Abstract
Human serum albumin (HSA) is the dominating protein in human plasma. Many bacterial species, especially streptococci, express surface proteins that bind HSA with high specificity and affinity, but the biological consequences of these protein-protein interactions are poorly understood. Group G streptococci (GGS), carrying the HSA-binding protein G, colonize the skin and the mucosa of the upper respiratory tract, mostly without causing disease. In the case of bacterial invasion, pro-inflammatory cytokines are released that activate the epithelium to produce antibacterial peptides, in particular the chemokine MIG/CXCL9. In addition, the inflammation causes capillary leakage and extravasation of HSA and other plasma proteins, environmental changes at the epithelial surface to which the bacteria need to respond. In this study, we found that GGS adsorbed HSA from both saliva and plasma via binding to protein G and that HSA bound to protein G bound and inactivated the antibacterial MIG/CXCL9 peptide. Another surface protein of GGS, FOG, was found to mediate adherence of the bacteria to pharyngeal epithelial cells through interaction with glycosaminoglycans. This adherence was not affected by activation of the epithelium with a combination of IFN-γ and TNF-α, leading to the production of MIG/CXCL9. However, at the activated epithelial surface, adherent GGS were protected against killing by MIG/CXCL9 through protein G-dependent HSA coating. The findings identify a previously unknown bacterial survival strategy that helps to explain the evolution of HSA-binding proteins among bacterial species of the normal human microbiota.
Keywords: Antimicrobial Peptides, Bacteria, Chemokines, Inflammation, Innate Immunity, Albumin, Mucosa
Introduction
Several streptococcal species, among them group G streptococci (GGS2; Streptococcus dysgalactiae ssp. equisimilis), colonize the human dermis and the mucosa of the upper airways. In addition to being part of the normal microbiota, GGS are opportunistic pathogens, causing both superficial infections, such as pharyngitis and erysipelas, and deep and severe infections (1, 2). In the event of bacterial invasion, an inflammatory host response is initiated where dendritic, natural killer, and T cells residing in subepithelial tissues recognize bacterial products by pattern recognition receptors (3). This results in the production of proinflammatory cytokines, among them IFN-γ and TNF-α, rendering an activated phenotype to adjacent cells, including the epithelial lining (4–6). As a consequence, epithelial cells start producing host defense molecules, e.g. the antibacterial chemokine MIG/CXCL9 (monokine-induced by gamma-interferon/CXC ligand 9) (7, 8). In addition, production of chemokines causes recruitment and activation of neutrophils (3). Release of neutrophil granule proteins, in particular heparin-binding protein, together with neurogenic mechanisms, contribute to extravasation of plasma constituents, e.g. albumin (9–11). These changes of the habitat at the epithelial surface force the bacteria to use adaptive countermeasures to survive, including the release of proteases and proteins that attenuate the activity of antibacterial peptides (12–15).
More than 30 years ago, it was reported that different streptococcal species bind human serum albumin (HSA) with high affinity (16). In GGS (and group C streptococci), protein G, an IgG-binding surface protein (17, 18), was found to bind also HSA (19). In protein G, so-called GA modules, located in the N-terminal region, bind HSA (20–22), whereas the IgG-binding C domains are found in the C-terminal half of the molecule (23, 24). The interactions with HSA and IgG occur independently, and the organization of protein G allows GGS to coat their surface with an inner layer of IgG and an outer layer of HSA, the two most abundant proteins of human plasma. Surface proteins containing HSA-binding GA modules are also present in strains of the anaerobic bacterium Finegoldia magna (22) and in a biofilm-generating protein (Embp) of Staphylococcus epidermidis (25, 26). The bacterial species that express proteins containing GA modules are all part of the normal human bacterial flora. However, in addition, they are opportunistic pathogens, and it is noteworthy that the GA-containing proteins increase the virulence of F. magna and S. epidermidis (26, 27). A more recently discovered surface protein in GGS, FOG, has also been ascribed a role in virulence by interfering with neutrophil function and binding to collagen (28, 29).
The central question addressed in this study is whether FOG and the HSA-binding property of protein G affect the survival of GGS at the epithelial surface when the conditions dramatically change under the influence of proinflammatory chemokines. The results show that FOG is essential for the adherence of GGS to both activated and non-activated epithelial cells and that the adsorption of HSA to the surface of adherent GGS via the GA modules of protein G provides the bacteria with a protective shield of HSA against antibacterial MIG/CXCL9 released by the activated epithelium.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Recombinant human MIG/CXCL9, CCL3, IFN-γ, TNF-α, and rabbit antibodies against MIG/CXCL9 were from PeproTech (Rocky Hill, NJ). Novicidin (KNLRRIIRKGIHIIKKYF), a synthetic peptide derived from the ovine cathelicidin SMAP-29 (30), was generously provided by Dr. Hans-Henrik Kristensen (Novozymes, Copenhagen, Denmark).
HSA was from Sigma (catalog no. A3782). The GA module protein (residues 213–265 of protein PAB) was produced as described (31). Dermatan sulfate (DS) 36 and heparan sulfate (HS) 6 were provided by Lars-Åke Fransson.
Bacterial Strains and Growth Conditions
The S. dysgalactiae ssp. equisimilis (GGS) strain G148 and the Streptococcus mutans strain α3201 were collected at the Department of Clinical Microbiology, Lund University Hospital. The GGS strain G45 was collected at the Royal Brisbane Hospital. Strain G45 carries the FOG protein on its surface, whereas strain G148 naturally lacks FOG. Streptococci were routinely grown in Todd Hewitt broth (Difco) containing yeast extract (2 g/liter; Oxoid Ltd.) in 5% CO2 at 37 °C. The isogenic mutants G45ΔFOG and G45ΔG, lacking protein FOG and protein G, respectively, were grown in the presence of erythromycin (1 μg/ml) to maintain the knocked out phenotype (32).
Bactericidal Assays
The GGS strains G148 and G45 and isogenic mutants of the latter were grown to mid-log phase in Todd Hewitt broth, washed, and diluted in incubation buffer. 50 μl of bacteria (2 × 106 colony-forming unit (cfu)/ml) were incubated with MIG/CXCL9 at various concentrations or with buffer alone for 1 h at 37 °C. To quantify bactericidal activity, serial dilutions of the incubation mixtures were plated on Todd Hewitt agar, and the number of cfu was determined after overnight incubation. To determine bactericidal activity on the surface of the pharyngeal epithelial cell line Detroit 562 (American Type Culture Collection, Manassas, VA), cells were grown in 24-well tissue culture plates (Costar) to near-confluence in minimal essential medium (Invitrogen) with heat-inactivated FBS (10%). Thereafter, cells were washed three times with minimal essential medium without FBS and incubated with serum-free minimal essential medium in the absence or presence of the indicated stimuli. This was followed by the addition of 25 μl of bacteria (AP1; 2 × 106/ml in PBS), layered on top of the epithelial cells. Centrifugation at 300 × g for 10 min was performed to promote cell-bacterium interaction, followed by incubation for 30 min at 37 °C. Trypsin (2.5 mg/ml in PBS) was used to detach the cells from the wells, and Triton X-100 (0.025% in PBS) was added to lyse the cells and release internalized bacteria. The number of cfu was determined by plating appropriate dilutions on culture plates. In some experiments, the mixture of cells and bacteria was detached using a rubber policeman, fixed, and embedded in Epon.
Surface Plasmon Resonance
HSA and the GA module were diluted in 10 mm sodium acetate (pH 4) at a concentration of 10 μg/ml, followed by immobilization via amine coupling to flow cells of a CM5 sensor chip (Biacore, Uppsala, Sweden). Binding and dissociation phases were monitored using a Biacore 2000 instrument. HSA and MIG/CXCL9 were injected at different concentrations (typically 31–500 nm) at a flow rate of 35 μl/min and a temperature of 25 °C over the flow cells using a running buffer containing 10 mm Hepes, 150 mm NaCl, 0.005% surfactant P20, and 3.4 mm EDTA (pH 7.5). To determine the association of MIG/CXCL9 with HSA in complex with the immobilized GA domain, an equal concentration of HSA (250 nm) was repetitively injected before injecting various concentrations of MIG/CXCL9 (31–500 nm) at a later time point. The association (Ka) and dissociation (Kd) rate constants were determined simultaneously using the equation for 1:1 Langmuir binding in the BIAevaluation Version 4.1 software (Biacore). The binding curves were fitted locally, and the equilibration dissociation constants (Kd) were calculated from mean values of the obtained rate constants.
HSA Adsorption Experiments
Bacteria (strain G148, 2 × 108 cfu/ml) were incubated in 100% saliva (collected and pooled from 12 healthy donors after >1 h of fasting using a SalivetteTM sponge device (Sarstedt, Nümbrecht, Germany)), 10% citrate-treated plasma, or HSA (4 mg/ml in PBS) at room temperature for 20 min. The bacteria were washed extensively with PBS, and bound proteins were eluted using 0.2 m glycine HCl (pH 2). Eluates were separated by SDS-PAGE under reducing conditions and visualized by Coomassie Brilliant Blue staining.
Preparation of Cytoplasts and Electron Microscopy
Cytoplasts (cell-derived vesicles free of nuclei) mimic the epithelial cell surface but are smaller and more stable than intact cells, making them suitable for negative staining and electron microscopy. To prepare cytoplasts, a suspension (7.5 × 107/ml) of washed Detroit 562 cells was pressurized with nitrogen for 5 min at 350 p.s.i. in a custom-made nitrogen cavitation bomb. The cavitate was collected, and a subcellular fraction of membranes was prepared by sequential centrifugation at 1000 × g for 10 min, followed by 10,000 × g for 20 min, after which the resulting pellet was collected. MIG/CXCL9 and albumin were labeled with 4- and 15-nm colloidal gold, respectively, as described (8). GGS in TBS (strain G148, 2 × 106 cfu/ml) were incubated for 20 min with 15-nm gold-labeled HSA. Thereafter, a suspension of the membrane fraction, dissolved in TBS, was incubated with gold-labeled MIG/CXCL9. An equimolar amount of gold-labeled HSA was added, and the incubation was allowed to proceed for an additional 20 min. The specimens were subjected to negative staining and examined in a JEOL 1200EX transmission electron microscope. In the case of Epon-embedded specimens, ultrathin sections were incubated with rabbit anti-MIG/CXCL9 antibodies (5 μg/ml) and visualized by a secondary step using goat anti-rabbit antibodies labeled with colloidal gold as described (33).
Labeling of Bacteria and Binding Assay
Bacteria were labeled with 125I using lactoperoxidase (Sigma). In brief, 1 ml of a 2% bacterial suspension (4 × 109 cfu/ml in PBS) was heat-killed by incubating the bacteria at 80 °C for 10 min. The bacteria were pelleted by centrifugation and resuspended in 100 μl of PBS, followed by the addition of 0.2 mCi of 125I. 2 μl of lactoperoxidase (2 mg/ml) were added together with 2 μl of freshly made 0.015% H2O2 in PBS, and the suspension was incubated for 10 min at room temperature. The bacteria were washed four times with cold PBS to remove unincorporated 125I and finally dissolved in 1 ml of PBS. Radiolabeling of DS36 and HS6 with 125I and binding to bacteria were performed and analyzed as described (17).
Statistical Analysis
Statistical significance was determined using Student's t test for paired observations.
RESULTS
Killing of GGS by MIG/CXCL9 Is Blocked by HSA
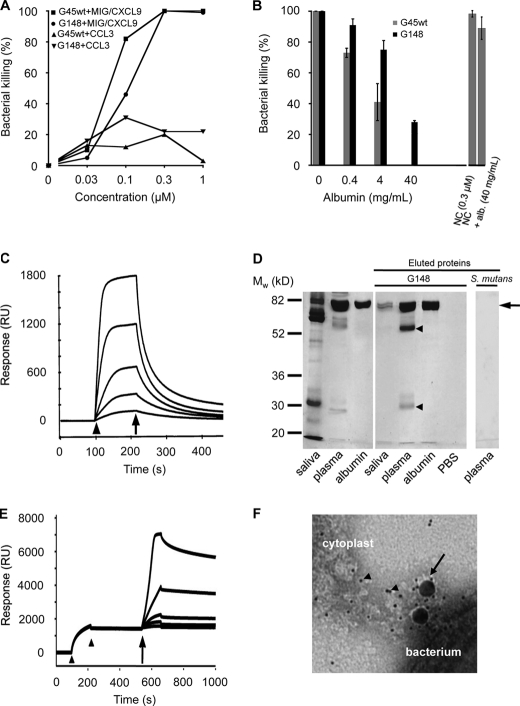
MIG/CXCL9 is produced by IFN-γ-activated pharyngeal epithelial cells and plays a key role in mediating bactericidal activity at the epithelial cell surface (8). Because GGS can cause pharyngitis, these bacteria will encounter MIG/CXCL9 in this context. Using a viable count assay, GGS (strains G45wt and G148) were dose-dependently killed upon exposure to this antibacterial protein. The CC chemokine CCL3 exerted low or no antibacterial activity at similar concentrations (Fig. 1A). Preincubation of MIG/CXCL9 with HSA for 20 min caused an efficient and dose-dependent inhibition of the antibacterial activity at HSA concentrations corresponding to those present in 1%, 10%, and 100% plasma (0.4, 4, and 40 mg/ml), respectively (Fig. 1B). In these experiments, the two GGS isolates mentioned above were used; strain G148 expresses HSA-binding protein G (19, 20) but lacks FOG, whereas strain G45wt expresses both protein G and FOG (19, 20, 28). In contrast to MIG/CXCL9, the antibacterial activity of the ovine cathelicidin-derived synthetic peptide novicidin was not significantly affected by HSA.
FIGURE 1.
Characterization of the interactions between the antibacterial chemokine MIG/CXCL9, GGS, and HSA. A, bactericidal activity of MIG/CXCL9 against GGS. Bacteria (strains G45wt and G148) were incubated with MIG/CXCL9 or CCL3 at the indicated concentrations or with buffer alone. To calculate percent killing, the cfu present after exposure to the polypeptides were compared with the cfu obtained after incubation in buffer alone. The data shown represent mean ± S.E. from three separate experiments. B, HSA attenuates the bactericidal activity of MIG/CXCL9. Bacteria were incubated with recombinant MIG/CXCL9 (0.3 μm) for 1 h at 37 °C or with MIG/CXCL9 that had been preincubated with HSA at 0.4, 4, or 40 mg/ml (corresponding to the HSA content of 1, 10, and 100% plasma, respectively) for 20 min prior to exposure to GGS (strains G45wt and G148) for 1 h at 37 °C. The number of cfu after incubation in buffer alone was set to 100%. The antibacterial activity of the peptide novicidin (NC; 0.3 μm) was not significantly affected (p = 0.25) by the presence of albumin (alb.; 40 mg/ml). The data shown represent mean ± S.E. from three separate experiments. C, HSA binds MIG/CXCL9 in surface plasmon resonance experiments. HSA was immobilized on a sensor chip, and increasing concentrations of MIG/CXCL9 were injected over the surface. The start and stop of injection are denoted by an arrowhead and arrow, respectively. RU, response units. D, GGS adsorb HSA from saliva and plasma. Bacteria were incubated with undiluted saliva, 10% plasma, or 4 mg/ml HSA for 20 min. After washing with PBS, bound proteins were eluted from bacteria with glycine HCl. These eluted proteins were separated by SDS-PAGE and visualized by Coomassie Brilliant Blue staining. Bands corresponding to albumin (arrow) are seen in all lanes, except the PBS control. The bands of ∼25 and ∼56 kDa in the lane with material eluted from bacteria incubated with plasma (arrowheads) correspond to light and heavy chains of IgG, respectively. S. mutans strain α3201 was incubated with plasma, but no bound proteins were visible after elution. E, binding of albumin to immobilized GA modules, followed by a dose-dependent increase in binding of MIG/CXCL9 as determined by surface plasmon resonance. GA modules were immobilized on a sensor chip, followed by injection (between the two arrowheads) and binding of HSA to the chip. Thereafter, increasing concentrations of MIG/CXCL9 were injected over the surface (indicated by the arrow), displaying a stable binding to the surface-bound GA·HSA complexes. F, electron micrograph showing the interaction between cytoplasts derived from pharyngeal epithelial cells, MIG/CXCL9 (labeled with 4-nm colloidal gold particles; arrowheads), HSA (labeled with 15-nm colloidal gold particles; arrow), and bacteria.
Analysis of the Interactions between GGS, HSA, and MIG/CXCL9
The binding between MIG/CXCL9 and HSA was investigated using surface plasmon resonance. MIG/CXCL9 showed a dose-dependent binding to immobilized HSA with a KD of 28 nm (Fig. 1C). To investigate the HSA-binding properties of GGS in physiological contexts, bacteria of the G148 strain were incubated with saliva (undiluted), plasma (10%), or HSA (4 mg/ml in PBS). After washing with PBS, bound proteins were dissociated from the bacterial surface by incubation with acid glycine, separated by SDS-PAGE, and stained with Coomassie Brilliant Blue (Fig. 1D). The results show that the bacteria adsorbed HSA when incubated with plasma or saliva from healthy donors. As judged from the SDS-PAGE bands, the amount of HSA in saliva under non-inflamed conditions was lower than in the diluted plasma sample and the HSA solution. The two bands at ∼25 and 56 kDa following incubation in plasma are explained by binding of IgG to the IgG-binding modules of protein G. They correspond to IgG light and heavy chains, respectively (SDS-PAGE was run under reducing conditions). An α-streptococcus (S. mutans) did not bind any plasma proteins as judged by SDS-PAGE, thus serving as a negative control. In addition, several other Gram-positive coccal species, such as Staphylococcus aureus and S. epidermidis, have been reported not to bind albumin (16).
Next, surface plasmon resonance was used to determine whether HSA in complex with the GA modules still binds MIG/CXCL9. GA modules were immobilized on a sensor chip, followed by injection of HSA (Fig. 1E), and a strong and stable binding of HSA to the chip was recorded (KD = 0.5 nm). In a subsequent step, increasing concentrations of MIG/CXCL9 were injected over the chip, and MIG/CXCL9 bound strongly to the GA·HSA complexes with a KD of 5 nm. No binding was obtained when MIG/CXCL9 was injected over GA alone (data not shown).
Similar to most chemokines, MIG/CXCL9 has glycosaminoglycan (GAG)-binding properties (8, 35). To visualize a possible simultaneous interaction between GAGs, MIG/CXCL9, HSA, and GGS, electron microscopy with negative staining was applied (Fig. 1F). Intact cells cannot be used for these studies, whereas cytoplasts (cell-derived vesicles free of nuclei) mimic the epithelial cell surface but are smaller and more stable. Cytoplasts were prepared from pharyngeal epithelial cells by nitrogen cavitation. GGS (strain G148) were washed and incubated with HSA labeled with 15-nm colloidal gold particles and MIG/CXCL9 labeled with smaller (4 nm) gold particles. At the interface between bacteria and cytoplasts, colloidal gold particles of different sizes co-localized, showing the presence of both MIG/CXCL9 and HSA. The large number of the smaller particles, corresponding to MIG/CXCL9, suggests a high local concentration of the chemokine at the interface.
Surface Protein FOG of GGS Promotes Adherence to Pharyngeal Epithelial Cells
A question raised by the results described above is whether GGS colonizing an epithelial surface are protected by protein G-bound HSA when antibacterial MIG/CXCL9 is produced in response to an inflammatory stimulus. Previous work has demonstrated that the adherence of another streptococcal species, Streptococcus pyogenes, to epithelial cells, is promoted by interactions between protein M (Ref. 36 and references therein) at the bacterial surface and GAGs of the epithelium (37), whereas the mechanisms promoting adherence of GGS to epithelial cells are poorly understood. Initial experiments demonstrated that in contrast to strain G45 (another clinical isolate of GGS), the G148 strain used in the experiments described above adhered less to epithelial cells and exhibited low or no interaction with highly sulfated GAGs (see below). For these reasons, the G45 strain was used in the subsequent experiments.
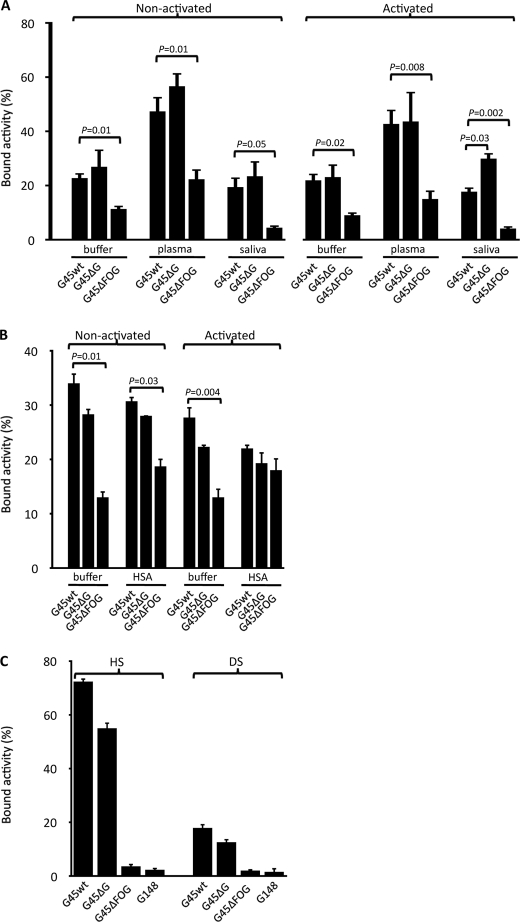
Apart from protein G, G45 bacteria express a fibrous protein M-like surface protein called FOG (28), which is not found in the G148 strain, suggesting that this protein could contribute to GAG binding and adherence. Heat-killed and 125I-labeled wild-type G45 bacteria and the isogenic mutants G45ΔFOG and G45ΔG, lacking protein FOG and protein G, respectively, were preincubated in PBS, citrated human plasma, saliva, or HSA solution, followed by washing with PBS. Thereafter, the bacteria were incubated with epithelial cells that were either non-activated or activated overnight with IFN-γ and TNF-α. The cell culture medium was discarded, and the bacteria were incubated with epithelial cells for 3 h. After washing, the radioactivity of the remaining attached bacteria was determined (Fig. 2, A and B). The adherence was consistently and significantly lower for bacteria lacking protein FOG (G45ΔFOG) during both non-activated and activated conditions. The binding of radiolabeled soluble GAGs (HS and DS) to G148, G45, and G45 mutant bacteria (Fig. 2C) showed that FOG was mainly responsible for the interaction with the GAGs. HS is the dominating GAG at epithelial surfaces, found in proteins such as CD44 and syndecan (38, 39), and the interaction was more pronounced with HS than with DS. A decrease in the binding of both GAGs, although to a much lesser degree, was also observed comparing wild-type G45 bacteria with the isogenic mutant lacking protein G, whereas strain G148, naturally lacking FOG, bound HS and DS in the order of G45ΔFOG. Taken together, the results demonstrate that FOG interacts with GAGs and promotes adhesion of GGS to both non-activated and activated epithelia.
FIGURE 2.
Binding of GGS to pharyngeal epithelial cells. A and B, GGS strain G45. Wild-type G45 and isogenic mutants G45ΔFOG and G45ΔG, lacking protein FOG and protein G, respectively, were killed by heat and labeled with 125I. Pharyngeal epithelial cells were cultured in the absence (non-activated) or presence (activated) of a combination of IFN-γ (100 units/ml) and TNF-α (10 ng/ml). 125I-Labeled G45wt, G45ΔFOG, and G45ΔG bacteria were incubated for 15 min in PBS, 10% human plasma, and whole saliva (A) or 0.4 mg/ml HSA (B); washed; and incubated with epithelial cells for 3 h at 37 °C. After washing, the radioactivity of the remaining pellets was calculated, and the radioactivity in percent of total added radioactivity was determined. Values are mean ± S.E. of at least three separate experiments, and Student's t test for paired observations was used to calculate p values. C, GGS bind highly sulfated GAGs. G45wt, G45ΔFOG, G45ΔG, and G148 bacteria were incubated with 125I-labeled HS or DS for 1 h at room temperature. After washing, the radioactivity of the bacterial pellets was determined and compared with the total radioactivity added. Data represent mean ± S.E. of four separate experiments. p values were calculated using Student's t test for paired observations.
Binding of HSA Protects GGS against Killing Also at the Surface of Cytokine-activated Pharyngeal Epithelial Cells
As shown above, the adherence of GGS to the epithelial cells was not influenced by cytokine activation, but these experiments did not address the question of whether the survival of the bacteria was affected. GGS were therefore incubated with resting or cytokine-activated pharyngeal epithelial cells. To investigate the role of HSA, the experiments were performed in the absence or presence of HSA and included both wild-type and isogenic GGS mutant bacteria lacking the HSA-binding protein G (G45ΔG). Wild-type and mutant bacteria were preincubated with HSA in PBS or with PBS alone, washed, and added to epithelial cells grown to confluence. After incubation for 3 h, non-adherent bacteria were discarded by washing with PBS, and the viability of the remaining adherent bacteria was determined by viable counts. Wild-type bacteria (strain G45wt) displayed decreased survival on cytokine-activated pharyngeal epithelial cells compared with non-activated epithelium. However, pretreatment with HSA significantly increased bacterial survival on the surface of activated cells. In contrast, the presence of HSA during the first incubation step did not influence the survival of the non-HSA-binding G45ΔG mutant on cytokine-activated epithelial cells (Fig. 3, A and B). BSA has a 2–3 orders of magnitude lower binding affinity for the GA module compared with HSA and was therefore included as an irrelevant control (30). Pretreatment of bacteria (strain G45wt) with BSA did not increase the survival on cytokine-activated epithelial cells. Taken together, these results suggest that the binding of HSA to the surface of GGS via protein G protects GGS from antibacterial activity on the activated epithelium.
FIGURE 3.
Bacterial survival on the surface of non-activated and activated pharyngeal epithelial cells. A, GGS (strain G45wt) were incubated with HSA (4 mg/ml) or PBS, washed, and added to non-activated or IFN-γ- and TNF-α-activated epithelial cells. Thereafter, bacteria and epithelial cells were co-incubated for 3 h. Following washing, the epithelial cells were detached and lysed, and the resulting debris was plated on agar plates. The number of cfu was calculated and related to the control (non-activated epithelial cells and strain G45wt pretreated with HSA). Values are mean ± S.E. from three separate experiments, and p values were calculated using Student's t test for paired observations. B, the same experiment as in A was performed with the isogenic mutant G45ΔG, devoid of protein G. Values represent mean ± S.E. of three separate experiments. C, electron microscopy of G45wt bacteria (GGS) incubated with non-activated pharyngeal epithelial cells (Ep). Ultrathin sections of cells and bacteria were incubated with anti-MIG/CXCL9 antibodies, followed by visualization of bound antibodies using secondary antibodies conjugated with 10-nm colloidal gold particles. The bacteria have a preserved integrity with a visible cell wall/plasma membrane. A few scattered gold particles are seen, indicating low or no presence of MIG/CXCL9. D, G45wt bacteria visualized at the surface of pharyngeal epithelial cells activated with a combination of IFN-γ and TNF-α. The integrity of bacteria is lost, and the cell wall/plasma membrane is no longer apparent. MIG/CXCL9 is seen both associated with the bacterial surface (arrows) and intracellularly (arrowheads). E, G45wt bacteria coated with HSA prior to exposure to activated pharyngeal epithelial cells. Bacterial integrity is preserved, and large amounts of MIG/CXCL9 are detected at the bacterial surface but not intracellularly (arrows). Scale bar = 1 μm. F, higher magnification (×10) of C showing the bacterial cell wall (W; indicated with a zigzag line) and the lipid bilayer of the plasma membrane (M) of a streptococcus in contact with a non-activated epithelial cell. G, disintegrated plasma membrane and cell wall with dispersed presence of MIG/CXCL9 in a streptococcus in contact with the cytokine-activated epithelium. H, higher magnification (×10) of A of a streptococcus coated with HSA. MIG/CXCL9, visualized as colloidal gold particles, accumulated at the cell wall, and the plasma membrane is intact.
Previous work has shown that the bactericidal activity of activated pharyngeal epithelial cells against S. pyogenes is attributable mainly to MIG/CXCL9 (8). To investigate if this is the case also with GGS, electron microscopy was used to visualize bacteria interacting with epithelial cells and MIG/CXCL9. Bacteria (strain G45wt) were incubated with either non-activated or cytokine-activated pharyngeal epithelial cells (Fig. 3, C–H). Bacteria adhering to the non-activated cells displayed a preserved integrity with a visible cell wall and plasma membrane (Fig. 3, A and F), and immunogold staining to detect MIG/CXCL9 indicated low or no presence of the chemokine. In contrast, GGS associated with the surface of cytokine-activated pharyngeal epithelial cells displayed a loss of integrity; cell walls and plasma membrane were no longer apparent (Fig. 3, D and G). MIG/CXCL9 was found both at bacterial surfaces and intracellularly, indicating uptake of the bactericidal protein. Bacteria incubated with HSA prior to exposure to activated pharyngeal epithelial cells had a preserved morphology with an intact plasma membrane and cell wall (Fig. 3, E and H). In the latter case, MIG/CXCL9 was seen accumulating at the peripheral parts of the cell wall corresponding to the location of protein G.
DISCUSSION
With regard to structure and protein-binding properties, protein G is one of the most well characterized surface proteins of Gram-positive bacteria. Especially the IgG-binding modules have been studied by several research groups, and from a functional point of view, the binding of IgG via the Fc region was shown to add selective advantage to the bacteria by blocking effector functions of IgG antibodies (Ref. 40 and references therein). The biological function of the HSA-binding so-called GA modules of protein G is less well understood, and the starting point for this investigation was an early experiment showing that GGS efficiently adsorbed HSA from the saliva of healthy humans, despite a low concentration of HSA. This finding suggested that GGS in the normal bacterial flora of the upper airways under physiological and non-inflammatory conditions bind HSA to their surface via the GA modules of protein G. It also indicated that the HSA coating of colonizing GGS should be more pronounced during inflammation and vascular leakage, leading to a high local concentration of HSA at the mucosa. However, the biological consequences of the interaction with HSA remained unclear; was bacterial adherence and colonization affected, and/or could HSA at the surface of GGS influence innate immune mechanisms?
A series of experiments showed that protein G and HSA had no or very small impact on the adherence of GGS to epithelial cells, despite some loss of HS and DS binding to bacteria lacking protein G. Instead, another surface protein, FOG, was found to play a crucial role in the adherence through interactions with highly sulfated GAGs such as HS. During inflammation, a plethora of GAG-binding proteins, among them MIG/CXCL9 and other antimicrobial peptides, are produced. Interestingly, FOG-mediated adhesion was not changed during inflammation, suggesting that the protein does not compete with chemokines for binding sites on GAGs. This implies that antimicrobial peptides (e.g. MIG/CXCL9) and FOG bind to different epitopes on GAGs or that FOG has a higher affinity for shared epitopes on GAGs, thus possibly displacing GAG-attached antimicrobial peptides. In both cases, a mechanism neutralizing the antimicrobial peptides close to the bacterial surface should be beneficial to the colonizing GGS.
The central finding of this study is that HSA bound to the surface of GGS via protein G protects the bacteria against MIG/CXCL9, a major and important bactericidal peptide released at the epithelial surface in response to an inflammatory stimulus (8). The utilization of a premade host protein present in large quantities in inflammatory exudates to form a protective shield against a powerful antibacterial polypeptide represents a novel bacterial survival strategy that is schematically depicted in Fig. 4. From a bacterial point of view, this mechanism appears as a rational and economic way to handle the harsh environment during inflammation. The observation that GGS adsorb HSA from saliva could indicate that these bacteria, as members of the indigenous flora of the oropharynx, also under normal and non-inflammatory conditions use HSA binding for nutrition (see below) and as a defense against constitutively expressed antibacterial peptides/proteins such as CCL28, histatins, and lysozyme (41, 42).
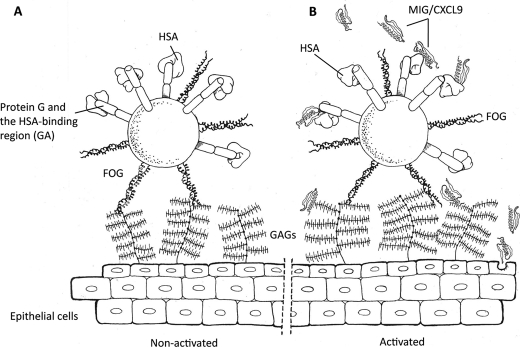
FIGURE 4.
Schematic and hypothetical representation of GGS at the epithelium of the oropharynx. A, under normal, non-activated conditions, GGS adhere to the epithelium via binding of FOG to highly sulfated GAGs. It is likely that the GA modules of protein G adsorb HSA from saliva, coating the bacteria also in the absence of an inflammatory stimulus causing vascular leakage and extravasation of HSA. B, in response to pro-inflammatory stimuli, epithelial cells produce antibacterial peptides, including MIG/CXCL9. This chemokine is associated with GAGs at the epithelial surface but is also present in a soluble form. HSA bound to protein G binds and attenuates the bactericidal activity of MIG/CXCL9. Also at the activated epithelial surface, the FOG protein remains an important mediator of bacterial adhesion.
The HSA-binding GA module (repeats of 45 amino acid residues each) was originally discovered in protein G (19, 20) but was given its name (GA stands for protein G-related albumin-binding module) when homologous modules were identified in an HSA-binding protein of F. magna (formerly Peptostreptococcus magnus) called PAB (22). F. magna is an anaerobic Gram-positive coccus found in the normal human bacterial flora at all non-sterile body surfaces, including the skin and oropharynx, and analogous to GGS, F. magna is also an opportunistic pathogen (Ref. 43 and references therein). So-called exon or module shuffling resulting in functional hybrid proteins has played a major role in the evolution of eukaryotic genes, and in this process, introns have been crucial by increasing the probability of favorable duplication and recombination events (44, 45). The known cases of module shuffling took place millions of years ago, and there were no contemporary examples (46) until the discovery of PAB revealed that this protein is the product of a transfer of the GA module from the protein G gene of GGS to the PAB gene of F. magna and that the conjugative plasmid pCF10 from Enterococcus faecalis participated in the transfer and recombination events resulting in the mosaic organization of PAB (22). The high degree of homology between the GA modules of proteins G and PAB and the short generation times in bacteria suggested that the shuffling of GA had occurred recently. Because prokaryotic genes as a rule lack introns, these results raised the question as to how multidomain bacterial proteins like PAB evolve. This was explained by the identification of recer sequences, a new kind of genetic element promoting interdomain in-frame recombination at the gene level and acting as structureless spacers in the corresponding protein (27). Another question relates to the evolutionary pressure behind the shuffling of the GA module between various bacterial species. F. magna strains expressing PAB are tetracycline-resistant (27), and the pCF10 plasmid mentioned above carries TetM, a common and important tetracycline resistance determinant (34), suggesting that the exposure to antibiotics has stimulated the rapid evolution of GA-containing proteins in bacterial species of the normal flora.
The binding of HSA to GA was shown to promote bacterial growth, presumably by making the free fatty acids transported by HSA (in plasma, 99% of these are bound to HSA) available for the bacteria (27), and this work presents another selective advantage of the GA module, especially for bacteria of the normal microbiota. Apart from GGS (and the closely related group C streptococci) and F. magna, a GA-containing protein (Embp) has also been identified in S. epidermidis (25). Embp is a giant protein (1 MDa) that is necessary for biofilm formation, and it contains 38 GA modules (26). GGS, F. magna, and S. epidermidis are all members of the normal flora, and they are often found in the same ecological niches (this is also true for E. faecalis, which contributes to the shuffling of GA with the pCF10 plasmid). When an inflammatory stimulus (for instance, a pathogen trying to establish itself on an epithelial surface inhabited by these commensals) results in the exudation of HSA and the production of antibacterial MIG/CXCL9, the binding of HSA to GA will provide both nutrients and protection against MIG/CXCL9. This efficient usage of the dominating human plasma protein emphasizes the power of bacterial adaptation and sheds further light on the rapid and ongoing evolution of GA-containing proteins among bacterial species of the normal human flora. A final aspect of the properties and spread of the GA module and the selective advantages it offers is related to virulence. Commensals expressing GA-containing proteins are also opportunistic pathogens, and there are numerous reports suggesting that they represent a growing medical problem. Thus, under antibiotic pressure, novel proteins are formed that tilt the delicate balance between bacteria of the normal flora and the human host toward virulence.
Acknowledgments
We thank Pia Andersson and Maria Baumgarten for excellent technical assistance, Rita Wallén for help with electron microscopy, and Annette Dahlström for the illustration in Fig. 4. The isogenic mutant G45 strain lacking the FOG protein was a gift from Dr. Inka Sastalla.
The work was supported by grants from the Swedish Research Council (Projects 7480 and 20674); the Swedish Heart and Lung Foundation (Project 20080246); the Medical Faculty at Lund University; the Swedish Government Funds for Clinical Research (ALF); and the Foundations of Torsten and Ragnar Söderberg, Bergh, Crafoord, Greta and Johan Kock, Marianne and Marcus Wallenberg, and Alfred Österlund.
- GGS
- group G streptococci
- HSA
- human serum albumin
- DS
- dermatan sulfate
- HS
- heparan sulfate
- cfu
- colony-forming unit
- GAG
- glycosaminoglycan.
REFERENCES
- 1. Hashikawa S., Iinuma Y., Furushita M., Ohkura T., Nada T., Torii K., Hasegawa T., Ohta M. (2004) J. Clin. Microbiol. 42, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sylvetsky N., Raveh D., Schlesinger Y., Rudensky B., Yinnon A. M. (2002) Am. J. Med. 112, 622–626 [DOI] [PubMed] [Google Scholar]
- 3. Sansonetti P. J. (2004) Nat. Rev. Immunol. 4, 953–964 [DOI] [PubMed] [Google Scholar]
- 4. Hyland K. A., Brennan R., Olmsted S. B., Rojas E., Murphy E., Wang B., Cleary P. P. (2009) FEMS Immunol. Med. Microbiol. 55, 422–431 [DOI] [PubMed] [Google Scholar]
- 5. Raeder R. H., Barker-Merrill L., Lester T., Boyle M. D., Metzger D. W. (2000) J. Infect. Dis. 181, 639–645 [DOI] [PubMed] [Google Scholar]
- 6. Kolls J. K., McCray P. B., Jr., Chan Y. R. (2008) Nat. Rev. Immunol. 8, 829–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sauty A., Dziejman M., Taha R. A., Iarossi A. S., Neote K., Garcia-Zepeda E. A., Hamid Q., Luster A. D. (1999) J. Immunol. 162, 3549–3558 [PubMed] [Google Scholar]
- 8. Egesten A., Eliasson M., Johansson H. M., Olin A. I., Morgelin M., Mueller A., Pease J. E., Frick I. M., Bjorck L. (2007) J. Infect. Dis. 195, 684–693 [DOI] [PubMed] [Google Scholar]
- 9. Gautam N., Olofsson A. M., Herwald H., Iversen L. F., Lundgren-Akerlund E., Hedqvist P., Arfors K. E., Flodgaard H., Lindbom L. (2001) Nat. Med. 7, 1123–1127 [DOI] [PubMed] [Google Scholar]
- 10. Edens H. A., Parkos C. A. (2003) Curr. Opin. Hematol. 10, 25–30 [DOI] [PubMed] [Google Scholar]
- 11. McDonald D. M. (1992) Am. Rev. Respir. Dis. 146, S40–S44 [DOI] [PubMed] [Google Scholar]
- 12. Schmidtchen A., Frick I. M., Andersson E., Tapper H., Björck L. (2002) Mol. Microbiol. 46, 157–168 [DOI] [PubMed] [Google Scholar]
- 13. Frick I. M., Akesson P., Rasmussen M., Schmidtchen A., Björck L. (2003) J. Biol. Chem. 278, 16561–16566 [DOI] [PubMed] [Google Scholar]
- 14. Nizet V. (2007) J. Allergy Clin. Immunol. 120, 13–22 [DOI] [PubMed] [Google Scholar]
- 15. Potempa J., Pike R. N. (2009) J. Innate Immun. 1, 70–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kronvall G., Simmons A., Myhre E. B., Jonsson S. (1979) Infect. Immun. 25, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Björck L., Kronvall G. (1984) J. Immunol. 133, 969–974 [PubMed] [Google Scholar]
- 18. Reis K. J., Boyle M. D., Ayoub E. M. (1984) J. Clin. Lab. Immunol. 13, 75–80 [PubMed] [Google Scholar]
- 19. Björck L., Kastern W., Lindahl G., Widebäck K. (1987) Mol. Immunol. 24, 1113–1122 [DOI] [PubMed] [Google Scholar]
- 20. Akerström B., Nielsen E., Björck L. (1987) J. Biol. Chem. 262, 13388–13391 [PubMed] [Google Scholar]
- 21. Nygren P. A., Eliasson M., Abrahmsén L., Uhlén M., Palmcrantz E. (1988) J. Mol. Recognit. 1, 69–74 [DOI] [PubMed] [Google Scholar]
- 22. de Château M., Björck L. (1994) J. Biol. Chem. 269, 12147–12151 [PubMed] [Google Scholar]
- 23. Fahnestock S. R., Alexander P., Nagle J., Filpula D. (1986) J. Bacteriol. 167, 870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guss B., Eliasson M., Olsson A., Uhlén M., Frej A. K., Jörnvall H., Flock J. I., Lindberg M. (1986) EMBO J. 5, 1567–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williams R. J., Henderson B., Sharp L. J., Nair S. P. (2002) Infect. Immun. 70, 6805–6810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christner M., Franke G. C., Schommer N. N., Wendt U., Wegert K., Pehle P., Kroll G., Schulze C., Buck F., Mack D., Aepfelbacher M., Rohde H. (2010) Mol. Microbiol. 75, 187–207 [DOI] [PubMed] [Google Scholar]
- 27. de Château M., Björck L. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 8490–8495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johansson H. M., Mörgelin M., Frick I. M. (2004) Microbiology 150, 4211–4221 [DOI] [PubMed] [Google Scholar]
- 29. Nitsche D. P., Johansson H. M., Frick I. M., Mörgelin M. (2006) J. Biol. Chem. 281, 1670–1679 [DOI] [PubMed] [Google Scholar]
- 30. Dorosz J., Gofman Y., Kolusheva S., Otzen D., Ben-Tal N., Nielsen N. C., Jelinek R. (2010) J. Phys. Chem. B 114, 11053–11060 [DOI] [PubMed] [Google Scholar]
- 31. Johansson M. U., Frick I. M., Nilsson H., Kraulis P. J., Hober S., Jonasson P., Linhult M., Nygren P. A., Uhlén M., Björck L., Drakenberg T., Forsén S., Wikström M. (2002) J. Biol. Chem. 277, 8114–8120 [DOI] [PubMed] [Google Scholar]
- 32. Nitsche-Schmitz D. P., Johansson H. M., Sastalla I., Reissmann S., Frick I. M., Chhatwal G. S. (2007) J. Biol. Chem. 282, 17530–17536 [DOI] [PubMed] [Google Scholar]
- 33. Bengtson S. H., Sandén C., Mörgelin M., Marx P. F., Olin A. I., Leeb-Lundberg L. M., Meijers J. C., Herwald H. (2008) J. Innate Immun. 1, 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christie P. J., Korman R. Z., Zahler S. A., Adsit J. C., Dunny G. M. (1987) J. Bacteriol. 169, 2529–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamel D. J., Sielaff I., Proudfoot A. E., Handel T. M. (2009) Methods Enzymol. 461, 71–102 [DOI] [PubMed] [Google Scholar]
- 36. Fischetti V. A. (1989) Clin. Microbiol. Rev. 2, 285–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frick I. M., Schmidtchen A., Sjöbring U. (2003) Eur. J. Biochem. 270, 2303–2311 [DOI] [PubMed] [Google Scholar]
- 38. Sampson P. M., Boyd R. B., Pietra G. G., Fishman A. P. (1984) J. Appl. Physiol. 57, 1648–1654 [DOI] [PubMed] [Google Scholar]
- 39. Bernfield M., Götte M., Park P. W., Reizes O., Fitzgerald M. L., Lincecum J., Zako M. (1999) Annu. Rev. Biochem. 68, 729–777 [DOI] [PubMed] [Google Scholar]
- 40. Boyle M. P. D. (1990) Bacterial Immunoglobulin-binding Proteins, Academic Press, San Diego, CA [Google Scholar]
- 41. Hieshima K., Ohtani H., Shibano M., Izawa D., Nakayama T., Kawasaki Y., Shiba F., Shiota M., Katou F., Saito T., Yoshie O. (2003) J. Immunol. 170, 1452–1461 [DOI] [PubMed] [Google Scholar]
- 42. Edgerton M., Koshlukova S. E. (2000) Adv. Dent. Res. 14, 16–21 [DOI] [PubMed] [Google Scholar]
- 43. Murdoch D. A. (1998) Clin. Microbiol. Rev. 11, 81–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gilbert W. (1978) Nature 271, 501. [DOI] [PubMed] [Google Scholar]
- 45. Patthy L. (1994) Curr. Opin. Struct. Biol. 4, 383–392 [Google Scholar]
- 46. Patthy L. (1991) Curr. Opin. Struct. Biol. 1, 351–361 [Google Scholar]