Abstract
Proteoglycan (PG) expression was studied in primary human umbilical vein endothelial cells (HUVEC). RT-PCR analyses showed that the expression of the PG serglycin core protein was much higher than that of the extracellular matrix PG decorin and the cell surface PG syndecan-1. PG biosynthesis was further studied by biosynthetic [35S]sulfate labeling of polarized HUVEC. Interestingly, a major part of 35S-PGs was secreted to the apical medium. A large portion of these PGs was trypsin-resistant, a typical feature of serglycin. The trypsin-resistant PGs were mainly of the chondroitin/dermatan sulfate type but also contained a minor heparan sulfate component. Secreted serglycin was identified by immunoprecipitation as a PG with a core protein of ∼30 kDa. Serglycin was furthermore shown to be present in perinuclear regions and in two distinct types of vesicles throughout the cytoplasm using immunocytochemistry. To search for possible serglycin partner molecules, HUVEC were stained for the chemokine growth-related oncogene α (GROα/CXCL1). Co-localization with serglycin could be demonstrated, although not in all vesicles. Serglycin did not show overt co-localization with tissue-type plasminogen activator-positive vesicles. When PG biosynthesis was abrogated using benzyl-β-d-xyloside, serglycin secretion was decreased, and the number of vesicles with co-localized serglycin and GROα was reduced. The level of GROα in the apical medium was also reduced after xyloside treatment. Together, these findings indicate that serglycin is a major PG in human endothelial cells, mainly secreted to the apical medium and implicated in chemokine secretion.
Keywords: Chemokines, Chondroitin Sulfate, Secretion, Sorting, Vesicles, Apical, Endothelial Cells, Proteoglycan, Serglycin
Introduction
Proteoglycans (PGs)4 are located in the extracellular matrix, on cell surfaces, and in intracellular storage granules (1–3). These highly polyanionic macromolecules have important functions as structural components of extracellular matrices in such different tissues as cartilage, loose connective tissue, and basement membranes. Cell surface PGs are important for cell attachment and as receptors for growth factors, enzymes, and chemokines. In addition, PGs present in storage granules, such as in mast cells, have important functions in storing enzymes, growth factors, and other bioactive compounds (3, 4).
The most prominent intracellular PG is serglycin (3–5). It was cloned and sequenced 3 decades ago (6) and has been regarded as a PG restricted to the hematopoietic cell lineages (7). However, using Northern blotting, it was demonstrated that human endothelial cells and smooth muscle cells expressed mRNA for serglycin (8). This was later confirmed in cultured human endothelial cells using an antibody against serglycin (9) and in cultured human smooth muscle cells using N-terminal sequencing (10). In endothelial cells, it was also shown that serglycin was located in intracellular vesicles, distributed throughout the cytoplasm, including perinuclear regions (9). Moreover, it was reported that serglycin showed co-localization with tissue-type plasminogen activator (tPA) (9). Endothelial cells contain several types of vesicles, and some of these have been shown to contain proinflammatory chemokines (11). The presence of serglycin in these vesicles has not been studied in detail.
Mice with an inactivated version of the serglycin gene have been generated, and it has been revealed that granule morphology (12, 13) and storage of several mast cell-specific proteases, histamine, and serotonin is greatly affected in these mice (12, 14). Other cell types, such as neutrophils (15), cytotoxic T-lymphocytes (16), and macrophages (17), are also affected by serglycin deficiency. To further study the biological implications of serglycin, we have made use of human umbilical vein endothelial cells (HUVEC). We have also made use of semipermeable filters to study polarized HUVEC, which is a useful in vitro model system with relevance to the in vivo situation. Endothelial cells have important functions related to diabetes, atherosclerosis, inflammation, and extravasation of tumor cells, but the synthesis and possible functions of PGs in these cells have only been studied to a limited extent. The results presented here show that serglycin is a major PG in HUVEC, secreted to the apical medium and present in secretory granules containing the chemokine growth-related oncogene α (GROα/CXCL1).
EXPERIMENTAL PROCEDURES
Endothelial Cell Culture
HUVEC were isolated enzymatically from infant umbilical cords of normal pregnancies as described (18). The cells were established in MCDB-131 medium (Sigma-Aldrich) with 7% fetal calf serum, epidermal growth factor (10 ng/ml), fibroblast growth factor (1 ng/ml), hydrocortisone (1 μg/ml), gentamicin (50 μg/ml), and fungizone (0.25 μg/ml). The cells were routinely grown to 80% confluence at 37 °C in 5% CO2, and cells from passages 2–4 were used in all experiments. Ethical approval for the use of human endothelial cells was obtained from the Regional Committees for Medical and Health Research Ethics.
To obtain polarized cells, HUVEC were trypsinized and seeded on Costar Transwell clear polyester membrane inserts with a pore size of 0.4 μm and growth surface area of 4.67 cm2 (Sigma-Aldrich) in 6-well plates. The cells were seeded at a density of 1 × 105 cells/cm2 with 2.6 ml of MCDB-131 medium in the basolateral compartment and 1.5 ml in the apical compartment. Medium was changed every second day until the start of the experiment, when a tight monolayer had been established. This was determined by measurement of transendothelial electrical resistance across the endothelial cell monolayer using the Millicell® Electrical Resistance System (Millipore).
For abrogation of chondroitin sulfate (CS)/dermatan sulfate (DS) biosynthesis, HUVEC were incubated for 20–24 h in 1 mm benzyl-β-d-xyloside (xyloside), a gift from Dr. H. C. Robinson (Monash University, Melbourne, Australia).
Real-time PCR
HUVEC were cultured in cell culture flasks as described above. The cells were washed with medium without serum three times, and total RNA was thereafter isolated using the 6100 Nucleic Acid PrepStation (Applied Biosystems) or the NucleoSpin RNA II kit (Macherey-Nagel). RNA was frozen at −70 °C before further analyses. First-strand cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) according to manufacturer's instructions with 5 μl of RNA and a final concentration of 7.5 ng/μl random hexamers (Invitrogen) in a total volume of 20 μl. The cDNA concentration was determined spectrophotometrically, and cDNA was diluted to 800 ng/μl. Real-time PCR was performed on an ABI PRISM 7900 HT using iQTMSYBR Green Supermix (Bio-Rad) in a total volume of 10 μl, containing 80 ng of cDNA and a final primer concentration of 200 nm. PCR cycling conditions included a 95 °C heating step of 10 min at the beginning of every run. The samples were then cycled 40 times at 95 °C for 30 s (denaturation), 58 °C for 20 s (annealing), and 72 °C for 20 s (extension). A melting curve from 60 to 90 °C was generated at the end of every run. Prior to experiments, the primer efficiency for each primer pair was determined with three different dilutions of the cDNA. The CT values were plotted against log concentrations of the dilutions, and primer efficiency was calculated according to the formula, efficiency = 10(−1/slope) − 1. The results were calculated by the comparative CT method (User Bulletin 2; ABI PRISM 770 sequence detection system (P7N 4303859)), using hypoxanthine guanine phosphoribosyltransferase as a housekeeping gene. For primers used and primer efficiency, see Table 1.
TABLE 1.
Primers used in real-time PCR
| Target | Sequence | Amplicon size | Efficiency |
|---|---|---|---|
| bp | |||
| β-Actin (forward) | 5′-CGC GAG AAG ATG ACC CAG AT-3′ | 150 | 0.97 |
| β-Actin (reverse) | 5′-GAT GGG CAC AGT GTG GGT G-3′ | ||
| Serglycin (forward) | 5′-CTA AGT TGG TCA TGA TGC AGA A-3′ | 105 | 0.96 |
| Serglycin (reverse) | 5′-TCC GCG TAG GAT AAC CTT GAA C-3′ | ||
| Syndecan-1 (forward) | 5′-TGC CGC AAA TTG TGG CTA C-3′ | 147 | 1.02 |
| Syndecan-1 (reverse) | 5′-GCT GCG TGT CCT TCC AAG TG-3′ | ||
| Decorin (forward) | 5′-ACT CTT CAG GAG CTG CGT GC-3′ | 105 | 1.09 |
| Decorin (reverse) | 5′-CGG ATT GGT GCC CAG TTC T-3′ |
Proteoglycans
Both polarized HUVEC and cells cultured in conventional cell culture flasks were labeled with [35S]sulfate. The cells were incubated with [35S]sulfate (0.1–0.2 mCi/ml; Montebello Diagnostics) in sulfate-free RPMI 1640 medium (Invitrogen) for 20–24 h. The conditioned media were collected and centrifuged to remove cell debris. Cell fractions were solubilized in 4 m guanidine hydrochloride with 2% Triton X-100 in 50 mm sodium acetate, pH 6.0.
In order to remove excess unincorporated [35S]sulfate and to isolate the 35S-macromolecules, 1 ml of each fraction was applied to a 4-ml column of Sephadex G-50 Fine (GE Healthcare) in 0.05 m Tris/HCl, pH 8.0, 0.05 m NaCl. The first 1 ml of eluate was discarded, and the following 1.5 ml, containing the 35S-macromolecules, was collected. For determination of the level of 35S-macromolecules, aliquots of both cell and medium fractions were counted for radioactivity using a Wallac scintillation counter (Wallac Oy, Turku, Finland). Aliquots of cell fractions were used for protein determination using the Uptima BC Assay protein quantification kit (Interchim).
The samples were concentrated using vacuum centrifugation and Vivaspin ultrafiltration devices with a molecular mass cut-off at 30 kDa (GE Healthcare). In order to depolymerize CS/DS GAG chains, samples from medium and cell fractions (∼5,000–10,000 cpm for each analysis) were incubated at 37 °C overnight with 0.01 units of chondroitin ABC lyase (cABC, EC 4.2.2.4, Sigma-Aldrich) in 0.05 m Tris-HCl, pH 8.0, containing 0.05 m sodium acetate and 0.02% BSA. In parallel samples, heparan sulfate (HS) was degraded with HNO2 (nitrous acid) deamination at pH 1.5, cleaving the polysaccharide at N-sulfated glucosamine units (19). Briefly, equal volumes of 0.5 m Ba(NO2)2 and 0.5 m H2SO4 were mixed and centrifuged for 5 min at 10,000 rpm to remove precipitated BaSO4. Equal volumes of the resulting HNO2 and samples from media of ∼5,000–10,000 cpm were mixed and incubated for 10 min at room temperature. Reaction was stopped by the addition of 2 m Na2CO3 to obtain neutral pH.
In order to test for protease-resistant PGs, isolated 35S-macromolecules from samples containing equal amounts of protein were treated with 5 μl of 0.25% trypsin-EDTA (Sigma-Aldrich) overnight and inactivated with soybean trypsin inhibitor (Sigma-Aldrich). Samples were either trypsin-treated or treated with cABC, HNO2, or a combination of both after trypsin treatment. All samples were further analyzed by SDS-PAGE and autoradiography.
Immunoprecipitation
HUVEC were incubated with [35S]sulfate as described. Medium from the apical and basolateral compartments were harvested, and cell fractions were washed in cold PBS and incubated at 4 °C in lysis buffer containing 1% Nonidet P-40, 50 mm Tris, pH 7.5, 2 mm EDTA, 150 mm NaCl, 35 μg/ml PMSF, and protease inhibitor mixture (Roche Applied Science; one tablet in 10 ml, added fresh along with PMSF). Isolated 35S-macromolecules from medium and cell fractions of HUVEC were subjected to Sephadex G-50 gel filtration as described above prior to concentration using Vivaspin ultrafiltration devices (molecular mass cut-off 30 kDa) and vacuum centrifugation. Protein content was determined in each sample after G-50 gel filtration, and equal amounts of protein from each sample were used in further analyses. As positive control, medium from the monocytic cell line THP-1 was subjected to the same treatment (20). PG samples from media were subjected to cABC treatment, and MgSO4 was added to a final concentration of 5 mm to prevent nonspecific binding of [35S]sulfate. The samples were then incubated with a rabbit anti-human serglycin antibody (a kind gift from Professor Niels Borregaard, Rikshospitalet, Copenhagen, Denmark) diluted 1:500 (stock solution of antibody was 2 μg/ml) overnight at 4 °C. 30 μg of protein A/G solution (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was then added, and the incubation was continued at 4 °C for 2 h. Next, the samples were centrifuged and washed two times with 0.05 m Tris-HCl with 0.15 m NaCl, 0.05% Triton X-100, and 1% BSA, followed by four washes in the same buffer but without BSA. Material bound to protein A/G was finally released by boiling for 5 min in sample buffer, centrifuged, and loaded on to 4–20% SDS-PAGE. Molecular mass protein markers (Amersham Biosciences) were run on all gels for mass determination. After electrophoresis, the gels were dried and subjected to autoradiography.
Western Blotting
Both polarized HUVEC and cells cultured in conventional cell culture flasks were subjected to Western blotting. HUVEC were incubated for 24 h in MCDB-131 as described above but with serum reduced to 2%. Medium fractions were harvested and centrifuged to remove cell debris. Cells were rinsed in ice-cold PBS and solubilized in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Triton X-100, 1% SDS, 1% sodium deoxycholate, 10 mm EDTA, 10 mm Na4PO2O7, and protease inhibitor mixture) and used for protein determination. PGs were purified by DEAE ion exchange chromatography. The samples were applied to individual DEAE-Sephacel polyprep columns equilibrated in 0.15 m Tris-HCl buffer, pH 8.0, with 0.15 m NaCl, washed in the same buffer, and thereafter washed with Tris buffer with 0.3 m NaCl before bound material was eluted in Tris-HCl buffer with 2 m NaCl. The eluate was concentrated using Vivaspin ultrafiltration devices (mass cut-off 30 kDa). To determine the presence of perlecan or decorin in the apical and basolateral media of polarized cells, identical percentages of the different fraction volumes were separated on SDS-PAGE and electroblotted to a PVDF membrane (Millipore) using the CriterionTM gel system (Bio-Rad). Primary antibodies against perlecan, which was provided to us by Prof. R. V. Iozzo, and decorin (R&D Systems) were used. The secondary antibodies used were HRP-linked donkey anti-rabbit IgG (GE Healthcare) and HRP-linked rabbit ant-goat IgG (R & D Systems), respectively. The membranes were developed using ECL Western blotting detection reagents (GE Healthcare) and finally exposed to films. Similarly, the expression of serglycin in HUVEC cultured on plastic was investigated by Western blotting using the primary antibody against serglycin and the secondary HRP-linked donkey anti-rabbit IgG.
Immunocytochemistry
Monolayers of HUVEC grown on Lab-Tek chamber slides (Nalge Nunc International, Rochester, NY) coated with 1% (w/v) gelatin type A from porcine skin were briefly submerged in PBS and fixed in 4% paraformaldehyde for 10 min before washing twice for 5 min each in PBS. For immunostaining, the fixed monolayers were incubated with mouse monoclonal primary antibody against EEA1 (early endosome antigen 1) (1.2 μg/ml clone 14; Transduction Laboratories), LAMP-2 (lysosome-associated membrane protein 2) (1:200 of F8/86; Dako), tPA (10 μg/ml clone ESP4; American Diagnostica), or GROα (1 μg/ml clone 20326; R&D Systems) overnight at 4 °C. Next we incubated with biotinylated horse anti-mouse IgG (1:200; Vector Laboratories) and rabbit anti-human serglycin (1:150) for 1.5 h at room temperature and finally with strepatividin-Cy3 conjugate (1:1000; The Jackson Laboratory) combined with Alexa-488 goat anti-rabbit IgG (1:400; Molecular Probes) for 1 h at room temperature. All antibodies and the streptavidin conjugate were diluted in PBS containing 1.25% rinderalbumin. Saponin (0.1%) was used for permeabilization in all steps except in the last washing. Irrelevant matched primary antibody was used as negative control for GROα and serglycin. Images were obtained using a confocal laser-scanning microscope (Leica TCS, Leica Microsystems or Olympus FluoView FV1000) equipped with argon (488 nm) and helium/neon (543 and 633 nm) lasers. Oil objectives, plan apochromat ×100/1.4 or ×60/1.35, were used. The fluorochromes were excited and detected sequentially.
Enumeration of GROα- and Serglycin-positive Cells
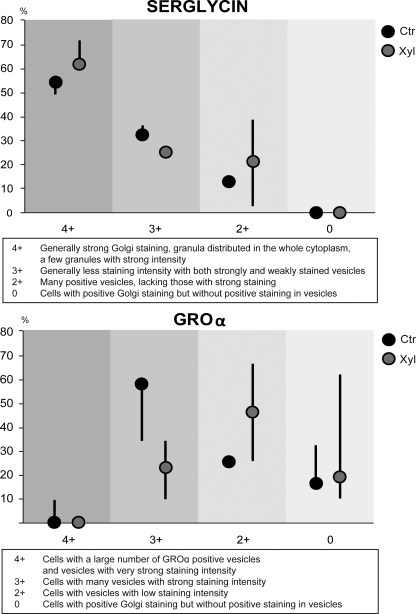
Immunostained HUVEC that were positive for GROα or serglycin were analyzed by scoring for the fluorescence intensity and presence in Golgi-associated structures and vesicles. The difference in fluorescence signals between control and xyloside-treated cells were more pronounced for GROα than for serglycin. The pattern of GROα and serglycin was therefore graded 4+, 3+, 2+, and 0. GROα grading was as follows: 4+, cells with a large number of GROα positive vesicles and vesicles with very strong staining intensity; 3+, cells with many vesicles with strong staining intensity; 2+, cells with vesicles with low staining intensity; 0, cells with positive Golgi staining but without positive staining in vesicles. Serglycin grading was as follows: 4+, generally strong Golgi staining, granula distributed in the whole cytoplasm, and a few granules with strong intensity; 3+, Generally less staining intensity with both strongly and weakly stained vesicles; 2+, many positive vesicles, lacking those with strong staining; 0, cells with positive Golgi staining but without positive staining in vesicles. Twelve fields of vision were counted and evaluated manually for both antibodies per experiment, and three experiments were included in the analysis.
ELISA
Conditioned media were obtained from polarized HUVEC cultured under serum-free conditions. The cells were incubated with or without 0.5 ng/ml interleukin-1β (IL-1β) to compare secretion of GROα in untreated and stimulated cells. Furthermore, these cells were also cultured with or without xyloside (1 mm) to investigate the effect of abrogating PG synthesis on GROα secretion. Medium fractions were collected after 20 h and centrifuged in order to remove cell debris. The cells were harvested in lysis buffer containing 1% Nonidet P-40, 0.05 m Tris-HCl, pH 7.5, 2 mm EDTA, 0.15 m NaCl, 35 μg/ml PMSF, and protease inhibitor mixture (Roche Applied Science). Both conditioned media and cell fractions were analyzed for content of GROα using the DuoSet ELISA Development kit (R&D Systems).
Statistics
The levels of GROα released into the apical and basolateral media from IL-1β-treated cells with and without xyloside were measured by ELISA and analyzed for possible statistical differences using an unpaired t test with PASW statistics 18 (IBM). p values of <0.05 were considered significant.
RESULTS
Proteoglycan Synthesis and Secretion in Polarized HUVEC
PG biosynthesis in HUVEC has been studied only to a limited extent (8, 9). To further investigate PG expression in endothelial cells, RNA was isolated, and real time quantitative RT-PCR analyses were performed to compare the expression levels of three PGs: the secretory PG decorin, the plasma membrane-associated syndecan-1, and the intracellular PG serglycin (primers specified in Table 1). The results in Table 2 are based on RNA isolated from two separate cultures of HUVEC and show that serglycin is by far the most highly expressed PG core protein of the three analyzed.
TABLE 2.
The expression of three different core proteins in two separate preparations of cultured HUVEC
| Core protein | -Fold expression of serglycin and decorin relative to syndecan-1 |
||
|---|---|---|---|
| Ct of medium | Ct calibrated for β-actin | -Fold increase | |
| Syndecan-1 | 29.93 | 13.91 | 1.0 |
| Serglycin | 19.09 | 3.06 | 1837.2 |
| Decorin | 26.85 | 10.82 | 8.5 |
| -Fold expression of serglycin and decorin relative to syndecan-1 |
|||
|---|---|---|---|
| Ct of medium | Ct calibrated for β-actin | -Fold increase | |
| Syndecan-1 | 28.02 | 12.00 | 1.0 |
| Serglycin | 19.08 | 3.06 | 451.3 |
| Decorin | 28.07 | 12.06 | 0.96 |
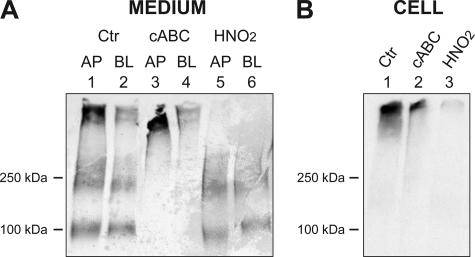
HUVEC were further cultured on semipermeable filters and biosynthetically labeled with [35S]sulfate to study de novo synthesis of sulfated macromolecules, such as PGs. This system makes it possible to study secretion to the apical and basolateral side of confluent endothelial monolayers. Interestingly, a major part of the 35S-labeled macromolecules synthesized by polarized HUVEC were secreted to the apical medium (Fig. 1A, lane 1). The secreted 35S-labeled macromolecules were clearly separated into three parts: one of high molecular mass (migrating at the top of the gel), a second with an approximate mass of 200–250 kDa, and a third species with a mass of ∼100 kDa. All three components were found as secretory products in both apical and basolateral fractions but in markedly higher amounts in the apical medium (compare lanes 1 and 2). The cell fraction, in contrast, contained mainly the high molecular mass component (Fig. 1B, lane 1). The recovered fractions were subjected to either cABC treatment, which leads to depolymerization of CS and DS, or to treatment with HNO2, pH 1.5, leading to degradation of heparin and HS. These experiments revealed that the high molecular mass 35S-macromolecules in the medium fractions were completely degraded by HNO2, indicating that these 35S-labeled macromolecules were entirely composed of PGs of the HS/heparin type (Fig. 1A, lanes 5 and 6). In contrast, the 200–250-kDa and 100-kDa components were resistant to HNO2 treatment but were degraded completely by cABC, indicating that also these 35S-macromolecules were PGs, although of the CS/DS type (Fig. 1A, lanes 3 and 4). The high molecular mass 35S-macromolecules found in the cell fraction were almost completely degraded by HNO2 and resistant to cABC, suggesting that these 35S-labeled macromolecules contained PGs of the HS/heparin type (Fig. 1B, lanes 2 and 3). Together, these data indicate that HUVEC synthesize and secrete large amounts of PGs and that most of the PG secretion occurs at the apical side of the cell.
FIGURE 1.
Secretion of 35S-PGs in polarized HUVEC. HUVEC were cultured on semipermeable supports and labeled with [35S]sulfate for 20–24 h after a tight monolayer had developed. Apical (AP) and basolateral (BL) medium fractions were harvested, and cell fractions were obtained after solubilization of the cell layer. Medium (A) and cell fractions (B) were subjected to gel chromatography followed by cABC treatment to degrade CS/DS, HNO2 to degrade HS, or no treatment (Ctr). The samples were finally analyzed by SDS-PAGE and autoradiography. Shown is one representative of six experiments. The migration positions of molecular mass markers (in kDa) are shown on the left.
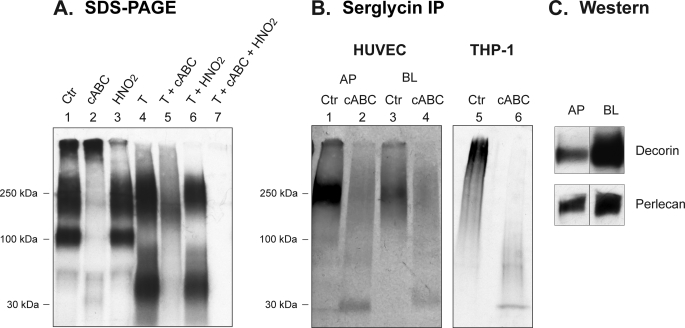
In serglycin, the GAG chains (heparin or CS type) are densely clustered in the central part of the core protein within a short repetitive Ser-Gly sequence (3). This tight clustering of GAG chains masks potential proteolytic cleavage sites, resulting in a “protease-resistant” PG (21). Notably, this feature can be utilized experimentally to distinguish serglycin from other PGs, with most other PG types being readily degraded by proteolytic treatment (e.g. by trypsin). Thus, to investigate the expression of serglycin in HUVEC, 35S-PGs from medium fractions were subjected to trypsin treatment or to combinations of trypsin, cABC, and HNO2 treatment. The 200–250-kDa 35S-PGs were resistant to trypsin treatment (Fig. 2A, lane 4), thus suggesting that this PG population contains serglycin. In contrast, the higher mass and 100-kDa PGs were susceptible (Fig. 2A, lane 4), indicating that these fractions contain PG species of the non-serglycin type. Furthermore, after trypsin treatment, a major band with mass of ∼50 kDa appeared (Fig. 2A, lane 4), most likely representing partially degraded PGs or free GAG chains liberated by the trypsin digestion of the high molecular mass and 100-kDa PGs. The trypsin-resistant 35S-PG was mainly of the CS/DS type, as judged by susceptibility to cABC treatment (Fig. 2A, lane 5) and relative resistance to HNO2 (Fig. 2A, lane 6). Notably, all of the trypsin-resistant 35S-PGs were degraded with a combination of cABC and HNO2 treatment (Fig. 2A, lane 7), suggesting that this PG fraction either contains distinct CS (major part) and HS/heparin (minor part) PGs or contains hybrid PG species having both CS and heparin/HS chains. Similar results were obtained with medium fractions from HUVEC cultured on plastic and semipermeable filters (data not shown).
FIGURE 2.
Characterization of 35S-PGs. A, 35S-PGs recovered from the medium were either untreated (Ctr; lane 1) or subjected to either cABC, HNO2, or trypsin treatment alone (lanes 2–4); to trypsin treatment in combination with either cABC (lane 5) or HNO2 (lane 6); or to a combination of trypsin, cABC, and HNO2 (lane 7). The products were subjected to SDS-PAGE and autoradiography. Shown is one representative of four experiments. B, apical (AP) and basolateral (BL) medium fractions were harvested from [35S]sulfate-labeled HUVEC. Recovered 35S-PGs were untreated or subjected to cABC digestion. These samples were immunoprecipitated (IP) using an anti-serglycin antibody, followed by SDS-PAGE. Intact serglycin PG (lanes 1 and 3) and serglycin core protein with 35S-labeled stubs (lanes 2 and 4) were detected after autoradiography. Shown is one representative of three experiments. 35S-PGs from THP-1 cells were included as a positive control (lanes 5 and 6). C, apical and basolateral medium fractions were subjected to Western blotting (Western) using antibodies against perlecan and decorin. Perlecan migrated with a molecular mass higher than the 250 kDa standard, and decorin migrated corresponding to the 100 kDa standard.
To further investigate the expression of serglycin by HUVEC, 35S-PGs from both the apical and basolateral media were subjected to immunoprecipitation with an anti-serglycin antibody, followed by SDS-PAGE. As a positive control, 35S-PGs from the monocyte cell line THP-1 were included. This cell line has been shown to secrete mainly serglycin (20). As shown in Fig. 2B, both the apical and the basolateral media contained the serglycin PG (lanes 1 and 3) and its corresponding core protein (lanes 2 and 4), the latter showing a migration velocity similar to that of the serglycin core protein immunoprecipitated from THP-1-conditioned medium (lane 6). In agreement with a more pronounced secretion of trypsin-resistant 35S-labeled PGs to the apical side, larger amounts of the serglycin PG (lanes 1 and 3) and core protein (lanes 2 and 4) were found in the apical than in the basolateral medium. Notably, the obtained molecular mass (∼30 kDa) is larger than the predicted mass (17 kDa) of the serglycin core protein, a finding that is explained by the presence of 35S-labeled hexasaccharides left on the core protein after cABC treatment, as reported by Schick and co-workers (9).
Apical and basolateral medium fractions were also subjected to Western blotting using antibodies against perlecan and decorin. Both of these PGs were predominantly secreted to the basolateral medium, as can be seen in Fig. 2C. From these experiments, we conclude that polarized HUVEC synthesize and secrete protease-resistant serglycin, mainly to the apical medium, and that HUVEC-derived serglycin is mainly of the CS/DS type.
Xyloside and PGs in HUVEC
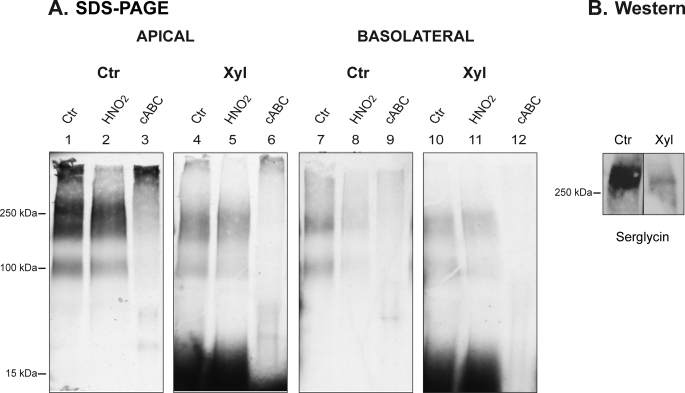
To further address the role of serglycin in polarized HUVEC, we used β-d-xylosides, agents that are known to inhibit the assembly of intact PGs (22–24). HUVEC were cultured with or without 1 mm xyloside and were biosynthetically labeled with [35S]sulfate. The apical and basolateral medium fractions were recovered and analyzed by SDS-PAGE before and after cABC or HNO2 treatment. As shown in Fig. 3, the xyloside treatment caused a reduction in the secretion of PGs both into the apical (compare lanes 1 and 4) and basolateral media (compare lanes 7 and 10). Further, the xyloside treatment resulted in the generation of low molecular mass CS/DS chains (Fig. 3, lanes 4 and 10), as judged by susceptibility to cABC treatment (Fig. 3, lanes 6 and 12). Accordingly, exposing HUVEC to xyloside decreased the biosynthesis of intact PGs and stimulated the generation of free CS/DS chains. Furthermore, Western blotting was also performed to investigate the effect of xyloside on serglycin specifically. From Fig. 3B, it is evident that secretion of serglycin is dramatically decreased after xyloside treatment.
FIGURE 3.
The effect of xyloside treatment on 35S-PGs in HUVEC. A, polarized HUVEC were cultured with (Xyl) or without (Ctr) 1 mm xyloside for 20–24 h in the presence of [35S]sulfate. Apical and basolateral medium fractions were harvested, subjected to gel chromatography, and thereafter not treated (Ctr; lanes 1, 4, 7, and 10) or treated with cABC (lanes 3, 6, 9, and 12) or HNO2 (lanes 2, 5, 8, 11), followed by SDS-PAGE and autoradiography. This is one representative of four experiments. B, medium fractions from control and xyloside-treated HUVEC cultured on conventional plastic were subjected to Western blotting using an anti-serglycin antibody. The migration positions of mass markers (in kDa) are shown on the left.
It is important to note that treatment of polarized HUVEC with 1 mm xyloside did not lead to a complete uncoupling of CS/DS PG synthesis. We used this concentration in order not to completely abrogate the PG biosynthesis because higher concentrations of xylosides may lead to dramatic, unwanted effects on vesicle integrity (25). The amount of released free CS/DS chains was almost equal in the two medium compartments, suggesting that the apical secretion of PGs carrying such chains depends on their respective core proteins. This may be a cell-specific phenomenon because polarized canine kidney epithelial cells secrete a major part of xyloside-generated CS/DS chains to the apical medium (26), whereas human colon epithelial cells secrete more of such chains to the basolateral medium (27).
Intracellular Localization of Serglycin
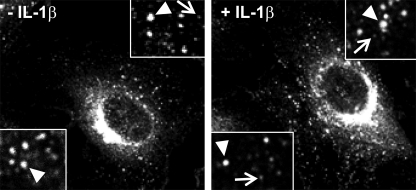
The localization of serglycin in several hematopoietic cells has been thoroughly investigated (3, 4), but the distribution of serglycin in endothelial cells has scarcely been studied (9). Proinflammatory cytokines can enhance the expression of serglycin mRNA in HUVEC (8). To address this issue further, we investigated the expression of PGs and the intracellular distribution of serglycin in IL-1β-stimulated cells. We first analyzed the effect of IL-1β on PG biosynthesis and secretion. Indeed, there was a 30% increase in 35S-PGs in both the cell and medium fractions from stimulated cells compared with controls (data not shown). We further performed immunostaining and examined the distribution of serglycin by confocal microscopy. We observed serglycin in perinuclear regions but also in numerous vesicles throughout the cytoplasm (Fig. 4, left). Immunostaining of the Golgi marker protein 58K-9 (clone 58-K9, data not shown) indicates that the strong signal in the perinuclear region originated from the Golgi complex. It was also evident that serglycin was present in two types of vesicles, of different sizes and of different fluorescence intensities, with the smaller vesicles showing the lowest fluorescence intensities. After IL-1β stimulation of HUVEC, the cells contained larger amounts of serglycin-containing vesicles per cell than did unstimulated cells, and the average size of the serglycin-positive vesicles was smaller than in unstimulated cells (Fig. 4, right).
FIGURE 4.
Distribution of serglycin in HUVEC. HUVEC were cultured in chamber slides and incubated without (left) or with (right) 0.5 ng/ml IL-1β for 24 h, fixed, and immunostained for serglycin (rabbit anti-serglycin). The immunofluorescence pictures were acquired with an original magnification of ×100 (Leica TCS confocal microscope). Corner insets show high magnification of areas from representative cells. Two morphologically distinct serglycin-positive vesicles were observed, the larger with relatively intense fluorescence signal (arrowhead) and the smaller vesicles showing lower fluorescence intensity (arrows).
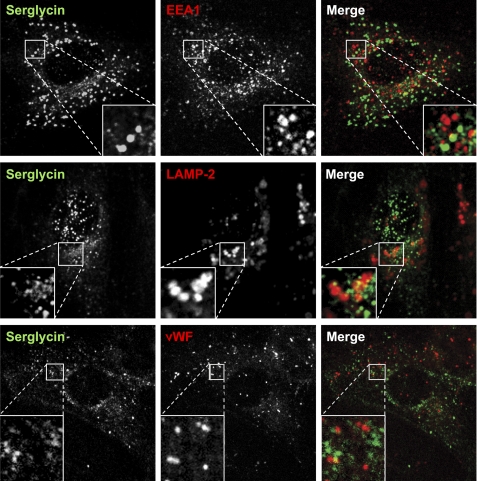
It has been demonstrated that serglycin can bind to cell surface receptors that are internalized (28, 29). Thus, serglycin may be endocytosed and transported into endosomes/late endosomes, and this could give rise to the positive staining for serglycin in HUVEC. To investigate if the vesicular staining of serglycin was due to receptor-mediated endocytosis of secreted serglycin, co-staining for serglycin and the endocytic markers EEA1 and LAMP-2 was performed. Although we observed scattered overlap for serglycin and the endocytic markers, there was no overt co-localization of either of these two markers with serglycin (Fig. 5). Based on these results, we propose that serglycin is localized in vesicles belonging to the secretory rather than the endocytic pathway. The most well studied granule of the secretory pathway in endothelial cells is the Weibel-Palade body, which can be identified by the presence of von Willebrand factor (vWF). However, we did not observe localization of serglycin in elongated vWF-positive Weibel-Palade bodies (Fig. 5).
FIGURE 5.
Serglycin and vesicles belonging to the endocytic pathway and the Weibel-Palade body. HUVEC were fixed and immunostained for serglycin together with the endosomal marker EEA1 (clone 14), the lysosomal marker LAMP-2 (clone H4B4), or the Weibel-Palade body marker vWF (F8/86). Corner insets show high magnification of framed areas (original magnification, ×100, Leica TCS confocal microscope). Green, serglycin; red, EEA1, LAMP-2, or vWF as indicated.
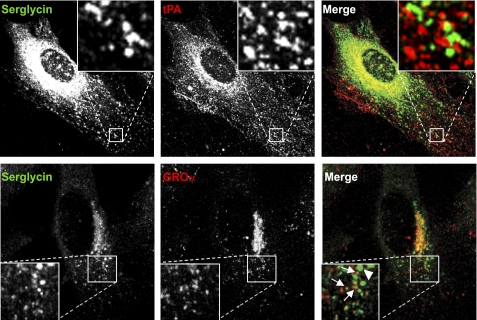
A previous study has suggested a possible co-localization of serglycin and tPA, using a serglycin antibody produced in chicken (9). To evaluate this notion, we investigated the possible co-localization of serglycin with tPA in HUVEC, using the serglycin antibody available for our studies, raised in rabbits. HUVEC were stimulated with N-butyrate to enhance the expression of tPA (30). As shown in Fig. 6 (top row, middle panel), immunostaining against tPA revealed strong signals in the Golgi region in addition to signals that distributed in a granular pattern throughout the cytoplasm. Whereas tPA distributed together with serglycin in the Golgi region, there was no apparent co-localization of tPA with serglycin-positive granules localized throughout the cytoplasm (Fig. 6, right).
FIGURE 6.
Serglycin and GROα- and tPA-containing vesicles. HUVEC were cultured in chamber slides and stimulated with 3 mm butyrate or 0.5 ng/ml IL-1β for 20 h before fixation and immunostaining against serglycin and tPA (clone ESP-4) or GROα (clone 20326), respectively. Corner insets show high magnification of framed areas. The immunofluorescence pictures of serglycin/tPA and serglycin/GROα were acquired by Olympus FluoView FV1000 (original magnification, ×60) and Leica TCS (original magnification, ×100) confocal microscopes, respectively. Green, serglycin; red, tPA or GROα. Lower panel, arrows indicate vesicles that are double positive for serglycin and GROα; the arrowhead indicates a vesicle that is serglycin-positive.
We have previously established that serglycin can be associated with the chemokines macrophage inflammatory protein 1α (MIP-1α/CCL3) and platelet factor 4 (PF4/CXCL4) (31, 32). Interestingly, the smallest serglycin-positive vesicles appeared to have a distribution and size similar to the chemokine-containing type 2 granules of HUVEC that contain the chemokines GROα/CXCL1 and monocyte chemoattractant chemokine-1 (MCP-1/CCL-2) (30). IL-1β up-regulates the expression of both GROα and MCP-1 in HUVEC (11). We therefore hypothesized that serglycin may be in type 2 granules, with the implication that serglycin may have a role in storage and/or secretion of chemokines in HUVEC. To test this hypothesis, we performed paired immunostaining against serglycin and GROα/CXCL1. To obtain detectable levels of GROα, HUVEC were stimulated with IL-1β (Fig. 6, lower row). Interestingly, several of the relatively small serglycin-positive vesicles were also positive for GROα, suggesting that serglycin and GROα indeed co-localize. However, those vesicles that were large and showed a bright fluorescent signal for serglycin were GROα-negative (indicated with an arrowhead in Fig. 6, lower row, right panel). Hence, these results indicate heterogeneity in vesicles of IL-1β-stimulated HUVEC with regard to serglycin and GROα content.
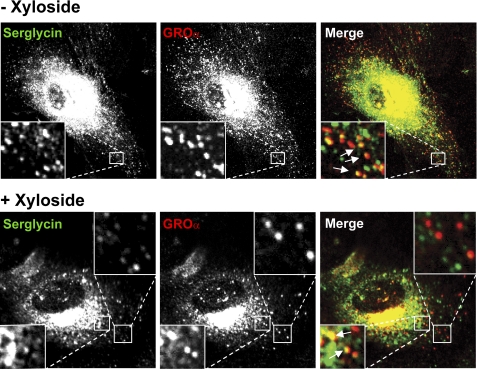
To further study the importance of serglycin for vesicle formation and content in HUVEC, cells were stimulated with IL-1β, with or without xyloside treatment, and stained for serglycin and GROα. As indicated in Fig. 7, the number of serglycin-positive vesicles was changed after xyloside treatment, both in the Golgi region and in other parts of the cytoplasm. HUVEC treated with a combination of IL-1β and xyloside contained GROα-positive vesicles (Fig. 7, lower row). However, the vesicles were lower in staining intensity and number as compared with control cells that had not been treated with xyloside (see Fig. 7, upper row). To obtain more detailed information on the effect of xylosides, the size and fluorescence intensity of the vesicles was scored as described under “Experimental Procedures.” This analysis supported our previous observation that the xyloside treatment caused alterations in the staining patterns both for serglycin and GROα (Fig. 8). Notably, abrogating PG biosynthesis with xyloside resulted in decreased content of GROα in HUVEC vesicles. The fluorescence intensity of the serglycin-positive vesicles was also reduced after xyloside treatment (Fig. 8). These changes were observed in three separate experiments, which all showed the same pattern. These observations are therefore compatible with a role for serglycin in the regulation of GROα levels in cytoplasmic vesicles.
FIGURE 7.
GROα-positive granules and xyloside treatment. HUVEC were cultivated in the presence of 0.5 ng/ml IL-1β, either without (−Xyloside) or with (+Xyloside) 1 mm xyloside for ∼24 h and fixed before paired immunostaining against serglycin and GROα. The pictures were acquired by an Olympus FluoView FV1000 microscope (original magnification, ×60). Corner insets show high magnification of framed areas. Green, serglycin; red, GROα.
FIGURE 8.
Distribution and staining intensity of serglycin and GROα in HUVEC after xyloside treatment. HUVEC were cultured in the presence of 0.5 ng/ml IL-β for ∼24 h, either in the absence (Ctr) or presence of 1 mm xyloside (Xyl) and stained for serglycin and GROα. The staining pattern, including staining intensity, was analyzed in whole cells in 12 fields of vision and evaluated as described under “Experimental Procedures.” The percentage of cells showing the respective staining patterns is depicted. Each point represents the median of three experiments, and the bars indicate the minimum and maximum values.
Secretion of Serglycin and GROα
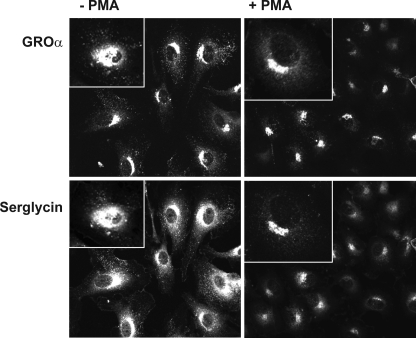
To further investigate whether serglycin is primarily associated with secretory vesicles, HUVEC were treated for 45 min with phorbol 12-myristate 13-acetate (PMA) to induce secretion (30). Cells were thereafter stained for serglycin and GROα, and the results in Fig. 9 show that, after PMA stimulation, there was a large decrease in cytoplasmic staining for both serglycin and GROα, suggesting that they are indeed associated with vesicles destined for secretion.
FIGURE 9.
Release of serglycin following PMA stimulation. HUVEC cultivated in the presence of 0.5 ng/ml IL-β for ∼24 h were incubated without or with 100 ng/ml PMA for 45 min before fixation and paired immunostaining against serglycin (green) and GROα (red) (original magnification, ×40). Corner insets show high magnification of representative individual cells (original magnification, ×60).
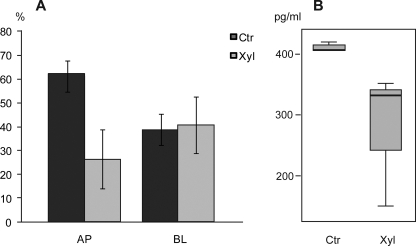
As shown in Figs. 7 and 8, the intracellular pattern of GROα localization in stimulated HUVEC depends, at least partly, on the expression of PGs. To substantiate this finding, we also examined whether the secretion of GROα was affected by xyloside treatment. After exposure of HUVEC to IL-1β with or without xyloside for 20–24 h, the apical and basolateral media were harvested. Analysis by ELISA indicated that the level of GROα in the apical medium was decreased in the presence of xyloside, whereas the basolateral level was not affected (Fig. 10). Hence, these data suggest that serglycin is implicated in GROα secretion in HUVEC.
FIGURE 10.
GROα secretion from IL-1β-stimulated HUVEC. HUVEC were cultured on filters in the presence of 0.5 ng/ml IL-1β with (Xyl) and without (Ctr) 1 mm xyloside for 20–24 h. Apical (AP) and basolateral (BL) media were recovered and analyzed for GROα using ELISA. A, secreted levels of GROα to the apical plus basolateral side in the control were set to 100%. Each bar represents mean values ± S.D. (error bars) of three experiments. B, the reduced secretion to the apical medium with xyloside was not statistically significant (p = 0.171) due to one very low value after xyloside treatment. If this low value is defined as an outlier, the decrease is statistically significant (p = 0.005).
DISCUSSION
In the present report, we have studied PG secretion in polarized HUVEC. Serglycin was found to be a major PG in these primary cells and to be secreted predominantly to the apical medium. In contrast, both perlecan and decorin were predominantly secreted to the basolateral medium. Further, immunocytochemical analysis revealed that serglycin was present in secretory vesicles, partly co-localizing with GROα. The apical secretion of serglycin is intriguing. Endothelial cells are attached to a basement membrane, where the PG perlecan is an important component (2, 33). Endothelial cells also express other PGs important for extracellular matrix formation, such as decorin, biglycan, and versican (34–36). The data presented here (Fig. 2C) on cultured polarized HUVEC are in agreement with such studies. Endothelial cells are exposed to a high degree of shear stress under in vivo conditions and therefore need continuous, firm attachment to their underlying support in order to function properly. One likely hypothesis would thus be that PGs in HUVEC would be secreted predominantly to the basolateral medium, as an in vitro reflection of secretion to the basement membrane in vivo. However, it is important to note that several studies have also shown the presence and importance of the endothelial apical glycocalyx, where PGs are important components (37). Syndecan-1 is one component of this glycocalyx, and is subject to shedding under stress conditions, such as heart surgery (37, 38). Studies on HUVEC have shown that the cells also have the capacity to form glycocalyx under in vitro conditions (39).
The predominant secretion of serglycin to the apical side of the HUVEC clearly suggests that HUVEC-derived serglycin is not primarily intended for deposition into the extracellular matrix supporting the endothelial cell layer but rather for functions at the apical side. Possibly, the apical serglycin may become, at least partly, deposited in the endothelial glycocalyx. Whether serglycin is also found in such a glycocalyx or whether it is released to the circulation in vivo has not been the subject of any studies. It has, however, been shown that the concentration of serglycin is elevated in plasma of patients with acute myeloid leukemia, suggesting that serglycin can be released to the circulation under certain pathological conditions (40). The latter study also suggests that serglycin may be used as a marker to distinguish these two types of leukemia. Elevated levels of serglycin have also been shown in 30% of bone marrow aspirates from myeloma patients (41). Furthermore, serglycin may possibly be involved in extravasation of NK cells in the human endometrium (42). Serglycin has also been studied in relation to parasite infections during pregnancy (43).
In the present study, we have made use of xylosides to interfere with serglycin biosynthesis. Furthermore, we have used trypsin resistance as a criterion to discern serglycin from other CSPGs. It can be argued that xylosides do not specifically interfere only with serglycin biosynthesis. A more definitive experiment to show specific inhibition of serglycin expression would be to use siRNA. This is currently the subject of further studies.
Our finding that serglycin is localized in vesicles together with GROα is compatible with a role for serglycin in regulating proinflammatory activities of endothelial cells, possibly by affecting the storage and/or secretion of chemokines. This would clearly be in line with the proposed proinflammatory role of serglycin in various hematopoietic cell types (3, 4). This is also supported by the finding that IL-1β increased the levels of PGs secreted from HUVEC, indicating that endothelial cell activation involves changes in PG expression. Furthermore, in HUVEC cultured on plastic surfaces, serglycin mRNA was up-regulated after stimulation with tumor necrosis factor-α and IL-1α (8, 9). The intracellular localization of serglycin in HUVEC has not been extensively studied (9, 43). Here, by using immunocytochemistry, we found that serglycin was distributed in two morphologically distinct vesicles: small vesicles with a relatively weak fluorescent signal and larger vesicles displaying a stronger fluorescence. The reduced number of serglycin-positive vesicles following stimulation with PMA strongly suggests that these vesicles belong to the secretory pathway. Also, our studies on 35S-PG secretion show that serglycin is predominantly secreted to the apical medium. Several types of granules have been described in endothelial cells, and a possible co-localization of serglycin and tPA in HUVEC has been reported (9). However, we observed no overt co-localization of serglycin and tPA. One potential explanation for this apparent discrepancy could be that Schick et al. (9) performed their studies using another experimental approach, including another anti-serglycin antibody.
In a previous study (30), we introduced the type 2 granule, a small (50–100 nm in diameter), electron-lucent, chemokine-containing granule negative for vWF and tPA. In the present study, we observed that serglycin co-localized with GROα, suggesting that serglycin may be present in such type 2 granules. These granules are released from endothelial cells upon brief incubation with PMA or histamine, suggesting that they are involved in the rapid secretory response following cellular activation (e.g. by proinflammatory agonists). We were previously unable to identify adaptors, such as Rab proteins associated with the type 2 granules, and they are so far only partly characterized (30). However, this study provides further insight into the composition of this type of granules by suggesting that they also contain serglycin. Importantly, serglycin is thereby the first non-chemokine molecule identified in type 2 granules. Interestingly, co-localization of serglycin with GROα was only observed after IL-1β stimulation, whereas serglycin-positive vesicles were observed in both unstimulated and stimulated cells. Serglycin PGs may promote the storage of chemokines within vesicles and aid in their secretion to the apical side of the endothelium. Notably, serglycin co-localized with GROα in some but not all GROα-containing vesicles, suggesting that serglycin is not essential for the presence of GROα in vesicles. Based on these findings, serglycin may have functions other than those related to sorting of GROα into these vesicles.
Treatment with xylosides reduced the amount of serglycin as well as GROα in the apical medium, compatible with a role for serglycin in the regulation of GROα secretion to the apical side. The function of serglycin in this process is intriguing. It has previously been shown that serglycin is involved in the maturation of storage granules in mast cells (44), and it therefore appears likely that serglycin may have a similar role in endothelial cells. However, in polymorphonuclear leukocytes, which contain several types of storage granules, serglycin is only present during the initial formation of azurophilic granules in myeloblasts and promyelocytes and is not found in granules of more mature cells. Accordingly, serglycin may have diverse and cell type-specific functions related to secretory vesicle formation and granule storage, and it is also possible that the exact role of serglycin may vary between different types of vesicles. In endothelial cells, serglycin may thus affect GROα functions in several ways (e.g. by promoting its storage in the secretory granules, by protecting it from degradation, or by presenting it at the correct site of delivery).
Together, the data presented here may have a functional impact on our understanding of GROα biology. Previous studies have shown that GROα can support monocyte arrest to the endothelium in models of inflammation and may thereby be relevant for the development of atherosclerosis (45). Moreover, enhanced plasma levels of GROα in patients with coronary artery disease have been demonstrated (46). Although these measurements were based on GROα expression in peripheral monocytes, it is tempting to speculate that activated endothelial cells could contribute significantly to the total expression and secretion of GROα. Accordingly, based on the data presented here, we may propose that serglycin can regulate the impact of GROα during such pathological conditions. Thus, serglycin may be involved in inflammatory conditions and development of diseases, like coronary artery disease, by its influence on GROα levels at the apical site of the endothelium.
In summary, the data presented in this study expand the current knowledge of serglycin biology by showing that serglycin is a major PG in polarized human endothelial cells and is secreted to the apical side, and, finally, our data implicate serglycin in the regulation of chemokine secretion.
Acknowledgments
We thank the Molecular Fluorescence Imaging Center (Oslo University Hospital-Rikshospitalet) and the Norwegian Molecular Imaging Consortium (Department of Molecular Biosciences, University of Oslo) for providing equipment for confocal microscopy.
This work was supported by grants from the Throne Holst Foundation, Helse Sør-Øst, the Norwegian Cancer Society and the Swedish Research Council.
- PG
- proteoglycan
- tPA
- tissue-type plasminogen activator
- HUVEC
- human umbilical vein endothelial cell(s)
- CS
- chondroitin sulfate
- DS
- dermatan sulfate
- cABC
- chondroitin ABC lyase
- HS
- heparan sulfate
- vWF
- von Willebrand factor
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1. Esko J. D., Selleck S. B. (2002) Annu. Rev. Biochem. 71, 435–471 [DOI] [PubMed] [Google Scholar]
- 2. Iozzo R. V., San Antonio J. D. (2001) J. Clin. Invest. 108, 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kolset S. O., Tveit H. (2008) Cell Mol. Life Sci. 65, 1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pejler G., Abrink M., Wernersson S. (2009) BioFactors 35, 61–68 [DOI] [PubMed] [Google Scholar]
- 5. Kolset S. O., Prydz K., Pejler G. (2004) Biochem. J. 379, 217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bourdon M. A., Oldberg A., Pierschbacher M., Ruoslahti E. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 1321–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kolset S. O., Gallagher J. T. (1990) Biochim. Biophys. Acta 1032, 191–211 [DOI] [PubMed] [Google Scholar]
- 8. Kulseth M. A., Kolset S. O., Ranheim T. (1999) Biochim. Biophys. Acta 1428, 225–232 [DOI] [PubMed] [Google Scholar]
- 9. Schick B. P., Gradowski J. F., San Antonio J. D. (2001) Blood 97, 449–458 [DOI] [PubMed] [Google Scholar]
- 10. Lemire J. M., Chan C. K., Bressler S., Miller J., LeBaron R. G., Wight T. N. (2007) J. Cell. Biochem. 101, 753–766 [DOI] [PubMed] [Google Scholar]
- 11. Øynebråten I., Bakke O., Brandtzaeg P., Johansen F. E., Haraldsen G. (2004) Blood 104, 314–320 [DOI] [PubMed] [Google Scholar]
- 12. Abrink M., Grujic M., Pejler G. (2004) J. Biol. Chem. 279, 40897–40905 [DOI] [PubMed] [Google Scholar]
- 13. Braga T., Grujic M., Lukinius A., Hellman L., Abrink M., Pejler G. (2007) Biochem. J. 403, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ringvall M., Rönnberg E., Wernersson S., Duelli A., Henningsson F., Abrink M., García-Faroldi G., Fajardo I., Pejler G. (2008) J. Allergy Clin. Immunol. 121, 1020–1026 [DOI] [PubMed] [Google Scholar]
- 15. Niemann C. U., Abrink M., Pejler G., Fischer R. L., Christensen E. I., Knight S. D., Borregaard N. (2007) Blood 109, 4478–4486 [DOI] [PubMed] [Google Scholar]
- 16. Grujic M., Christensen J. P., Sørensen M. R., Abrink M., Pejler G., Thomsen A. R. (2008) J. Immunol. 181, 1043–1051 [DOI] [PubMed] [Google Scholar]
- 17. Zernichow L., Abrink M., Hallgren J., Grujic M., Pejler G., Kolset S. O. (2006) J. Biol. Chem. 281, 26792–26801 [DOI] [PubMed] [Google Scholar]
- 18. Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. (1973) J. Clin. Invest. 52, 2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shively J. E., Conrad H. E. (1976) Biochemistry 15, 3932–3942 [DOI] [PubMed] [Google Scholar]
- 20. Oynebråten I., Hansen B., Smedsrød B., Uhlin-Hansen L. (2000) J. Leukoc. Biol. 67, 183–188 [DOI] [PubMed] [Google Scholar]
- 21. Stevens R. L., Otsu K., Austen K. F. (1985) J. Biol. Chem. 260, 14194–14200 [PubMed] [Google Scholar]
- 22. Kolset S. O., Sakurai K., Ivhed I., Overvatn A., Suzuki S. (1990) Biochem. J. 265, 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lugemwa F. N., Esko J. D. (1991) J. Biol. Chem. 266, 6674–6677 [PubMed] [Google Scholar]
- 24. Suzuki D., Yagame M., Kim Y., Sakai H., Mauer M. (2002) Nephron 92, 564–572 [DOI] [PubMed] [Google Scholar]
- 25. Kolset S. O., Zernichow L. (2008) Glycoconj. J. 25, 305–311 [DOI] [PubMed] [Google Scholar]
- 26. Kolset S. O., Vuong T. T., Prydz K. (1999) J. Cell Sci. 112, 1797–1801 [DOI] [PubMed] [Google Scholar]
- 27. Prydz K., Vuong T. T., Kolset S. O. (2009) Glycoconj. J. 26, 1117–1124 [DOI] [PubMed] [Google Scholar]
- 28. Falkowska-Hansen B., Oynebråten I., Uhlin-Hansen L., Smedsrød B. (2006) Mol. Cell Biochem. 287, 43–52 [DOI] [PubMed] [Google Scholar]
- 29. Toyama-Sorimachi N., Kitamura F., Habuchi H., Tobita Y., Kimata K., Miyasaka M. (1997) J. Biol. Chem. 272, 26714–26719 [DOI] [PubMed] [Google Scholar]
- 30. Øynebråten I., Barois N., Hagelsteen K., Johansen F. E., Bakke O., Haraldsen G. (2005) J. Immunol. 175, 5358–5369 [DOI] [PubMed] [Google Scholar]
- 31. Kolset S. O., Mann D. M., Uhlin-Hansen L., Winberg J. O., Ruoslahti E. (1996) J. Leukoc. Biol. 59, 545–554 [DOI] [PubMed] [Google Scholar]
- 32. Graham G. J., Wilkinson P. C., Nibbs R. J., Lowe S., Kolset S. O., Parker A., Freshney M. G., Tsang M. L., Pragnell I. B. (1996) EMBO J. 15, 6506–6515 [PMC free article] [PubMed] [Google Scholar]
- 33. Whitelock J. M., Murdoch A. D., Iozzo R. V., Underwood P. A. (1996) J. Biol. Chem. 271, 10079–10086 [DOI] [PubMed] [Google Scholar]
- 34. Raats C. J., Luca M. E., Bakker M. A., Van Der Wal A., Heeringa P., Van Goor H., Van Den Born J., De Heer E., Berden J. H. (1999) J. Am. Soc. Nephrol. 10, 1689–1699 [DOI] [PubMed] [Google Scholar]
- 35. Han S. Y., Jee Y. H., Han K. H., Kang Y. S., Kim H. K., Han J. Y., Kim Y. S., Cha D. R. (2006) Nephrol. Dial. Transplant. 21, 2406–2416 [DOI] [PubMed] [Google Scholar]
- 36. Cattaruzza S., Schiappacassi M., Ljungberg-Rose A., Spessotto P., Perissinotto D., Mörgelin M., Mucignat M. T., Colombatti A., Perris R. (2002) J. Biol. Chem. 277, 47626–47635 [DOI] [PubMed] [Google Scholar]
- 37. Rehm M., Bruegger D., Christ F., Conzen P., Thiel M., Jacob M., Chappell D., Stoeckelhuber M., Welsch U., Reichart B., Peter K., Becker B. F. (2007) Circulation 116, 1896–1906 [DOI] [PubMed] [Google Scholar]
- 38. Svennevig K., Hoel T., Thiara A., Kolset S., Castelheim A., Mollnes T., Brosstad F., Fosse E., Svennevig J. L. (2008) Perfusion 23, 165–171 [DOI] [PubMed] [Google Scholar]
- 39. Chappell D., Jacob M., Paul O., Rehm M., Welsch U., Stoeckelhuber M., Conzen P., Becker B. F. (2009) Circ. Res. 104, 1313–1317 [DOI] [PubMed] [Google Scholar]
- 40. Niemann C. U., Kjeldsen L., Ralfkiaer E., Jensen M. K., Borregaard N. (2007) Leukemia 21, 2406–2410 [DOI] [PubMed] [Google Scholar]
- 41. Theocharis A. D., Seidel C., Borset M., Dobra K., Baykov V., Labropoulou V., Kanakis I., Dalas E., Karamanos N. K., Sundan A., Hjerpe A. (2006) J. Biol. Chem. 281, 35116–35128 [DOI] [PubMed] [Google Scholar]
- 42. Yasuo T., Kitaya K., Yamaguchi T., Fushiki S., Honjo H. (2008) J. Reprod. Immunol. 78, 1–10 [DOI] [PubMed] [Google Scholar]
- 43. Valiyaveettil M., Achur R. N., Muthusamy A., Gowda D. C. (2004) Mol. Biochem. Parasitol. 134, 115–126 [DOI] [PubMed] [Google Scholar]
- 44. Braga T., Ringvall M., Tveit H., Abrink M., Pejler G. (2009) Mol. Immunol. 46, 422–428 [DOI] [PubMed] [Google Scholar]
- 45. Liu D., Razzaque M. S., Cheng M., Taguchi T. (2001) Histochem. J. 33, 621–628 [DOI] [PubMed] [Google Scholar]
- 46. Breland U. M., Halvorsen B., Hol J., Øie E., Paulsson-Berne G., Yndestad A., Smith C., Otterdal K., Hedin U., Waehre T., Sandberg W. J., Frøland S. S., Haraldsen G., Gullestad L., Damås J. K., Hansson G. K., Aukrust P. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1005–1011 [DOI] [PubMed] [Google Scholar]