Abstract
Dictyostelium discoideum myosin II heavy chain kinase A (MHCK A), a member of the atypical α-kinase family, phosphorylates sites in the myosin II tail that block filament assembly. Here we show that the catalytic activity of A-CAT, the α-kinase domain of MHCK A (residues 552–841), is severely inhibited by the removal of a disordered C-terminal tail sequence (C-tail; residues 806–841). The key residue in the C-tail was identified as Thr825, which was found to be constitutively autophosphorylated. Dephosphorylation of Thr825 using shrimp alkaline phosphatase decreased A-CAT activity. The activity of a truncated A-CAT lacking Thr825 could be rescued by Pi, phosphothreonine, and a phosphorylated peptide, but not by threonine, glutamic acid, aspartic acid, or an unphosphorylated peptide. These results focused attention on a Pi-binding pocket located in the C-terminal lobe of A-CAT. Mutational analysis demonstrated that the Pi-pocket was essential for A-CAT activity. Based on these results, it is proposed that autophosphorylation of Thr825 activates ACAT by providing a covalently tethered ligand for the Pi-pocket. Ab initio modeling studies using the Rosetta FloppyTail and FlexPepDock protocols showed that it is feasible for the phosphorylated Thr825 to dock intramolecularly into the Pi-pocket. Allosteric activation is predicted to involve a conformational change in Arg734, which bridges the bound Pi to Asp762 in a key active site loop. Sequence alignments indicate that a comparable regulatory mechanism is likely to be conserved in Dictyostelium MHCK B-D and metazoan eukaryotic elongation factor-2 kinases.
Keywords: Cytoskeleton, Dictyostelium, Myosin, Protein Kinases, Protein Phosphorylation, α-Kinase Family, Allosteric Site, Autophosphorylation
Introduction
Dictyostelium discoideum MHCK A3 is a highly specialized protein kinase that targets three threonine residues located in the α-helical coiled-coil tail of myosin II (1–4). Phosphorylation of these sites results in the disassembly of myosin II bipolar filaments and inhibits processes, such as cytokinesis, that depend on myosin II contractile activity (5, 6). MHCK A is 130-kDa in size and consists of an N-terminal α-helical coiled-coil domain, a central kinase domain, and a C-terminal WD-repeat domain (7). The coiled-coil domain assembles into trimers or tetramers, binds, and cross-links actin filaments and is responsible for targeting MHCK A to actin-rich cellular protrusions (8–10). The WD-repeat domain interacts with filamentous myosin II and is required for MHCK A to efficiently phosphorylate myosin II (10, 11). The kinase domain of MHCK A bears no sequence similarity to the superfamily of “conventional” eukaryotic protein kinases but instead belongs to a small but widespread family of atypical protein kinases termed the α-kinases (12).
In addition to MHCK A, D. discoideum expresses five proteins with α-kinase domains. Three of the proteins, termed MHCK B, C, and D, are closely related to MHCK A and at least two to them, MHCK B and C, function cooperatively to regulate myosin II filament assembly in vivo (13–15). The other two α-kinases, AK1 and VwkA, have domain structures unrelated to MHCK A. AK1 contains an Arf GTPase-activating protein domain and VwkA contains an N-terminal von Willebrand factor A-like domain. The function of AK1 is not known, whereas VwkA is involved in the regulation of contractile vacuole function (16, 17). Mammals express six multidomain proteins with α-kinase domains (12). These include TRPM6 and TRPM7, which function as divalent cation channels and phosphorylate the tail of non-muscle myosin II (18, 19) and eEF2K, which regulates protein synthesis (20).
The isolated α-kinase domain of MHCK A, termed A-CAT, displays a high level of protein kinase activity and strongly prefers to phosphorylate threonine residues (21, 22). The x-ray crystal structure of A-CAT reveals that it is composed of an N-terminal and C-terminal lobe with the active site situated in a cleft at the interface between the two lobes (23). An invariant catalytic residue in the active site (Asp766) can be phosphorylated, suggesting that the α-kinase catalytic mechanism differs from that of conventional eukaryotic protein kinases. A-CAT contains a tightly bound zinc atom that is required for stability and binds two Mg2+ ions, one in an active site pocket and one at the center of the glycine-rich N/D-loop. The Mg2+ binding sites are regulatory, because millimolar concentrations of Mg2+, in excess of that required to bind to ATP, are required for A-CAT to exhibit maximal catalytic activity (22). A-CAT also binds a Pi molecule at a highly basic site in the C-terminal lobe that we term the Pi-pocket (23).
The catalytic activity of MHCK A is enhanced at least 50-fold by autophosphorylation (24). The cellular signaling mechanisms that activate MHCK A remain to be elucidated, but in vitro the rate of autophosphorylation is stimulated by actin filaments, myosin II, and negatively charged compounds, such as DNA and acidic phospholipids (25, 26). MHCK A incorporates up to 10 mol of phosphate/mol, but 3 mol of phosphate are sufficient for maximal activation (24). Here we identify Thr825 as a key autophosphorylation site that is required for the activity of A-CAT and MHCK A. Thr825 is located within a disordered sequence that links the α-kinase domain to the WD-repeat domain. We further demonstrate that the Pi-pocket functions as a positive allosteric binding site, and propose a model in which the phosphorylated Thr825 (Thr(P)825) activates A-CAT by providing a covalently tethered ligand for the Pi-pocket. Sequence alignments indicate that the proposed regulatory mechanism is conserved in the other MHCKs and the metazoan eEF2Ks.
EXPERIMENTAL PROCEDURES
Materials
Peptides used in this study were synthesized by the Sheldon Biotechnology Facility, McGill University. ATP, MBP, aspartic acid, glutamic acid, threonine, and phosphothreonine were purchased from Sigma and [γ-32P]ATP was obtained from PerkinElmer Life Sciences.
Plasmid Constructs
DNA manipulations were carried out using standard methods (27). Truncation and deletion constructs of the MHCK A α-kinase domain (A-CAT; residues 552–841) were created using PCR (22). DNA constructs encoding site-directed mutants of A-CAT were generated using the QuikChange XL site-directed mutagenesis system (Stratagene). Constructs were cloned in-frame into the NcoI and XhoI sites of the pET-28a vector (Novagen) to add an N-terminal His tag and a TEV protease site as described (23). Full-length MHCK A constructs (NCBI accession XP_635600) were cloned into the plasmid pTX-FLAG vector (28) that had been adapted to GATEWAY technology (Invitrogen). Briefly, the parent plasmid pTX-FLAG was digested by BamHI and XhoI and blunted with T4 DNA polymerase. The Gateway recombination cassette (reading frame cassette B) was then inserted to create destination vector pTX-FLAG-GATE. Expression constructs encoding MHCK A were then created using pTX-FLAG-GATE according to the manufacturer's instructions.
Protein Expression and Purification
Wild-type and mutant forms of A-CAT were expressed in Escherichia coli BL21(DE3) and purified by chromatography over a His-Bind column (Novagen) as described (supplemental Fig. S1) (23, 29). FLAG-tagged MHCK A was expressed in the D. discoideum AX3 cell line (30). Cells were cultured on 9-cm plastic Petri dishes in HL5 medium (31) supplemented with 10,000 units/ml of penicillin and 10 mg/ml of streptomycin (Sigma). Plasmids were introduced into AX3 cells by electroporation at 0.85 KV and 25 microfarads using a Bio-Rad Gene Pulser II Electroporation System (32). Clonal cell lines expressing FLAG-tagged MHCK A were produced by dilution plating and selection in 20 μg/ml of geneticin (G418) (Invitrogen). Cells were lysed by homogenization in 500 mm NaCl, 1 mm EDTA, 0.3% Triton X-100, and 50 mm Tris-HCl, pH 8.0, containing one complete mini protease inhibitor tablet (Roche Applied Diagnostics) per 50 ml of buffer at 4 °C. The supernatant obtained following centrifugation at 12,000 × g for 1 h was passed over a column of anti-FLAG M2 Affinity gel (Sigma) (33). FLAG-MHCK A was eluted with TBS buffer (150 mm NaCl, 50 mm Tris-HCl, pH 7.4) containing 200 μg/ml of FLAG peptide and dialyzed against 20 mm NaCl and 50 mm Tris-HCl, pH 7.4.
Kinase, ATPase, and Autophosphorylation Assays
ATPase and kinase activity was assayed at 22 °C in kinase buffer (2 mm MgCl2, 1 mm dithiothreitol, 0.25 mm ATP, and 20 mm TES, pH 7.0) containing [γ-32P]ATP at a specific activity of 5–500 cpm/pmol of ATP. Kinase assays included MBP or the MH-3 synthetic peptide (RKKFGEAEKTKAKEFL) at a concentration of 30 or 300 μm, respectively. Other additions to the kinase and ATPase assays are described in the figure legends. Assays were initiated by the addition of 1.45 μm A-CAT or 38.8 nm MHCK A. Aliquots were removed at 1, 2, 3, 4, and 5 min for kinase assays and 5, 10, 20, and 40 min for ATPase assays. In some cases kinase assays were performed at 4 °C and aliquots were removed at 20, 40, 60, and 90 s. ATP hydrolysis was measured by following the release of 32P (34). Kinase activity was measured by spotting 20-μl aliquots onto squares of Whatman P81 phosphocellulose paper (35). The squares were washed in 1% phosphoric acid, immersed in ScintiVerse Universal LS Mixture (Fisher Scientific), and counted using a Beckman LS 9000 scintillation counter. Over the assay time courses linear rates of ATPase activity and phosphate incorporation were obtained. Under the assay conditions used here A-CAT exhibited an ATPase activity of 0.022 ± 0.002 s−1 and a kinase activity of 0.047 ± 0.004 s−1 and 0.101 ± 0.0029 s−1 toward MBP and MH-3, respectively. MHCK A exhibited a kinase activity of 0.19 ± 0.033 s−1 toward MBP. Autophosphorylation assays contained 14.5 μm A-CAT-5xA and were carried out in kinase buffer containing [γ-32P]ATP at a specific activity of 500 cpm/pmol of ATP. Aliquots of 20 μl were taken at 1, 5, 10, 20, 40, and 60 min and subjected to SDS-PAGE. After staining with Coomassie Blue the A-CAT-5xA band was excised and counted in scintillation fluid to measure incorporation of 32P. For some assays A-CAT-5xA was dephosphorylated by treatment at room temperature for 1 h with 10 units of calf intestinal alkaline phosphatase-agarose beads (Sigma) or for 5 h with 10 units of SAP (Fermentas). A total of 200 μg of A-CAT-RRGT or A-CAT-RRGS were incubated with 0.5 μg of recombinant PKA catalytic subunit (Active Motif) for 30 min at 22 °C in kinase buffer. Following dialysis against 20 mm Tris-HCl, pH 7.4, kinase assays were carried out using the MH-3 peptide as described above. Data were fit to a hyperbolic equation by nonlinear regression analysis using the program SigmaPlot (Systat Software Inc.). Where shown, mean ± S.D. are derived from 3 to 6 determinations.
Crystallization Procedures and Data Collection and Analysis
A-CAT truncated at Leu809 (A-CAT-Δ809) was concentrated to 230 μm using a 10,000 molecular weight cut-off Ultrafree-4 Centrifugal Filtration Unit (Millipore). The His tag was cleaved off by incubation overnight at 4 °C with AcTEV protease (Invitrogen), and the protease was removed by chromatography over a His-Bind column. A 1-μl aliquot of A-CAT-Δ809 was mixed with 1 μl of reservoir solution consisting of 0.1 m Tris-HCl, pH 8.5, 0.2 m NaH2PO4, and 18% (w/v) PEG 8000. Crystallization was performed by the hanging drop vapor diffusion method at 4 °C. Crystals of A-CAT-Δ809 appeared after a few days and grew to their maximum size in 10 days. Crystals were flash frozen in a stream of liquid nitrogen after dipping them in a cryoprotectant solution that consisted of the mother liquor component and 25% (w/v) ethylene glycol. A 1.9-Å dataset for the A-CAT-Δ809 crystal was collected at the F1 beamline at the Cornell High Energy Synchrotron Source (CHESS). The dataset was processed and scaled with DENZO and SCALEPACK or with the HKL2000 suite program (36) and solved by molecular replacement with the initially solved A-CAT structure (23) as the search model with PHASER software (37). CNS and REFMAC5 were used to build and refine the final model (38, 39).
Modeling of the C-tail
Ab initio structure prediction methods were used to generate a three-dimensional model of the C-terminal tail sequence (C-tail) with Thr825 at the Pi-pocket or the active site. Residues 806 to 830 were first added in an extended conformation (±135° φ/ψ angles) to the crystal structure of A-CAT (Protein Data Bank code 3LLA, chain B). The side chains of the starting model were prepacked using the Rosetta-fixed backbone design/packing application (using the parameters -ex1, -ex2, use_input_sc). A three-stage modeling protocol was then applied. In the first step the FloppyTail protocol was used to generate 5500 structural models of the C-tail using a Rosetta fragment library for 3-mer (40). In the second step, the positions of residues 823–830 from the two top scoring FloppyTail models were refined by applying the FlexPepDock protocol, which accurately models the conformation of peptides that fold upon binding to their receptors (41). In the last step, the Rosetta loop refinement protocol over residues 806–824 was used to connect the FlexPepDock model with the rest of the FloppyTail model (42). To model the C-tail at the Pi-pocket, the protocol was constrained to force the phosphate group of Thr825 to create similar hydrogen bonds and salt bridges as the original Pi molecule solved in the crystal structure. To model the C-tail at the active site a soft constraint was imposed in the initial low resolution step that required that the Cβ atom of Thr825 be within 3–6 Å of the Cβ atom of Asp756, which is presumed to act as the catalytic base in the phosphotransferase reaction (23). This constraint was removed in the subsequent high-resolution modeling steps of all three stages. An ATP molecule at the active site was included, starting from its orientation in the A-CAT-D766A structure (PDB code 3LMI).
RESULTS
The Disordered C-terminal Sequence Is Required for A-CAT Activity
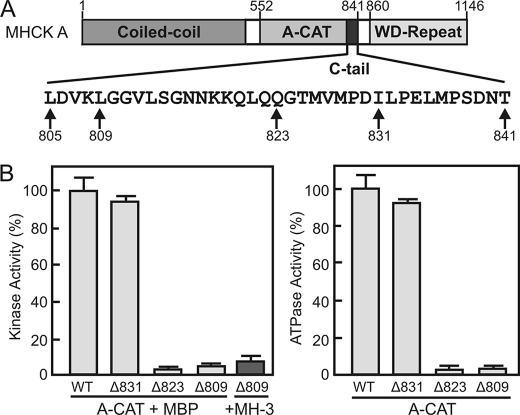
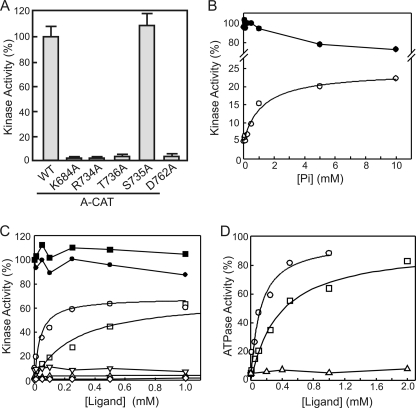
A-CAT is a catalytically active fragment of MHCK A that encompasses the α-kinase domain (residues 552–805) as well as part of the flexible linker that connects the α-kinase domain to the WD-repeat domain (residues 806–841) (Fig. 1A) (22, 23). In the x-ray crystal structure of A-CAT, residues 806–841 lack defined electron density and thus form a disordered C-terminal tail (C-tail) (23). Truncation of the C-tail at residue 831 did not alter the kinase activity of A-CAT, but truncation at residues 823 or 809 resulted in the loss of 90–95% of kinase activity (Fig. 1B, left panel). The severe loss of kinase activity was observed with both myelin basic protein and the MH-3 peptide, which corresponds to the Thr2029 site in the myosin II tail, as substrate (24). In the absence of a protein or peptide substrate A-CAT exhibits a basal rate of ATPase activity (23). Truncation of the C-tail at residues 823 or 809 reduced the ATPase activity of A-CAT by ∼95% (Fig. 1B, right panel). A section of the C-tail between residues 823 and 831 is therefore required for both the kinase and ATPase activities of A-CAT.
FIGURE 1.
Truncation of the C-tail inhibits the kinase and ATPase activities of A-CAT. A, schematic diagram showing the domain organization of MHCK A. A-CAT encompasses the entire α-kinase domain and part of the unstructured sequence (C-tail) linking the WD-repeat domain. The amino acid sequence of the C-tail is shown with the experimental sites of truncation indicated. B, the kinase (left panel) and ATPase (right panel) activities of wild-type A-CAT (WT) and A-CAT truncated at residues 831 (Δ831), 823 (Δ823), or 809 (Δ809) were determined. Removal of residues 823 to 831 from the C-tail resulted in a large decrease in kinase and ATPase activities. Activities were determined from time courses performed as described under “Experimental Procedures” using either MBP or the MH-3 peptide as substrate as indicated. Activities are reported as a percentage of the wild-type activity. Error bars represent the standard deviation.
An Essential Threonine Residue Is Conserved in the C-tail
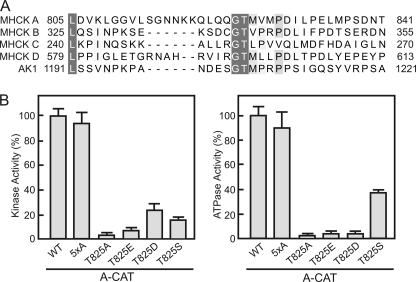
A multiple sequence alignment of the C-tail regions of D. discoideum MHCK A-D and AK1 reveals a conserved Gly-Thr-hydrophobic motif (Fig. 2A). In A-CAT the conserved threonine residue is Thr825, which lies within the region defined by truncation analysis (residues 823–831) as being critical for A-CAT activity. Thr825 has been identified by mass spectrometry as one of six autophosphorylation sites in A-CAT (23). The other five autophosphorylation sites (Ser553, Thr612, Thr613, Thr614, and Thr634) are located within the N-terminal lobe. Mutation of Ser553, Thr612, Thr613, Thr614, and Thr634 to alanine (A-CAT-5xA) had little effect on the kinase or ATPase activities of A-CAT (Fig. 2B). In contrast, mutation of Thr825 to alanine decreased kinase and ATPase activities by 95% (Fig. 2B). This result suggests that autophosphorylation of Thr825 is critical for the activity of A-CAT. Mutation of Thr825 to glutamic acid or aspartic acid resulted in the loss of ∼90 and 75% of kinase activity, respectively, indicating that a negatively charged residue only weakly compensates for the loss of phosphothreonine (Fig. 2B). A mutant with serine in place of Thr825 also exhibited low kinase and ATPase activity (Fig. 2B). Serine residues are poor substrates for A-CAT, suggesting that the low activity may reflect the incomplete autophosphorylation of Ser825 (21, 29).
FIGURE 2.
The conserved Thr825 residue in the C-tail is required for A-CAT activity. A, a multiple sequence alignment of the C-tail regions of D. discoideum MHCK A, MHCK B, MHCK C, MHCK D, and AK1. Conserved residues are shaded in gray. A Gly-Thr motif (residues 824 and 825 in MHCK A) is present in all of the C-tail sequences. The NCBI accession numbers are: MHCK A, XP_635119; MHCK B, XP_636368; MHCK C, XP_635600; MHCK D, XP_640080; and AK1, XP_629868. B, the kinase (left panel) and ATPase (right panel) activities of wild-type A-CAT (WT), A-CAT-5xA, and the indicated Thr825 mutants were determined. Mutation of Thr825 severely inhibited both the kinase and ATPase activities of A-CAT. Activities were determined from time courses performed as described under “Experimental Procedures” and the activities are reported as a percentage of A-CAT activity. Error bars represent the standard deviation.
Phosphorylation of the C-tail Activates A-CAT
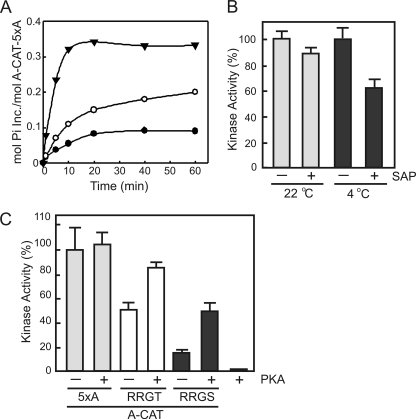
Incubation of A-CAT-5xA with [γ-32P]ATP resulted in the incorporation of less than 0.1 mol of phosphate/mol (Fig. 3A). The amount of phosphate incorporated into A-CAT-5xA increased to 0.2 mol/mol after a 1-h treatment with calf intestinal alkaline phosphatase and to 0.33 mol/mol after a 5-h treatment with SAP (Fig. 3A). We interpret these results to indicate that Thr825 is nearly fully phosphorylated in bacterially expressed A-CAT-5xA and that it is only partially dephosphorylated even after extensive phosphatase treatment. The phosphorylated state of Thr825 was confirmed by mass spectrometry (data not shown). Kinase assays showed that SAP treatment produced only a small decrease in the kinase activity of A-CAT-5xA (Fig. 3B). It was reasoned that this may reflect the ability of A-CAT-5xA to rapidly autophosphorylate Thr825 in the kinase assay (Fig. 3A). When kinase assays were carried out at 4 °C to reduce the rate of Thr825 autophosphorylation, a significant decrease in the activity of SAP-treated A-CAT-5xA could be detected (Fig. 3B). These results are consistent with the conclusion that phosphorylation of Thr825 is required for A-CAT activity.
FIGURE 3.
Phosphorylation of the C-tail activates A-CAT. A, time course of the autophosphorylation of A-CAT-5xA prior to treatment (●) and following treatment for 1 h with calf intestinal alkaline phosphatase (○) or for 5 h with SAP (▾). The low level of 32P incorporation indicates that Thr825 is highly phosphorylated and is resistant to dephosphorylation by calf intestinal alkaline phosphatase and SAP. Autophosphorylation assays were performed by incubating A-CAT-5xA with [γ-32P]ATP as described under “Experimental Procedures.” B, the kinase activity of A-CAT-5xA incubated for 5 h in the presence or absence of SAP was assayed at 22 and 4 °C. The assays at 4 °C, which limits the ability of A-CAT-5xA to autophosphorylate Thr825, showed that dephosphorylation of Thr825 by SAP decreased the kinase activity of A-CAT-5xA. Activities were determined from time courses performed as described under “Experimental Procedures” and are reported as a percentage of the untreated A-CAT-5xA activity. Error bars represent the standard deviation. C, the kinase activities of A-CAT-5xA (5xA) or A-CAT-5xA with the QQGT sequence in the C-tail mutated to RRGT or RRGS were determined before and after incubation with PKA as indicated. Activities were determined from time courses performed as described under “Experimental Procedures” with the MH-3 peptide as substrate. The MH-3 peptide was not a substrate for PKA. Incubation with PKA activated the RRGT and RRGS mutants, but not A-CAT-5xA, showing that phosphorylation of the Thr825 site enhances kinase activity. Activities are reported as a percentage of A-CAT-5xA activity. Error bars represent the standard deviation.
The ability of Thr825 phosphorylation to activate A-CAT-5xA was further examined by mutating Gln822 and Gln823 to arginine to convert Thr825 into a PKA phosphorylation site (i.e. QQGT to RRGT). A mutant with serine in place of Thr825 (RRGS) was also created. A-CAT-RRGS exhibited a lower initial activity than A-CAT-RRGT, which is consistent with serine being a poorer autophosphorylation site than threonine. Incubation with PKA and MgATP increased the activity of A-CAT-RRGT by 60% and A-CAT-RRGS by more than 3-fold (Fig. 3C). The increase in activity was accompanied by the incorporation of 0.25 mol of Pi/mol into A-CAT-RRGS by PKA. Control experiments showed that the activity of A-CAT-5xA was not altered by incubation with PKA and that the MH-3 peptide was not a substrate for PKA (Fig. 3C). This result provides additional evidence that A-CAT activity depends on the phosphorylation of the Thr825 (or Ser825) site in the C-tail.
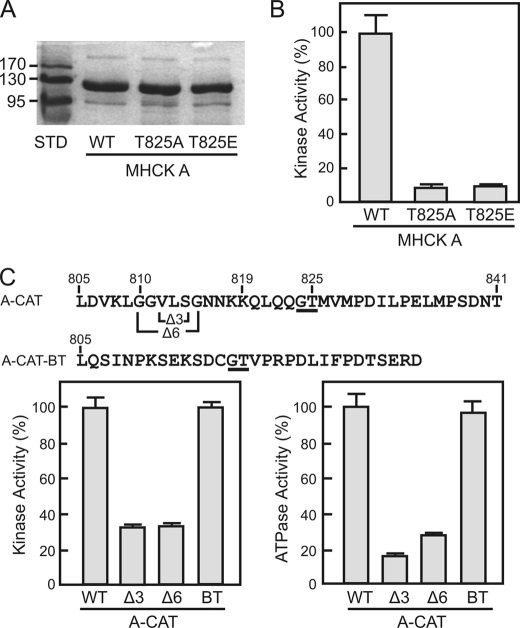
To examine whether autophosphorylation of Thr825 is required for the activation of full-length MHCK A, wild-type MHCK A and T825A and T825E mutants were expressed in D. discoideum as FLAG-tagged proteins (Fig. 4A). The kinase activities of the MHCK A T825A and T825E mutants were less than 10% that of wild-type MHCK A (Fig. 4B). This shows that autophosphorylation of Thr825 is a necessary step in the activation of MHCK A.
FIGURE 4.
Regulation of A-CAT and MHCK A by Thr825. A, the Coomassie Blue-stained SDS gel shows wild-type MHCK A (WT) and the MHCK A T825A and T825E mutants purified from D. discoideum as described under “Experimental Procedures.” B, the kinase activities of MHCK A (WT) and the MHCK A T825A and T825E mutants were assayed as described under “Experimental Procedures.” Mutation of Thr825 severely inhibited the kinase activity of MHCK A. Activities are reported as a percentage of MHCK A activity. Error bars represent the standard deviation. C, the top line shows the sequence of the A-CAT C-tail and indicates the residues deleted to generate the Δ3 and Δ6 constructs. The second line shows the sequence of the MHCK B C-tail (residues 326–354) that was fused to Leu805 of A-CAT to generate the A-CAT-BT chimera. The conserved Gly-Thr sequence is underlined. The kinase (left panel) and ATPase (right panel) activities of wild-type A-CAT (WT), the Δ3 and Δ6 constructs, and the A-CAT-BT chimera (BT) were determined from time courses performed as described under “Experimental Procedures.” The C-tail of MHCK B rescues the activity of the truncated A-CAT. Activities are reported as a percentage of wild-type A-CAT activity. Error bars represent the standard deviation.
The effect of moving Thr825 closer to the α-kinase domain was examined by deleting three (A-CAT-Δ3) or six (A-CAT-Δ6) residues from the intervening linker sequence (Fig. 4C). A-CAT-Δ3 and A-CAT-Δ6 were considerably less active than A-CAT, indicating that the function of Thr825 depends to some extent on its position within the C-tail (Fig. 4C). The conserved nature of the Gly-Thr-hydrophobic sequence suggested that the C-tails of AK1 and MHCK B-D may have functions comparable with that of MHCK A (Fig. 2A). To test this possibility the C-tail of MHCK B (residues 326–354) was fused to the C terminus of A-CAT truncated at Leu805 (Fig. 4C). The C-tail of MHCK B fully rescued both the kinase and ATPase activity of the truncated A-CAT (Fig. 4C). This result supports the view that the MHCK C-tail regions perform analogous regulatory functions.
X-ray Crystal Structure of A-CAT-Δ809
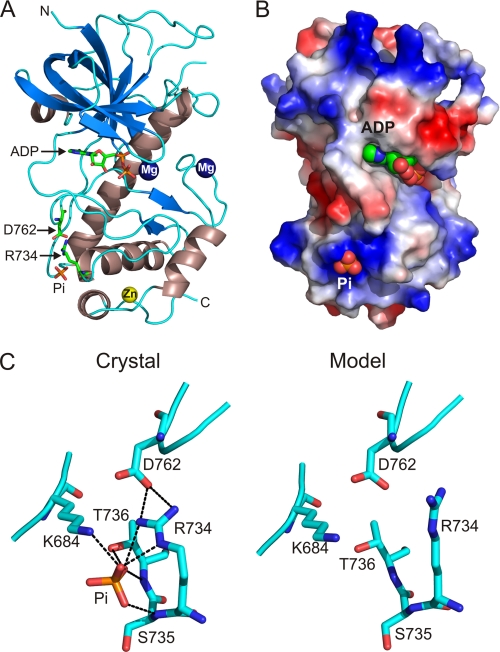
A-CAT truncated at residue 809 (A-CAT-Δ809) was purified in yields comparable with that of A-CAT, showing that the loss of the C-tail does not compromise the stability of the core α-kinase domain. Indeed, A-CAT-Δ809 formed diffraction quality crystals much more readily than A-CAT, indicating that the C-tail impairs crystal formation. The x-ray crystal structure of A-CAT-Δ809 was solved to a resolution of 1.8 Å (Fig. 5A and supplemental Table S1). When the Cα atoms of A-CAT-Δ809 and A-CAT were superimposed, a root mean square difference of 0.37 Å was obtained, which indicates that the two structures are virtually identical. The structure of A-CAT-Δ809 further shows that the absence of the C-tail did not alter the binding of ligands. A-CAT-Δ809, like A-CAT, contained a zinc atom bound to the C-terminal lobe, a Pi molecule bound to the Pi-pocket and Mg2+ ions bound at the active site and at the center of the N/D-loop (23). The active site of A-CAT-Δ809 contained ADP, even though crystallization was carried out in a buffer containing ATP (Fig. 5A). This result is surprising given the low ATPase activity exhibited by A-CAT-Δ809 (Fig. 1B) and raises the possibility that the crystallization conditions induce A-CAT-Δ809 to adopt a catalytically active conformation.
FIGURE 5.
X-ray crystal structure of A-CAT-Δ809. A, the structure of A-CAT-Δ809 is shown with β-strands in blue and α-helices in strawberry. Mg2+ ions are rendered as dark blue spheres and the zinc atom as a yellow sphere. Arg734, Asp762, ADP, and Pi are shown as sticks with the C, O, N, and P atoms colored green, red, blue, and orange, respectively. N, N terminus; C, C terminus. B, the distribution of electrostatic potential is shown on the molecular surface of A-CAT-Δ809, with blue indicating areas of net positive charge and red areas of net negative charge. ADP and Pi are rendered as spheres. The view is rotated 40° to the right from the view in panel A. C, detailed view of the Pi-pocket. The crystal structure of A-CAT (left panel) shows that the Pi molecule interacts with the side chains of Arg734, Lys684, and Thr736 and the main chain carbonyl of Ser735 (dotted lines). The bent Arg734 side chain bridges the Pi molecule to Asp762 in an active site loop. Ab initio modeling of the Pi-pocket in the absence of Pi (right panel) shows that the side chain of Arg734 can switch from the bent to a straight configuration that does not interact with Asp762. Although the straight conformation of Arg734 can occur when modeling in the presence of a ligand, it is favored by an empty Pi-pocket. The conformational change in Arg734 is postulated to act as an allosteric switch that controls the activity of A-CAT in response to Pi binding.
The Pi-pocket Regulates A-CAT Activity
It was reasoned that Thr(P)825 must bind to a site in the core α-kinase domain to stimulate catalytic activity. The Pi-pocket, which is located near to the bottom of the C-terminal lobe, provides an obvious candidate for a phosphate-dependent binding site (Fig. 5B). The Pi molecule makes electrostatic interactions with the side chains of Lys684, located in the loop following α-helix C, and Arg734, located at the start of α-helix D. The Pi molecule also forms hydrogen bonds with the main chain carbonyl of Ser735 and the main chain carbonyl and side chain hydroxyl group of Thr736 (Fig. 5C and supplemental Fig. S2). Mutation of Lys684, Arg734, and Thr736 to alanine reduced the kinase activity of A-CAT by 90–95% showing that an intact Pi-pocket is essential for activity (Fig. 6A). A mechanism by which Pi binding may influence the conformation of the active site is apparent from the structure of A-CAT, which shows that Arg734 forms a salt bridge with Asp762 in one of the principal catalytic loops (Fig. 5C). Mutation of Asp762 to alanine drastically reduced catalytic activity, demonstrating that the salt bridge with Arg734 is of functional importance (Fig. 6A).
FIGURE 6.
Ligand binding to the Pi-pocket activates A-CAT. A, the kinase activity of wild-type A-CAT (WT) and the indicated Pi-pocket mutants were determined as described under “Experimental Procedures.” Error bars represent the standard deviation. Mutation of residues that bind Pi resulted in a severe loss in kinase activity. B, the kinase activity of A-CAT (closed symbols) and A-CAT-Δ809 (open symbols) were assayed in the presence of NaH2PO4. The addition of Pi enhanced the kinase activity of A-CAT-Δ809. A hyperbolic curve fit to the A-CAT-Δ809 data yielded a Ka = 440 ± 150 μm and a Vmax = 22 ± 3%. Activities are reported as a percentage of the A-CAT activity in the absence of Pi. C, the kinase activity of A-CAT (closed symbols) and A-CAT-Δ809 (open symbols) were assayed in the presence of phosphothreonine (squares), the QQG(p)TMVMPD peptide (circles), threonine (triangles), and the unphosphorylated QQGTMVMPD peptide (inverted triangles). The A-CAT-Δ809-R734A mutant was assayed in the presence of phosphothreonine (diamonds). Hyperbolic curves fit to the A-CAT-Δ809 data yielded a Ka = 260 ± 80 μm and a Vmax = 65 ± 6% for phosphothreonine and a Ka = 35 ± 12 μm and a Vmax = 64 ± 3% for the QQG(p)TMVMPD peptide. Activities are reported as a percentage of the A-CAT activity in the absence of any additions. D, the ATPase activity of A-CAT-Δ809 was assayed in the presence of phosphothreonine (squares), QQG(p)TMVMPD peptide (circles), or threonine (triangles). Hyperbolic curves fit to the data yielded a Ka = 360 ± 40 μm and a Vmax = 93 ± 2% for phosphothreonine and a Ka = 120 ± 40 μm and a Vmax = 97 ± 8% for the QQG(p)TMVMPD peptide. The kinase or ATPase activity shown for each ligand concentration in panels B–D was determined from a time course performed as described under “Experimental Procedures.”
The possibility that Pi might be able to compensate for the loss of Thr825 by providing a ligand for the Pi-pocket was tested by adding NaH2PO4 to the kinase assays. The addition of Pi stimulated the kinase activity of A-CAT-Δ809 by ∼4-fold, to a level ∼20% that of A-CAT (Fig. 6B). Half-maximal activation was achieved at a Pi concentration of 440 ± 150 μm. At higher concentrations of Pi a slight inhibition of A-CAT activity was observed, likely because of the increased ionic strength (1). Phosphothreonine proved to be a better activator than Pi, stimulating the kinase activity of A-CAT-Δ809 to a level approaching 65% that of A-CAT (Fig. 6C). Half-maximal activation occurred at a phosphothreonine concentration of 250 ± 80 μm. No activation of A-CAT-Δ809 occurred when threonine was added to the kinase assays (Fig. 6C). Glutamic acid and aspartic acid also did not activate (not shown). A peptide with a sequence corresponding to residues 823–830 of the C-tail (QQGTMVMPD) had no effect on the activity of A-CAT-Δ809, whereas the phosphorylated version of the peptide (QQG(p)TMVMPD) was as effective an activator as phosphothreonine (Fig. 6C). Half-maximal activation by the QQG(p)TMVMPD peptide was achieved at a concentration of 40 ± 15 μm. Mutation of Arg734 to alanine abolished the ability of phosphothreonine to activate A-CAT-Δ809 (Fig. 6C) as did mutation of Asp762 to alanine (not shown). These results confirm that activation of A-CAT-Δ809 is the result of ligand binding to the Pi-pocket.
Phosphothreonine and the QQG(p)TMVMPD peptide also restored the ATPase activity of A-CAT-Δ809 (Fig. 6D). Both activators increased the ATPase activity of A-CAT-Δ809 to a level approaching 95% that of A-CAT. Half-maximal activation was achieved at QQG(p)TMVMPD and phosphothreonine concentrations of 120 ± 40 and 360 ± 40 μm, respectively. Taken together, the data show that phosphorylated ligands dramatically and specifically rescue the catalytic activity of A-CAT-Δ809.
Modeling of the C-tail at the Pi-pocket
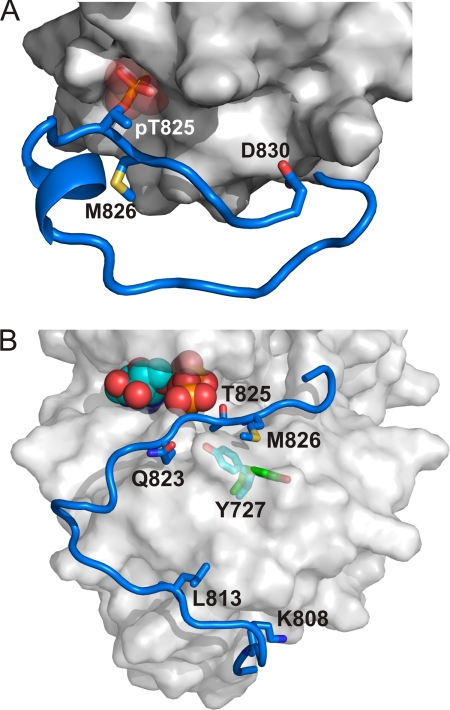
Analytical ultracentrifugation studies show that A-CAT is a monomer in solution (23). To determine whether it is feasible for Thr(P)825 to act as an intramolecular ligand for the Pi-pocket, residues 806–830 of the C-tail were modeled using the Rosetta FloppyTail and FlexPepDock protocols (40, 41). The only constraint on the model was that the Thr825 phosphoryl group was forced to adopt the same binding interactions as the Pi molecule in the crystal structure. The modeling process resulted in 5,500 independent models. The two top-scoring models converged on a single binding mode that allows Thr(P)825 to dock into the Pi-pocket (Fig. 7A and supplemental Fig. S3). Reassuringly, the backbone and side chain conformations of residues 823–830 are nearly identical in both independent models. The model predicts that residues 806–817 form a random coil and make little or no contact with A-CAT, which is consistent with multiple sequence alignments that show this section of the C-tail to be poorly conserved (Fig. 2A). Residues 818–823 in the model form a short α-helix that, again, makes no contact with A-CAT. The conserved Gly824 allows the polypeptide chain to make a sharp turn that places the Thr825 phosphoryl group into the Pi-pocket in the same orientation as the Pi in the crystal structure. Residues on the C-terminal side of Thr825 take up an extended conformation and are involved in interactions with A-CAT. This stretch of sequence corresponds to the C-tail peptide (QQGTMVMPD) used in the A-CAT-Δ809 activation experiments described above. Most notably, Met826 makes hydrophobic contacts with Pro683 and Val799.
FIGURE 7.
Models of the C-tail bound to the Pi-pocket and active site. The Rosetta FloppyTail and FlexPepDock protocols were used to model the C-tail (residues 806–830) using the procedure described under “Experimental Procedures.” A, model of the C-tail with the phosphorylated Thr825 bound into the Pi-pocket. The C-tail is colored blue, key residues in the C-tail that interact with the α-kinase domain are shown in stick representation, and the original Pi in the crystal structure is represented as transparent spheres. The surface of A-CAT (PBD code 3LLA, chain B) is shown in gray. The model shows that the Thr825 phosphoryl group can act as an intramolecular ligand for the Pi-pocket. B, model of the C-tail with the unphosphorylated Thr825 at the active site. The C-tail is colored blue, residues in the C-tail that interact with the α-kinase domain are shown in stick representation, and ATP (from PBD code 3LMI) is shown as spheres. The surface of A-CAT is shown in gray and is transparent to illustrate the position of Tyr727 predicted by the model (cyan sticks) and in the crystal structure (green sticks). The model illustrates that it is feasible for Thr825 to be autophosphorylated via an intramolecular mechanism.
Interestingly, the modeling studies predict that the side chain of Arg734 in the Pi-pocket can switch from the bent conformation present in the A-CAT crystal structure to a straight conformation (Fig. 5C). The conformational change moves the Arg734 guanidino group 3 to 6 Å away from the Pi pocket and thus disrupts the electrostatic interactions with the Pi molecule and Asp762. The tendency of Arg734 to adopt the two orientations was evaluated by repacking the side chain conformations in the region of the Pi-pocket 200 times, both in the absence and presence of the C-tail. In the free state, 99.5% of the models had the side chain of Arg734 in the straight conformation, whereas when Thr(P)825 is bound a significant fraction of the models (42.3%) had the Arg734 side chain in the bent conformation. These studies suggest that binding of a phosphorylated ligand induces a conformational change in Arg734 that promotes formation of a salt bridge with Asp762.
Modeling of the C-tail in the Active Site
The same three-stage modeling protocol was carried out to examine whether it is feasible for Thr825 to interact in an intramolecular manner with the A-CAT active site. A soft constraint that required the Cβ atom of Thr825 to be within 3–6 Å of the Cβ atom of Asp756 (the presumed catalytic base) was applied in the initial low-resolution modeling using the FloppyTail protocol but was then removed in the subsequent high-resolution modeling steps. The modeling studies clearly indicate that the flexible C-tail allows Thr825 to access the active site (Fig. 7B). Although there was some convergence near the active site, the top-scoring models were not identical, indicating that the flexible C-tail is likely to be able to reach the catalytic site via an ensemble of conformations (supplemental Fig. S4). The top scoring model provides one possible snapshot of this ensemble and is stabilized by several favorable interactions, including a salt bridge between Lys808 and Glu746, hydrophobic interactions between Leu813 and Thr793, and a hydrogen bond between Gln822 and the main chain amide of Lys722 (Fig. 7B). In addition, the model predicts that the side chain of Tyr727 rotates from its position in the crystal structure toward the C-tail so that it can form a hydrogen bond with the side chain of Gln823 and participate in hydrophobic interactions with Met826.
DISCUSSION
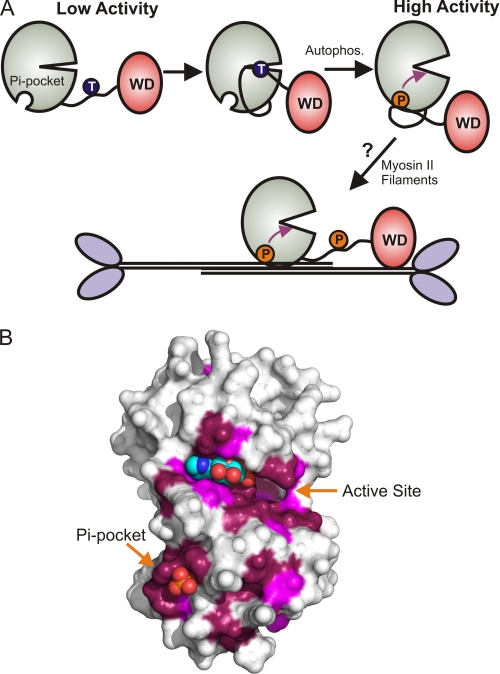
MHCK A is potently activated by autophosphorylation but the identity of the autophosphorylation sites and the mechanisms involved in the activation process have remained obscure. Previous studies have localized several MHCK-A autophosphorylation sites to a segment (amino acids 499–550) immediately N-terminal to the α-kinase domain (22). Studies on A-CAT, the isolated α-kinase domain of MHCK A, show that autophosphorylation of the N-terminal sites stimulates kinase activity by about 3-fold, yet deletion of the N-terminal segment has little effect on activity (22). These results were interpreted to indicate that the N-terminal segment may harbor an autoinhibitory sequence that is relieved by autophosphorylation. Here, we focus on the autophosphorylation of Thr825, which is located in an unstructured sequence C-terminal to the α-kinase domain. Autophosphorylation of Thr825 is shown to be essential for the catalytic activity of A-CAT. We propose that Thr(P)825 activates A-CAT by folding back onto the α-kinase domain, where it provides a ligand for an allosteric binding site, termed the Pi-pocket, that specifically recognizes phosphoamino acids (Fig. 8A).
FIGURE 8.
A conserved activation mechanism for the MHCK/eEF2K family. A, model for the activation of MHCK A. Only the α-kinase (green) and WD-repeat (red) domains of MHCK A are shown. MHCK A is in a low-activity state when the Pi-pocket in the α-kinase domain is empty. The intramolecular autophosphorylation of Thr825 in the disordered sequence connecting the α-kinase and WD-repeat domains provides a ligand for the Pi-pocket and induces a conformational change (purple arrow) that converts MHCK A to a high activity state. MHCK A is targeted to its physiological substrate, myosin II filaments, by the WD-repeat domain. It is speculated that the Pi-pocket could bind Thr(P) residues in the myosin II tail and promote the phosphorylation of adjacent threonine residues. B, the ConSurf server was used to map the conservation of amino acids, derived from a multiple sequence alignment of 20 MHCK/eEF2K homologues, onto the surface structure of A-CAT (PDB code 3LKM). Invariant residues (conservation score of 9) are colored purple and highly conserved residues (conservation score of 8) are colored violet. The Pi-pocket, the active site, and the N/D-loop represent the most highly conserved parts of the surface.
Autophosphorylation of Thr825
A-CAT purifies as a constitutively active monomer with full protein kinase activity (22, 23). Until the C-terminal truncation and mutagenesis studies described here, the key role played by autophosphorylation of Thr825 in promoting the activity of A-CAT had escaped detection. This can be attributed to the observation that Thr825 is constitutively phosphorylated in bacterially expressed A-CAT and that Thr(P)825 is resistant to phosphatases, perhaps due to its ability to dock into the Pi-pocket. Thr825 does not have the features expected for a good A-CAT substrate. Most importantly, the p + 1, p + 2, p + 3, and p + 4 positions on the C-terminal side of Thr825 are occupied by hydrophobic residues instead of the lysine or arginine residues strongly preferred by A-CAT (21, 29). Indeed, we did not detect phosphorylation of the QQGTMVMPD peptide, which corresponds to the Thr825 phosphorylation site, when it was included in a kinase assay with A-CAT at a concentration of 0.5 mm (data not shown). The rapid autophosphorylation of Thr825 in the context of the C-tail is therefore likely to depend on a unimolecular autophosphorylation mechanism, in which Thr825 is presented to the active site at a very high effective concentration (Ceff) (Fig. 7B). A Ceff of 2.3 mm can be calculated for the tethered Thr825 based on the volume of a hemisphere with radius r, where r is the average (root mean square) length of the linker (∼7 nm) connecting Thr825 to the α-kinase domain (43). The actual Ceff of Thr825 at the catalytic site might well be much greater than this as the result of favorable binding interactions between the C-tail and A-CAT (Fig. 7B). Although the top scoring model provides only one possible snapshot for how the C-tail may access the active site, it suggests some specific interactions that could be tested experimentally in future work. In particular, the hydrogen bond between the side chain of Gln823 in the p − 2 position and the side chain of Tyr727 provides a rationale for why A-CAT prefers to phosphorylate peptides with a hydrogen bond-forming residue (serine, threonine, or tyrosine) in the p − 2 position (29). However, the absence of basic residues means that the p + 1 to p + 4 positions in the C-tail will not bind to A-CAT in the same manner as good peptide substrates. Indeed, it can be speculated that the residues C-terminal to Thr825 may have evolved to favor binding at the Pi-pocket rather than at the active site.
The Pi-pocket Provides a Binding Site for Thr(P)825 and Communicates with the Active Site
The results reported here show that loss of Thr825 severely inhibits both the kinase and ATPase activities of A-CAT. This rules out the possibility that Thr(P)825 acts by facilitating the binding of peptide or protein substrates and instead points to a direct effect on the active site. The proposal that the Pi-pocket is the binding site for Thr(P)825 is based on the following evidence: (i) the crystal structure of A-CAT identifies the Pi-pocket as a Pi-binding site; (ii) mutations that disrupted the Pi-pocket dramatically inhibited the activity of A-CAT; (iii) A-CAT-Δ809, which lacks the C-tail and Thr825, could be specifically activated by phosphorylated ligands including Pi, phosphothreonine, and a phosphorylated peptide; (iv) activation by phosphorylated ligands was abolished by mutations (R734A, D762A) that disrupted the Pi-pocket; and (v) modeling studies demonstrated that it is feasible for Thr(P)825 to provide an intramolecular ligand for the Pi-pocket.
Importantly, a molecular mechanism that allows the Pi-pocket to communicate directly with the active site is apparent from the crystal structure of A-CAT and gains support from the modeling studies (Fig. 5C). The mechanism hinges on a salt bridge that is formed between Arg734 in the Pi-pocket and Asp762 in the active site loop that harbors catalytic residues Asp756 and Asp766. Asp766 accepts the γ-phosphate from ATP to form an aspartylphosphate intermediate and Asp756 is presumed to be the catalytic base in the phosphotransferase reaction (23, 44). By anchoring the catalytic loop, the salt bridge between Asp762 and Arg734 helps to fix the positions of Asp756 and Asp766 within the active site. The modeling studies indicate that the bent conformation of the Arg734 side chain that bridges the Pi molecule to Asp762 is not energetically favorable, especially when the Pi-pocket is empty. Thus, a plausible allosteric model can be proposed in which the binding of a phosphorylated ligand to the Pi-pocket stabilizes the bent conformation of the Arg734 side chain, which in turn promotes the electrostatic interaction with Asp762 and helps to properly align the Asp756–Asp766 catalytic loop within the active site. It is interesting that interactions involving the Asp756–Asp766 loop have also been implicated in the activation of TRPM7 by dimer assembly (45). The results suggest that regulatory mechanisms that stabilize what may be an inherently mobile Asp756–Asp766 catalytic loop may provide a common pathway by which to activate the α-kinases.
In several experiments the kinase and ATPase activities of A-CAT were found to diverge. Phosphothreonine and the QQG(p)TMVMPD peptide restored about 60–65% of the kinase activity of A-CAT-Δ809 but close to 95% of the ATPase activity. Differential effects on the kinase and ATPase activities of A-CAT were also noted with the T825D, T825S, and Δ3 mutants (Figs. 2B and 4C). It can be speculated that the balance between the two enzymatic activities may depend on the active site conformation, which is influenced by the ligand for the Pi-pocket. Whether or not the ATPase (and ADPase) activities exhibited by A-CAT are physiologically relevant is currently not clear (23).
The identification of the Pi-pocket as a positive allosteric binding site provides a rationale for why A-CAT-Δ809 and A-CAT have virtually identical crystal structures. Both A-CAT and A-CAT-Δ809 were crystallized from buffers containing 0.2 m NaH2PO4 and in both crystal structures Pi occupies the Pi-pocket (23). It can be concluded that in the case of A-CAT, Pi in the crystallization buffer displaced Thr(P)825 from the Pi-pocket, causing the C-tail to take up a disordered conformation that is not visible in the crystal structure. In the case of A-CAT-Δ809, Pi provided a ligand for the Pi-pocket in the absence of the C-tail and induced A-CAT-Δ809 to adopt the active conformation. This analysis highlights the need to identify crystallization conditions that lack Pi. In the absence of Pi, it may be possible to obtain a structure of the inactive state of A-CAT-Δ809 with an empty Pi-pocket and a structure of A-CAT in the autoactivated state with Thr(P)825 docked into the Pi-pocket.
Substrate Specificity of the Pi-pocket
Glutamic acid and aspartic acid did not activate A-CAT-Δ809, although glutamic acid, and especially aspartic acid, supported a low level of catalytic activity when substituted for Thr835 in the C-tail. This suggests that the Pi-pocket exhibits a weak affinity for carboxyl groups. The primary interaction with the Pi-pocket is through the phosphoryl group. However, Pi was less effective than phosphothreonine in stimulating the activity of A-CAT-Δ809, indicating that the amino acid side chain plays some role in the binding interaction. The QQG(p)TMVMPD peptide activated A-CAT-Δ809 at a 3–5-fold lower concentration than phosphothreonine, which is consistent with the prediction from modeling studies that Met826 can act as a secondary recognition site to promote binding of the C-tail at the Pi-pocket (Fig. 7A). The importance of the potential secondary interactions in mediating the binding of Thr825 to the Pi-pocket can be tested experimentally in future work. The estimated binding affinity of the phosphorylated QQG(p)TMVMPD peptide for the Pi-pocket is in the range of 35–120 μm. This is well below the Ceff for the tethered Thr825 of 2.3 mm calculated above, and implies that autophosphorylation of Thr825 would be sufficient to saturate the Pi-pocket and fully switch on kinase activity.
A Pi-binding site has been identified in casein kinase I and glycogen synthase kinase 3β (46–48). In both cases, the Pi-binding site is located within the active site cleft and is responsible for the recognition of substrates that contain a phosphoamino acid. It is interesting to speculate that the Pi-pocket might act, along with the WD-repeat domain, to target MHCK A to bipolar myosin II filaments (Fig. 8A). Multiple threonine residues within each myosin II monomer need to be phosphorylated before the filament falls apart. Once one threonine residue in the filament is phosphorylated, it could act as a ligand for the Pi-pocket and enhance the rate of phosphorylation of nearby threonine residues (Fig. 8A). Such a mechanism could result in a more efficient disassembly of bipolar filaments and may provide an explanation for the ability of myosin II filaments to activate MHCK A (24). Negatively charged compounds, including DNA and vesicles composed of phosphatidylinositol, also activate MHCK A in vitro (26). The mechanism of activation is not known, but could involve the Pi-pocket. It will be important to establish whether the Pi-pocket is able to recognize phosphorylated lipids, nucleotides, and other phosphorylated molecules, or whether it is specific for phosphopeptides.
Conservation of the Pi-pocket in the MHCKs and eEF2Ks
Phylogenetic analysis demonstrates that the α-kinase domains of the Dictyostelium MHCKs and metazoan eEF2Ks group together to form one branch of the α-kinase family (12, 23). A multiple sequence alignment of the MHCKs and eEF2Ks shows that the residues that bind Pi (Lys684, Arg734, and Thr736) and the aspartic acid that forms a salt bridge with Arg734 (Asp762) are invariant (supplemental Fig. S5). The highly conserved nature of the Pi-pocket can be visualized by mapping residues conserved in the multiple sequence alignment onto the surface of A-CAT (Fig. 8B) (49). This result implies that an allosteric regulatory mechanism dependent on the binding of phosphorylated ligands may underlie the regulation of the entire MHCK/eEF2K family. Although the C-tail sequences of the MHCK/eEF2K family members are divergent, a threonine residue followed by a hydrophobic residue is always present (supplemental Fig. S5). The conserved nature of the hydrophobic residue in the p + 1 position is consistent with the observation that its predicted binding partner, Pro683, is an invariant residue in the MHCK/eEF2K family (supplemental Fig. S5). eEF2K rapidly autophosphorylates multiple serine and threonine residues via an intramolecular mechanism but the sites have not been mapped (50). Several residues in the C-tail of eEF2K are targets for exogenous protein kinases and their phosphorylation can either inhibit or activate kinase activity (20). Further studies will be required to determine whether any of the C-tail phosphorylation sites regulate eEF2K by acting as a ligand for the Pi-pocket.
In summary, we have demonstrated that the α-kinase domain of MHCK A contains a previously undetected allosteric binding site for phosphorylated peptides. The allosteric binding site provides a mechanism to switch on the activity of MHCK A in response to autophosphorylation and is likely to play a central role in regulating the cellular activities of MHCK A and its close homologues, including eEF2K.
Supplementary Material
Acknowledgments
We thank David McLeod of the Protein Function Discovery Facility, Queen's University for carrying out mass spectrometry experiments. Portions of this research were carried out at the Cornell High Energy Synchrotron Source.
This work was supported in part by Canadian Institutes of Health Research Grant MOP8603 and Heart and Stroke Foundation of Ontario Grant T6054 (to G. P. C.), Natural Sciences and Engineering Research Council of Canada Grant 203705 (to Z. J.), the Israel Science Foundation funded by Israel Academy of Science and Humanities ISF Grant 306/6, and USA-Israel Bi-national Science Foundation Grant 2009418 (to O. S.-F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S5.
The atomic coordinates and structure factors (code 3PDT) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- MHCK
- D. discoideum myosin II heavy chain kinase
- A-CAT
- the α-kinase domain of MHCK A
- AK1
- D. discoideum α-kinase 1
- eEF2K
- eukaryotic elongation factor-2 kinase
- MBP
- myelin basic protein
- TRPM
- transient receptor potential melastatin
- Pi
- inorganic phosphate
- SAP
- shrimp alkaline phosphatase
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid.
REFERENCES
- 1. Côte G. P., Bukiejko U. (1987) J. Biol. Chem. 262, 1065–1072 [PubMed] [Google Scholar]
- 2. De La Roche M. A., Smith J. L., Betapudi V., Egelhoff T. T., Côté G. P. (2002) J. Muscle Res. Cell Motil. 23, 703–718 [DOI] [PubMed] [Google Scholar]
- 3. Vaillancourt J. P., Lyons C., Côté G. P. (1988) J. Biol. Chem. 263, 10082–10087 [PubMed] [Google Scholar]
- 4. Lück-Vielmetter D., Schleicher M., Grabatin B., Wippler J., Gerisch G. (1990) FEBS Lett. 269, 239–243 [DOI] [PubMed] [Google Scholar]
- 5. Kolman M. F., Futey L. M., Egelhoff T. T. (1996) J. Cell Biol. 132, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Egelhoff T. T., Lee R. J., Spudich J. A. (1993) Cell 75, 363–371 [DOI] [PubMed] [Google Scholar]
- 7. Futey L. M., Medley Q. G., Côté G. P., Egelhoff T. T. (1995) J. Biol. Chem. 270, 523–529 [DOI] [PubMed] [Google Scholar]
- 8. Steimle P. A., Licate L., Côté G. P., Egelhoff T. T. (2002) FEBS Lett. 516, 58–62 [DOI] [PubMed] [Google Scholar]
- 9. Russ M., Croft D., Ali O., Martinez R., Steimle P. A. (2006) Biochem. J. 395, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolman M. F., Egelhoff T. T. (1997) J. Biol. Chem. 272, 16904–16910 [DOI] [PubMed] [Google Scholar]
- 11. Steimle P. A., Naismith T., Licate L., Egelhoff T. T. (2001) J. Biol. Chem. 276, 6853–6860 [DOI] [PubMed] [Google Scholar]
- 12. Middelbeek J., Clark K., Venselaar H., Huynen M. A., Van Leeuwen F. N. (2010) Cell Mol. Life Sci. 67, 875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yumura S., Yoshida M., Betapudi V., Licate L. S., Iwadate Y., Nagasaki A., Uyeda T. Q., Egelhoff T. T. (2005) Mol. Biol. Cell 16, 4256–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagasaki A., Itoh G., Yumura S., Uyeda T. Q. (2002) Mol. Biol. Cell 13, 4333–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang W., Licate L., Warrick H., Spudich J., Egelhoff T. (2002) BMC Cell Biol. 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Betapudi V., Mason C., Licate L., Egelhoff T. T. (2005) Mol. Biol. Cell 16, 2248–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Betapudi V., Egelhoff T. T. (2009) Traffic 10, 1773–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark K., Langeslag M., van Leeuwen B., Ran L., Ryazanov A. G., Figdor C. G., Moolenaar W. H., Jalink K., van Leeuwen F. N. (2006) EMBO J. 25, 290–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark K., Middelbeek J., Lasonder E., Dulyaninova N. G., Morrice N. A., Ryazanov A. G., Bresnick A. R., Figdor C. G., Van Leeuwen F. N. (2008) J. Mol. Biol. 378, 790–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Proud C. G. (2007) Biochem. J. 403, 217–234 [DOI] [PubMed] [Google Scholar]
- 21. Luo X., Crawley S. W., Steimle P. A., Egelhoff T. T., Cote G. P. (2001) J. Biol. Chem. 276, 17836–17843 [DOI] [PubMed] [Google Scholar]
- 22. Côté G. P., Luo X., Murphy M. B., Egelhoff T. T. (1997) J. Biol. Chem. 272, 6846–6849 [DOI] [PubMed] [Google Scholar]
- 23. Ye Q., Crawley S. W., Yang Y., Côté G. P., Jia Z. (2010) Sci. Signal. 3, ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medley Q. G., Gariépy J., Côté G. P. (1990) Biochemistry 29, 8992–8997 [DOI] [PubMed] [Google Scholar]
- 25. Egelhoff T. T., Croft D., Steimle P. A. (2005) J. Biol. Chem. 280, 2879–2887 [DOI] [PubMed] [Google Scholar]
- 26. Medley Q. G., Bagshaw W. L., Truong T., Côté G. P. (1992) Biochim. Biophys. Acta 1175, 7–12 [DOI] [PubMed] [Google Scholar]
- 27. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 28. Levi S., Polyakov M., Egelhoff T. T. (2000) Plasmid 44, 231–238 [DOI] [PubMed] [Google Scholar]
- 29. Crawley S. W., Côté G. P. (2008) Biochim. Biophys. Acta 1784, 908–915 [DOI] [PubMed] [Google Scholar]
- 30. Hadwiger J. A., Firtel R. A. (1992) Genes Dev. 6, 38–49 [DOI] [PubMed] [Google Scholar]
- 31. Sussman M. (1987) Methods Cell Biol. 28, 9–29 [DOI] [PubMed] [Google Scholar]
- 32. Knecht D. A., Shelden E. (1995) Dev. Biol. 170, 434–444 [DOI] [PubMed] [Google Scholar]
- 33. Crawley S. W., de la Roche M. A., Lee S. F., Li Z., Chitayat S., Smith S. P., Côté G. P. (2006) J. Biol. Chem. 281, 6307–6315 [DOI] [PubMed] [Google Scholar]
- 34. Pollard T. D., Korn E. D. (1973) J. Biol. Chem. 248, 4682–4690 [PubMed] [Google Scholar]
- 35. Casnellie J. E. (1991) Methods Enzymol. 200, 115–120 [DOI] [PubMed] [Google Scholar]
- 36. Otwinowski Z., Minor W. (1997) in Methods in Enzymology (Carter C., Sweet R. eds) Vol. 276, pp. 307–326, Academic Press, New York: [DOI] [PubMed] [Google Scholar]
- 37. McCoy A. J., Grosse-Kunstleve R. W., Storoni L. C., Read R. J. (2005) Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 [DOI] [PubMed] [Google Scholar]
- 38. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 39. Murshudov G. N., Vagin A. A., Lebedev A., Wilson K. S., Dodson E. J. (1999) Acta Crystallogr. D Biol. Crystallogr. 55, 247–255 [DOI] [PubMed] [Google Scholar]
- 40. Kleiger G., Saha A., Lewis S., Kuhlman B., Deshaies R. J. (2009) Cell 139, 957–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raveh B., London N., Schueler-Furman O. (2010) Proteins 78, 2029–2040 [DOI] [PubMed] [Google Scholar]
- 42. Wang C., Bradley P., Baker D. (2007) J. Mol. Biol. 373, 503–519 [DOI] [PubMed] [Google Scholar]
- 43. Kramer R. H., Karpen J. W. (1998) Nature 395, 710–713 [DOI] [PubMed] [Google Scholar]
- 44. Yamaguchi H., Matsushita M., Nairn A. C., Kuriyan J. (2001) Mol. Cell 7, 1047–1057 [DOI] [PubMed] [Google Scholar]
- 45. Crawley S. W., Côté G. P. (2009) Biochem. J. 420, 115–122 [DOI] [PubMed] [Google Scholar]
- 46. Xu R. M., Carmel G., Sweet R. M., Kuret J., Cheng X. (1995) EMBO J. 14, 1015–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dajani R., Fraser E., Roe S. M., Young N., Good V., Dale T. C., Pearl L. H. (2001) Cell 105, 721–732 [DOI] [PubMed] [Google Scholar]
- 48. Longenecker K. L., Roach P. J., Hurley T. D. (1996) J. Mol. Biol. 257, 618–631 [DOI] [PubMed] [Google Scholar]
- 49. Glaser F., Pupko T., Paz I., Bell R. E., Bechor-Shental D., Martz E., Ben-Tal N. (2003) Bioinformatics 19, 163–164 [DOI] [PubMed] [Google Scholar]
- 50. Redpath N. T., Proud C. G. (1993) Eur. J. Biochem. 212, 511–520 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.