Abstract
The intra-S phase checkpoint protein complex Tof1/Csm3 of Saccharomyces cerevisiae antagonizes Rrm3 helicase to modulate replication fork arrest not only at the replication termini of rDNA but also at strong nonhistone protein binding sites throughout the genome. We investigated whether these checkpoint proteins acted either antagonistically or synergistically with Rrm3 in mediating other important functions such as maintenance of genome stability. High retromobility of a normally quiescent retrovirus-like transposable element Ty1 of S. cerevisiae is a form of genome instability, because the transposition events induce mutations. We measured the transposition of Ty1 in various genetic backgrounds and discovered that Tof1 suppressed excessive retromobility in collaboration with either Rrm3 or the F-box protein Dia2. Although both Rrm3 and Dia2 are believed to facilitate fork movement, fork stalling at DNA-protein complexes did not appear to be a major contributor to enhancement of retromobility. Absence of the aforementioned proteins either individually or in pair-wise combinations caused karyotype changes as revealed by the altered migrations of the individual chromosomes in pulsed field gels. The mobility changes were RNase H-resistant and therefore, unlikely to have been caused by extensive R loop formation. These mutations also resulted in alterations of telomere lengths. However, the latter changes could not fully account for the magnitude of the observed karyotypic alterations. We conclude that unlike other checkpoint proteins that are known to be required for elevated retromobility, Tof1 suppressed high frequency retrotransposition and maintained karyotype stability in collaboration with the aforementioned proteins.
Keywords: Checkpoint Control, DNA Helicase, DNA Replication, Gene Amplification, Gene Transposable Elements, Intra-S Checkpoint, Karyotype Stability, Pulse Field Gel, Replication Fork, Retrotransposon
Introduction
Checkpoint pathways are signal transduction mechanisms that ensure that cell division is prevented from proceeding to completion until all of the necessary, preceding steps have been successfully completed. The S-phase checkpoint pathway in budding yeast consists of sensor kinases such as Mec1 that recognizes single-stranded DNA coated with the binding protein RPA at stalled and collapsed forks. It transmits the signal by phosphorylating a mediator protein such as Mrc1 that in turn activates the effecter kinase Rad53 by phosphorylation. The effecter controls the expression of downstream genes necessary to halt cell cycle progression until the stalled forks have been restarted (1, 2). Tof1 and Csm3 of Saccharomyces cerevisiae are also intra-S phase checkpoint mediators and are homologs of the mammalian “Timeless” and “TIPIN” family of proteins (3–6). In addition to their role in the maintenance of fork stability, the mammalian homologs also mediate the circadian response (4, 5). Tof1 and Csm3 proteins form a fork protection complex with Mrc1 (7–10). This family of proteins also perform other important functions such as promotion of sister chromatid cohesion (11–13). Rrm3 is known to promote fork movement by removing nonhistone proteins from in front of replication forks throughout the yeast genome (14). Tof1 and Csm3 protect the binding of nonhistone proteins to DNA by antagonizing their displacement by the helicase Rrm3 during fork passage (15, 16). Dia2 is an F-box protein, which is a component of the ubiquitin E3 ligase complex involved in substrate recognition by polyubiquitination that marks the substrate for degradation (17). Tof1, Rrm3, and Dia2 are associated with the replisome and appear to facilitate unimpeded fork passage through the chromatin (7, 14, 18, 19).
In cells that are subjected to genotoxic stress, e.g. by hydroxyurea (HU),2 absence of Tof1 or Mrc1, or Csm3 causes dissociation of the replisomal complex (20). Although the three proteins are stably bound to each other (6), only Tof1 and Csm3, but not Mrc1, are involved in protecting the Fob1-Ter complex from transient dissociation by Rrm3 “sweepase” during fork passage, thereby ensuring stable polar fork arrest at the replication termini (15, 21, 22). The Tof1/Csm3 complex also promotes controlled recombination at the Ter sites located in the nontranscribed spacers of rDNA, thereby providing a mechanism for sequence homogenization of the rDNA repeats (16).
Ty1 is one of 5 species of retrotransposable elements present in budding yeast. These retrovirus-like elements are flanked by long terminal repeats and contain gag and pol genes that encode the structural components of the virus-like particles that coat the retrotransposon, and the reverse transcriptase/integrase, respectively. The retrotransposon is copied from an RNA intermediate into DNA (23, 24). Integration of the provirus like DNA into the host chromosome is either catalyzed by the integrase or by the RAD52-dependent recombination pathway (25) (see Fig. 1A). In WT cells the Ty1 elements remain quiescent and transpose infrequently because of the activities of several host-encoded repressors. In the absence of the repressors of Ty1 transposition (RTT) the frequency of transposition (retromobility) is enhanced (26). The transposition events are potentially mutagenic and therefore are detrimental to the maintenance of genome stability.
FIGURE 1.
Simultaneous deletions of TOF1 and RRM3 lead to ultrahigh frequency of retromobility. A, schematic view of the split HIS3 retromobility assay; the details are described in the text; B, bar diagrams showing the relative frequency of genomic Ty1his3AI retromobility in WT, tof1Δ, rrm3Δ, rrm3Δtof1Δ (three independently constructed clones), csm3Δ, and rrm3Δcsm3Δ strains; C, plates showing complementation of rrm3Δtof1Δ strain by TOF1 or RRM3 carried in plasmid vectors; WT strain carried a blank vector whereas the rrm3Δtof1Δ strain carried a vector alone, one with TOF1 or RRM3; D, bar diagrams showing the frequencies of Ty1his3AI retrotransposition after complementation of WT strain with a vector and the rrm3Δtof1Δ strain with either a vector or with a vector containing TOF1 or RRM3.
The present work was undertaken to investigate the possible role of the Tof1/Csm3 complex in the maintenance of genome stability. We also wished to determine whether the two protein components, while acting together to counteract Rrm3 helicase, individually have different functions. We used two criteria for the measurement of genome stability namely suppression of excessive Ty1 retromobility and maintenance of the WT karyotype. Here, we report that Tof1 but not Csm3 prevented promiscuous Ty1 retrotransposition by synergistically collaborating with two fork-associated proteins, namely Rrm3 and Dia2. Whereas the solo rrm3Δ or dia2Δ strains showed a 20–30-fold increase in Ty1 retromobility over that of the WT strain, rrm3Δ tof1Δ or dia2Δ tof1Δ double mutants enhanced the process by a factor of 100–350-fold over that of the WT strain; this is the highest frequency of retrotransposition reported thus far. We also show that Tof1, Csm3, Rrm3, and Dia2 proteins contributed to the maintenance of karyotype stability either individually or in various pair-wise combinations.
EXPERIMENTAL PROCEDURES
Yeast Strains
All the yeast strains used in the study are described in Table 1.
TABLE 1.
List of strains used in this study
| Strains | Genotype | Source |
|---|---|---|
| 1. JC2326 | MATΔ-ura3, cir0, ura3–167, leu::hisG, his3Δ200, Ty1his3AI-270, Ty1NEO-588, Ty1 (tyb::lacz)-146 | (29) |
| 2. JC2326NK1 | rrm3Δ:: KanMX | This study |
| 3. JC2326NK2 | tof1Δ:: KanMX | This study |
| 4. JC2326NK3 | rrm3Δ::KanMX, tof1Δ:: Leu2 clone1 | This study |
| 5. JC2326NK4–8 | rrm3Δ:: KanMX, tof1Δ::Phe clones 2–6 | This study |
| 6. JC2326NK9 | csm3Δ::Kan MX | This study |
| 7. JC2326NK10–12 | rrm3Δ::KanMX, csm3Δ::Ade2 clones 1–3 | This study |
| 8. JC2326 NK13 | dia2Δ::KanMX | This study |
| 9. JC2326NK14 | dia2Δ::KanMX, tof1Δ:: Phe | This study |
| 10. JC2326NK15 | dia2Δ::KanMX, csm3Δ::Phe | This study |
Two-dimensional Agarose Gel Analysis of Replication Intermediates
The two-dimensional gel analysis of replication intermediates of the WT, tof1Δ, rrm3Δ, rrm3Δtof1Δ, csm3Δ, rrm3Δcsm3Δ, dia2Δ, dia2Δ tof1Δ, and dia2Δcsm3Δ strains were carried out as described previously (15, 27).
Ty1 Retromobility Assay
The retromobility assay was performed essentially as described in Ref. 28. The assay was carried out using a yeast strain in which a single Ty1 element is marked by an indicator gene HIS3AI (25). The reporter gene HIS3 ORF contains an artificial intron (AI) in an orientation opposite to that of HIS3 ORF (open reading frame) (Fig. 1A) (25). When this marked Ty1 element undergoes transcription followed by splicing, reverse transcription, and finally, integration into genomic locations, it generates HIS3-positive prototrophs. The quantitative measurement of the number of HIS3-positive colonies is considered as a measure of retromobility by transposition or by cDNA-mediated recombination.
Briefly, a single colony of WT yeast strain (JC2326) or its deletion derivatives, containing a copy of the Ty1his3AI was grown overnight in 10 ml of YPD. The overnight cultures were re-inoculated into fresh 5 ml of YPD at a 1:1000 dilutions and grown at 20 °C up to saturation (for 144 h). After growth, the cultures were diluted and plated on YPD for viable counts of colonies, and 1 ml of each saturated culture was plated on minimal media (SD/His−) plates. The plates were incubated at 30 °C for 3–7 days before counting the His+ colonies to determine transposition frequency.
Integration Assay for Ty1 Element at tRNAgly Sites
Integration assay for Ty1 cDNA element after transposition at tRNAgly site was performed as described in Refs. 29, 30. Each culture was grown for induction of transposition as described in the previous section. The total genomic DNA of all the strains was prepared as in (15). To detect Ty1 integration events at genomic tRNAgly loci, oligonucleotides TYB-OUT2 (5′-GTGATGACAAAACCTCTTCCG-3′) and SUF16–2 (5′-GGCAACGTTGGATTTTACCAC-3′) were used for PCR. This pair of primers was used to amplify the unselected Ty1 element integrated at tRNAgly loci. The PCR conditions were followed as exactly mentioned in (30). As an endogenous control the TEC1 ORF was amplified in separate tubes using same amount of genomic DNA. The PCR products were fractionated by agarose gel electrophoresis (1.5% agarose) and transferred to nylon membrane. The blots were probed with Ty1 LTR region specific probe (5′-GTGGAAGCTGAAACGCAAGGATTGAT-3′) labeled by [γ-32P]ATP as described in Ref. 30.
Pulsed Field Gel Electrophoresis (PFGE)
Karyotype analysis of the strains used in this study was carried out by PFGE using the Contour-clamped Homogeneous Electric Field (CHEF) gel apparatus CHEF DRII (Bio-Rad). All strains were grown at the 30 °C for 48 h in YPD, and they were used as un-induced culture. For induction of transposition the un-induced culture was inoculated in fresh YPD at 1:1000 dilutions and grown to saturation at 20 °C for 144 h. 3 ml of each culture was then harvested at 4 °C. Cells were washed twice with 50 mm EDTA (pH 8.0). Agarose gel plugs were prepared as described in the instruction manual of the manufacturer of CHEF gel (Bio-Rad). Electrophoresis of the samples was carried out in 1.2% agarose gels in 0.5× TBE at 6 V/cm for 26 h at 8 °C. The pulse times were 60–120 s. After electrophoresis, the gels were stained with ethidium bromide and photographed.
Measurement of Telomere Length
Genomic DNA was prepared from the yeast cultures grown at 30 °C for 24 h. 10 μg of genomic DNA was digested with PstI or XhoI overnight at 37 °C. The digested DNA was fractioned by electrophoresis in 1% agarose gels at 60 V and transferred to Nytran membrane by Southern transfer. The blots were probed with the 32P-labeled telomere specific probe generated using primers containing the following sequences: Forward 5′-ACACACTCTCTCACATCTACC-3′ and reverse 5′-TTGCGTTCCATGACGAGCGC-3′ from the plasmid pYT14 (31), a kind gift from Dr. Thomas Petes. The relative length of telomeres of each strain was compared with that of the WT strain.
RESULTS
Deletions of Both TOF1 and RRM3 Induced Ultrahigh Frequency Retrotransposition
Replication stress is known to induce Ty1 retromobility (32). Because Tof1 helps to maintain replisomal integrity and Rrm3 promotes fork passage through DNA-protein complexes, we wished to find out whether the absence of both of these proteins would cause a stronger replication stress that could be manifested in greatly increased rates of Ty1 retromobility. We also wished to determine whether both Tof1 and its binding partner Csm3 would function similarly in this regard. Previously, the highest frequency of Ty1 retromobility reported in rrm3Δ strains was of the order of ∼30-fold above the WT level (33). We measured Ty1 transposition frequency (retromobility) in WT, rrm3Δ, tof1Δ, csm3Δ, rrm3Δtof1Δ, and rrm3Δcsm3Δ strains using the split HIS3 reporter assay shown in Fig. 1A. The substrate contained a HIS3 gene split by an artificial intron. The intron was removed in vivo by splicing and the “proviral DNA” was integrated into the host chromosomes either by the Ty1-encoded integrase or the RAD52-dependant general recombination pathway (25). The frequency of appearance of His+ colonies in various mutants in comparison with the isogenic WT strain therefore was a measure of the magnitude of retromobility (Fig. 1A).
Using this assay we discovered that tof1Δ individually did not show detectable enhancement of retromobility. Cells with rrm3Δ, as reported before, showed a ∼30-fold enhancement of retromobility over that of the WT cells (33). However, a double deletion of TOF1 and RRM3 synergized ultrahigh frequency Ty1 retromobility (up to ∼350-fold over the WT levels; Fig. 1, B and C). This is the highest frequency of retrotransposition observed to date. These observations have been made from three independently constructed rrm3Δ tof1Δ strains that showed retromobility frequencies ranging from 100× to ∼350× over that of the WT strain whereas in contrast, the rrm3Δcsm3Δ strain elicited frequencies equal to that of the rrm3Δ strain (Fig. 1B). We attempted to confirm the results by complementation of the rrm3Δ tof1Δ double mutant with a plasmid-borne TOF1 and found, as expected, that it caused a reduction of the Ty1 retromobility back to the level of the solo rrm3Δ strain (Fig. 1, C and D). Similarly, complementation with a RRM3 containing plasmid reduced the frequency of retromobility to that of the tof1Δ level. The complementation data showed that tof1Δ and not some other cryptic mutation(s) was responsible for generating the very high level of retromobility in an rrm3Δ background. (Fig. 1, C and D).
It is known that Tof1 collaborates with Csm3 to modulate fork arrest at Ter sites (15). However, as contrasted with tof1Δ, a csm3Δ strain manifested no detectable enhancement of retromobility, either by itself or in an rrm3Δ background. In an rrm3Δcsm3Δ strain there was the same ∼30-fold increase in retromobility that is the characteristic of an rrm3Δ mutation. Csm3, unlike Tof1, did not appear to modulate Ty1 transposition. Therefore, we concluded that the two interacting proteins have different individual functions (Fig. 1B). The third component of the fork protection complex is Mrc1. Because mrc1Δ is synthetically lethal when present in combination with either tof1Δ, dia2Δ or rrm3Δ (34, 35), we could not investigate its possible contribution to retromobility in a rrm3Δ or dia2Δ background.
Absence of Tof1 Promoted Ty1 Transposition Preferentially to tRNAgly Loci whereas Rrm3 Counteracted This Tendency
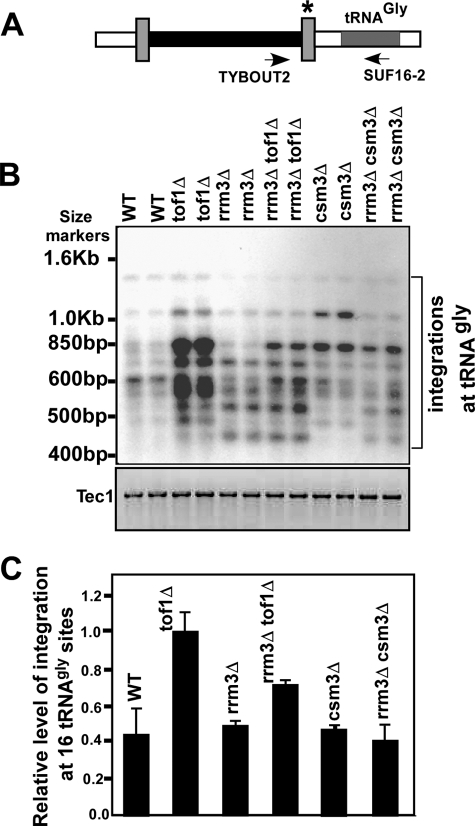
Although the double deletion strain rrm3Δtof1Δ showed quantitatively very high frequency of retromobility, we wished to determine whether qualitatively, the spectrum of integration sites was different in the individual deletions in comparison with the double deletions. We also wished to determine what effect, if any, one deletion had over that of the other, in comparison with the WT cells. It is already known that Ty1 has some preference for integration into DNA sequences encoding some but not all tRNA-encoding sequences in WT cells (30). We analyzed the frequency of integration into the 16 tRNAgly loci by performing polymerase chain reaction (PCR) using a primer pair, one of which was complementary to the tRNAgly sequence and the other to Ty1 (Fig. 2A). Southern blot analyses of PCR products of WT and rrm3Δ cells showed a lower level of integration at the tRNAgly encoding locus (Fig. 2, B and C). The tof1Δ cells, in contrast, showed distinctly enhanced integration (by a factor of 2–2.5) into the loci, whereas a double deletion of rrm3Δtof1Δ showed a marked reduction, although the level was still slightly higher than that of the WT cells. Cells containing csm3Δ showed preferential integration at two chromosomal sites in the tRNAgly loci, one of which was abolished in the rrm3Δcsm3Δ double mutant. These data suggested that in the rrm3Δtof1Δ double mutant, not only the retromobility was quantitatively enhanced, but also the integration sites were qualitatively altered in comparison with the WT and the single mutant strains. We have measured random integration into the WT, tof1Δ and rrm3Δtof1Δ cells after fractionation of chromosomes from single HIS+ integrants but have not observed significantly different integration patterns (not shown). It has been previously shown that not all tRNA-encoding loci are preferred sites of Ty1 integration; only some of the tRNAgly loci are known to be hot spots of integration (36). We looked at integration into 8 tRNAthr and 5 tRNA ser loci and observed that tof1Δ cells showed similar levels of integration in comparison with the WT and the other genotypes (supplemental Fig. S6). We also performed integration into 5 S DNA and discovered that not only these were not hot spots of integration but also there was no enhancement of retromobility in tof1Δ cells (supplemental Fig. S6). We conclude from the data that enhancement of Ty1 retromobility in a tof1Δ strain is sequence context-dependent.
FIGURE 2.
TOF1 deletion unmasked the tRNAgly site for integration of Ty1 cDNA. A, schematic view of the integrated Ty1 element at 16 tRNAgly loci; TYBOUT2 and SUF16–2 were primers from Ty1 element and tRNAgly region, respectively used for PCR amplification to analyze Ty1 integration; (*) indicates the LTR region used as probe for detection of Ty1 element amplified by PCR; B, autoradiogram of a representative Southern blot showing integration of Ty1 cDNA in WT, tof1Δ, rrm3Δ, rrm3Δtof1Δ, csm3Δ, and rrm3Δcsm3Δ strains (shown in duplicate); C, bar diagrams showing relative frequencies of Ty1 integration in WT, tof1Δ, rrm3Δ, rrm3Δtof1Δ, csm3Δ, and rrm3Δcsm3Δ strains.
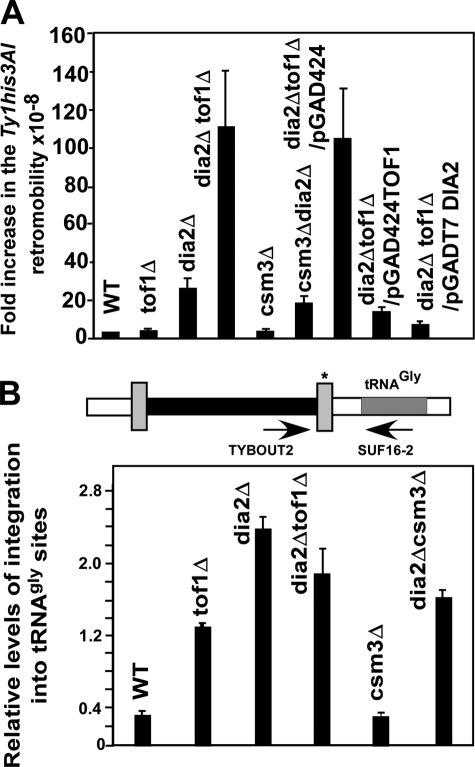
A Combination of dia2Δ and tof1Δ Also Promoted High Frequency Retromobility
Is the ultrahigh frequency of retromobility unique to rrm3Δtof1Δ or can one replace rrm3Δ by deletion of another gene (e.g. DIA2) that is also known to promote fork propagation and observe the same order of high frequency retromobility? We surmised that Dia2 protein might be an important player in this context for the following reasons. The protein physically interacts with Mrc1 at its tricotertapeptide containing N-terminal domain and the interaction regulates replisome activity (19). It travels with the replisome by binding to Mrc1, which in turn is loaded onto DNA by Tof1(18). We used WT, dia2Δ, tof1Δ, csm3Δ, dia2Δtof1Δ, and dia2Δ csm3Δ strains to investigate this question using the assay described in Fig. 1. The data showed that, although dia2Δ by itself enhanced Ty1 retromobility by ∼30-fold over that of the WT cells, the double deletion dia2Δtof1Δ showed a ∼110-fold enhancement (Fig. 3A). In contrast a dia2Δ csm3Δ strain showed a ∼20-fold enhancement in retromobility, which was similar to that shown by a dia2Δ single mutant. Here also, TOF1 but not its interacting partner CSM3 was able to suppress promiscuous Ty1 retromobility in collaboration with DIA2. In confirmatory experiments, introduction of a plasmid-borne TOF1 into the dia2Δtof1Δ strain reduced the frequency of retromobility close to that of the dia2Δ single mutant (Fig. 3A). We also looked at the integration frequency into the 16 loci encoding tRNAgly and found that dia2Δ, tof1Δ, dia2Δtof1Δ, and dia2Δcsm3Δ strains showed higher frequency of integration into the targets as contrasted with the WT and csm3Δ strains (Fig. 3B). The significance of this observation is that the higher frequency of integration into tRNAgly is probably not due to the removal of a protein barrier. As shown in the next section, while rrm3Δtof1Δ double mutant was able to arrest forks at Ter sites and several other sites in the rDNA, a dia2Δ tof1Δ double mutant was unable to arrest forks at Ter and at the other sites yet both showed very high frequencies of retromobility. Control experiments using tRNAthr, tRNAser, and 5S loci did not show enhanced integration (supplemental Fig. S6).
FIGURE 3.
Simultaneous deletions of TOF1 and DIA2 lead to high frequency of Ty1his3AI retromobility. A, bar diagrams showing frequencies of Ty1his3AI retromobility in WT, tof1Δ, dia2Δ, dia2Δtof1Δ, csm3Δ, and dia2Δcsm3Δ strains, and in a dia2Δtof1Δ strain complimented with blank vector and that containing TOF1 or DIA2. B, role of Tof1 and Dia2 in suppression of Ty1 cDNA integration at tRNAgly sites; schematic diagram showing tRNAgly site and the primers used to analyze cDNA integration (top panel); (*) indicates the LTR region used as probe for detection of Ty1 element amplified by PCR from genomic DNA Bar diagrams showing the relative levels of integrated Ty1 at tRNAgly sites in WT, tof1Δ dia2Δ, dia2Δ tof1Δ, csm3Δ, and dia2Δ csm3Δ strains (bottom panel).
Several factors such as high levels of transcription of Ty1 and enhanced cDNA synthesis are known to be causative factors of higher frequency of retromobility. We analyzed Ty1 transcription and cDNA generation in the various mutants described above and compared them with that of the WT strain. The results are described in detail in the supplemental data (supplemental results and supplemental Figs. S1, S2, S3). The results showed that none of the mutant strains especially the rrm3Δtof1Δ and dia2Δ tof1Δ, revealed significant increases in either Ty1 transcription or cDNA generation that would be commensurate with the ultrahigh frequency of retromobility.
High Retromobility of Ty1 in rrm3Δtof1Δ and dia2Δtof1Δ Strains Occurred Independently of Fork Arrest at Ter and at Other DNA-Protein Barriers
Does ultrahigh frequency retromobility require fork arrest at Ter and fork stalling elsewhere on the chromosomes? We addressed this question by comparing replication fork progression in the aforementioned genotypes with that in the dia2Δtof1Δ and rrm3Δtof1Δ cells (with appropriate controls). As contrasted with the WT DNA that showed a single termination spot (formed by two merged spots corresponding to closely spaced pair of Ter sites) at Ter (solid arrow), the two-dimensional gel pattern of rrm3Δ cells not only showed enhanced fork arrest at Ter (solid arrow) but also fork stalling at 3 other sites (open arrowhead). As expected, in both tof1Δ and csm3Δ cells, fork arrest was mostly abolished. However, in rrm3Δtof1Δ and rrm3Δcsm3Δ cells, fork arrest at Ter and at 2 other sites was partially restored. These data, confirmed previously published work (15). In contrast two-dimensional gel patterns of replication intermediates of dia2Δ revealed no abolition of fork arrest at Ter. As contrasted with rrm3Δtof1Δ and rrm3Δcsm3Δ cells, replication intermediates of dia2Δtof1Δ and dia2Δcsm3Δ showed neither restoration of fork arrest at Ter nor fork stalling at the other sites. Because both rrm3Δtof1Δ and dia2Δtof1Δ cells caused high frequency of retromobility, we concluded that enhancement of retromobility could not be ascribed to fork stalling at Ter and at the other barriers. Furthermore, although the rrm3Δtof1Δ cells showed ∼10-fold greater retromobilty in comparison with an rrm3Δ strain the former had distinctly lower levels of fork arrest in comparison with the latter strain (Figs. 1 and 4).
FIGURE 4.
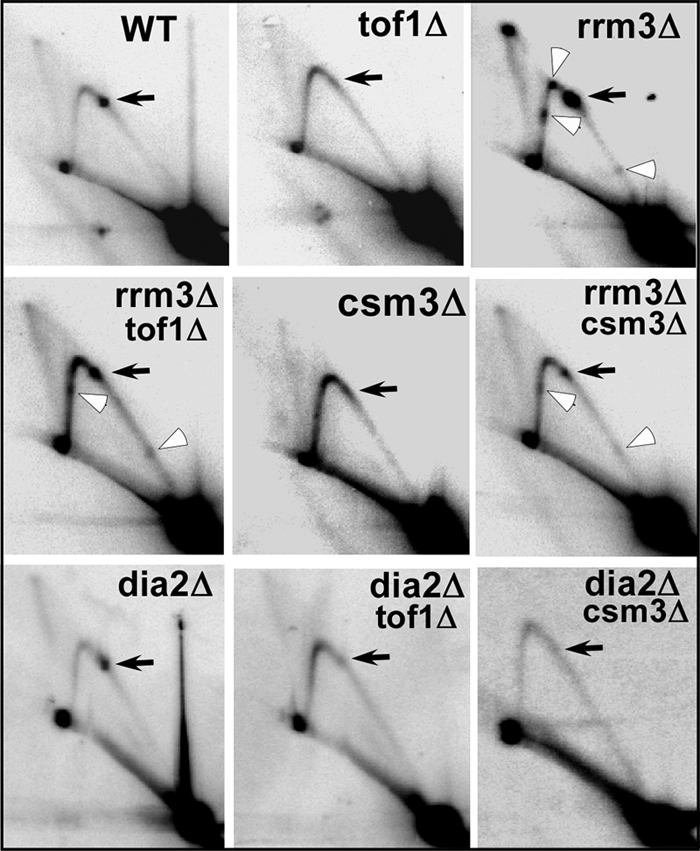
High frequency of Ty1 his3AI retromobility was independent of DNA-binding protein-mediated replication fork arrest. Autoradiograms of two-dimensional agarose gels of replication intermediates from the rDNA Ter region of chromosome XII showing that in the wild-type strain (WT) DNA replication fork were arrested at the Ter sites (black arrow), the arrest was abolished in tof1Δ and csm3Δ strains (panels marked tof1Δ and csm3Δ, respectively); in contrast, in the rrm3Δ strain replication fork arrest occurs at Ter sites is enhanced over that of the WT strain (rrm3Δ) and fork arrest occurred at 3 other locations (open arrows); in both rrm3Δ tof1Δ and rrm3Δ csm3Δ strains the fork arrest was partially restored at Ter and elsewhere; in the dia2Δ strain the replication fork arrest at Ter sites was unaffected; the dia2Δtof1Δ and dia2Δcsm3Δ cells did not show any restoration of fork arrest at the Ter sites as contrasted with rrm3Δtof1Δ and rrm3Δcsm3Δ strains.
Tof1, Csm3, Rrm3, and Dia2 Promoted Karyotype Stability
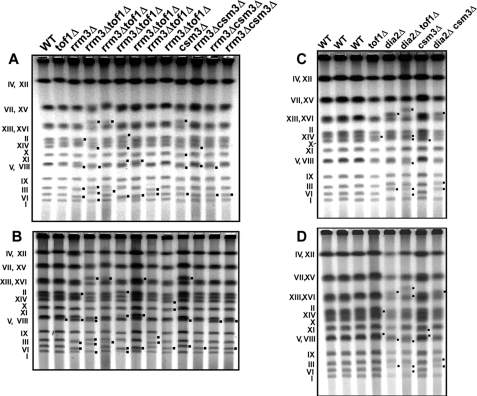
The large increase in the retromobility frequency in the rrm3Δtof1Δ and dia2Δtof1Δ strains prompted us to explore genome instability by using another criterion namely karyotype stability. We carried out a pulsed field gel-based karyotype analysis of the uninduced and induced (for retromobility) WT and the various mutant strains. To our surprise, we discovered that several strains such as tof1Δ, rrm3Δ, csm3Δ and dia2Δ showed chromosomal size variations even if the cells were not induced for retrotransposition (Fig. 5, A and C). In the mutant backgrounds several chromosomes showed the altered mobility in comparison to WT cells. The alterations were easier to detect in the smaller chromosomes because these were better resolved in the CHEF gels in comparison with the larger ones (Fig. 5, A–D). In tof1Δ cells the changes in mobility of the individual chromosomes differed from that of the csm3Δ cells (Fig. 5, A–D). However, it would be difficult to conclude at this time, without analysis of a much larger set of data, as to whether one particular deletion (e.g. tof1Δ) targeted a specific set of chromosome in comparison with another deletion strain (e.g. csm3Δ). We analyzed three independent clones of rrm3Δcsm3Δ (Fig. 5A) and observed a relatively more stable karyotype in comparison with the rrm3Δtof1Δ (six independent clones) and the WT cells. The karyotype of uninduced dia2Δ, dia2Δcsm3Δ and dia2Δtof1Δ cells also showed mobility changes in several chromosomes (Fig. 5C). To determine quantitatively the extent of karyotype changes in the various mutants in comparison with the WT we plotted the mobilities of the chromosomes as a function of their molecular masses using the Quantity OneTM software (Bio-Rad). Excepting rrm3Δ that showed reductions in chromosome size all of the other mutants showed increase in chromosome size, in the order of 25 to 40 Mb.
FIGURE 5.
Absence of the replication fork associated factors (RRM3, TOF1, CSM3, and DIA2) lead to karyotypic instability. A, ethidium bromide strained pulse field agarose gel showing karyotype changes in uninduced WT, tof1Δ, rrm3Δ, rrm3Δtof1Δ, csm3Δ, and rrm3Δcsm3Δ strains; B, same as in A, excepting that the cultures were induced for Ty1 retrotransposition by growing at 20 °C for 144 h; the un-induced culture of tof1Δ, rrm3Δ, and rrm3Δtof1Δ, csm3Δ and rrm3Δcsm3Δ strains showed size variations of different chromosomes as described in the text; C and D, panels showing the karyotype changes of WT, tof1Δ, dia2Δ, dia2Δtof1Δ, csm3Δ, and dia2Δcsm3Δ strains in uninduced and induced cultures, respectively; the dia2Δ, dia2Δtof1Δ and dia2Δcsm3Δ cells showed size variations in different chromosomes that are described in the text.
Although the precise molecular mechanism leading to the karyotype variations remains unknown at the present time, we considered the possibility that the altered mobility of the chromosomes could be due to trapping of RNA transcripts, resulting in extensive R-loop formation. If the size variations observed in PFGE gel were due to DNA:RNA hybrid formation then exhaustive RNase H treatment of the samples should restore the normal mobility pattern (seen in WT cells) by removing the RNA strands. We extensively digested the samples with RNase H for 12 h and washed with 50 mm EDTA. The RNase H-treated chromosomes along with the untreated chromosomes were fractionated in agarose gel by PFGE and stained with ethidium bromide. We did not observe any difference between the untreated and RNase H-treated intact chromosomes with regard to their mobility patterns in PFGE gel (supplemental Fig. S4). It is also unlikely that the karyotype changes could have been caused by trapped replication bubbles because DNA extracted from cells in the stationary phase showed the same karyotypic changes.
Regulation of Telomere Length Homeostasis Was Dependent on Tof1, Csm3, Rrm3, and Dia2
It has been reported that reduction in telomere length leads to enhancement of Ty1 transposition frequency (28). We wished to investigate whether the mutations used in this work namely tof1Δ, csm3Δ, dia2Δ and rrm3Δ either individually or in pair-wise combinations caused telomere length alterations that could possibly account for the high retromobility that we observed in certain strains. We grew the WT, tof1Δ, rrm3Δ, rrm3Δtof1Δ, and csm3Δ, rrm3Δcsm3Δ, dia2Δ, dia2Δtof1Δ and dia2Δcsm3Δ strains at 30 °C for 24–48 h; genomic DNA was prepared, digested with XhoI and PstI, fractionated by agarose gel, blotted on to NytranTM membranes and probed with a labeled telomere-specific DNA probe as described under “Experimental Procedures.” As shown in Fig. 6 the average telomere length increased in the absence of TOF1, CSM3, and RRM3. In the dia2Δ strain the telomere length did not show any detectable difference from that of the WT cells. Interestingly, the average telomere length in rrm3Δtof1Δ, rrm3Δcsm3Δ, dia2Δtof1Δ and dia2Δcsm3Δ strains was shorter than that of the WT cells (Fig. 6). Furthermore, the rrm3Δtof1Δ strain showed a greater degree of shortening of telomeres than the dia2Δtof1Δ strain. We also observed in the rrm3Δtof1Δ and dia2Δtof1Δ cells, that showed high frequency of retromobility, shortened telomeres in comparison with the rrm3Δcsm3Δ and dia2Δcsm3Δ cells that did not show significant changes in telomere length (Fig. 6). We suggest that it is unlikely that the small changes in telomere lengths caused by the mutations could have accounted for either the high retromobility or the relatively large karyotype changes observed, although partial loss of telomere length homeostasis could have contributed in some degrees to genome instability.
FIGURE 6.
The replication fork-associated factors TOF1, RRM3, CSM3, and DIA2 contribute to the maintenance of telomere length homeostasis. Autoradiogram of a Southern blot showing the relative length of telomeres in the WT, dia2Δ, dia2Δtof1Δ, dia2Δcsm3Δ, tof1Δ, csm3Δ, rrm3Δ, rrm3Δtof1Δ, and rrm3Δcsm3Δ strains; telomere lengths were analyzed by restriction digestion of genomic DNA by XhoI or PstI restriction enzymes and developed by hybridization to a labeled telomere-specific probe.
Tof1, Csm3, Rrm3, and Dia2 travel with the replisome and play key roles in the activation of replication checkpoint induced by genotoxic stress induced by HU. Could checkpoint induction account for the enhanced retromobility? We analyzed the sensitivity of the WT, tof1Δ, rrm3Δ, rrm3Δtof1Δ, csm3Δ, rrm3Δcsm3Δ, dia2Δ, dia2Δtof1Δ, and dia2Δcsm3Δ strains to 200 mm HU by spotting 10-fold serially diluted log phase cultures. We observed that the single deletion strains, namely tof1Δ, csm3Δ, and dia2Δ cells, showed HU sensitivity albeit to different degrees (supplemental Fig. S5, A and B). However, the rrm3Δ cells were not sensitive to HU although this strain has been reported to cause Rad53 phosphorylation without any genotoxic treatment (37). The csm3Δ cells were less sensitive to HU than tof1Δ cells. Interestingly, the rrm3Δtof1Δ and rrm3Δcsm3Δ cells were less sensitive to HU than their single-deletion counterparts suggesting that Tof1 and Csm3 counteracted the effect of Rrm3 in inducing HU sensitivity. In contrast to rrm3Δ cells (Fig. S5A), the dia2Δ cells were sensitive to HU stress (supplemental Fig. S5B). Interestingly, the dia2Δtof1Δ and dia2Δcsm3Δ cells showed increased sensitivity to HU in comparison to their single-deletion counterparts suggesting that Tof1 and Csm3 function in parallel with Dia2 in the maintenance of genome stability. The data, along with other observations reported above, supported the conclusion that Tof1 and Csm3 appear to have both shared and distinct functions and that Rrm3, Dia2, and Tof1 act in parallel pathways in controlling retromobility and genome stability. However, we did not observe a direct 1:1 correspondence between HU resistance and the extent of Ty1 retromobility in the various mutant strains examined in this work.
DISCUSSION
This work reports a new pathway of maintenance of genome stability that involves Tof1 of budding yeast acting together with two other proteins namely Rrm3 and Dia2 that are repressors of Ty1 transposition (RTT). Tof1 in combination with either of the two proteins suppressed highly promiscuous Ty1 retromobility. This is in sharp contrast with other mediators and effectors of checkpoint signaling such as Rad9 and Rad53 that promoted promiscuous retromobilty in a cell having a mutation in an RTT gene (26). The work also reports that the interacting partner of Tof1 namely, Csm3 did not promote repression of retromobility. Therefore, the two protein components, while working together to catalyze certain functions such as counteraction of the Rrm3 helicase activity (15, 16), also individually mediated different functions. This separation of function is supported by the observation that tof1Δ cells do not grow in plates containing 200 mm HU whereas csm3Δ cells were only partial sensitive to HU. Furthermore, tof1Δ, unlike csm3Δ, promoted preferential insertions of Ty1 into tRNAgly loci. This activity was sequence context-dependent and was not observed at either tRNAthr, 5S region, or tRNAser loci.
Tof1, Csm3, and Mrc1 form a fork protection complex (7). Although we were able to investigate the contributions of Tof1 and Csm3 to genome stability we could not do so for Mrc1 because its deletion is synthetically lethal with either rrm3Δ or dia2Δ (34, 38, 39).
How does tof1Δ synergize high frequency retromobility in either an rrm3Δ or dia2Δ cells? Although unraveling this mechanism is likely to take a considerable amount of additional work in the future, our results already show that it is unlikely to be due to an impact of the mutations on Ty1 transcription, cDNA synthesis or enhanced integration upstream of tRNAgly genes. Rather, we suggest that it is more likely to be due to alteration in the status (e.g. state of the chromatin) of the targets of transposition. Tof1 functionally interacts with the Les4 subunit of the Ino80 chromatin remodeling complex (40). Because the target of Ty1 is probably chromatin, it stands to reason that the transposition events should require chromatin remodeling to generate nucleosome free integration sites. It is therefore possible that Tof1, at least partially controls retrotransposition by modulating chromatin remodeling.
Our data show further that in a tof1Δ strain, integration of Ty1 at or near several loci encoding tRNAgly was significantly enhanced. Because a tof1Δ strain did not show an overall increase in the Ty1 retromobility in comparison with the WT strain it is expected that high frequency integration at tRNAgly sites could be accompanied by corresponding reduction of integration at other sites so that there is no net increase in retromobility. It has been reported that in a rrm3Δ strain there was a ∼30-fold increase in retromobility. This increase was probably caused by multiple insertions of Ty1 into a given site of integration (41). We hypothesize that a rrm3Δtof1Δ double mutant might be synergistically enhancing this process. We anticipate that a more detailed analysis of the mechanism would probably require paired-end, massively parallel sequencing of the Ty1 integrants present in selected chromosomes of rrm3Δtof1Δ cells in comparison with the WT and single deletion controls. This is a major project by itself that is planned for the future.
A second mechanism that might contribute to a reduction of genome stability is suggested by the unexpected observation that tof1Δ, csm3Δ, rrm3Δ and dia2Δ either individually or in pairs altered the karyotype of budding yeast mostly by additions of extra sequences, or in the case of rrm3Δ, by introducing deletions. The changes in the mobility of the chromosomes in pulse field gels could not be attributed to extensive R loop formation because the mobility alterations were resistant to extensive digestion with RNase H. It is also unlikely that the mobility changes can be ascribed to chromosomes containing replication bubbles or branches because such chromosomes fail to enter pulse field gels (42). All of these gene products are known to help maintenance of fork integrity and to facilitate its navigation through tightly bound nonhistone protein barriers (7, 14, 15). Dia2 binds to replication origins, is present at replication forks and interacts with Mrc1, that is a component of the fork protection complex (18, 19, 43). Therefore, it is reasonable to expect that in the absence of these proteins replication forks are likely to be more fragile and error-prone to induce addition or deletions of base pairs. It is not clear at the present time exactly which types of sequences (e.g. repeats, purine stretches etc.) are the sites for the alterations that lead to the observed karyotype changes. This will be investigated further in the near future by paired-end massively parallel sequencing of a few selected chromosomes that show the alteration in mobility in pulsed field gels.
It has been reported that mutations that perturb telomere length homeostasis create replication stress that in turn promotes a higher frequency of retromobility (28). It has been reported that TOF1 and CSM3 can cause contraction of (CNG)n trinucleotide repeats and TOF1 along with several checkpoint-related proteins can cause trinucleotide repeat expansion (44). It is possible that such a mechanism could account for the observed changes in telomere length. Although, the small changes in telomere length observed could contributes to some increase in retromobility, it is unlikely to be the sole cause for the much larger changes in chromosome lengths that are of the order of 20–40 kb. Also the modest changes in telomere length, we suggest, are unlikely to fully account for the ultrahigh frequency retrotransposition.
The high frequency of Ty1 retromobility observed in the (3 independently isolated) rrm3Δtof1Δ strains would be expected to generate spontaneous mutations that could act as suppressors. In fact we have isolated one such clone that might include a possible suppressor(s). This will be a subject of future investigation.
Finally, epistasis analyses reported in this work showed that TOF1 contributed to the maintenance of genome stability in a pathway that is parallel to ones in which RRM3 or DIA2 are functional. Our conclusion, summarized in Fig. 7, has taken into consideration the known synthetic lethality of dia2Δrrm3Δ that indicates that the latter two genes also function in parallel pathways.
FIGURE 7.
A model showing relative roles played by replication fork associated proteins TOF1, CSM3, RRM3, and DIA2 in the maintenance of genome stability. The model, based on epistasis analysis and biochemical assays described in the text, suggest that TOF1 and RRM3 on the one hand and TOF1 and DIA2 on the other, act in parallel pathways to contribute to genome stability. The model has taken into consideration the fact that dia2Δrrm3Δ is synthetically lethal and therefore RRM3 and DIA2 are likely to function in parallel pathways.
Supplementary Material
Acknowledgment
We thank Dr. Pascale Lesage for plasmids and strain.
This work was supported, in whole or in part, by National Institutes of Health Grants GM049264 and GM049264-17S1 (to D. B.) and by GM52072 (to M. J. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- HU
- hydroxyurea
- PFGE
- pulsed field gel electrophoresis
- CHEF
- contour-clamped homogeneous electric field.
REFERENCES
- 1. Sancar A., Lindsey-Boltz L. A., Unsal-Kaçmaz K., Linn S. (2004) Annu. Rev. Biochem. 73, 39–85 [DOI] [PubMed] [Google Scholar]
- 2. Elledge S. J. (1996) Science 274, 1664–1672 [DOI] [PubMed] [Google Scholar]
- 3. Foss E. J. (2001) Genetics 157, 567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gotter A. L., Manganaro T., Weaver D. R., Kolakowski L. F., Jr., Possidente B., Sriram S., MacLaughlin D. T., Reppert S. M. (2000) Nat. Neurosci. 3, 755–756 [DOI] [PubMed] [Google Scholar]
- 5. Gotter A. L. (2003) J. Mol. Biol. 331, 167–176 [DOI] [PubMed] [Google Scholar]
- 6. Mayer M. L., Pot I., Chang M., Xu H., Aneliunas V., Kwok T., Newitt R., Aebersold R., Boone C., Brown G. W., Hieter P. (2004) Mol. Biol. Cell 15, 1736–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katou Y., Kanoh Y., Bando M., Noguchi H., Tanaka H., Ashikari T., Sugimoto K., Shirahige K. (2003) Nature 424, 1078–1083 [DOI] [PubMed] [Google Scholar]
- 8. Kemp M. G., Akan Z., Yilmaz S., Grillo M., Smith-Roe S. L., Kang T. H., Cordeiro-Stone M., Kaufmann W. K., Abraham R. T., Sancar A., Unsal-Kaçmaz K. (2010) J. Biol. Chem. 285, 16562–16571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unsal-Kaçmaz K., Sancar A. (2004) Mol. Cell. Biol. 24, 1292–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alcasabas A. A., Osborn A. J., Bachant J., Hu F., Werler P. J., Bousset K., Furuya K., Diffley J. F., Carr A. M., Elledge S. J. (2001) Nat. Cell Biol. 3, 958–965 [DOI] [PubMed] [Google Scholar]
- 11. Chan R. C., Chan A., Jeon M., Wu T. F., Pasqualone D., Rougvie A. E., Meyer B. J. (2003) Nature 423, 1002–1009 [DOI] [PubMed] [Google Scholar]
- 12. Xu H., Boone C., Klein H. L. (2004) Mol. Cell. Biol. 24, 7082–7090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Warren C. D., Eckley D. M., Lee M. S., Hanna J. S., Hughes A., Peyser B., Jie C., Irizarry R., Spencer F. A. (2004) Mol. Biol. Cell 15, 1724–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ivessa A. S., Lenzmeier B. A., Bessler J. B., Goudsouzian L. K., Schnakenberg S. L., Zakian V. A. (2003) Mol. Cell 12, 1525–1536 [DOI] [PubMed] [Google Scholar]
- 15. Mohanty B. K., Bairwa N. K., Bastia D. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 897–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mohanty B. K., Bairwa N. K., Bastia D. (2009) Eukaryotic Cell 8, 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cardozo T., Pagano M. (2004) Nat. Rev. Mol. Cell Biol. 5, 739–751 [DOI] [PubMed] [Google Scholar]
- 18. Morohashi H., Maculins T., Labib K. (2009) Curr. Biol. 19, 1943–1949 [DOI] [PubMed] [Google Scholar]
- 19. Mimura S., Komata M., Kishi T., Shirahige K., Kamura T. (2009) EMBO J. 28, 3693–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lengronne A., Katou Y., Mori S., Yokobayashi S., Kelly G. P., Itoh T., Watanabe Y., Shirahige K., Uhlmann F. (2004) Nature 430, 573–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calzada A., Hodgson B., Kanemaki M., Bueno A., Labib K. (2005) Genes Dev. 19, 1905–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tourrière H., Versini G., Cordón-Preciado V., Alabert C., Pasero P. (2005) Mol. Cell 19, 699–706 [DOI] [PubMed] [Google Scholar]
- 23. Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. (1985) Cell 40, 491–500 [DOI] [PubMed] [Google Scholar]
- 24. Garfinkel D. J., Boeke J. D., Fink G. R. (1985) Cell 42, 507–517 [DOI] [PubMed] [Google Scholar]
- 25. Curcio M. J., Garfinkel D. J. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 936–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Curcio M. J., Kenny A. E., Moore S., Garfinkel D. J., Weintraub M., Gamache E. R., Scholes D. T. (2007) Mol. Cell. Biol. 27, 8874–8885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brewer B. J., Fangman W. L. (1987) Cell 51, 463–471 [DOI] [PubMed] [Google Scholar]
- 28. Scholes D. T., Kenny A. E., Gamache E. R., Mou Z., Curcio M. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 15736–15741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scholes D. T., Banerjee M., Bowen B., Curcio M. J. (2001) Genetics 159, 1449–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mou Z., Kenny A. E., Curcio M. J. (2006) Genetics 172, 2157–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shampay J., Szostak J. W., Blackburn E. H. (1984) Nature 310, 154–157 [DOI] [PubMed] [Google Scholar]
- 32. Beauregard A., Curcio M. J., Belfort M. (2008) Annu. Rev. Genet 42, 587–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stamenova R., Maxwell P. H., Kenny A. E., Curcio M. J. (2009) Genetics 182, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tong A. H., Lesage G., Bader G. D., Ding H., Xu H., Xin X., Young J., Berriz G. F., Brost R. L., Chang M., Chen Y., Cheng X., Chua G., Friesen H., Goldberg D. S., Haynes J., Humphries C., He G., Hussein S., Ke L., Krogan N., Li Z., Levinson J. N., Lu H., Ménard P., Munyana C., Parsons A. B., Ryan O., Tonikian R., Roberts T., Sdicu A. M., Shapiro J., Sheikh B., Suter B., Wong S. L., Zhang L. V., Zhu H., Burd C. G., Munro S., Sander C., Rine J., Greenblatt J., Peter M., Bretscher A., Bell G., Roth F. P., Brown G. W., Andrews B., Bussey H., Boone C. (2004) Science 303, 808–813 [DOI] [PubMed] [Google Scholar]
- 35. Pan X., Ye P., Yuan D. S., Wang X., Bader J. S., Boeke J. D. (2006) Cell 124, 1069–1081 [DOI] [PubMed] [Google Scholar]
- 36. Bachman N., Eby Y., Boeke J. D. (2004) Genome Res. 14, 1232–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bessler J. B., Zakian V. A. (2004) Genetics 168, 1205–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torres J. Z., Schnakenberg S. L., Zakian V. A. (2004) Mol. Cell. Biol. 24, 3198–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schmidt K. H., Kolodner R. D. (2004) Mol. Cell. Biol. 24, 3213–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morrison A. J., Kim J. A., Person M. D., Highland J., Xiao J., Wehr T. S., Hensley S., Bao Y., Shen J., Collins S. R., Weissman J. S., Delrow J., Krogan N. J., Haber J. E., Shen X. (2007) Cell 130, 499–511 [DOI] [PubMed] [Google Scholar]
- 41. Bashir A., Volik S., Collins C., Bafna V., Raphael B. J. (2008) PLoS Computational Biology 4, e1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Christman M. F., Dietrich F. S., Levin N. A., Sadoff B. U., Fink G. R. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 7637–7641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koepp D. M., Kile A. C., Swaminathan S., Rodriguez-Rivera V. (2006) Mol. Biol. Cell 17, 1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Razidlo D. F., Lahue R. S. (2008) DNA Repair 7, 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.