Abstract
Objective
To explore the association of the DRD4 exon 3 7-repeat allele with clinically significant levels of autistic symptoms among children and adolescents with DSM-IV Attention-Deficit/Hyperactivity Disorder (ADHD).
Methods
Subjects included in the main analysis were 954 Missouri-born twins from a study of the genetic epidemiology of ADHD with complete data on DSM-IV ADHD diagnosis, DRD4 genotype and the parent-rated Social Responsiveness Scale (SRS). Logistic regression was used to investigate the association of the DRD4 7-repeat allele with clinically elevated SRS score.
Results
Among individuals with DSM-IV ADHD (any subtype), the DRD4 7-repeat allele was associated with high SRS score. The distribution of raw SRS scores appeared bimodal among subjects with at least one copy of the DRD4 7-repeat allele, suggesting a possible interaction between this DRD4 genotype and other, unmeasured variables.
Conclusions
The DRD4 7-repeat allele may increase the risk for clinically elevated autistic symptoms in children and adolescents with ADHD. Further studies are needed to confirm this finding and explore the role of specific gene-gene and gene-environment interactions in the development of autistic symptoms and other co-occurring psychopathology among individuals with ADHD.
Keywords: Attention-Deficit/Hyperactivity Disorder, Autism Spectrum Disorder, DRD4, genetic association, comorbidity
Résumé
Objectif
Étudier la relation qui existe entre l’allèle à 7 répétitions dans l’exon 3 du gène DRD4 et les notes significatives au niveau clinique des symptômes autistiques chez des enfants et adolescents souffrant de TDAH tel que défini par le DSM IV.
Méthodologie
Neuf cent cinquante quatre jumeaux nés au Missouri ont participé à une vaste étude épidémiologique sur la génétique du TDAH. Les données portaient sur le TDAH, le génotype du récepteur de la dopamine DRD4 et la Social Responsiveness Scale—SRS (Échelle sociale de réponse) remplie par les parents. La corrélation entre l’allèle à 7 répétitions du gène DRD4 et une note clinique SRS élevée a été étudiée par régression logistique.
Résultats
L’allèle à 7 répétitions du récepteur de la dopamine DRD4 était associé, chez les enfants qui avaient reçu un diagnostic de TDAH (tous types confondus), à une note clinique SRS élevée. On a constaté une répartition bimodale des notes SRS brutes chez les sujets qui avaient au moins une copie de l’allèle à 7 répétitions du gène DRD4, ce qui laisse penser à une interaction possible entre le génotype du DRD4 et d’autres variables non mesurées.
Conclusion
Il est possible que l’allèle à 7 répétitions du DRD4 augmente le risque d’obtention d’une note clinique élevée lors de l’évaluation des symptômes autistiques chez les enfants et les adolescents atteints de TDAH. D’autres travaux devront confirmer cette hypothèse en étudiant, de manière plus approfondie, le rôle des interactions précises gène-gène et gène-environnement dans les symptômes autistiques, ainsi que les psychopathologies concomitantes chez les sujets souffrant de TDAH.
Mots clés: trouble du déficit d’attention avec hyperactivité, trouble envahissant du développement, gène DRD4, corrélation génétique, comorbidité
Introduction
A number of clinic- and population-based studies demonstrate the frequent co-occurrence of Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) symptoms and diagnoses (Gadow, DeVincent, & Pomeroy, 2006; Goldstein & Schwebach, 2004; Hattori et al., 2006; Holtmann, Bolte, & Poustka, 2007; Reiersen, Constantino, Volk, & Todd, 2007; Santosh & Mijovic, 2004; Sturm, Fernell, & Gillberg, 2004). Twin studies suggest a substantial amount of genetic overlap between these two disorders (Reiersen, Constantino, Grimmer, Martin, & Todd, 2008; Ronald, Edelson, Asherson, & Saudino, 2010; Ronald, Simonoff, Kuntsi, Asherson, & Plomin, 2008). Smalley and Colleagues reported statistically significant common linkage peaks between studies of ASD and ADHD (Smalley, Loo, Yang, & Cantor, 2005). A recent Quantitative Trait Locus (QTL) Linkage analysis using the ADHD-enriched International Multi-Center ADHD Genetics Study (IMAGE) sample suggests that a peak at 15q24 may be pleiotropic for ADHD and autistic symptoms (Nijmeijer et al., 2010). Despite the evidence for genetic overlap, very little progress has been made in discovering which specific genetic polymorphisms can influence both types of symptoms.
Exon 3 of the DRD4 gene (which encodes the D4 dopamine receptor) contains a 48 base pair tandem repeat polymorphism that has been extensively studied in ADHD research. The 7-repeat variant was associated with ADHD in multiple studies (Faraone et al., 2005). Previously reports from the Missouri Twin Study evidenced an interaction between the DRD4 7-repeat allele and prenatal nicotine exposure on the risk for a severe combined form of ADHD defined using latent class analysis (Neuman et al., 2007). Along with additional results involving other dopamine system genes, these findings led to a synapse-based hypothesis that may explain the mechanism of interaction between dopamine system genes and prenatal smoking in producing ADHD (Todd & Neuman, 2007). Although the authors did not specifically test the hypothesis, further examination of these data suggests that while subjects with the DRD4 7-repeat allele had elevated ADHD symptoms if exposed to prenatal nicotine, subjects with this same genotype had lower mean ADHD symptoms than those without the 7-repeat allele if there was no prenatal exposure to nicotine (Pluess, Belsky, & Neuman, 2009). The authors hypothesize that DRD4 may actually be a “plasticity gene” rather than an ADHD vulnerability gene. This would be consistent with a hypothesis previously proposed by Belsky and colleagues, who suggest some genetic alleles (including DRD4 7-repeat allele) may cause individuals to be more responsive to both positive and negative environmental influences (Belsky et al., 2009).
Using the same ADHD-enriched Missouri Twin Study sample, we found that individuals with ADHD have elevated levels of autistic symptoms as measured by the Social Responsiveness Scale (SRS), and that mean SRS scores differed depending on ADHD subtype: the DSM-IV combined type and a severe combined ADHD latent class showed the highest levels of autistic symptoms (Reiersen et al., 2007). Some other DSM-IV and latent class subtypes showed elevated mean SRS scores compared to unaffected subjects, but the mean scores for these groups were substantially lower than the combined type ADHD phenotypes and not necessarily suggestive of clinically significant autistic symptoms. Children and adolescents with ADHD whose parents reported motor coordination problems on the CBCL were particularly likely to have clinically elevated SRS scores (Reiersen, Constantino, & Todd, 2008). To investigate whether the genes associated with combined type ADHD in this sample also increased risk for autistic symptoms, we previously explored potential main and interaction effects of dopamine system genes and prenatal nicotine exposure on the presence of clinically elevated autistic symptoms (Reiersen, Neuman et al., 2008). We did not find main or interaction effects of maternal smoking during pregnancy, DAT genotype, or DRD4 genotype on autistic symptoms when the sample was analyzed as a whole (including those with and without ADHD), or when latent class ADHD subtypes (other than severe combined) were analyzed separately. However, among individuals in the severe combined ADHD latent class (n=79), there was a statistically significant association between the DRD4 7-repeat allele and clinically elevated autistic symptoms defined using the SRS (OR=3.27, 95% CI: 1.25–8.56; p=0.016). There was no evidence for gene-environment interaction between maternal smoking and either candidate gene polymorphism in producing ASD within the severe combined latent class.
Here, we report a similar analysis using the same Missouri Twin Sample, but using DSM-IV ADHD criteria instead of latent class methods to define the ADHD phenotype. Although latent class methods may have particular value for genetic studies, the use of DSM-IV ADHD diagnoses may be more relevant to current clinical practice. Latent class analysis is a form of mixture modeling that can be used to identify latent groups of individuals within a sample based on patterns of symptom endorsement. Based upon a hypothesis that they are more phenotypically and genetically homogeneous and more consistent with the natural clustering of symptoms in the population, latent class subtypes may be particularly useful for genetic studies. DSM-IV subtypes are instead defined using specific symptom count cutoffs and additional requirements such as impairment in multiple settings and onset by age 7 years. Information regarding the relationship between latent class and DSM-IV subtypes is reported elsewhere (Volk, Todorov, Hay, & Todd, 2009). In the current sample, the clinically relevant latent class subtypes generally include a larger number of individuals than the most equivalent DSM-IV subtype (Neuman et al., 2005; Reiersen et al., 2007; Volk, Neuman, & Todd, 2005). Because our severe combined latent class has the most overlap with DSM-IV combined subtype, we expect to see evidence of an association between the DRD4 7-repeat allele and high SRS score among individuals with DSM-IV combined type ADHD. While we do not expect to have the power to detect any clear effects of gene-gene or gene-environment interaction on SRS scores in this sample, examination for differences in SRS score distributions depending on DRD4 genotype and subtype may provide preliminary evidence of such interactions.
Method
Subjects and Measures
All study protocols were approved by the Washington University School of Medicine Human Studies Committee. Written informed consent was obtained from all adult-age subjects. Assent was obtained from youths younger than 18 years and informed consent, from their legal guardians. The present analysis uses a subset (n=954) of the population-based Missouri Twin Study (MOTWIN) sample with complete data for DSM-IV ADHD diagnoses, SRS score and DRD4 genotype. The overall MOTWIN sample (1,608 twins in 804 families) was enriched approximately four fold for ADHD through the use of parent-rated screening questions regarding inattentive symptoms (Neuman et al., 2005; Volk et al., 2005). It is virtually entirely Caucasian (>95%), and a majority of subjects (62%) are male as a result of enrichment for ADHD. DSM-IV ADHD diagnoses were obtained using the Missouri Assessment of Genetics Interview for Children, whose validity and reliability has been established (Todd, Joyner, Heath, Neuman, & Reich, 2003). The Social Responsiveness Scale (SRS) is a 65-item questionnaire measuring parent-reported autistic behaviors (Constantino & Gruber, 2005). While its emphasis is on deficits in reciprocal social behavior, it also includes some items related to communication and and stereotyped/repetitive behaviors.
Data Analysis
Subjects were classified as having a clinically elevated SRS score (“high SRS”) if their score was 2.5 standard deviations above a sex-specific mean obtained from an epidemiological sample of twins (Constantino & Todd, 2003). The rationale for this method of dichotomizing high vs. low SRS score in the current twin sample is described elsewhere (Reiersen et al., 2007). A binary variable indicating whether subjects had “high SRS” was used as the dependent variable in logistic regression analyses examining the relationship between DRD4 genotype and high SRS score. The main analysis focused on the association between the DRD4 7-repeat allele (risk allele), and presence of high SRS score among subjects with DSM-IV ADHD. Standard errors were adjusted for familial clustering (non-independence within twin pairs) using the “cluster” option available in STATA. We also examined the distribution of SRS scores among subjects with DSM-IV ADHD who did or did not carry the risk allele using kernel density estimation.
Results
Sample characteristics are summarized in Table 1. After adjusting for age and gender, the DRD4 7-repeat allele was associated with high SRS score in individuals with a DSM-IV ADHD diagnosis (n=142, OR= 3.04, 95% CI: 1.28–7.24, p = 0.012); but not in individuals without DSM-IV ADHD (n = 812, OR= 1.15, 95% CI: 0.52–2.52, p = 0.722).
Table 1.
Sample characteristics
| Entire sample N=954 | No ADHD N=812 | Inattentive N=77 | Hyperactive N=14 | Combined N=51 | |
|---|---|---|---|---|---|
| Male | 583 (61.1%) | 464 (57.1%) | 63 (81.8%) | 11 (78.6%) | 45 (88.2%) |
| Female | 371 (38.9%) | 348 (42.9%) | 14 (18.2%) | 3 (21.4%) | 6 (11.8%) |
| Age (± SD) | 14.1 (± 4.2) | 14.2 (± 4.2) | 13.7 (± 4.6) | 13.3 (± 5.1) | 12.9 (± 3.5) |
| High SRS* | 76 (7.8%) | 41 (5.1%) | 15 (19.5%) | 0 (0%) | 20 (39.2%) |
| Mean SRS (± SD) | 36.8 (± 26. 6) | 32.5 (± 22.8) | 55.3 (± 31.4) | 47.8 (± 23.1) | 74.4 (± 33.2) |
| DRD4-7 carriers | 251 (26.3%) | 212 (26.1%) | 17 (22.1%) | 3 (21.4%) | 19 (37.2%) |
| Non-carriers | 703 (73.7%) | 600 (73.9%) | 60 (77.9%) | 11 (78.9%) | 32 (62.8%) |
Defined as 2.5 SD above gender-specific norms (see data analysis section).
Further, the effect is strongest in the DSM-IV combined subtype (n=51, OR= 4.31, 95% CI: 1.25–14.8, p = 0.021). Although the inattentive subtype has a higher prevalence of high SRS than controls (20% on n = 80; compared to 5.2% on n=844; χ2 = 26.1, p < 0.0001), the difference is not mediated by DRD4 genotype (n = 77, OR= 1.83, 95% CI: 0.51–6.60, p = 0.352).
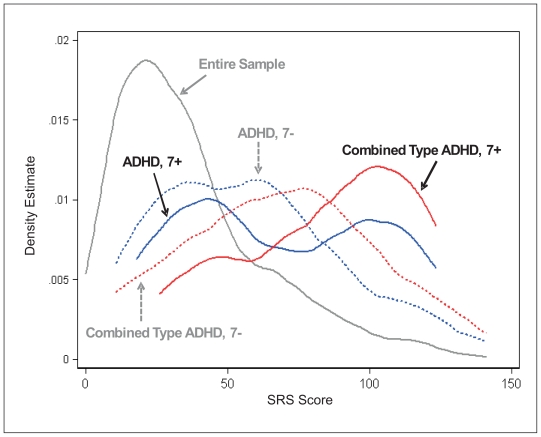
We then examined the distribution of SRS scores as a function of DSM-IV diagnosis and DRD4 genotype. In individuals with ADHD (any type), this distribution is distinctly bimodal for carriers of the DRD4 7-repeat allele (Figure 1). The source of this bimodality is driven primarily by the combined subtype. In that subgroup, the distribution is clearly shifted toward higher SRS scores, and even more so for carriers of the DRD4 7-repeat allele.
Figure 1.
In the sample as a whole (N = 954), SRS scores cluster in the normal range (roughly, 0 to 50; gray line). There is a noticeable shift towards higher elevated SRS scores in the presence of ADHD (any type, N=142) in both carriers (solid blue line, N=39) and non-carriers (dashed blue line, N=103) of the DRD4 7-allele, with a suggestion of bi-modality amongst carriers. Finally, the trend towards higher scores is especially salient in children with DSM-IV combined type ADHD for both 7-repeat allele carriers (solid red line, N=19) and non-carriers (dashed red line, N=32). The data suggest that the bimodality observed in the SRS scores for ADHD children as a whole is driven by the subset of 19 children who present with combined type ADHD and carry the DRD4 7-allele.
Discussion
In this sample, the DRD4 7-repeat allele is associated with clinically elevated autistic traits (high SRS score) among individuals with DSM-IV ADHD, consistent with previous findings in the same sample that used population-defined criteria (Reiersen, Neuman et al., 2008). It may be that the DRD4 gene influences the severity of autistic social impairment among individuals who have ADHD but has little effect on social behaviors in people who lack ADHD symptoms. Forms of ADHD that are associated with the DRD4 7-repeat allele might generally be characterized by an increased level of autistic social impairment. However, not all ADHD subjects with the 7-repeat allele had clinically elevated SRS scores. The distribution of SRS scores is bimodal among children with DSM-IV ADHD who have the DRD4 7-repeat allele. It is possible that this is an artifact of the ascertainment procedure and relatively small sample size, so replication in a larger sample is important. However, the bimodal distribution is consistent with the interaction of DRD4 genotype with additional, unmeasured factors (including, perhaps, gene-gene interactions). The bimodal distribution is also consistent with suggestions by others that DRD4 may actually be a “plasticity gene” rather than a vulnerability gene (Belsky et al., 2009). According to this hypothesis, the 7-repeat allele could have either positive or negative influences on behavior, depending on environmental and other genetic background. In the specific case of the relationship between DRD4 and SRS score, the presence of the 7-repeat allele may tend to push the level of social functioning toward the more competent range in the presence of favorable conditions, or toward the social impairment range in the presence of adverse conditions. Of note, a previous study by Grady and colleagues found no evidence for increased frequency of rare DRD4 exon 3 alleles in a sample of 68 probands with autism (Grady et al., 2005). However, that study required probands to meet criteria for autistic disorder on the ADOS-G and ADI-R. It is possible that DRD4 polymorphisms have more influence on milder forms of ASD such as those that are likely to be present in our own sample. We do not have a measure of ASD other than the SRS, so cannot be certain whether any of our study subjects met criteria for autistic disorder. However, the study protocol would have excluded subjects whose parents reported the child had been diagnosed with autistic disorder, so we assume that most if not all ASD cases in our sample have a disorder that is more consistent with Asperger’s Disorder or Pervasive Developmental Disorder Not Otherwise Specified than autistic disorder. Future studies investigating the association of DRD4 with ASD symptoms among subjects with ADHD should include more detailed clinical assessment for ASD and should also attempt to disentangle true autistic social deficits from abnormalities of social behavior that may be a direct result of ADHD symptoms or other co-occurring psychopathology.
This study has some limitations. Most importantly, the number of subjects with complete data and an ADHD diagnosis is too small to allow detailed statistical analysis of gene-gene interaction, gene-environment interaction, and allelic heterogeneity effects, which are potential explanations for the bimodality observed in the SRS score distribution among ADHD subgroups carrying the 7-repeat allele. Because the sample was enriched for ADHD through screening questions assessing inattention, the results could also be affected by sampling artifacts. Also, we did not have any measure of ASD other than the SRS, so it is not possible to confirm whether individual subjects actually met criteria for an autism spectrum disorder. We can only use the proxy of high SRS score to estimate the number who have a level of autistic symptoms that is consistent with ASD.
In the search for mechanisms for the co-occurrence of ADHD along with ASD and other forms of psychopathology, it is important to consider the possibility that some genetic polymorphisms may influence overall stability of nervous system development. Thus, the presence of a particular “susceptibility gene” may increase the risk for multiple different disorders, and the particular disorder(s) that appear may depend on random events during development, epistatic interactions with other genes, and/or environmental influences. ADHD is not the only neurodevelopmental disorder that shows evidence of genetic overlap with autism - recent studies suggest that ADHD, tic disorders, and developmental coordination disorders may be influenced by genetic and environmental factors that also contribute to the presence of ASD symptoms (Lichtenstein, Carlstrom, Rastam, Gillberg, & Anckarsater, 2010). In addition to single nucleotide and variable number of tandem repeat polymorphisms, some specific copy number variations (insertions or deletions that may encompass multiple genes in a region) can be variably expressed as multiple types of neurodevelopmental disorders (Elia et al., 2009). While some genetic polymorphisms may affect general susceptibility to multiple disorders, others may be much more specific in causing a particular disorder (or disorder subtype). When conducting linkage or association studies focusing on the mechanisms of comorbidity, it may be very helpful to compare the results obtained for phenotypes based on symptoms of just one disorder with the results obtained using a combined symptom phenotype. For example, in a QTL linkage study of Autistic symptoms in the ADHD-enriched IMAGE sample, Nijmeijer and colleagues found differences in some QTL linkage peaks depending on whether they used a phenotype considering autistic symptoms alone vs. a phenotype that removed the effect of ADHD symptoms (Nijmeijer et al., 2010). They hypothesized that one of their peaks might be pleiotropic for ADHD and ASD because the peak showed reduced height when ADHD symptoms were controlled for. Other peaks were thought to be relatively specific for ASD symptoms since they had not been found in previous studies of ADHD linkage and remained as significant regardless of whether ADHD symptoms were included as a covariate.
Also, the use of DSM-defined behavioral symptoms may not be adequate to fully define phenotypes for genetic studies. Even when we have used methods such as latent class analysis in an attempt to reduce phenotypic and genetic heterogeneity, the resulting subtypes are not very stable across time (Todd et al., 2008), so may not be ideal for family genetic studies in which we would like to classify subjects as affected or unaffected by lifetime ADHD. Including symptoms of multiple disorders in a latent class analysis (Nestadt et al., 2009; Volk et al., 2005) and consideration of longitudinal course may produce useful complex phenotypes for genetic study. For some neurodevelopmental disorders, the inclusion of potential endophenotypes such as cognitive performance measures and neuroimaging findings may be critical in the definition of phenotypes for genetic study.
Along with other research suggesting etiological overlap between disorders, the current work is relevant to the diagnostic nosology of co-occurring disorders. On one hand, giving separate diagnoses due to the presence of two or more types of symptoms may incorrectly imply that the disorders are entirely separable or have different causes; but on the other hand, forbidding co-diagnosis in cases where both types of symptoms could be due to a single etiology may lead to an incomplete description of the problem and failure to address those symptoms that are not part of the more prominent disorder’s definition. Based on the frequent co-occurrence of ADHD and ASD symptoms, we previously recommended that the DSM exclusion criterion forbidding co-diagnosis of ADHD and ASD be abandoned (Reiersen & Todd, 2008), and continue to support this view. However, even if this is done, we may not have an ideal method of classifying individuals who present with a complex mixture of neurodevelopmental symptoms. Some children with a combination of ADHD plus ASD, motor coordination problems, mood disorders, and/or other issues will be considered as having multiple comorbid DSM diagnoses. Other individuals with impairing symptoms in multiple areas (but not enough symptoms in one diagnostic category for a major DSM diagnosis) might be classified as having multiple sub-threshold “not otherwise specified” diagnoses; however, this type of classification ignores the possibility that such a complex set of symptoms may some-times represent a single, but multifaceted, neurodevelopmental disorder. A number of strategies have been proposed to categorize children with complex symptoms, including Deficits in Attention, Motor Control, and Perception (DAMP) (Gillberg, 2003), Multiple Complex Developmental Disorder (Towbin, Dykens, Pearson, & Cohen, 1993), Multidimensionally impaired (Kumra et al., 1998), various “dysregulation” syndromes (Althoff, Rettew, Ayer, & Hudziak, 2010; Brotman et al., 2006), and “atypical brain development”. (Gilger & Kaplan, 2001; B. Kaplan, Crawford, Cantell, Kooistra, & Dewey, 2006; B. J. Kaplan, Dewey, Crawford, & Wilson, 2001). Some of these are partially overlapping constructs, so it may not make sense to include every one of them in the same diagnostic nosology, but all represent attempts to describe how multiple neurodevelopmental and/or psychiatric symptoms have a tendency to occur together. Rather than creating several new complex clinical diagnostic categories, another strategy is to routinely assess children with any neurodevelopmental disorder using quantitative measures of severity in multiple symptom domains. In order to increase the usefulness of this strategy for directing appropriate treatments, it would be helpful to see more randomized controlled treatment trials that target various symptom dimensions in children with complex presentations. Treatment studies that focus on one disorder and exclude individuals with multiple disorders do not give us the information we need to treat children with a more complex set of symptoms.
The results presented here support the practice of assessing for multiple types of developmental abnormalities and psychopathology in children and adolescents who present with ADHD or ASD. It is not known at the moment why some children with ADHD and the DRD4 7-repeat allele have clinically elevated SRS scores and others do not, but if hypotheses regarding the effects of plasticity genes (Belsky et al., 2009) are correct, the same children who have increased susceptibility to developing poor social functioning under certain conditions, might also have increased response to enriched environment or clinical interventions that may improve social competence. Thus, it should not be assumed that individuals with ADHD plus ASD symptoms will have a poor prognosis regardless of treatment interventions. Further work is needed to determine whether such individuals respond best to specific types of intervention.
Acknowledgements / Conflicts of Interest
Supported by grants R01-MH-052813, R01-MH-083823 and K08-MH-080287 from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health. The authors have no financial relationships to disclose.
References
- Althoff RR, Rettew DC, Ayer LA, Hudziak JJ. Cross-informant agreement of the Dysregulation Profile of the Child Behavior Checklist. Psychiatry Research. 2010;178(3):550–555. doi: 10.1016/j.psychres.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, et al. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biological Psychiatry. 2006;60(9):991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Manual. Los Angeles, California: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of General Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Elia J, Gai X, Xie HM, Perin JC, Geiger E, Glessner JT, et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Molecular Psychiatry. 2010;15(6):637–46. doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. Journal of Autism and Developmental Disorders. 2006;36(2):271–283. doi: 10.1007/s10803-005-0060-3. [DOI] [PubMed] [Google Scholar]
- Gilger JW, Kaplan BJ. Atypical brain development: a conceptual framework for understanding developmental learning disabilities. Developmental Neuropsychol. 2001;20(2):465–481. doi: 10.1207/S15326942DN2002_2. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Deficits in attention, motor control, and perception: a brief review. Archives of Diseases in Childhood. 2003;88(10):904–910. doi: 10.1136/adc.88.10.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Schwebach AJ. The comorbidity of Pervasive Developmental Disorder and Attention Deficit Hyperactivity Disorder: results of a retrospective chart review. Journal of Autism and Developmental Disorders. 2004;34(3):329–339. doi: 10.1023/b:jadd.0000029554.46570.68. [DOI] [PubMed] [Google Scholar]
- Grady DL, Harxhi A, Smith M, Flodman P, Spence MA, Swanson JM, et al. Sequence variants of the DRD4 gene in autism: further evidence that rare DRD4 7R haplotypes are ADHD specific. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;136B(1):33–35. doi: 10.1002/ajmg.b.30182. [DOI] [PubMed] [Google Scholar]
- Hattori J, Ogino T, Abiru K, Nakano K, Oka M, Ohtsuka Y. Are pervasive developmental disorders and attention-deficit/hyperactivity disorder distinct disorders? Brain and Development. 2006;28(6):371–374. doi: 10.1016/j.braindev.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Bolte S, Poustka F. Attention deficit hyperactivity disorder symptoms in pervasive developmental disorders: association with autistic behavior domains and coexisting psychopathology. Psychopathology. 2007;40(3):172–177. doi: 10.1159/000100007. [DOI] [PubMed] [Google Scholar]
- Kaplan B, Crawford S, Cantell M, Kooistra L, Dewey D. Comorbidity, co-occurrence, continuum: What’s in a name? Child: Care, Health and Development. 2006;32(6):723–731. doi: 10.1111/j.1365-2214.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Dewey DM, Crawford SG, Wilson BN. The term comorbidity is of questionable value in reference to developmental disorders: data and theory. Journal of Learning Disabilities. 2001;34(6):555–565. doi: 10.1177/002221940103400608. [DOI] [PubMed] [Google Scholar]
- Kumra S, Jacobsen LK, Lenane M, Zahn TP, Wiggs E, Alaghband-Rad J, et al. “Multidimensionally impaired disorder”: is it a variant of very early-onset schizophrenia?”. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37(1):91–99. doi: 10.1097/00004583-199801000-00022. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Carlstrom E, Rastam M, Gillberg C, Anckarsater H. The Genetics of Autism Spectrum Disorders and Related Neuropsychiatric Disorders in Childhood. American Journal of Psychiatry. 2010;167(11):1357–63. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Di CZ, Riddle MA, Grados MA, Greenberg BD, Fyer AJ, et al. Obsessive-compulsive disorder: subclassification based on co-morbidity. Psychological Medicine. 2009;39(9):1491–1501. doi: 10.1017/S0033291708004753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biological Psychiatry. 2007;61(12):1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Neuman RJ, Sitdhiraksa N, Reich W, Ji TH, Joyner CA, Sun LW, et al. Estimation of prevalence of DSM-IV and latent class-defined ADHD subtypes in a population-based sample of child and adolescent twins. Twin Research and Human Genetics. 2005;8(4):392–401. doi: 10.1375/1832427054936646. [DOI] [PubMed] [Google Scholar]
- Nijmeijer JS, Arias-Vasquez A, Rommelse NN, Altink ME, Anney RJ, Asherson P, et al. Identifying loci for the overlap between attention-deficit/hyperactivity disorder and autism spectrum disorder using a genome-wide QTL linkage approach. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(7):675–685. doi: 10.1016/j.jaac.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J, Neuman RJ. Prenatal smoking and attention-deficit/hyperactivity disorder: DRD4-7R as a plasticity gene. Biological Psychiatry. 2009;66(4):e5–6. doi: 10.1016/j.biopsych.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Grimmer M, Martin NG, Todd RD. Evidence for shared genetic influences on self-reported ADHD and autistic symptoms in young adult Australian twins. Twin Research and Human Genetics. 2008;11(6):579–585. doi: 10.1375/twin.11.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Todd RD. Co-occurrence of motor problems and autistic symptoms in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(6):662–672. doi: 10.1097/CHI.0b013e31816bff88. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. Journal of Child Psychology and Psychiatry. 2007;48(5):464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Neuman RJ, Reich W, Constantino JN, Volk HE, Todd RD. Intersection of autism and ADHD: Evidence for a distinct syndrome influenced by genes and by gene-environment interactions. In: Hudziak JJ, editor. Developmental Psychopathology and Wellness: Genetic and Environmental Influences. Arlington, VA: American Psychiatric Publishing, Inc; 2008. pp. 191–208. [Google Scholar]
- Reiersen AM, Todd RD. Co-occurrence of ADHD and autism spectrum disorders: phenomenology and treatment. Expert Review of Neurotherapeutics. 2008;8(4):657–669. doi: 10.1586/14737175.8.4.657. [DOI] [PubMed] [Google Scholar]
- Ronald A, Edelson LR, Asherson P, Saudino KJ. Exploring the relationship between autistic-like traits and ADHD behaviors in early childhood: findings from a community twin study of 2-year-olds. Journal of Abnormal Child Psychology. 2010;38(2):185–196. doi: 10.1007/s10802-009-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of Child Psychology and Psychiatry. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- Santosh PJ, Mijovic A. Social impairment in Hyperkinetic Disorder - relationship to psychopathology and environmental stressors. European Child and Adolescent Psychiatry. 2004;13(3):141–150. doi: 10.1007/s00787-004-0372-4. [DOI] [PubMed] [Google Scholar]
- Smalley SL, Loo SK, Yang MH, Cantor RM. Toward localizing genes underlying cerebral asymmetry and mental health. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2005;135(1):79–84. doi: 10.1002/ajmg.b.30141. [DOI] [PubMed] [Google Scholar]
- Sturm H, Fernell E, Gillberg C. Autism spectrum disorders in children with normal intellectual levels: associated impairments and subgroups. Developmental Medicine and Child Neurology. 2004;46(7):444–447. doi: 10.1017/s0012162204000738. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Todorov AA, Neuman RJ, Reiersen AM, Henderson CA, et al. Predictors of stability of attention-deficit/hyperactivity disorder subtypes from childhood to young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(1):76–85. doi: 10.1097/chi.0b013e31815a6aca. [DOI] [PubMed] [Google Scholar]
- Todd RD, Joyner CA, Heath AC, Neuman RJ, Reich W. Reliability and stability of a semistructured DSM-IV interview designed for family studies. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(12):1460–1468. doi: 10.1097/00004583-200312000-00013. [DOI] [PubMed] [Google Scholar]
- Todd RD, Neuman RJ. Gene-environment interactions in the development of combined type ADHD: evidence for a synapse-based model. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2007;144B(8):971–975. doi: 10.1002/ajmg.b.30640. [DOI] [PubMed] [Google Scholar]
- Towbin KE, Dykens EM, Pearson GS, Cohen DJ. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32(4):775–782. doi: 10.1097/00004583-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Volk HE, Neuman RJ, Todd RD. A systematic evaluation of ADHD and comorbid psychopathology in a population-based twin sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(8):768–775. doi: 10.1097/01.chi.0000166173.72815.83. [DOI] [PubMed] [Google Scholar]
- Volk HE, Todorov AA, Hay DA, Todd RD. Simple identification of complex ADHD subtypes using current symptom counts. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(4):441–450. doi: 10.1097/CHI.0b013e31819996ba. [DOI] [PMC free article] [PubMed] [Google Scholar]