136/gut.40.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu CJ, Mac Ardle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 17.Chang JX, Chen S, Ma LP, et al. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11:5485–5491. doi: 10.3748/wjg.v11.i35.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deitch EA. Multiple organ failure: pathophysiology and potential future. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granger DN. Role of xanthine oxidase and neutrophils in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 20.Hierholzer C, Kalff JG, Billiar TR, Bauer AJ, Tweardy DJ, Harbrecht BG. Induced nitric oxide promotes intestinal inflammation following hemorrhagic shock. Am J Physiol. 2004;286:G225–G233. doi: 10.1152/ajpgi.00447.2002. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci USA. 2000;97:1772–1777. doi: 10.1073/pnas.97.4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–456. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 23.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 24.Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1–C19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- 25.Hills BA, Butler BD, Lichtenberger LM. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983;244:G561–G568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- 26.Goddard PJ, Kao YJ, Lichtenberger LM. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 1990;98:361–370. doi: 10.1016/0016-5085(90)90826-m. [DOI] [PubMed] [Google Scholar]
- 27.Albanese CT, Cardona M, Smith S, et al. Role of intestinal mucus in transepithelial passage of bacteria across the intact ileum in vitro. Surgery. 1994;116:76–82. [PubMed] [Google Scholar]
- 28.Maxson RT, Dunlap JP, Tryka F, Jackson RJ, Smith SD. The role of the mucus gel layer in intestinal bacterial translocation. J Surg Res. 1994;57:682–686. doi: 10.1006/jsre.1994.1201. [DOI] [PubMed] [Google Scholar]
- 29.Katayama M, Xu D, Specian RD, Deitch EA. Role of bacterial adherence and the mucus barrier on bacterial translocation: effects of protein malnutrition and endotoxin in rats. Ann Surg. 1997;225:317–326. doi: 10.1097/00000658-199703000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, Zaborina O, Zahorin A, et al. High-molecular weight polyethylene glycol prevents lethal sepsis due to intestinal Pseudomonas aeruginosa. Gastroenterology. 2004;126:488–498. doi: 10.1053/j.gastro.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Nimmerfall F, Rosenthaler J. Significance of the goblet-cell mucin layer, the outermost luminal barrier to passage through the gut wall. Biochem Biophys Res Commun. 1980;94:960–966. doi: 10.1016/0006-291x(80)91328-5. [DOI] [PubMed] [Google Scholar]
- 32.Qin X, Caputo FJ, Xu D, Deitch EA. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock. 2008;29:372–376. doi: 10.1097/shk.0b013e3181453f4e. [DOI] [PubMed] [Google Scholar]
- 33.Iiboshi Y, Nezu R, Cui L, et al. Adhesive mucous gel layer and mucus release as intestinal barrier in rats. J Parenter Enteral Nutr. 1996;20:98–104. doi: 10.1177/014860719602000298. [DOI] [PubMed] [Google Scholar]
- 34.Hills BA, Butler BD, Lichtenberger LM. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983;244:G561–G568. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- 35.Lugea A, Salas A, Casalot J, Guarner F, Malagelada JR. Surface hydrophobicity of the rat colonic mucosa is a defensive barrier against macromolecules and toxins. Gut. 2000;46:515–521. doi: 10.1136/gut.46.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grisham MB, Von Ritter C, Smith BF, Lamont JT, Granger DN. Interaction between oxygen radicals and gastric mucin. Am J Physiol. 1987;253:93–96. doi: 10.1152/ajpgi.1987.253.1.G93. [DOI] [PubMed] [Google Scholar]
- 37.Forstner JF. Intestinal mucins in health and disease. Digestion. 1978;17:234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- 38.Kemper AC, Specian RD. Rat small intestinal mucins: a quantitative analysis. Anat Rec. 1991;229:219–226. doi: 10.1002/ar.1092290209. [DOI] [PubMed] [Google Scholar]
- 39.Cohen DB, Magnotti LJ, Lu Q, et al. Pancreatic duct ligation reduced lung injury following trauma and hemorrhagic shock. Ann Surg. 2004;240:885–891. doi: 10.1097/01.sla.0000143809.44221.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caputo FJ, Rupani B, Watkins AC, et al. Pancreatic duct ligation abrogates the trauma hemorrhage-induced gut barrier failure and the subsequent production of biologically active intestinal lymph. Shock. 2007;28:441–446. doi: 10.1097/shk.0b013e31804858f2. [DOI] [PubMed] [Google Scholar]
- 41.Deitch EA, Chu H, Xu DZ. Organ blood flow and the central hemodynamic response is better preserved in female, as opposed to male rats, after trauma-hemorrhagic shock. J Trauma. 2008;65:566–572. doi: 10.1097/TA.0b013e31814b2c38. [DOI] [PubMed] [Google Scholar]
- 42.Ba ZF, Kuebler JF, Rue LW, Bland KI, Wang P, Chaudry IH. Gender dimorphic tissue perfusion response after acute hemorrhage and resuscitation: role of vascular endothelial cell function. Am J Physiol Heart Circ Physiol. 2003;284:H2162–H2169. doi: 10.1152/ajpheart.00724.2002. [DOI] [PubMed] [Google Scholar]
- 43.Menárguez M, Pastor LM, Odeblad E. Morphological characterization of different human cervical mucus types using light and scanning electron microscopy. Hum Reprod. 2003;18:1782–1789. doi: 10.1093/humrep/deg382. [DOI] [PubMed] [Google Scholar]
- 44.Choi HJ, Chung YS, Kim HJ, et al. Signal pathway of 17beta-estradiol-induced MUC5B expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2009;40:168–178. doi: 10.1165/rcmb.2007-0377OC. [DOI] [PubMed] [Google Scholar]
- 45.Yu HP, Hsieh YC, Suzuki T, et al. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J Leukoc Biol. 2007;82:774–780. doi: 10.1189/jlb.0307182. [DOI] [PubMed] [Google Scholar]
- 46.Hsu JT, Kan WH, Hsieh CH, et al. Mechanism of estrogen-mediated intestinal protection following trauma-hemorrhage: p38 MAPK-dependent upregulation of HO-1. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1825–R1831. doi: 10.1152/ajpregu.00112.2008. [DOI] [PubMed] [Google Scholar]
- 47.Homma H, Hoy E, Xu D, Lu Q, Feinman R, Deitch EA. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am J Physiol Gastrointest Liver Physiol. 2005;288:G466–G472. doi: 10.1152/ajpgi.00036.2004. [DOI] [PubMed] [Google Scholar]