Abstract
Objective
The aim of the current study was to investigate the sex and age effects on the mean levels and the genetic architecture of adolescent self-reported emotional and behavioral problems.
Method
Survey data on psychopathology as assessed by the Youth Self Report (YSR; Achenbach & Rescorla, 2001) were collected in a large sample of Dutch adolescent twins and their non-twin siblings (6381 twins and 1195 siblings from 3511 families) aged 12 to 20 years. Sex and age effects on the levels of emotional and behavioral problems and on the genetic architecture were investigated using genetic structural equation modeling.
Results
For all syndrome scales of the YSR (except for Aggressive Behavior) and for the broadband scales Internalizing and Externalizing sex-differences in mean levels were found. Females score higher than males on Internalizing problems and its subscales (Anxious/Depressed and Withdrawn/Depressed), while males score higher than females on Externalizing behavior and its subscale Rule-Breaking. Age-effects on mean levels vary in strength and direction by syndrome and sex. An increase in problems with increasing age was seen for Anxiety/Depressed, while a decrease was observed for Somatic Complaints, Aggressive Behavior, and Social and Thought Problems. Significant genotype interactions with age and with sex were found for most scales of the YSR. These effects vary in strength and direction, resulting in differences in genetic architecture between males and females and developmental changes in genetic architecture throughout adolescence. For example the heritability for Anxious/Depressed and Withdrawn/Depressed behavior increases and the heritability of Externalizing behavior decreases throughout adolescence.
Conclusions
Age and sex are found to be important moderators of both mean levels and the heritability of self-reported adolescent emotional and behavioral problems. Differences between adolescents in YSR syndrome and broadband scales are accounted for by genetic and non-shared environmental influences. We observed no influence of shared environment in this large sample. Clinical implications of the age and sex effects on the genetic architecture are discussed.
Keywords: heritability, psychopathology, adolescence, youth self report, age
Résumé
Objectif
Étudier le rôle du sexe et de l’âge sur le niveau moyen et l’architecture génétique des problèmes affectifs et comportementaux signalés par des sujets des deux sexes.
Méthodologie
Évaluation de la psychopathologie d’un échantillon de 6 381 jumeaux néerlandais et de 1 195 frères et sœurs de ceux-ci. Les sujets, âgés de 12 à 20 ans et issus de 3 511 familles, ont rempli le Youth Self Report (Achenbach & Rescorla, 2001). L’influence du sexe et de l’âge des sujets sur la sévérité et l’architecture génétique des problèmes a été étudiée au moyen de la modélisation génétique par équations structurelles.
Résultats
On constate des différences liées au sexe dans la note moyenne de toutes les échelles du Youth Self Report (à l’exception de l’échelle d’agressivité) et des échelles d’intériorisation et d’extériorisation à bande large. La note d’évaluation des troubles intériorisés et des sous-échelles Anxiété/dépression et de Repli sur soi/dépression était plus élevée chez les adolescentes que chez les adolescents, tandis que la note d’évaluation des troubles extériorisés et de la sous-échelle Non-respect des règlements était plus élevée chez les adolescents que chez les adolescentes. L’influence de l’âge sur la note moyenne variait en intensité et en direction selon le syndrome et le sexe des sujets. L’anxiété et la dépression s’aggravaient avec l’âge, tandis que les douleurs somatiques, l’agressivité, les difficultés de socialisation et de conceptualisation s’atténuaient. La plupart des échelles du Youth Self Report indiquaient une interaction significative du génotype avec l’âge et le sexe. L’intensité et la direction de ces interactions variaient: l’architecture génétique différait entre adolescents et adolescentes, et était sujette à des modifications développementales tout au long de l’adolescence; pendant cette période, l’héritabilité de l’anxiété/de la dépression et du repli sur soi/de la dépression était plus marquée que l’héritabilité des troubles extériorisés.
Conclusions
L’âge et le sexe sont des modérateurs importants de la note moyenne des problèmes affectifs et comportementaux, et de l’héritabilité des problèmes signalés dans le Youth Self Report. Les différences constatées dans les syndromes et les échelles d’intériorisation et d’extériorisation à bande large s’expliquent par la génétique et les influences environnementales non partagées. L’environnement partagé n’avait aucune influence sur ce vaste échantillon. L’article présente les implications cliniques dues à l’âge et au sexe sur l’architecture génétique des problèmes étudiés.
Mots clés: héritabilité, psychopathologie, adolescence, Youth Self Report, âge
Introduction
Over the years ample research has been dedicated to unravel the sources of individual differences in childhood problem behavior. For most behavioral and emotional problems, except for Attention Problems, estimates of the influences of genetic factors vary across development (e.g. Bartels et al., 2007; Hudziak et al., 2003; Legrand et al., 1999; Kendler et al., 2008). Changes in the magnitude of genetic influences seem inversely related to changes in the importance of environmental influences shared by children growing up in the same family. Non-shared environmental influences (influences unique to a child) remain more or less constant. Furthermore, longitudinal studies indicate that stability of emotional and behavioral problems throughout childhood are mainly accounted for by genetic factors (Bartels et al, 2004 & 2007; Boomsma et al., 2005; Haberstick et al., 2005; Hoekstra et al., 2008; Van Beijsterveldt, 2004; Van Grootheest et al., 2004; Rietveld et al., 2004).
Less attention so far has been paid to causes of individual differences in behavioral and emotional problems during one of the most important age periods in human life; adolescence. This is surprising since it has been revealed that the peak age of onset for many psychiatric disorders is adolescence (e.g. Paus et al., 2008). Adolescence is the starting point of emerging, mainly individual specific, environmental opportunities, which could result in an increase of non-shared environmental variance and a decrease of shared environmental and possibly genetic variance. We observed this phenomenon for Attention Problems (Van den Berg et al., 2006) for which the heritability decreased from ~75% in childhood to around 40% in adolescence. Furthermore, the emotionally and biologically challenging period of adolescence with both changes in endocrine levels and changes in the environment may lead to changes in gene-expression, which could alter the heritability of a trait.
The extent to which these changes lead to changes in heritability estimates and the standardized influence of shared and non-shared environment on a phenotype at a certain age can be explored in genetically informative designs such as employed in twin-family studies. Mechanisms, such as gene-environment correlation (genetic control over environmental exposure), may also result in changing heritabilities over age. Gene-environment (GE) correlations in childhood are probably the result of passive (the association between the genotype a child inherits from her parents and the environment in which the child is raised) and reactive (an individual’s behavior evokes an environmental response) GE correlations, while adolescents and adult environments are probably increasingly the result of active (an individual possesses a heritable propensity to select environmental exposure) GE correlation (Plomin et al., 1977; Scarr & McCartney, 1983). In a recent meta-analysis (Bergen et al., 2007) the pattern of heritability estimates for several adolescent emotional and behavioral problems was studied. For most problem behaviors, heritability estimates increased throughout adolescence and the active rGE theory is proposed as part of the explanation, while accumulating effects of gene-expression or the expression of novel gene sets (e.g. due to maturation processes) is not ruled out. Other studies focusing on adolescent behavior support the results from this meta-analysis. For example, Van Grootheest et al., (2008) found that the influences of shared environment on the Obsessive Compulsive scale of the YSR decrease from age 12 to 16, with a parallel increase in heritability estimates. A recent study by Lamb et al. (2010) on the change in genetic architecture of Anxious/Depressed and Withdrawn/Depressed Behavior also revealed that shared environmental influences are significant at age 12 but diminish at age 14 and 16, with a parallel increase of genetic influences. The presence or absence of shared environmental influences on childhood and adolescent psychopathology is currently under debate. Burt (2009) concluded in a recent meta-analysis that shared environmental influences generally range from 10 to 30%, with the largest effects of shared environment in mid-childhood and diminishing effects during the transition from childhood to adolescence. This implies that samples of sufficient size are required to detect the effects of shared environment (e.g. Martin, Eaves, Kearsey, & Davies, 1978; Rietveld et al., 2003; Posthuma & Boomsma, 2000).
Adolescence is, besides a period that is associated with increased vulnerability to psychopathology, also a time of increased behavioral divergence between males and females (Paus, Keshavan, & Giedd, 2008). This is, for example, reflected in a disparity in rates of onset, course, and symptomatology of common psychiatric disorders. Prior to adolescence prevalence of Anxious/Depressed Behavior is more or less similar for boys and girls. With the onset of puberty, prevalences in females double, while in males prevalences stay approximately the same (Angold, Costello, & Worthman, 1998). Additionally to these sex-differences in mean levels, puberty with its biological changes, probably also induces sex-differences in the genetic architecture of emotional and behavioral problems. These effects, though, will be syndrome specific. For example, Lamb et al, (2010) found besides the increasing divergence in prevalence between males and females during adolescence, no sex-differences in heritability of Anxious/Depressed Behavior. In contrast, for Externalizing behavior during late adolescence, Hicks and colleagues (2007) revealed increasing genetic variation and heritability for males but a trend toward decreasing genetic variation and increasing environmental effects for females.
In the current study, we add to previous studies in adolescents, by investigating the effects of age and sex on the levels and the genetic architecture of adolescent emotional and behavioral problems in a large sample of adolescents twins and their non-twin silbings. Self-report data on the eight syndrome scales (Anxious/Depressed, Withdrawn/Depressed, Somatic Complaints, Social Problems, Thought Problems, Attention Problems, Rule-Breaking, Aggressive Behavior) and the two broadband scales (Internalizing and Externalizing) of the Youth Self Report (Achenbach and Rescorla, 2001) were analyzed. The large genetically informative sample consisted of Dutch adolescent twins and their non-twin siblings (6381 twins and 1195 siblings from 3511 families), aged 12 to 20. We investigated for all YSR syndrome and broadband scales:
Differences in levels as a function of age and sex
Qualitative sex differences in the genetic architecture;
Genotype by age interactions;
Genotype by sex interactions.
Genetic structural equation modeling was used to formulate a comprehensive framework for hypothesis testing. We expect that age and sex modify both mean levels as well as the genetic architecture of psychopathology throughout adolescence. We furthermore expect that these effects are syndrome specific, meaning that the sex and/or age effects vary by syndrome and are significant for some YSR scales but not for all scales.
Methods
Subjects
Participants were registered at birth with the Netherlands Twin Registry (NTR), established by the Department of Biological Psychology at the VU University in Amsterdam. In 2005, the Y-NTR started to collect data among adolescent twins and their non-twin siblings (Boomsma et al., 2006; Bartels et al., 2007a). During adolescence, 14-, 16-, and 18-years old twins, and their non-twin siblings (aged 12 to 25) received a self-report survey. Before inviting twins and their non-twin siblings to provide self-report data, parents were contacted to ask for written permission to send their children a self-report survey and to register non-twin siblings of the twins. Upon parental consent, questionnaires were sent to the twins and their non-twin siblings. In the beginning the survey was presented in a paper and pencil version. In 2009 survey collection started with an online version of the questionnaire and the paper and pencil version was used as a second reminder.
For the current study, data from surveys collected in adolescent twins (born in cohorts 1984–1997) and their non-twin siblings (aged 12 to 20 years), were analyzed. Overall response rate was 47%. The sample consisted of 7616 individuals (6381 twins and 1195 siblings from 3511 families) with a 45%–55% boy–girl distribution. Total sample constitution and sample constitution as a function of zygosity is described in table 1. Mean age of the sample is 16.47 (1.66), with age ranging from 12 to 20. Siblings were included in the analyses with a maximum of 2 siblings (1 brother and 1 sister) per family. Some families (n=30 families) had data of more than one sibling of the same sex (e.g. two non-twin sisters or two non-twin brothers). From these families data from the sibling closest in age to the twin was selected for analyses. 2459 individuals participated more than once in the longitudinal survey study. Of these individuals the most recent survey is used in the analyses. Zygosity was determined for 960 same-sex twin pairs by DNA analysis or blood group polymorphisms. For all other same-sex twin pairs, zygosity was determined by discriminant analysis, using longitudinal questionnaire items from the previously collected parental report. Agreement between zygosity assignment by the replies to the longitudinal questionnaires and zygosity determined by DNA markers/blood typing was around 93% (Rietveld et al. 2000).
Table 1.
Constitution and zygosity distribution of the sample
| Family constitution | Number of families | Zygosity | Number of families |
|---|---|---|---|
| One of the twin, no sibling | 417 | MZM | 551 |
| Twin Pair, no sibling | 1864 | DZM | 476 |
| One of the twin with a brother | 21 | MZF | 792 |
| One of the twin with a sister | 60 | DZF | 571 |
| One of the twin with a brother and a sister | 3 | DOSmf | 576 |
| Twin pair with a brother | 499 | DOSfm | 545 |
| Twin pair with a sister | 577 | In total 3511 families Cohort: 1984–1997 Mean age 16.47 (1.66) |
|
| Brother only (no twin) | 24 | ||
| Sister only (no twin) | 40 | ||
| Brother and Sister only (no twin) | 4 | ||
Non-response analyses
The moderate response rate of 47% requires non-response analyses in order to rule out nonrandom response and drop-out. Several characteristics, such as parental report history, family SES, parental education, religiousness, childhood problems behavior and educational achievement were compared for twin who returned the survey and the twins who did not. Results are summarized in table 2.
Table 2.
Prevalence and Means (SD) of childhood survey history, family, parental, and childhood variables for twins who completed the self-report survey compared to those who did not complete the self-report survey
| Non-respondents | Respondents | |
|---|---|---|
| Childhood survey history | ||
| % Returned = 6 childhood surveys | 59 | 78 |
| Family characteristics | ||
| % Religious | 67 | 67 |
| % Low/average/high SES | 4/65/31 | 3/54/43 |
| Parental characteristics | ||
| % Lower vocational education (mothers/fathers) | 38/35 | 31/32 |
| % Intermediate vocational education (mothers/fathers) | 39/39 | 42/31 |
| % Higher/scientific education (mothers/fathers) | 22/26 | 27/37 |
| % Smoking during pregnancy (mothers/fathers) | 27/41 | 20/30 |
| % alcohol consumption during pregnancy (mothers) | 22 | 21 |
| Twin characteristics during childhood | ||
| Mean level (standard deviation) of internalizing at age 3a | 4.8 (3.9) | 4.6 (3.8) |
| Mean level (standard deviation) of internalizing at age 7a | 5.4 (4.8) | 4.8 (4.8) |
| Mean level (standard deviation) of externalizing at age 12a | 4.6 (5.4) | 4.2 (4.9) |
| Mean level (standard deviation) of externalizing at age 3a | 17.4 (10.2) | 16.4 (9.8) |
| Mean level (standard deviation) of externalizing at age 7a | 8.6 (7.3) | 7.4 (6.6)* |
| Mean level (standard deviation) of Externalizing at age 12a | 6.5 (6.8) | 5.0 (5.3)* |
| % little unhappy or unhappy at age 3/7/12 | 3/9/3 | 4/7/4 |
| Mean (standard deviation) CITO scorea | 536.4 (8.8) | 538.3 (8.5)* |
| % Language/arithmetic: insufficient or weak | 9/8 | 8/5 |
| % Language/arithmetic: sufficient | 35/34 | 34/34 |
| % Language/arithmetic: good or very good | 56/58 | 58/61 |
Note. Significant difference in mean score between non-respondents and respondents (p < .01);
Because results of ANOVAs did not differ between first- and second born twins, means and standard deviation are only presented for first born twins.
Non-response analyses showed that parents of twins who did not return the survey have in the past been also less willing to participate in survey research of the Y-NTR compared parents of twins that participated. Other parental and familial characteristics, such as SES, parental education, religion, and smoking and alcohol consumption during pregnancy were similar for families of non-responding twins compared to families of responding twins. Small, but significant differences were found for Externalizing behavior at ages 7 and 12 with non-responders scoring higher. In addition responders scored significantly higher on a general test of educational achievement at age 12, but again the difference is very small. No differences are found for Internalizing problems at ages 3, 7, and 12 and Externalizing problems at age 3. Furthermore, no differences were found for specific educational achievement tests for language and arithmetic. It can be concluded that response bias does not constitute a serious threat to the validity of the results in this study.
Measures
The Dutch Health Behavior Questionnaire (DHBQ) is a self-report instrument targeting multiple domains, such as health, lifestyle, subjective wellbeing, and emotional and behavioral problems. It includes the Youth Self Report (Achenbach and Rescorla, 2001) to assess adolescent behavioral and emotional problems.
The Youth Self-Report is a screening tool for behavioral and emotional problems in adolescents that comprise the Achenbach System of Empirically Based Assessments (ASEBA). Adolescents are asked to fill out 118 items (112 items of the 2001 version supplemented with 6 items for the older version of the YSR) on a 3-point scale based on the occurrence of the behavior during the preceding 6 months: 0 if the problem item was not true, 1 if the item was somewhat or sometimes true, and 2 if it was very true or often true. The syndrome scales (ANX: Anxious/Depressed, SOM: Somatic complaints, WITH: Withdrawn/Depressed, SOC: Social Problems, THOUGHT: Thought Problems, AP: Attention Problems, RB: Rule-Breaking, AGG: Aggressive Behavior) were composed according to the 2001 profile (Achenbach & Rescorla, 2001). In addition, the broadband scales Internalizing behavior (INT: Anxious/Depressed, Somatic Complaints, and Withdrawn/Depressed) and Externalizing behavior (EXT: Rule-Breaking and Aggressive Behavior) were computed. Dutch syndrome scales and comparability with the syndrome scales developed by Achenbach are reported in Verhulst, Van der Ende, and Koot (1997).
To simplify interpretation of age effects, age was transformed into a z-score. The mean age of the sample (16.47) is represented by a z-score of 0.
Analyses
Means, variances, twin, and twin-sibling correlations were estimated for each of the 5 sex by zygosity groups (i.e. Monozygotic Male: MZM, Dizygotic Male: DZM, Monozygotic Female: MZF, Dizygotic Females: DZF, and Dizygotic Opposite sex: DOS) using the software package Mx (Neale et al., 2006). This was done in a so called saturated model which simply specifies for each sex by zygosity group that the data from the first- and second-born twin and the non-twin siblings are correlated without attempting to model these correlations as a function of genes and shared environment. Means and the covariance structure were estimated conditional on sex and age to take these variables into account. Age was included as a fixed effect (covariate) in the means model and on the covariance structure. We tested whether constraining the regression weight of age at zero led to a significant deterioration of model fit. Sex differences in means and variances for emotional and behavioral problems were tested by constraining means and variances to be equal between males and females and evaluating the decrease in goodness of fit.
Genetic analyses
Monozygotic (MZ) twins derive from a single fertilized zygote and are (nearly always) genetically identical. Less than perfect MZ twin correlations (rMZ < 1) therefore indicate environmental effects that are not shared between children growing up in the same family. Dizygotic (DZ) twins develop from two zygotes and, like non-twin siblings, share on average 50% of their segregating genes. A higher resemblance of MZ versus DZ twin pairs and twin-sibling correlations therefore may reflect the higher genetic similarity and indicates genetic effects. The design allows the estimation of environmental influences common to or shared by twins and siblings growing up in the same family. These shared environmental influences are implied if the resemblance between twin pairs and twin-siblings exceeds the resemblance expected on the basis of quantitative genetic theory (Falconer and Mackay, 1996). Data of twins and siblings provide the opportunity to decompose the variance of a trait into additive and non-additive genetic, shared environmental, and unique environmental components. Additive genetic variance (A) represents the sum of the effects of alleles over all loci that influence the trait. Non-additive genetic effects (D) comprise interaction between alleles at the same locus (dominance) or interaction between alleles at the different loci (epistasis). Shared environmental variance (C) is the part of the variance that is shared by members of a family and non-shared environmental variance (E) is the part of the total variance that is unique to a certain individual. Non-additive genetic effect and shared environmental effects are confounded in the classical twin (sibling) design and are therefore estimated in separate models. Based on the literature (e.g. Burt, 2009) and the correlations we from hereon continue with models considering additive genetic, shared environmental, and non-shared environmental effects only.
Age and sex moderation of Genetic and Environmental Variance
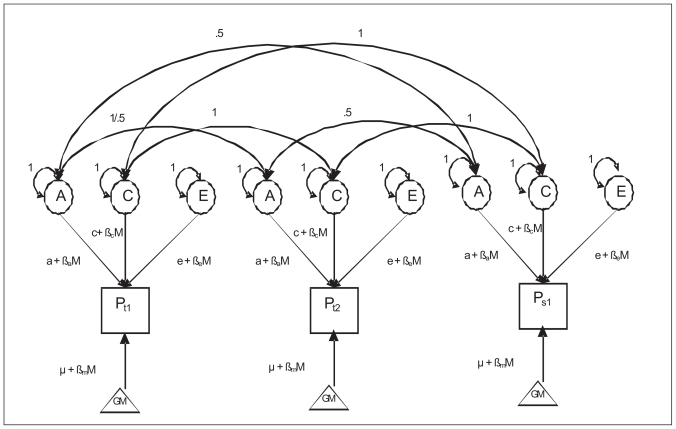
In order to test for sex-differences and developmental changes of the genetic architecture of adolescent emotional and behavioral problems a moderator model (Purcell et al., 2002) was expanded to use in an extended twin-sibling design with 5 zygosity groups (See Figure I).
Figure 1.
The moderator model. Squares represent measured variables, and circles represent latent, unobserved factors. The triangle represents the mean. The additive genetic, shared and non-shared environmental value is a linear function of the moderator M, for A represented by the equation a + βaM, where βa is the unknown parameters to be estimated from the data, representing the magnitude of the age effects.
To test for age moderation, age is included as a moderator on the genetic and environmental paths. The influence of A (additive genetic influences), C (shared environmental influences) and E (non-shared environmental influences) are represented by paths a + βaM, c + βcM, and e + βeM in which M represents the age of an individual. The value of M changes from subject to subject, taking on the value of age variable for that subject. If the β’s are significantly different from zero, there is evidence for a moderating effect on the variance components and if the β’s are significantly different for males and females, sex-differences in age-moderation is present. In other words, the moderation model allows a test whether the importance of additive genetic effects (a), shared environmental (c), and unique environmental effects (e) are changing as a linear function of age and are sex-specific. The un-moderated estimates of a, c, and e represent the path-loadings used to calculate the variance components A, C, and E at the mean age in the sample. The pathway μ + βxmM models a main effect of the moderator variable on the outcome, i.e. the effect of age on mean levels of the YSR scales.
The 5 group design enables testing for qualitative sex-differences by testing if in the opposite sex twins the genetic correlation is significantly lower than .5 and/or if the shared environmental correlation (if C matters for the phenotype) is lower than 1. Quantitative sex differences in the genetic architecture of emotional and behavioral problems were assessed by allowing the genetic (a), shared environmental (c), and non-shared environmental (e) parameter estimates to differ for males and females and we tested whether constraining these parameter estimates to be equal for males and females resulted in a significant deterioration of model fit. The statistical significance of the variance component C was assessed by testing whether fixing the corresponding parameter estimate (i.e. c) to zero resulted in a significant deterioration of model fit.
Genetic structural equation modeling in Mx (Neale et al., 2006) was used with the raw-data ML procedure for estimation of parameters. Nested sub-models (models that contain the same terms but are different in parsimony) were compared by hierarchic χ2 tests. The χ2 statistic is computed by subtracting −2LL (log-likelihood) for a reduced model from that for the full model (χ2 = −2LL0—(−2LL1)). Given that the reduced model is correct, this statistic is χ2 distributed with degrees of freedom (df) equal to the difference in the number of parameters estimated in the two models (Δdf = df0—df1).
Results
Sex and age effects on levels of behavioral problems
Means and standard deviations of the YSR syndrome and broadband scales are presented in table 3 for males and females. For all syndrome scales (except for Aggressive Behavior) and for the broadband scales Internalizing and Externalizing sex-differences in mean levels were found. Females score higher than males on Internalizing problems and its subscales (Anxious/Depressed, Withdrawn/Depressed, and Somatic Complaints), and on the Attention Problems and Thought Problems subscales, while males score higher than females on Externalizing behavior and its subscale Rule-Breaking, and on the Social Problems subscale
Table 3.
Number of individuals, grand means (at the mean age of 16.47), standard deviations, and age moderation for the YSR scales
| Males |
Females |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | M | sd | β | N | M | sd | β | |
| Anxious/depressed | 3029 | 3.13* | 3.35 | .1248 | 3726 | 4.97* | 4.48 | .1248 |
| Withdrawn/depressed | 3049 | 2.14* | 1.96 | .2391** | 3749 | 2.56* | 2.20 | ns** |
| Somatic complaints | 2979 | 1.60* | 1.93 | −.2294** | 3663 | 2.73* | 2.51 | −.1124** |
| Social problems | 3042 | 2.10* | 1.84 | ns** | 3742 | 2.01* | 1.81 | −.1873** |
| Thought problems | 3042 | 1.42* | 1.64 | ns** | 3748 | 1.65* | 1.72 | −.1341** |
| Attention problems | 3039 | 4.68* | 2.69 | ns | 3731 | 4.84* | 2.78 | ns |
| Rule-Breaking behavior | 3058 | 2.91* | 1.99 | .1403** | 3757 | 2.46* | 1.87 | ns** |
| Aggressive behavior | 3045 | 6.33 | 4.47 | −.2166 | 3738 | 6.33 | 3.99 | −.2166 |
| Internalizing | 2952 | 6.72* | 5.67 | ns | 3631 | 10.02* | 7.45 | ns |
| Externalizing | 3045 | 9.28* | 5.85 | −.1509 | 3736 | 8.69* | 5.27 | −.1509 |
mean levels (without age effect) are significantly different for males and females (p < .05)
age-effect is significant different between males and females (p < .05)
N = number of individuals; M = grand mean; sd = standard deviation; β = age moderation
Age-effects on mean levels vary by syndrome and sex and are positive for some syndromes while negative for others. The largest age effect is for Withdrawn/Depressed behavior in males (β=.2391), indicating that Withdrawn/Depressed behavior increases throughout adolescence in males. Internalizing behavior is found to be stable in this age period. For Anxious/Depressed a similar increase in problems is observed for males and females throughout adolescence, with females having a higher syndrome score over the whole period. For Withdrawn/Depressed a sex-by-age interaction is observed with females scoring higher than males at age 12, but the rise in Withdrawn/Depressed in males during adolescence results in higher levels of Withdrawn/Depressed behavior in males at the age of 20. Somatic Complaints is the only Internalizing subscale that shows a decrease in scores over age with a small sex-by-age interaction effect resulting in a steeper decline in males than females. Externalizing behavior shows a decrease over age in both males and females. This decline is driven by a decrease in Aggressive Behavior. Rule-breaking behavior is stable for females and shows a slight increase for males. Attention Problems in males and females and Thought and Social Problems in males do not change over age. A small sex-difference is observed for Attention Problems with slightly higher scores for females. For Thought Problems and Social Problems in females a decline in score is observed.
Twin-sibling correlations
Twin-sibling correlations, corrected for age, and their 95% confidence intervals for the syndrome scales are presented in table 4. The correlations indicate that genetic and non-shared environmental influences will be the expected underlying sources of individual differences in adolescent emotional and behavioral problems. This is based on the observation that the MZ correlations are significantly higher than the DZ correlations, but significantly lower than 1. Twin-sibling correlations could be constrained to be equal to the dizygotic twin correlations. Overall, except for Rule-Breaking, the opposite sex-correlation is not different from the same-sex dizygotic correlations providing no evidence for the presence of different genetic of environmental influences (qualitative sex-differences).
Table 4.
Twin-sibling correlations and their 95% confidence intervals for the YSR syndrome and broadband scales at the mean age of 16.47
| MZM | DZM/twin-brother | MZF | DZF/twin-sister | DOS/brother-sister | |
|---|---|---|---|---|---|
| Anxious/depressed | .54 (.46–.61) | .23 (.11–.33) | .43 (.37–.48) | .26 (.21–.32) | .21 (.15–.26) |
| Withdrawn/depressed | .41 (.32–.48) | .10 (.00–.19) | .43 (.37–.49) | .20 (.37–.49) | .18 (.13–.23) |
| Somatic complaints | .45 (.33–.54) | .14 (.00–.28) | .41 (.34–.47) | .19 (.13–.24) | .12 (.06–.18) |
| Social problems | .43 (.35–.51) | .09 (.00–.16) | .47 (.41–.53) | .18 (.09–.26) | .19 (.14–.23) |
| Thought problems | .39 (.30–.47) | .14 (.00–.21) | .57 (.52–.62) | .22 (.15–.28) | .17 (.11–.22) |
| Attention problems | .50 (.43–.56) | .12 (.02–.20) | .52 (.46–.57) | .28 (.21–.34) | .15 (.09–.20) |
| Rule-breaking behavior | .49 (.42–.55) | .25 (.19–.32) | .53 (.47–.58) | .33 (.26–.39) | .16 (.11–.21) |
| Aggressive behavior | .51 (.45–.57) | .16 (.10–.22) | .57 (.51–.62) | .26 (.19–.33) | .23 (.17–.27) |
| Internalizing | .57 (.50–.64) | .28 (.17–.38) | .48 (.42–.53) | .28 (.22–.34) | .22 (.16–.27) |
| Externalizing | .53 (.46–.58) | .21 (.15–.27) | .60 (.54–.64) | .32 (.25–.38) | .22 (.18–.28) |
Sex-specific Genetic analyses with Age-moderation
Detailed genetic model-fitting results for all eight subscales and the two broadband scales of the YSR are available as supplementary material at www.tweelingenregister.org (see publications > supplementary material). A summary of the overall findings is presented here.
Qualitative sex differences
For all syndrome scales no evidence was found for qualitative sex-differences in genetic influences since the genetic correlation could be constrained at .5 without a deterioration of model-fit. The absence of significant influences of shared environment for most of the syndromes made the test for qualitative sex-differences in shared environmental influences (rc <1) redundant.
Quantitative sex-differences
All syndrome and broadband scales, except for Social Problems, show quantitative sex-differences, indicating that the heritability and influences of the environment are different for males and females during adolescence. For the syndrome scales ANX, WITH, SOM, and THOUGHT heritabilities are higher for females than males, while for AP, RB, and AGG heritabilities are higher for males than females. Influences of shared environment are only observed for AP, RB, INT and EXT, but for females solely.
Age moderation on the parameter estimates for A, C, and E
There was no significant age moderation on the variance components for SOM in females and for THOUGHT, AP, and RB in males and females, indicating that the genetic architecture of these behavioral problems is stable throughout adolescence. For the other scales significant age effects on the genetic architecture are found. The age effects are different in males and females for Somatic Complaints (no age effect in females) solely, meaning that when the genetic architecture of the other syndrome scales changes over age this change is generally similar in males and females.
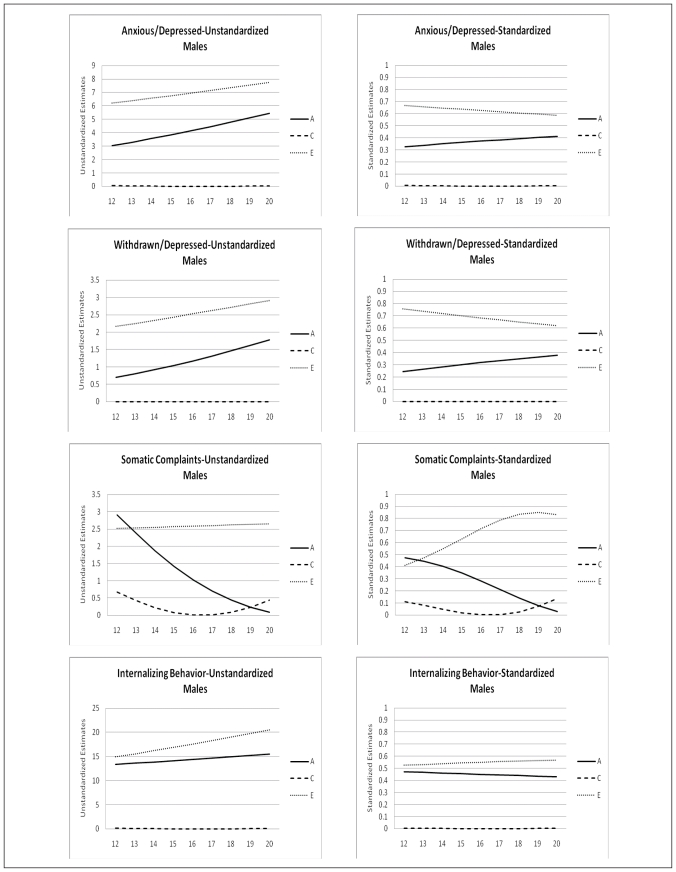
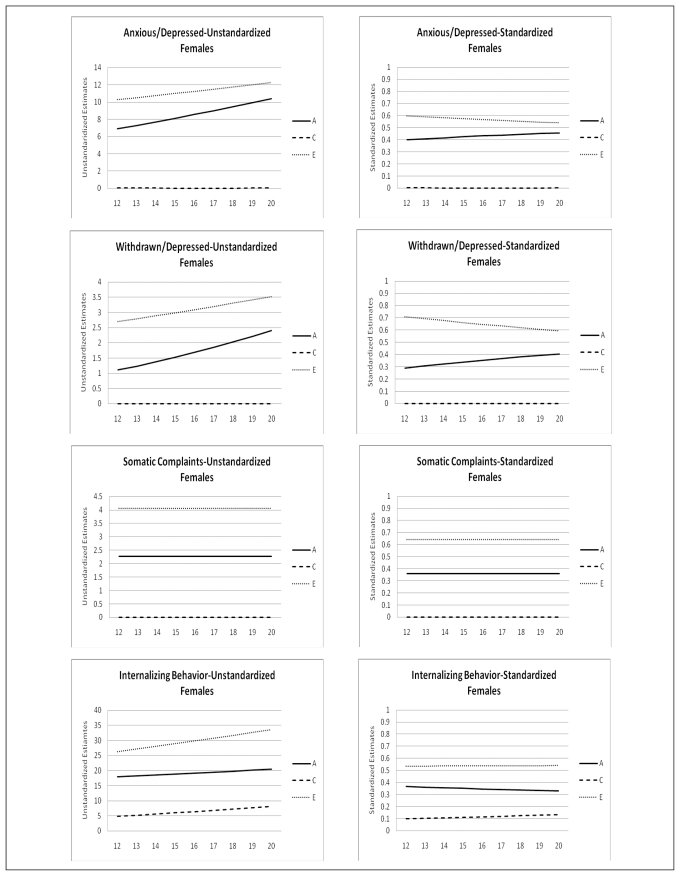
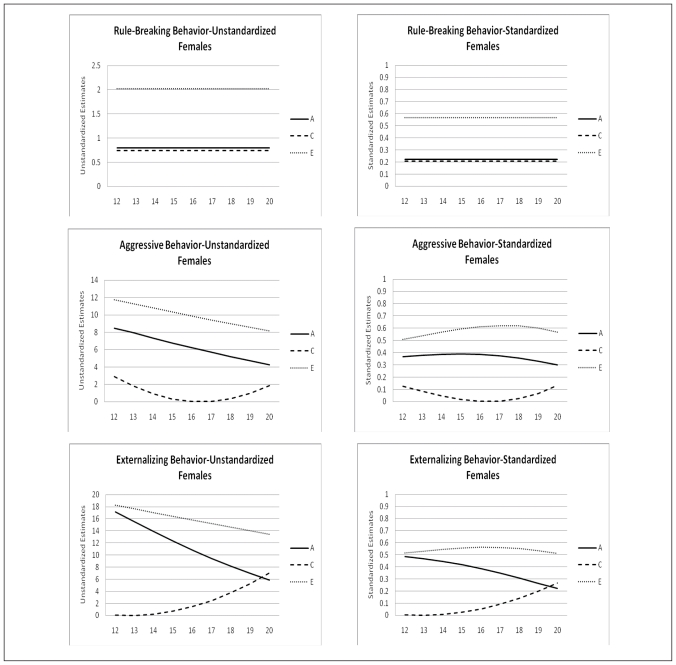
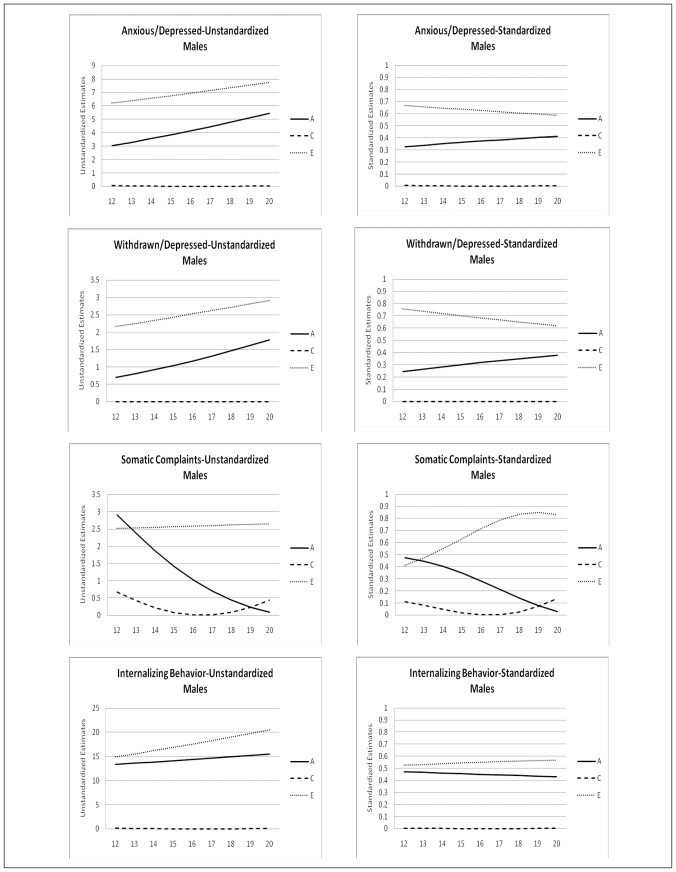
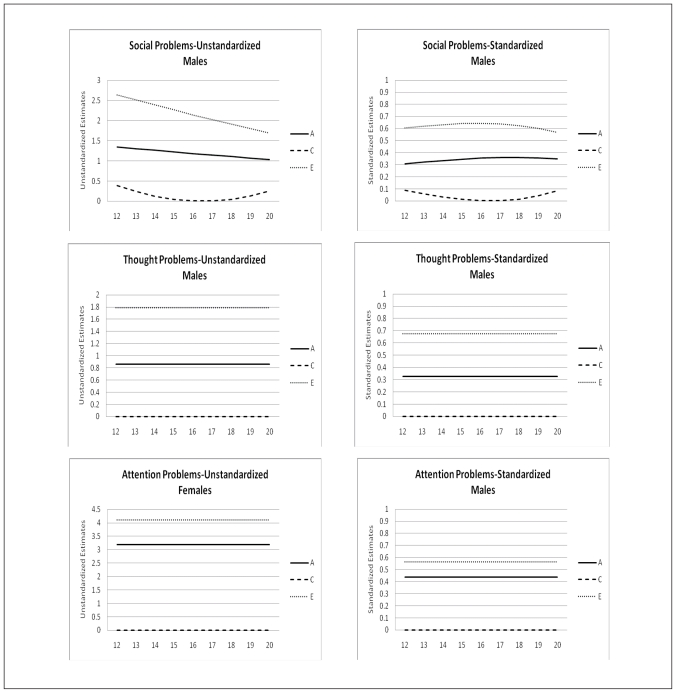
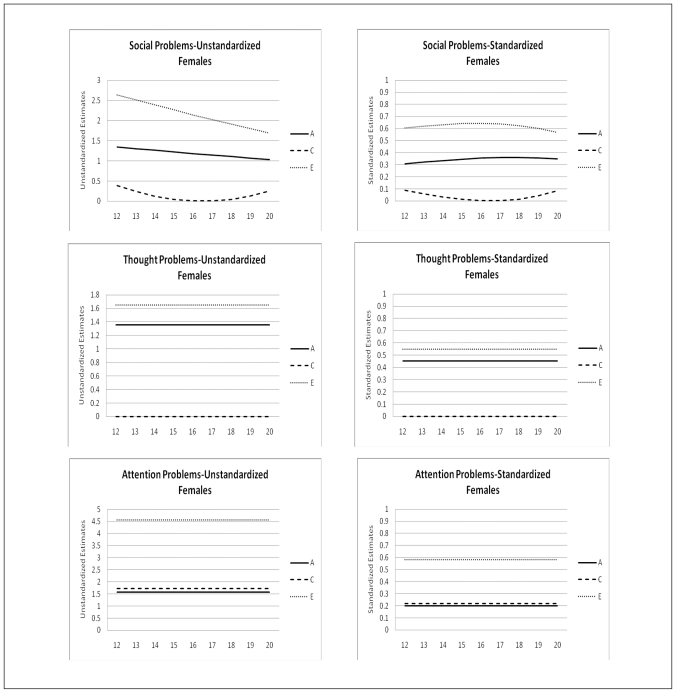
The change of the genetic architecture over age, both unstandardized as well as standardized, is visualized in figures 2a, b, and c. In the figures with unstandardized estimates it can be observed that the total variance of Internalizing behavior and its subscales (AD, WITH, and SOM) increases over age, while the total variance of Externalizing behavior and its subscales (RB and AGG) decreases over age. In general the increase and decrease can be attributed to change in both genetic variance as well as non-shared environmental variance. Remarkable is the change over age of the genetic and environmental variance of Somatic Complaints in males (variance in females is stable over age). Total variance of this syndrome decreases which is almost fully accounted for by a sharp decrease of genetic variance. Age by shared environmental influences interaction is observed for SOM, AGG, EXT, and SOC. These effects of C show the importance of including effects of age in genetic analyses, since shared environmental influences change over age with varying patterns.
Figure 2a.
Age moderation of unstandardized and standardized estimates of genetic, shared environmental, and non-shared environmental influences on Internalizing Behavior and its subscales for males and females
Figure 2b.
Age moderation of Unstandardized and Standardized Estimates of genetic, shared environmental, and non-shared environmental influences on Externalizing Behavior and its subscales for males and females
Figure 2c.
Age moderation of Unstandardized and Standardized Estimates of genetic, shared environmental, and non-shared environmental influences on Social Problems, Thought Problems and Attention Problems for males and females
Unstandardized and Standardized estimates
In table 5 standardized estimates of A, C, and E, with their 95% confidence intervals at the mean age of 16.47 are presented. For males variance is accounted for by genetic influences and non-shared environmental influences. For females a similar patterns is observed with the exception for AP, RB, INT, and EXT for which shared environmental influences play a small but significant role. Change of standardized estimates of genetic and environmental influences are visualized in figures 2a, b, and c. The effect of the change in total variance and the effect of age on the genetic architecture is clearly visible. For example for ANX and WITH these effects result in a increase in heritability and a decrease in non-shared environmental influences, while both unstandardized parameters were increasing. In line with the pattern of unstandardized estimates, the pattern of standardized estimates for SOM in males is remarkable. Heritability decreases over age and approach zero in young adulthood, while non-shared environmental influence show a parallel increase. Furthermore, remarkable is the pattern of heritability and non-shared environmental effects on AGG and EXT. Non-shared environmental influences seem to gradually take over the role of being most important factor in explaining individual differences in young adulthood in both males and females.
Table 5.
Standardized estimates (95%CI) of genetic, shared environmental and non-shared environmental influences on the YSR scales at the mean age of 16.47
| A | C | E | A | C | E | |
|---|---|---|---|---|---|---|
| Anxious/depressed | .38 (.30–.45) | - | .62 (.55–.70) | .44 (.39–.48) | - | .56 (.52–.61) |
| Withdrawn/depressed | .32 (.29–.40) | - | .68 (.60–.75) | .36 (.31–.41) | - | .64 (.59–.69) |
| Somatic complaints | .25 (.17–.33) | - | .74 (.67–.83) | .36 (.30–.41) | - | .64 (.59–.70) |
| Social problems | .36 (.31–.41) | - | .64 (.59–.69) | .36 (.31–.41) | - | .64 (.59–.69) |
| Thought problems | .33 (.25–.40) | - | .67 (.60–.75) | .45 (.40–.50) | - | .55 (.50–.60) |
| Attention problems | .44 (.36–.51) | - | .56 (.49–.64) | .20 (.10–.32) | .22 (.10–.32) | .58 (.54–.63) |
| Rule-breaking behavior | .51 (.44–.57) | - | .49 (.43–.56) | .22 (.12–.34) | .21 (.10–.30) | .57 (.52–.62) |
| Aggressive behavior | .50 (.43–.56) | - | .50 (.44–.57) | .38 (.33–.43) | - | .62 (.57–.67) |
| Internalizing | .45 (.38–.51) | - | .55 (.49–.62) | .34 (.22–.48) | .12 (.001–.23) | .54 (.49–.59) |
| Externalizing | .53 (.46–.59) | - | .47 (.41–.54) | .37 (.25–.46) | .07 (.001–.19) | .56 (.51–.61) |
Discussion
In the current study we investigate the effects of age and sex on the levels and the genetic architecture of adolescent emotional and behavioral problems. For all syndrome scales of the YSR (except for Aggressive Behavior) and for the broadband scales Internalizing and Externalizing, sex-differences in mean levels were found. Females score higher than males on Internalizing problems and its subscales (Anxious/Depressed and Withdrawn/Depressed), while males score higher than females on Externalizing behavior and its subscale Rule-Breaking. Differences between males and females have been consistently shown in epidemiological and clinical studies (Earls, 1987; Eme, 1979; Hartung & Widiger, 1998). Origins of male and female problems are likely to be rooted in biological, physical, cognitive, and social-emotional differences in males and females that can precede the expression of clinical problems (Rutter et al., 2003; Zahn-Waxler et al., 2008).
Besides sex-differences, developmental changes in levels of psychopathology have been subject of study for many years. We found that in our large sample of adolescent twins and siblings age-effects vary in strength and direction by syndrome and sex. An increase in problems with increasing age was seen for Anxious/Depressed, while a decrease was observed for Somatic Complaints, Aggressive Behavior, and Social and Thought problems. These developmental changes may result from changes brain region and systems that are key to regulation of perception and evaluation of risk and reward and arousal and motivation that seem under development during adolescence (Steinberg, 2005; Paus et al., 2008). Adolescence is a vulnerable period which is also reflected by the fact that the peak of onset for any mental disorder is age 14 (Kessler, 2005). Finally, longitudinal studies have shown sex-differences in brain developments, with for example, females reaching peak values of brain volume earlier than males (Lenroot & Giedd, 2010), which fits our findings of difference developmental patterns of YSR syndrome scales for males and females.
Besides sex and age effect on levels of emotional and behavioral problems, significant genotype interactions with age and with sex were found for most scales of the YSR. These effects vary in strength and direction, resulting in differences in genetic architecture between males and females and developmental changes in genetic architecture throughout adolescence. For the syndrome scales ANX, WITH, SOM, and THOUGHT heritabilities are higher for females than males, while for AP, RB, and AGG heritabilities are higher for males than females. Heritability by age and sex interactions might be related to the timing of gene expression and the timing of biological maturation. Differences in heritability in different age groups may also reflect the cumulative effects of experience. The current study, though, shows that these effects of increasing genetic and environmental variance by age cannot be generalized to all syndromes. For example, Internalizing behavior and its subscales Anxious/Depressed and Withdrawn/Depressed indeed show a rather sharp increase in both non-shared environmental and genetic variance. However, Aggressive Behavior and its broadband scale Externalizing Behavior show a rather sharp decrease in both non-shared environmental as well as genetic variance throughout adolescence. Mixed results are also reported by Hicks et al. (2008) even within the domain of externalizing behaviors. They found an increase in phenotypic variance, due to an increase in genetic and non-shared environmental variance for substance uses disorder, but reported a decrease in phenotypic variance for antisocial behavior. Changes in standardized estimates of genetic and environmental influence also appear to be syndrome specific. While a recent meta-analysis (Bergen et al., 2009), using a meta-analytic approach to examine the change of genetic and environmental variance in behavioral measures, reported significant heritability increase for externalizing behavior, anxiety symptoms, depressive symptoms, social attitudes, and IQ, we found these patterns to vary by syndrome. An increase in heritability and a parallel decrease in non-shared environmental influences is found for Anxious/Depressed and Withdrawn/Depressed behavior in both males and females in the current study. However, heritability estimates for Aggressive Behavior and Externalizing behavior seem to increase from age 12 to age 16 but decrease again between age 17 and 20. No effects of age on the genetic architecture are found for Somatic Complaints, Rule-Breaking behavior, Thought Problems and Attention Problems. Most remarkable is the development of genetic and environmental influences on Somatic Complaints. Genetic influences seem to diminish throughout adolescence while non-shared environmental influences remain stable.
The mix of results indicates the importance of investigating the effects of age and sex on the genetic architecture. Several other phenotypes have been subject of developmental studies over the past years, with most studies pointing into the direction of increasing heritabilities over age. For example increasing influences of genes have been settled for IQ (Haworth et al., 2009; Bartels et al., 2002), but also for less studied phenotypes, such as sports participation (van der Aa et al., 2010; Stubbe et al., 2005) an increase in genetic influences has been found.
Our study also adds to the recent discussion on the presence or absence of shared environmental influences on adolescent psychopathology (see Burt, 2009 & 2010 and Woods et al., 2010). One of the concerns with most studies on genetic and environmental, and especially shared environmental, influences on emotional and behavioral problems is the lack of power to detect small effects of C. In the current study small but significant influences of shared environment are found for Attention Problems, Rule-Breaking Behavior, Internalizing and Externalizing behavior in females. No effects of C have been detected for males and for the remaining YSR scales in females.
Power analyses (Posthuma & Boomsma, 2000) revealed that with our sample of 7616 individuals (6381 twins and 1195 siblings) we would have been able to detect shared environmental influences as small as 10%, indicating that we had enough power to detect C if present.
The significance of shared environmental influences on Attention Problems is remarkable. Genetic studies (family, twin, and adoption studies) demonstrated a strong genetic component in the etiology of the disorder in children (Faraone and Doyle, 2001; Rietveld et al., 2004), but substantially less is known about the heritability of Attention Problems in Adolescents. Most studies that include adolescents are not adolescence limited and despite large age-ranges of the samples not corrected for age (for a review see Freitag et al., 2010), providing no clear picture of the genetic architecture of Attention Problems in adolescents.
The results of this study should be interpreted in the context of several potential limitations. First, information on adolescent behavioral and emotional problems is based on self-report on the YSR. Although self-report sometimes is considered to be less reliable, it has been shown that behavioral problems can reliably be assessed using self-report (Verhulst et al., 1997). Second, this study is based on a normal population sample of twins and their non-twin siblings while using a scale that is primarily developed for use in clinical practice. This results in skewed distributions for the YSR syndrome and broadband scales. In most studies skewed distribution would have been transformed. In the current study, however, we decided to analyze untransformed data since Derks et al (2004) showed that when fitting an ACE model to censored data, unbiased estimates of the additive genetic effect will be obtained. Furthermore, the underestimation of shared environmental effects and the overestimation of non-shared environmental effects that are a result of the censored data, is not removed by transformation.
Clinical implications of our results are that identification or diagnosis of a specific syndrome is essential for proper intervention. For some syndromes or clusters of syndromes, age and/or sex specific intervention is essential, while for other syndromes sex and age are not of importance. However the evidence for effectiveness of sex-specific therapy, is lacking. Prevention strategies should also be developed with the effects of sex and age in mind. Some syndromes show increasing influence of non-shared environment, while these effects are stable or decreasing for other syndromes. Since adolescence is considered one of the most vulnerable periods in human life for the onset and development of mental problems, it is important to pin point the exact age by sex and by syndrome interaction in order to inform prevention and intervention strategies. Future studies are warranted in order to replicate our findings.
To summarize, significant effects of sex and age have been found on levels and genetic architecture of the syndrome scales and broadband scales of the YSR. The effects are syndrome specific, indicating that sex and age should be taken into account when studying levels and causes of individual differences in adolescent emotional and behavioral problems.
Acknowledgements/Conflicts of Interest
Funding was obtained from the following grants: “Spinozapremie” (NWO/SPI 56-464-14192); Genetics of Mental Illness: European Research Council (ERC-230374); “Twin-family database for behavior genetics and genomics studies” (NWO 480-04-004); “Genetic and Family influences on Adolescent Psychopathology and Wellness” (NWO 463-06-001); “A twin-sib study of adolescent wellness” (NWO-VENI 451-04-034 Bartels). M. Bartels is financially supported by a senior fellowship of the EMGO+ Institute for Health and Care. Niels van de Aa is financially supported by a WCRF International research grant. C.M. Middeldorp is financially support by the Netherlands Organisation for Scientific Research (NWO-VENI grant 916-76-125).
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Department of Psychiatry; 2001. [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28(1):51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- Bartels M, Rietveld MJH, Van Baal GCM, Boomsma DI. Genetic and Environmental Influences on the Development of Intelligence. Behavior Genetics. 2002;32(4):237–249. doi: 10.1023/a:1019772628912. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van den Oord EJCG, Hudziak JJ, Rietveld MJH, van Beijsterveldt CEM, Boomsma DI. Genetic and environmental mechanisms underlying stability and change in problem behaviors at ages 3, 7, 10, and 12. Developmental Psychology. 2004;40(5):852–867. doi: 10.1037/0012-1649.40.5.852. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van Beijsterveldt CEM, Derks EM, Stroet TM, Polderman JC, Hudziak JJ, et al. Young Netherlands Twin Register (Y-NTR): A longitudinal multiple informant study of problem behavior. Twin Research and Human Genetics. 2007a;10:3–11. doi: 10.1375/twin.10.1.3. [DOI] [PubMed] [Google Scholar]
- Bartels M, Boomsma DI, Hudziak JJ, van Beijsterveldt CEM, van den Oord EJCG. Twins and the Study of Rater (Dis)Agreement. Psychological Methods. 2007b;12(4):451–66. doi: 10.1037/1082-989X.12.4.451. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Research and Human Genetics. 2007;10(3):423–33. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Beijsterveldt van CEM, Hudziak JJ. Genetic and environmental influences on Anxious/Depression during childhood: A study from the Netherlands Twin Register. Genes, Brain and Behavior. 2005;4(8):466–481. doi: 10.1111/j.1601-183X.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Geus de EJC, Vink JM, Stubbe JH, Distel MA, Hottenga JJ, et al. Netherlands Twin Register: From Twins to Twin Families. Twin Research and Human Genetics. 2006;9:849–857. doi: 10.1375/183242706779462426. [DOI] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: a meta-analysis of shared environmental influences. Psychological Bulletin. 2009;135(4):608–37. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Burt SA. Are there shared environmental influences on attention-deficit/hyperactivity disorder? Reply to Wood, Buitelaar, Rijsdijk, Asherson, and Kunsti (2010) Psychological Bulletin. 2009;136(3):341–3. doi: 10.1037/a0019116. [DOI] [PubMed] [Google Scholar]
- Derks EM, Dolan CV, Boomsma DI. Effects of Censoring on Parameter Estimates and Power in Genetic Modelling. Twin Research. 2004;7(6):659–669. doi: 10.1375/1369052042663832. [DOI] [PubMed] [Google Scholar]
- Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatric Developments. 1987;5(1):1–23. [PubMed] [Google Scholar]
- Eme RF. Sex differences in childhood psychopathology: a review. Psychological Bulletin. 1979;86(3):574–95. [PubMed] [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. Essex: Longman Scientific and Technical; 1996. [Google Scholar]
- Faraone SV, Doyle AE. The nature and heritability of attention-deficit/hyperactivity disorder. Child and Adolescent Psychiatric Clinics of North America. 2001;10:299–316. viii–ix. [PubMed] [Google Scholar]
- Freitag CM, Rohde LA, Lempp T, Romanos M. Phenotypic and measurement influences on heritability estimates in childhood ADHD. European Child and Adolescent Psychiatry. 2010;19(3):311–23. doi: 10.1007/s00787-010-0097-5. [DOI] [PubMed] [Google Scholar]
- Hartung CM, Widiger TA. Gender differences in the diagnosis of mental disorders: Conclusions and controversies of the DSM-IV. Psychological Bulletin. 1998;123(3):260–78. doi: 10.1037/0033-2909.123.3.260. [DOI] [PubMed] [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, Bartels M, Posthuma D, Boomsma DI, Davis OS, Kovas Y, Corley RP, Defries JC, Hewitt JK, Olson RK, Rhea SA, Wadsworth SJ, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, Plomin R. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry. 2009 Jun 2; doi: 10.1038/mp.2009.55. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. Journal of Abnormal Psychology. 2007;116(3):433–47. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, van Beijsterveldt CEM, Boomsma DI. Genetic and Environmental influences on the stability of withdrawn behavior in children: A longitudinal, multi-informant twin study. Behavior Genetics. 2008;38(5):447–461. doi: 10.1007/s10519-008-9213-4. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, van Beijsterveldt CEM, Bartels M, Derks E, Boomsma DI. Individual differences in aggression in young children: cross-sectional analyses in Dutch twins. Behavior Genetics. 2003;33(5):575–589. doi: 10.1023/a:1025782918793. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, van Beijsterveldt CEM, Althoff RR, Stanger C, Rettew DC, Nelson EC, Todd RD, Bartels M, Boomsma DI. Genetic and Environmental Contributions to the Child Behavior Checklist Obsessive –Compulsive Scale. Archives of General Psychiatry. 2004;61:608–616. doi: 10.1001/archpsyc.61.6.608. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: evidence for genetic innovation and attenuation. Psychological Medicine. 2008;38(11):1567–75. doi: 10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Lamb DJ, Middeldorp CM, Beijsterveldt van CEM, Bartels M, Aa van der N, Polderman TJC, Boomsma DI. Heritability of anxious-depressive and withdrawn behavior: Age-related changes during adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(3):248–255. [PubMed] [Google Scholar]
- Legrand LN, McGue M, Iacono WG. A twin study of state and trait anxiety in childhood and adolescence. Journal of Child Psychology and Psychiatry. 1999;40(6):953–8. [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. 2010;72(1):46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Kearsey MJ, Davies P. The power of the classical twin study. Heredity. 1978;40(1):97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 7th ed. Richmond: VCU, Department of Psychiatry; 2006. [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence. Nature Review Neuroscience. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84(2):309–22. [PubMed] [Google Scholar]
- Posthuma D, Boomsma DI. A Note on the Statistical Power in Extended Twin Designs. Behavior Genetics. 2000;30:147–158. doi: 10.1023/a:1001959306025. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research. 2002;5(6):554–71. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rietveld MJ, van Der Valk JC, Bongers IL, Stroet TM, Slagboom PE, Boomsma DI. Zygosity diagnosis in young twins by parental report. Twin Research. 2000;3:134–141. doi: 10.1375/136905200320565409. [DOI] [PubMed] [Google Scholar]
- Rietveld MJ, Posthuma D, Dolan CV, Boomsma DI. ADHD: sibling interaction or dominance: an evaluation of statistical power. Behavior Genetics. 2003;33(3):247–55. doi: 10.1023/a:1023490307170. [DOI] [PubMed] [Google Scholar]
- Rietveld MJH, Hudziak JJ, Bartels M, van Beijsterveldt CEM, Boomsma DI. Heritability of attention problems in children: II. Longitudinal results from a study of twins, age 3 to 12. Journal of Child Psychology and Psychiatry. 2004;45(3):577–588. doi: 10.1111/j.1469-7610.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. Journal of Child Psychology and Psychiatry. 2003;44(8):1092–115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Development. 1983;54(2):424–35. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Stubbe JH, Boomsma DI, Geus de EJC. Sports participation during adolescence: a shift from environmental to genetic factors. Medicine & Science in Sports & Exercise. 2005;37(4):563–570. doi: 10.1249/01.mss.0000158181.75442.8b. [DOI] [PubMed] [Google Scholar]
- Van Beijsterveldt CEM, Bartels M, Hudziak JJ, Boomsma DI. Causes of stability in childhood aggression: Longitudinal genetic Analyses in Dutch twins. Behavior Genetics. 2003;33(5):591–605. doi: 10.1023/a:1025735002864. [DOI] [PubMed] [Google Scholar]
- Van den Berg SM, Willemsen G, de Geus EJ, Boomsma DI. Genetic etiology of stability of attention problems in young adulthood. American Journal Medical Genetics B: Neuropsychiatric Genetics. 2006;141B(1):55–60. doi: 10.1002/ajmg.b.30251. [DOI] [PubMed] [Google Scholar]
- Van der Aa N, Geus de EJC, van Beijsterveldt CEM, Boomsma DI, Bartels M. Genetic influences on individual differences in exercise behavior during adolescence. International Journal of Pediatrics. 2010 doi: 10.1155/2010/138345. Epub 2010 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Grootheest DS, Bartels M, Cath DC, Beekman AT, Hudziak JJ, Boomsma DI. Longitudinal genetic and environmental contributions to childhood obsessive-compulsive behavior. Biological Psychiatry. 2007;61:308–315. doi: 10.1016/j.biopsych.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Van Grootheest DS, Bartels M, van Beijsterveldt CE, Cath DC, Beekman AT, Hudziak JJ, Boomsma DI. Genetic and environmental contributions to self-report obsessive-compulsive symptoms in Dutch adolescents at ages 12, 14, and 16. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(10):1182–8. doi: 10.1097/CHI.0b013e3181825abd. [DOI] [PubMed] [Google Scholar]
- Verhulst FC, Van der Ende J, Koot HM. Handleiding voor de Youth Self-Report (YSR) Manual for the Youth Self-Report (YSR)] Rotterdam: Afdeling Kinder-en Jeugdpsychiatrie, Sophia Kinderziekenhuis/Academisch Ziekenhuis Rotterdam/Erasmus Universiteit Rotterdam; 1997. [Google Scholar]
- Wood AC, Buitelaar J, Rijsdijk F, Asherson P, Kuntsi J. Rethinking shared environment as a source of variance underlying attention-deficit/hyperactivity disorder symptoms: comment on Burt (2009) Psychological Bulletin. 2010;136(3):331–40. doi: 10.1037/a0019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: gender and psychopathology. Annual Review of Clinical Psychology. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]