Abstract
Salt-inducible kinase (SIK), one of the AMP-activated kinase (AMPK)-related kinases, has been suggested to play important functions in glucose homeostasis by inhibiting the cAMP-response element-binding protein (CREB)-regulated transcription coactivator (CRTC). To examine the role of SIK in vivo, we generated Drosophila SIK mutant and found that the mutant flies have higher amounts of lipid and glycogen stores and are resistant to starvation. Interestingly, SIK transcripts are highly enriched in the brain, and we found that neuron-specific expression of exogenous SIK fully rescued lipid and glycogen storage phenotypes as well as starvation resistance of the mutant. Using genetic and biochemical analyses, we demonstrated that CRTC Ser-157 phosphorylation by SIK is critical for inhibiting CRTC activity in vivo. Furthermore, double mutants of SIK and CRTC became sensitive to starvation, and the Ser-157 phosphomimetic mutation of CRTC reduced lipid and glycogen levels in the SIK mutant, suggesting that CRTC mediates the effects of SIK signaling. Collectively, our results strongly support the importance of the SIK-CRTC signaling axis that functions in the brain to maintain energy homeostasis in Drosophila.
Keywords: AMP-activated Kinase (AMPK), CREB, Drosophila, Metabolism, Serine Threonine Protein Kinase, Signal Transduction, AMPK-related Kinases, CRTC, SIK, TORC
Introduction
Salt-inducible kinase (SIK)2 was first cloned from adrenal glands of rats fed with a high salt diet (1), and its transcription was strongly induced by membrane depolarization in the brain (2). Recent studies unexpectedly demonstrated that SIK1 also plays important functions in liver glucose homeostasis (3) and survival of myocytes (4). Furthermore, SIK1 is involved in TGF-β signaling (5) and histone deacetylase regulation (6), supporting highly diverse functions in mammalian systems.
Through genomic database analyses, SIK2 (Qin-induced kinase (QIK)) and SIK3 (QSK) were identified as SIK family members based on their structural homologies to SIK1 (7). Interestingly, SIK family kinases belong to AMP-activated protein kinase (AMPK)-related kinases, which are activated by LKB1 (8, 9). Based on this conserved regulation of SIK and other AMPK-related kinases, SIK is believed to have highly specialized downstream targets to differentially function in the cell. However, due to the lack of loss-of-function animal models, the in vivo functions of SIK remain elusive, in contrast to the well characterized functions of AMPK.
The members of CREB-regulated transcription coactivator (CRTC), also known as TORC (transducer of regulated CREB), are newly identified CREB co-activators (10, 11). Under the basal condition, phosphorylated CRTC is sequestered in the cytoplasm. However, in response to increased levels of calcium and cAMP, CRTC is dephosphorylated and subsequently translocated to the nucleus, which leads to the activation of cAMP-response element (CRE)-mediated gene transcription by association with CREB (12, 13). Recent studies demonstrated that one of the family members, CRTC2, has a crucial function in the gluconeogenic program in the liver (3, 14). SIK and AMPK repress CRE transcriptional responses by phosphorylating CRTC2 at Ser-171 and keep the transcription factor in the cytoplasm (3, 13). In the case of Drosophila, increases in insulin signaling inhibit CRTC through phosphorylation under the fed condition (15). Also, activated Drosophila CRTC can stimulate CREB target genes (15), supporting evolutionarily highly conserved functions of CRTC in metazoans.
In this study, we describe the generation and characterization of SIK-deficient flies and demonstrate that SIK activity in the brain is required for the regulation of lipid and glycogen level in the body. We also show that SIK mutation increases starvation resistance. Finally, we demonstrate that CRTC and CREB dominantly mediate these in vivo functions of SIK.
EXPERIMENTAL PROCEDURES
Fly Stocks
The following fly stocks were obtained from the Bloomington Stock Center: hs-Gal4, gmr-Gal4, elav-Gal4, and cg-Gal4. CRTC25-3 was kindly provided by Dr. M. Montminy (15). UAS-CREBRNAi (line 101512) and UAS-AMPKαRNAi (line 1827) were obtained from the Vienna Drosophila RNAi Center. SIKΔ41 was generated by the imprecise excision of the SIKG366 line (Korea Advanced Institute of Science and Technology (KAIST) Drosophila Library Facility, Daejeon, Korea). All flies were grown on standard cornmeal-yeast-agar medium at 25 °C.
Generation of Transgenic Fly Strains
To generate UAS-SIK flies, SIK EST cDNA (Berkeley Drosophila Genome Project accession number RH42017) was cloned into the Myc-tagged pUAST vector and microinjected into W1118 embryos. Full-length CRTC cDNA was generated by reverse transcription-PCR (RT-PCR). The PCR-cloned CRTC was then subcloned into the NotI-XhoI site of the FLAG-tagged pUAST vector.
Site-directed Mutagenesis
For site-directed mutagenesis, the QuikChangeTM kit (Stratagene) was used. For generation of a kinase-dead mutant SIK (K170M, SIKKM), 5′-GAACGAGGTGGCTATCATGATCATTGACAAGTCGC-3′ and 5′-GCGACTTGTCAATGATCATGATAGCCACCTCGTTC-3′ primers were used. For generation of a CRTC mutant non-phosphorylatable by SIK (S157A, CRTCS157A), 5′-GCGGCGGTCCAGCGCCGATTCGGCGC-3′ and 5′-GCGCCGAATCGGCGCTGGACCGCCGC-3′ primers were used. For generation of a CRTC mutant mimicking SIK-dependent phosphorylation (S157D, CRTCS157D), 5′-GTGGCGGCGGTCCAGCGACGATTCGGCGC-3′ and 5′-GCGCCGAATCGTCGCTGGACCGCCGCCAC-3′ primers were used.
Quantitative Real-time PCR
Dissected tissues of larvae and adults were collected, and RNA was extracted using an RNeasy mini kit (Qiagen). Total RNA (1 mg) was reverse-transcribed by M-MLV reverse transcriptase (Promega), and the generated cDNA was used for real-time RT-PCR (Bio-Rad iQ5 real-time PCR detection system) using 2 ng of cDNA template and a 500 nm primer concentration. The following primers were used to amplify the SIK transcript: 5′-CTCGCGTCTTGTCCGACCCAATG-3′ and 5′-GTATGCCAGCCAAGGAGAGATCTTCG-3′ (kinase domain); 5′-TCGGAGAAGAAAGTTCT-3′ and 5′-GCCACTGGACGAGCTACTACT-3′ (C-terminal domain). Values were normalized to rp49. Results are expressed as arbitrary units, with the each value of salivary gland and abdomen considered as 1 unit.
Staining with Nile Red
The abdominal regions of female flies up to 5 days old were manually opened, and floating fat body cells were released into mounting medium (50% glycerol/PBS, 0.1% Triton X-100, Nile Red 1:55,000 (Sigma N3013)). Cells were analyzed within 4 h following mounting using a Zeiss LSM 510.
Immunoblot Analysis
Fly heads were lysed in lysis buffer (20 mm Tris-HCl (pH 7.5), 1% Triton X-100, 1 mm EDTA, 5 mm EGTA, 150 mm NaCl, 20 mm NaF, 1 μg/ml leupeptin, and 1 mm PMSF) for 30–60 min on ice. After centrifugation at 13,000 rpm for 15 min, the supernatant was reserved for protein determination and SDS-PAGE analysis. The antibodies used were anti-phospho-CRTC1 (Cell Signaling Technology, 3359), anti-FLAG-M2 (Cell Signaling Technology, 2368), anti-Myc (Cell Signaling Technology, 2272), and anti-β-tubulin (Developmental Studies Hybridoma Bank, E7).
Starvation Assay
For each genotype, 3–5-day-old female flies were transferred to vials of 1% agar/PBS with filter papers soaked with distilled water, and dead flies were scored every 4 h.
Lipid and Glycogen Measurement
Total lipid and glycogen of adult females were measured using previously described methods (15–18).
Capillary Feeder Assay
We used previously reported experimental procedures with minor modifications (19).
Quantification Analysis
Student's t tests were used for comparisons between two groups. p < 0.05 was considered statistically significant. The p values given in the survival data are the result of a log rank test using GraphPad Prism 5 software.
RESULTS
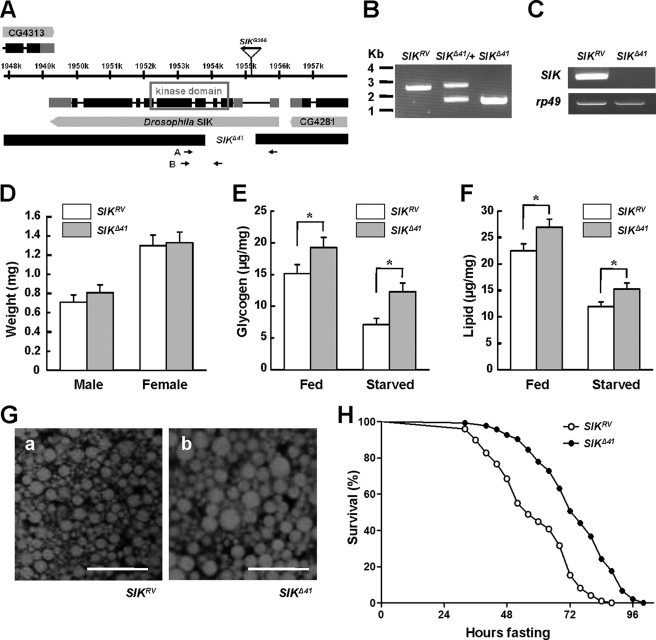
Drosophila SIK shares considerable sequence homology with mammalian SIK1 and SIK2 in its kinase domain (supplemental Fig. 1). To assess the in vivo role of SIK, SIK loss-of-function mutants were generated by mobilizing the P element from SIKG366. We found one allele, SIKΔ41, with a 1,148-bp (X1953962–1955112) deletion in the SIK encoding region including the translation start site and the ATP-binding site of the kinase domain (Fig. 1, A and B). By precisely excising the P element, we obtained a revertant allele, SIKRV, and used it as a genetically matched control for phenotypic analyses. SIK mRNA was not detected in SIKΔ41 (Fig. 1C and supplemental Fig. 2), confirming that it is a null mutant.
FIGURE 1.
SIK mutants are resistant to starvation. A, genomic region of SIK locus. Exons of SIK are indicated by boxes, and coding regions are colored black. The deleted regions for SIK-null mutants (SIKΔ41) are also presented. The gene expression of CG4313 and CG4281 is not affected in SIKΔ41 deletion (data not shown). B, PCR revealed deletion of genomic DNA in SIK revertants (SIKRV), heterozygous SIK mutants (SIKΔ41/+), and SIKΔ41 using the A primer set in A. C, RT-PCR analysis of SIK mRNA in SIKRV and SIKΔ41 using the B primer set in A. rp49 was used as a loading control. D, average weight of SIKRV and SIKΔ41 adult flies (mean ± S.D., n = 60). E, total glycogen contents, expressed as μg/mg of body weight, in fed or 24-h starved SIKRV and SIKΔ41 flies (mean ± S.D., n = 10; *, p < 0.05, Student's t test). F, total lipid contents in fed and 24-h starved SIKRV and SIKΔ41 flies (mean ± S.D., n = 10; *, p < 0.05, Student's t test). G, Nile Red staining of fat bodies from SIKRV (panel a) and SIKΔ41 (panel b) flies. Scale bar, 50 μm. H, relative survival rate in response to starvation of SIKRV and SIKΔ41 female flies. The percentage of survival at different times is shown. n = 60; p < 0.05 (log rank test). Experiments were performed in triplicate.
To examine the role of SIK in energy homeostasis, we characterized SIKΔ41 under feeding and fasting conditions. We found that 1-week-old SIKΔ41 mutant flies had higher amounts of lipid and stored glycogen when compared with those in SIKRV controls, but the difference became more apparent after 24 h of starvation (Fig. 1, E and F). Furthermore, Nile Red staining of dissected fat body tissues displayed larger lipid droplets in SIKΔ41 mutants than those of SIKRV controls (Fig. 1G). In contrast to increased lipid and glycogen storage, SIKΔ41 flies showed unnoticeable difference in body weight (Fig. 1D) and similar food intake when compared with SIKRV controls according to the capillary feeder method (supplemental Fig. 3).
Previous studies in Drosophila have shown that starvation resistance correlates considerably with lipid contents (20). Therefore, we wondered whether the higher lipid and glycogen contents of SIKΔ41 mutant flies provide a better chance of survival during starvation. Under starvation conditions, the median and maximum survival times increased by 26 and 20%, respectively, in SIKΔ41 flies when compared with SIKRV controls (Fig. 1H), supporting the idea that loss of SIK dramatically increased starvation resistance.
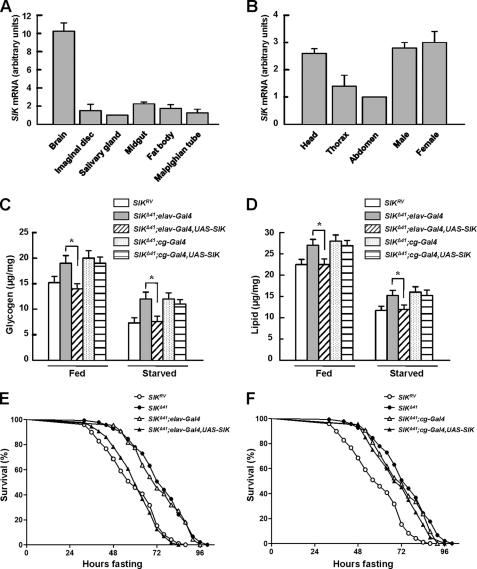
To identify the tissue distribution of SIK, we assessed the level of SIK transcripts in the third instar larvae. Interestingly, SIK expression levels were much higher in the brain than any other tissues (Fig. 2A). We also measured the expression pattern of SIK in adult body segments and also between adult male and female. Consistent with larval gene expression, SIK was highly expressed in the head and did not show sex-specific expression patterns (Fig. 2B). Collectively, these data suggest a role of Drosophila SIK in the nervous system.
FIGURE 2.
Neuronal SIK expression rescues starvation responses. A and B, the expression level of SIK was measured by quantitative real-time PCR analysis in larval (A) and adult tissues (B). Results are representative of three independent experiments and are expressed as arbitrary units (mean ± S.D.). C, total glycogen contents in fed and 24-h starved SIKRV and SIKΔ41 flies, with and without driving expression of SIK constructs using elav-Gal4 or cg-Gal4 (mean ± S.D., n = 10; *, p < 0.05, Student's t test). D, total lipid contents in fed and 24-h starved SIKRV and SIKΔ41 flies, with and without driving expression of SIK constructs using elav-Gal4 or cg-Gal4 (mean ± S.D., n = 10; *, p < 0.05, Student's t test). E, relative survival rate in response to starvation of SIKRV, SIKΔ41, SIKΔ41;elav-Gal4 control, and SIKΔ41;elav-Gal4,UAS-SIK female flies. n = 60; p < 0.05 (log rank test). F, relative survival rate in response to starvation of SIKRV, SIKΔ41, SIKΔ41;cg-Gal4 control, and SIKΔ41;cg-Gal4,UAS-SIK female flies. n = 60.
To further study the gene expression patterns of SIK, we examined which body part of SIK expression can mediate metabolic regulation. We used elav-Gal4 and cg-Gal4 to drive pan-neuronal and fat body-specific expression of UAS-SIK, respectively. As shown in supplemental Fig. 4, A and B, exogenous SIK protein was successfully expressed in a tissue-specific manner.
Intriguingly, neuronal overexpression of SIK in a SIKΔ41 background reduced glycogen and lipid contents to a level similar to those of SIKRV control fly in both fed and starved conditions (Fig. 2, C and D, respectively). However, fat body expression of Drosophila SIK did not alter either of the metabolic contents (Fig. 2, C and D), suggesting that there could be specific roles of SIK in neurons.
To determine whether SIK expression is required in the nervous system or in the fat body regarding mediation of starvation resistance in SIK mutants, we performed genetic rescue experiments. A dramatic decrease in starvation resistance to the level of SIKRV controls was observed in SIK-null mutants after expressing exogenous SIK under the control of elav-Gal4, a neuronal driver, but not under the control of cg-Gal4, a fat body driver (Fig. 2, E and F, respectively).
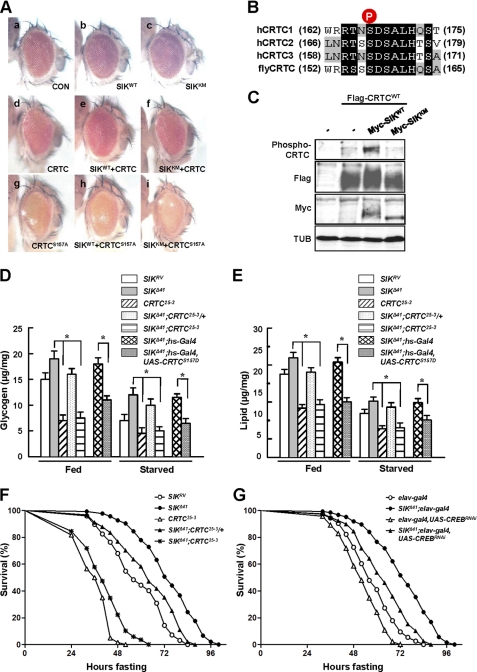
Because mammalian SIK regulates target gene expression by directly phosphorylating CRTC and consequently inhibiting CRTC nuclear translocation (3, 13), we performed ommatidial assays to examine functional interactions and genetic epistasis between Drosophila SIK and CRTC. Previous studies have successfully used ommatidial assays to discover the relationship between CRTC and CREB in Drosophila (15). Although expression of the SIK wild type or kinase-dead form under the control of gmr-Gal4, an eye-specific driver, in wild type genetic background did not show any apparent phenotypes (Fig. 3A, panels b and c, respectively), overexpression of CRTC using the gmr-Gal4 driver in wild type background resulted in a rough eye phenotype with ommatidial loss (Fig. 3A, panel d). Interestingly, co-overexpression of SIK with CRTC caused partial rescue of the CRTC phenotype, restoring ommatidial structure and eye size (Fig. 3A, panel e), whereas the kinase-dead form of SIK did not (Fig. 3A, panel f), indicating that the kinase activity-dependent interaction between SIK and CRTC is conserved in Drosophila.
FIGURE 3.
CRTC and CREB mediate the starvation responses of SIK. A, panels a–I, effects of indicated genotype expression in Drosophila eye. Genotypes are: gmr-Gal4/+ (panel a), gmr-Gal4/UAS-SIKWT (panel b), gmr-Gal4/UAS-SIKKM (panel c), gmr-Gal4/UAS-CRTCWT (panel d), gmr-Gal4,UAS-CRTCWT/UAS-SIKWT (panel e), gmr-Gal4,UAS-CRTCWT/UAS-SIKKM (panel f), gmr-Gal4,UAS-CRTCS157A/+ (panel g), gmr-Gal4,UAS-CRTCS157A/UAS-SIKWT (panel h), and gmr-Gal4,UAS-CRTCS157A/UAS-SIKKM (panel i). CON, control. B, amino acid alignment of human CRTC1–3 and Drosophila CRTC at the SIK phosphorylation site (circled P). C, immunoblot analysis showing the effect of wild type and kinase-dead SIK on Ser-157 phosphorylation of CRTC protein in adult flies. TUB, tubulin. D, total glycogen contents in fed and 24-h starved SIKRV, SIKΔ41, CRTC25-3, SIKΔ41;CRTC25-3/+, SIKΔ41;CRTC25-3, SIKΔ41;hs-Gal4, and SIKΔ41;hs-Gal4,UAS-CRTCS157D flies (mean ± S.D., n = 10; *, p < 0.05, Student's t test). E, total lipid contents in fed and 24-h starved SIKRV, SIKΔ41, CRTC25-3, SIKΔ41;CRTC25-3/+, SIKΔ41;CRTC25-3, SIKΔ41;hs-Gal4, and SIKΔ41;hs-Gal4,UAS-CRTCS157D flies (mean ± S.D., n = 10; *, p < 0.05, Student's t test). F, relative survival rates in response to starvation of SIKRV, SIKΔ41, CRTC25-3, SIKΔ41;CRTC25-3/+, and SIKΔ41;CRTC25-3 female flies. n = 60; p < 0.05 (log rank test). G, relative survival rate in response to starvation of elav-Gal4, SIKΔ41;elav-Gal4, elav-Gal4,UAS-CREBRNAi, and SIKΔ41;elav-Gal4,UAS-CREBRNAi female flies. n = 30; p < 0.05 (log rank test).
To confirm these genetic epistasis experiments further, we expressed the constitutive active CRTC S157A mutation, the counterpart of mammalian CRTC2 S171A (Fig. 3B), in the developing eye. Overexpression of CRTC S157A caused a more severe eye phenotype with loss of ommatidia and mechanosensory bristles (Fig. 3A, panel g) than the phenotypes found in wild type CRTC expression (Fig. 3A, panel d). Expectedly, co-overexpression of CRTC S157A with SIK failed to display any rescue of the defective ommatidia or mechanosensory bristles (Fig. 3A, panel h), indicating that this CRTC mutant does not respond to SIK signaling. To further confirm the interaction between SIK and CRTC, we examined the phosphorylation status of CRTC using fly head extracts from the same lines used in Fig. 3A, panels d–f. Expression of SIK increased Ser-157 phosphorylation of CRTC in a kinase activity-dependent manner (Fig. 3C), demonstrating that SIK induces CRTC phosphorylation at the conserved Ser-157.
We also generated SIK and CRTC double mutants (SIKΔ41; CRTC25-3) to genetically confirm their functional interactions. Although removal of one copy of the CRTC gene decreased lipid and glycogen levels in fed and starved SIKΔ41 mutant, removal of both copies of CRTC in the SIK mutant background showed further decreased lipid and glycogen contents similar to CRTC-null mutant (Fig. 3, D and E).
To test whether Ser-157 residue of CRTC is critical in regulating lipid and glycogen content by SIK, we expressed CRTC S157D, which mimics the phosphorylated CRTC by SIK. Remarkably, CRTC S157D reduced lipid and glycogen levels in fed and starved SIKΔ41 mutant flies (Fig. 3, D and E).
Consistent with these lipid and glycogen phenotypes, as CRTC level decreased in the SIK mutant background, the flies became sensitive to starvation (Fig. 3F). Taken together, these data indicate that CRTC mediates the metabolic and starvation responses of SIK signaling.
CREB has been characterized to mediate SIK-CRTC signaling in mammals (21), and CREB activity is decreased in Drosophila CRTC mutant (15). We conducted genetic interaction experiments between SIK and CREB by knocking down neuronal CREB expression in the SIK-null background. As CREB level decreased in a SIK mutant background, the increased lipid stores and starvation resistance of the SIK mutant were strongly inhibited (Fig. 3G and supplemental Fig. 5, B and C). These results provide strong evidence to support that CREB is the critical target of SIK-CRTC signaling in Drosophila.
Finally, to figure out which hormonal or humoral factors regulate fat and glycogen contents, and supposedly Drosophila energy homeostasis in SIK signaling (supplemental Fig. 8), we conducted genome-wide microarray analyses on head mRNAs from fed and starved SIKΔ41 mutant flies relative to those of SIKRV controls. This study revealed that 181 transcripts (fed condition) and 144 transcripts (starved condition) were significantly up-regulated in the mutant (≥2.0-fold) (supplemental Tables 1 and 2). Some of the affected genes are involved in stress responses and lipid metabolism. Many of the genes contain full CRE (TGACGTCA) and/or half-CRE (TGACG/CGTCA) consensus-binding site(s) within 3 kb upstream of the translational start site (supplemental Tables 1 and 2). These results again strongly support that CRTC-CREB-dependent gene expression is important to mediate SIK signaling in Drosophila.
DISCUSSION
SIK, one of the AMPK-related kinases, is involved in controlling fasting metabolism (3, 22). There is a single Drosophila orthologue for mammalian SIK1 and SIK2 (supplemental Fig. 1). Using the Drosophila model system, we discovered that neuronal SIK regulates metabolic indices, such as body contents of glycogen and lipid, by inhibiting CRTC transcriptional coactivator.
Mammalian SIK represses transcriptional responses mediated by CREB through phosphorylation and cytoplasmic retention of CRTCs (3, 13). Our results consistently demonstrated that CRTC Ser-157 residue, the counterpart of mammalian CRTC2 Ser-171 residue, is structurally and functionally conserved for SIK-dependent phosphorylation (Fig. 3B). Because SIK expression did not influence CRTC S157A-induced phenotypes and SIK mutants became more sensitive to starvation by reducing gene dosages of CRTC (Fig. 3), we concluded that CRTC mediates starvation responses of SIK signaling in Drosophila. These studies also support that the functional interaction between SIK and CRTC is highly conserved throughout the evolution.
In starved condition, SIK and CRTC double mutants showed a higher starvation resistance than CRTC mutants (Fig. 3F). Based on our microarray data and previously published data, the regulated genes of SIK and CRTC are not completely overlapped (supplemental Table 2) (15). The results suggest that the unknown function of SIK can mediate the difference between SIK/CRTC double mutants and CRTC mutants.
CRTC-CREB functional interaction is also highly conserved in Drosophila (15). As decreased neuronal CREB level strongly suppressed increased lipid stores and starvation resistance of SIK-null mutant (Fig. 3F and supplemental Fig. 5, B and C), we suggest CREB as a main target for SIK signaling in Drosophila. Consistently, recent studies have shown that neuronal CREB activity regulates energy stores and starvation responses (23). Combining these results, neuronal CREB should be the key target of SIK-CRTC signaling in Drosophila (supplemental Fig. 8).
Because AMPK has been suggested as an upstream kinase of CRTC from mammalian studies (3, 24), we conducted experiments to examine whether neuronal AMPK is also related to the phenotypes of the SIK mutant. Interestingly, our experiments showed that neuron-specific knockdown of AMPK induced no significant effect on lipid and glycogen storage (supplemental Fig. 6, B and C). In addition, AMPK RNAi flies were not resistant to starvation (supplemental Fig. 6D), which is consistent with previous reports by others (25, 26). These results suggest that SIK but not AMPK is the dominant upstream regulator of CRTC-CREB signaling in Drosophila brain.
Metabolic storage is modulated by a variety of tissue-specific processes (27). Surprisingly, we found that the main gene expression pattern of SIK appears in the brain rather than in other metabolic tissues including the fat body, the functional equivalent of the mammalian liver and adipose tissue in Drosophila (Fig. 2A). Furthermore, SIK expression in neuronal tissue almost fully rescued increased storage of lipid and glycogen phenotypes of SIK-null mutants (Fig. 2, C and D). These results strongly support that neuronal SIK controls glycogen and lipid storage in some remote tissues such as the fat body (supplemental Fig. 8).
Based on our current data, it is important to figure out the communication method between brain and fat/glycogen under SIK regulation (supplemental Fig. 8). Drosophila brain has neurosecretory cells that secret Drosophila insulin-like peptides (Dilps) and adipokinetic hormone (AKH, the insect glucagon) into the hemolymph (the insect blood) (28, 29). Recent studies showed that flies with mutations in the insulin signaling pathway have a moderate increase in lipid stores and are resistant to starvation (18, 29). In addition, AKH receptor mutant flies are impaired in fat storage and become resistant to starvation (30). Therefore, we have conducted quantitative PCR experiments to measure Dilps and AKH levels from SIK mutant head. Interestingly, we could not detect significant changes in Dilps and AKH expression (supplemental Fig. 7 and data not shown). Therefore, we suggest that neuronal SIK expression can change metabolic indices by regulating other hormones or humoral factors in Drosophila (supplemental Fig. 8).
In summary, we have demonstrated here that SIK is important in energy metabolism and starvation responses in vivo. Thereby SIK may be a potential therapeutic target for treating obesity and other metabolic diseases.
Supplementary Material
Acknowledgments
We thank Ayoung Kwak and Taeyoon Kyung for critical reading of the manuscript. We are grateful to M. Montminy for kindly providing reagents. We also thank the Bloomington Stock Center and Drosophila Genomics Research Center for kindly providing materials.
This work was supported by National Creative Research Initiatives Grant 2010-0018291 from the Korean Ministry of Education, Science and Technology.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–8, Tables 1–2, and Experimental Procedures.
- SIK
- salt-inducible kinase
- CRE
- cAMP-response element
- CREB
- cAMP-response element-binding protein
- CRTC
- CREB-regulated transcription coactivator
- AMPK
- AMP-activated protein kinase
- Dilp
- Drosophila insulin-like peptide
- AKH
- adipokinetic hormone.
REFERENCES
- 1. Wang Z., Takemori H., Halder S. K., Nonaka Y., Okamoto M. (1999) FEBS Lett. 453, 135–139 [DOI] [PubMed] [Google Scholar]
- 2. Feldman J. D., Vician L., Crispino M., Hoe W., Baudry M., Herschman H. R. (2000) J. Neurochem. 74, 2227–2238 [DOI] [PubMed] [Google Scholar]
- 3. Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 4. Berdeaux R., Goebel N., Banaszynski L., Takemori H., Wandless T., Shelton G. D., Montminy M. (2007) Nat. Med. 13, 597–603 [DOI] [PubMed] [Google Scholar]
- 5. Kowanetz M., Lönn P., Vanlandewijck M., Kowanetz K., Heldin C. H., Moustakas A. (2008) J. Cell Biol. 182, 655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Linden A. M., Nolan K. M., Sengupta P. (2007) EMBO J. 26, 358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okamoto M., Takemori H., Katoh Y. (2004) Trends Endocrinol. Metab. 15, 21–26 [DOI] [PubMed] [Google Scholar]
- 8. Hardie D. G., Carling D. (1997) Eur. J. Biochem. 246, 259–273 [DOI] [PubMed] [Google Scholar]
- 9. Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conkright M. D., Canettieri G., Screaton R., Guzman E., Miraglia L., Hogenesch J. B., Montminy M. (2003) Mol. Cell 12, 413–423 [DOI] [PubMed] [Google Scholar]
- 11. Iourgenko V., Zhang W., Mickanin C., Daly I., Jiang C., Hexham J. M., Orth A. P., Miraglia L., Meltzer J., Garza D., Chirn G. W., McWhinnie E., Cohen D., Skelton J., Terry R., Yu Y., Bodian D., Buxton F. P., Zhu J., Song C., Labow M. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12147–12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bittinger M. A., McWhinnie E., Meltzer J., Iourgenko V., Latario B., Liu X., Chen C. H., Song C., Garza D., Labow M. (2004) Curr. Biol. 14, 2156–2161 [DOI] [PubMed] [Google Scholar]
- 13. Screaton R. A., Conkright M. D., Katoh Y., Best J. L., Canettieri G., Jeffries S., Guzman E., Niessen S., Yates J. R., 3rd, Takemori H., Okamoto M., Montminy M. (2004) Cell 119, 61–74 [DOI] [PubMed] [Google Scholar]
- 14. Dentin R., Hedrick S., Xie J., Yates J., 3rd, Montminy M. (2008) Science 319, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 15. Wang B., Goode J., Best J., Meltzer J., Schilman P. E., Chen J., Garza D., Thomas J. B., Montminy M. (2008) Cell Metab. 7, 434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Handel E. (1985) J. Am. Mosq. Control Assoc. 1, 302–304 [PubMed] [Google Scholar]
- 17. Van Handel E. (1985) J. Am. Mosq. Control Assoc. 1, 299–301 [PubMed] [Google Scholar]
- 18. Broughton S. J., Piper M. D., Ikeya T., Bass T. M., Jacobson J., Driege Y., Martinez P., Hafen E., Withers D. J., Leevers S. J., Partridge L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3105–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ja W. W., Carvalho G. B., Mak E. M., de la Rosa N. N., Fang A. Y., Liong J. C., Brummel T., Benzer S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 8253–8256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Djawdan M., Chippindale A. K., Rose M. R., Bradley T. J. (1998) Physiol. Zool. 71, 584–594 [DOI] [PubMed] [Google Scholar]
- 21. Katoh Y., Takemori H., Lin X. Z., Tamura M., Muraoka M., Satoh T., Tsuchiya Y., Min L., Doi J., Miyauchi A., Witters L. A., Nakamura H., Okamoto M. (2006) FEBS J. 273, 2730–2748 [DOI] [PubMed] [Google Scholar]
- 22. Alessi D. R., Sakamoto K., Bayascas J. R. (2006) Annu. Rev. Biochem. 75, 137–163 [DOI] [PubMed] [Google Scholar]
- 23. Iijima K., Zhao L., Shenton C., Iijima-Ando K. (2009) PLoS One 4, e8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dentin R., Liu Y., Koo S. H., Hedrick S., Vargas T., Heredia J., Yates J., 3rd, Montminy M. (2007) Nature 449, 366–369 [DOI] [PubMed] [Google Scholar]
- 25. Tohyama D., Yamaguchi A. (2010) Biochem. Biophys. Res. Commun. 394, 112–118 [DOI] [PubMed] [Google Scholar]
- 26. Johnson E. C., Kazgan N., Bretz C. A., Forsberg L. J., Hector C. E., Worthen R. J., Onyenwoke R., Brenman J. E. (2010) PLoS One 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leopold P., Perrimon N. (2007) Nature 450, 186–188 [DOI] [PubMed] [Google Scholar]
- 28. Lee G., Park J. H. (2004) Genetics 167, 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rulifson E. J., Kim S. K., Nusse R. (2002) Science 296, 1118–1120 [DOI] [PubMed] [Google Scholar]
- 30. Grönke S., Müller G., Hirsch J., Fellert S., Andreou A., Haase T., Jäckle H., Kühnlein R. P. (2007) PLoS Biol. 5, e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.