Abstract
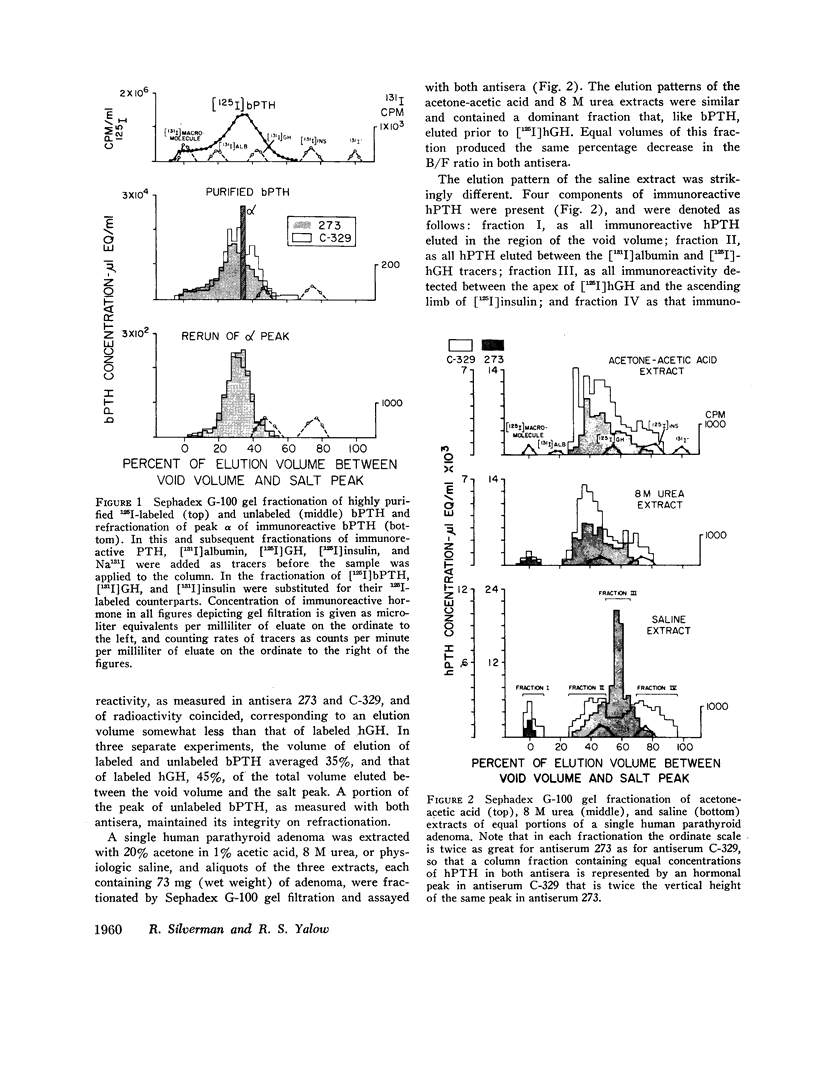
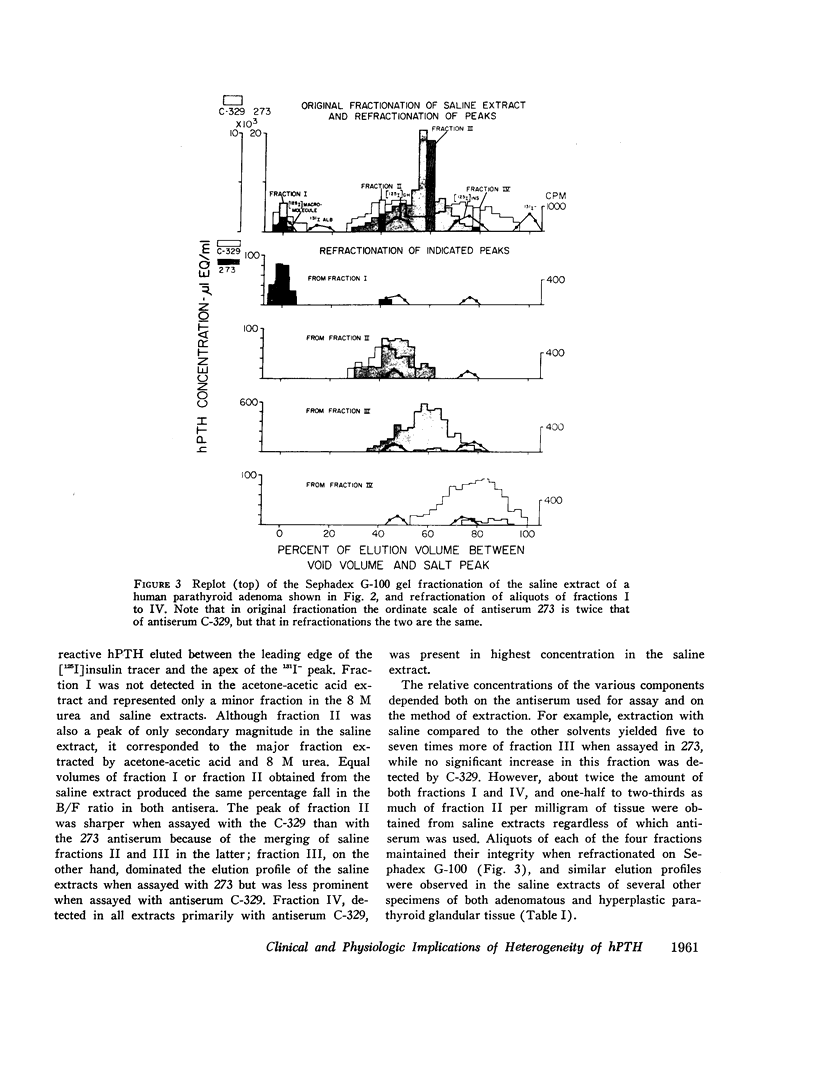
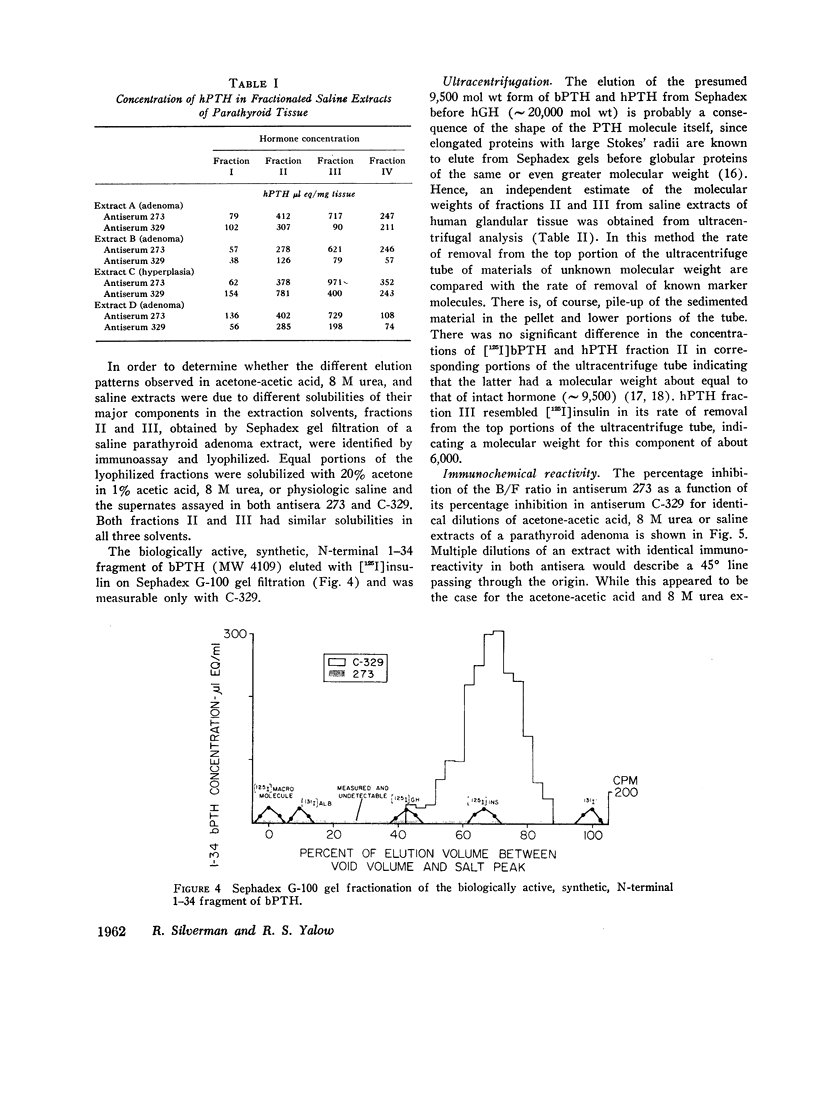
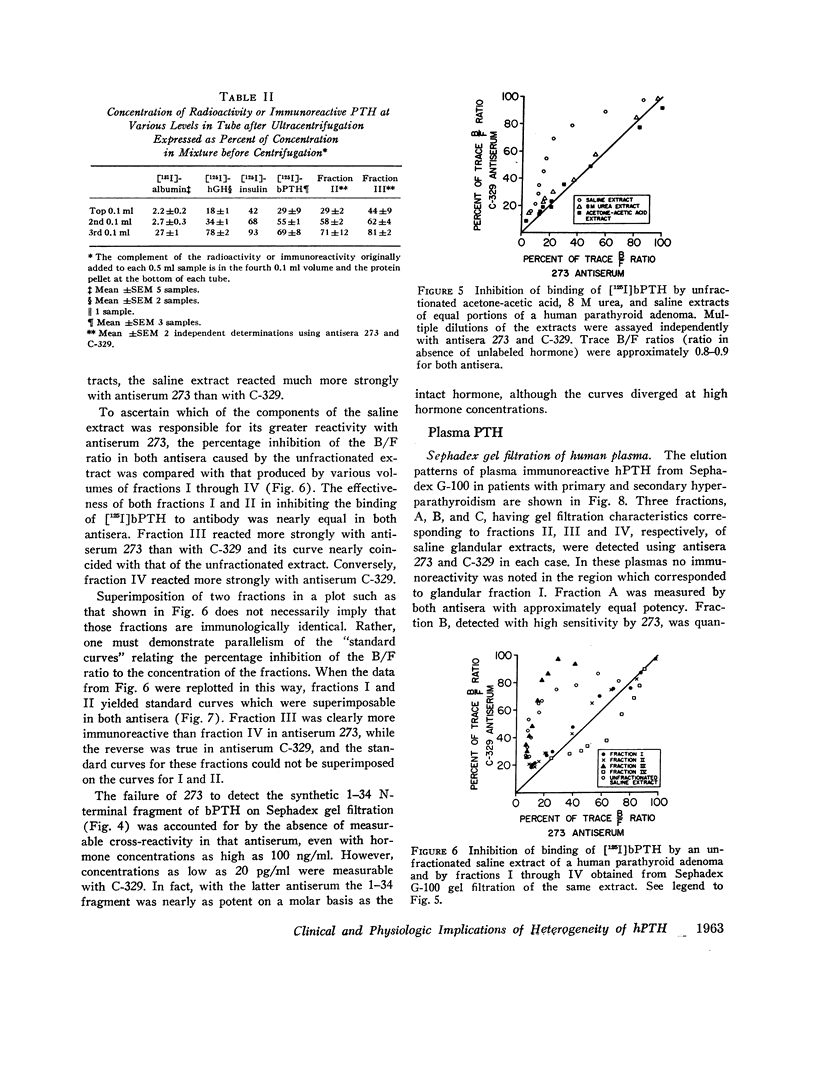
When immunoreactive human parathyroid hormone (hPTH), extracted by three different solvents (20% acetone in 1% acetic acid, 8 M urea, or normal saline) from parathyroid glandular tissue was subjected to Sephadex G-100 gel filtration and immunoassay using two different antisera (273 and C-329), four distinct fractions were observed. The first (I), a void volume peak, was detected by both antisera with similar immunoreactivity, as was a second (II), which had the elution and sedimentation properties of highly purified bovine parathyroid hormone (bPTH); a third (III) eluted between [125I]growth hormone and [125I]insulin, sedimented with the velocity of a molecule of approximately 6,000 mol wt, and was detected primarily by antiserum 273; a final fraction (IV), detected primarily by C-329, eluted just prior to [125I]insulin. The elution profiles of the acetone-acetic acid and 8 M urea extracts were similar and contained fraction II as their major component. In saline extracts, however, fraction III predominated.
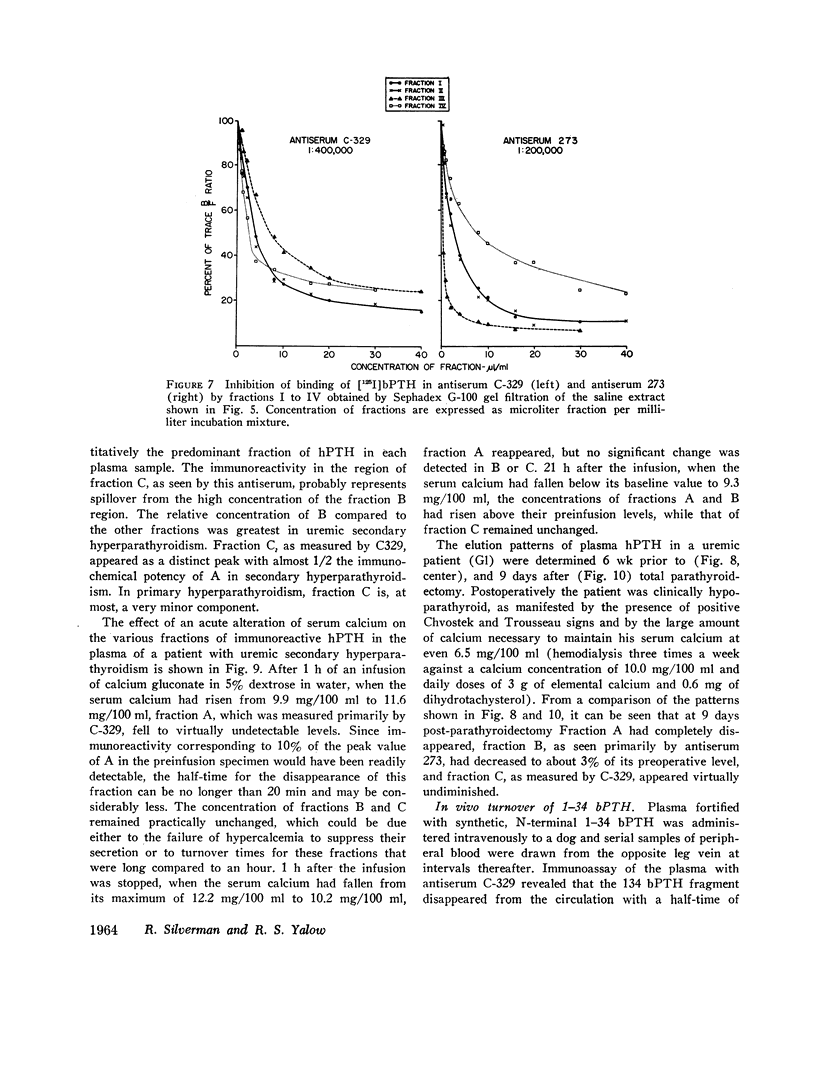
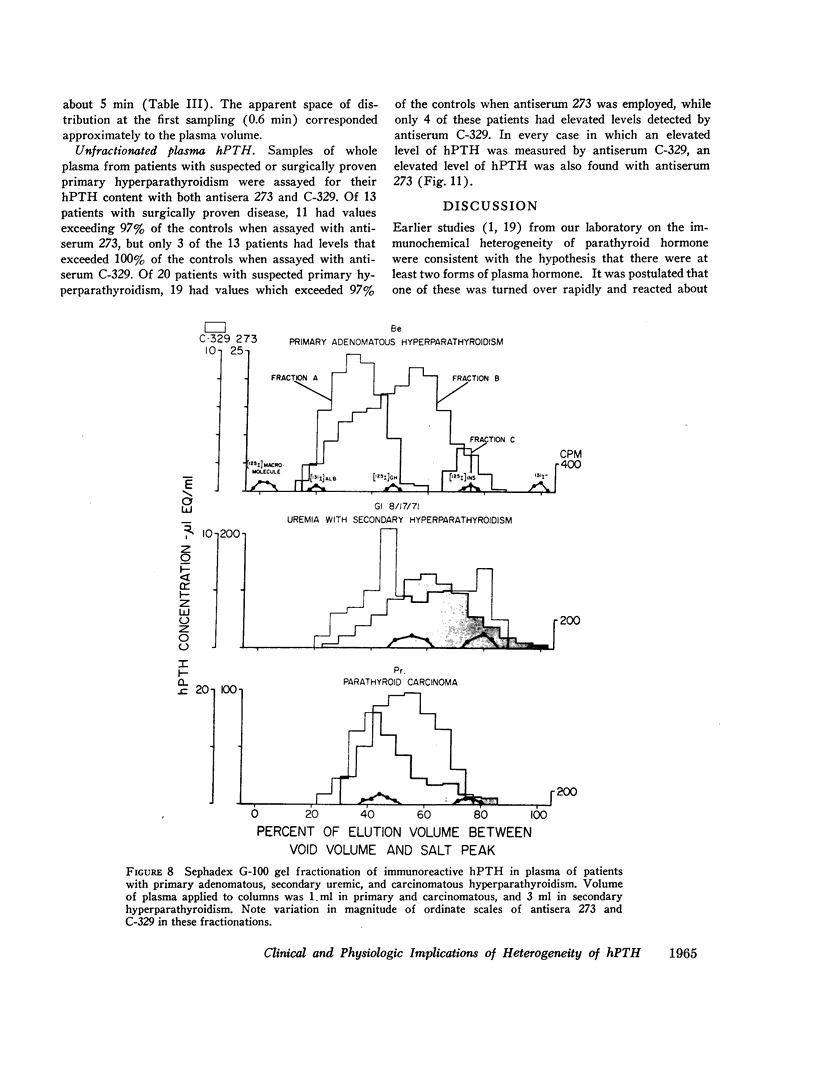
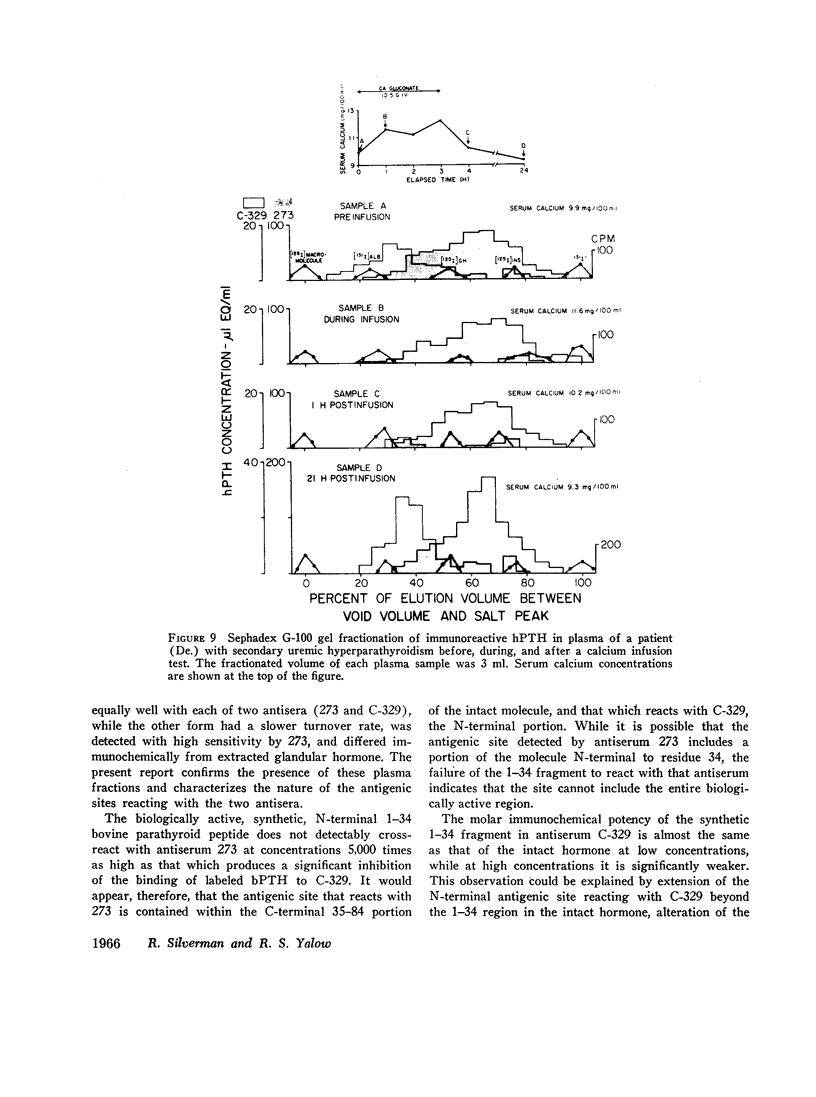
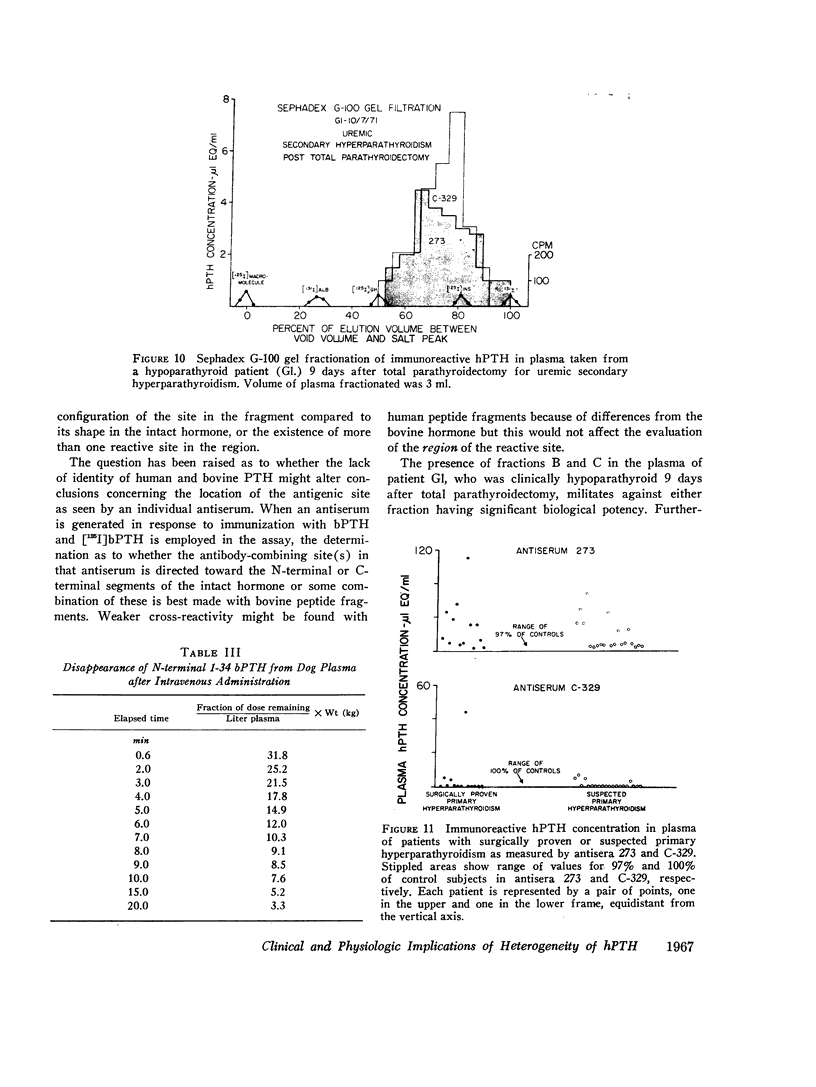
Three fractions, having gel filtration and immunologic characteristics similar to fractions II, III, and IV, respectively, of saline glandular extracts, were detected in the plasma of patients with both primary (adenomatous or carcinomatous) and secondary hyperparathyroidism. The predominant component in every plasma was the intermediate fraction that, like III, was detected primarily by antiserum 273, while the least abundant form was consistently the final fraction, detected primarily by antiserum C-329. The first fraction, like II, was detected with about equal potency by both antisera and had an elution volume on Sephadex corresponding to that of intact bPTH. It bore a reciprocal relationship to serum calcium and disappeared from the plasma of a uremic patient during calcium infusion or following parathyroidectomy with a half-time of no more than 20 min. This component therefore probably represents biologically active hormone. The intermediate and final fractions had turnover times in the plasma of a uremic patient more than 100 times greater than the active form, remained elevated even in the presence of post-parathyroidectomy hypoparathyroidism in this patient and were presumed to be biologically inactive. The ratio of biologically inactive fragments to the active form was greater in secondary hyperparathyroidism. The evidence presented favors a glandular origin for the fragments.
Comparison of hormonal assays with the two antisera reveals a striking advantage in the preoperative diagnosis of primary hyperparathyroidism with antiserum 273 that is due to the enhanced sensitivity occasioned by its detection of a biologically inactive as well as the biologically active hormonal form.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaud C. D., Sizemore G. W., Oldham S. B., Fischer J. A., Tsao H. S., Littledike E. T. Human parathyroid hormone: glandular and secreted molecular species. Am J Med. 1971 May;50(5):630–638. doi: 10.1016/0002-9343(71)90118-5. [DOI] [PubMed] [Google Scholar]
- Arnaud C. D., Tsao H. S., Oldham S. B. Native human parathyroid hormone: an immunochemical investigation. Proc Natl Acad Sci U S A. 1970 Sep;67(1):415–422. doi: 10.1073/pnas.67.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurbach G. D., Keutmann H. T., Niall H. D., Tregear G. W., O'Riordan J. L., Marcus R., Marx S. J., Potts J. T., Jr Structure, synthesis, and mechanism of action of parathyroid hormone. Recent Prog Horm Res. 1972;28:353–398. [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Clinical applications of radioimmunoassay of plasma parathyroid hormone. Am J Med. 1971 May;50(5):623–629. doi: 10.1016/0002-9343(71)90117-3. [DOI] [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Immunochemical heterogeneity of parathyroid hormone in plasma. J Clin Endocrinol Metab. 1968 Jul;28(7):1037–1047. doi: 10.1210/jcem-28-7-1037. [DOI] [PubMed] [Google Scholar]
- Canterbury J. M., Reiss E. Multiple immunoreactive molecular forms of parathyroid hormone in human serum. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1393–1398. doi: 10.3181/00379727-140-36681. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Macgregor R. R., Chu L. L., Kimmel J. R., Hamilton J. W. Calcemic fraction-A: biosynthetic peptide precursor of parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1521–1525. doi: 10.1073/pnas.69.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. A., Oldham S. B., Sizemore G. W., Arnaud C. D. Calcium-regulated parathyroid hormone peptidase. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2341–2345. doi: 10.1073/pnas.69.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith R. S., Furszyfer J., Johnson W. J., Fournier A. E., Sizemore G. W., Arnaud C. D. Etiology of hyperparathyroidism and bone disease during chronic hemodialysis. 3. Evaluation of parathyroid suppressibility. J Clin Invest. 1973 Jan;52(1):173–180. doi: 10.1172/JCI107161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Kemper B., Potts J. T., Jr, Rich A. Proparathyroid hormone: biosynthesis by human parathyroid adenomas. Science. 1972 Nov 10;178(4061):630–633. doi: 10.1126/science.178.4061.630. [DOI] [PubMed] [Google Scholar]
- Kemper B., Habener J. F., Potts J. T., Jr, Rich A. Proparathyroid hormone: identification of a biosynthetic precursor to parathyroid hormone. Proc Natl Acad Sci U S A. 1972 Mar;69(3):643–647. doi: 10.1073/pnas.69.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keutmann H. T., Aurbach G. D., Dawson B. F., Niall H. D., Deftos L. J., Potts J. T., Jr Isolation and characterization of the bovine parathyroid isohormones. Biochemistry. 1971 Jul 6;10(14):2779–2787. doi: 10.1021/bi00790a020. [DOI] [PubMed] [Google Scholar]
- O'Riordan J. L., Potts J. T., Aurbach G. D. Isolation of human parathyroid hormone. Endocrinology. 1971 Jul;89(1):234–239. doi: 10.1210/endo-89-1-234. [DOI] [PubMed] [Google Scholar]
- Rosselin G., Assan R., Yalow R. S., Berson S. A. Separation of antibody-bound and unbound peptide hormones labelled with iodine-131 by talcum powder and precipitated silica. Nature. 1966 Oct 22;212(5060):355–357. doi: 10.1038/212355a0. [DOI] [PubMed] [Google Scholar]
- Sherwood L. M., Lundberg W. B., Jr, Targovnik J. H., Rodman J. S., Seyfer A. Synthesis and secretion of parathyroid hormone in vitro. Am J Med. 1971 May;50(5):658–669. doi: 10.1016/0002-9343(71)90121-5. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Wong E. T., Lindall A. W., Schultz A. L. Human parathyroid gland immunoreactive peptides--evidence for proparathormone. J Lab Clin Med. 1971 Nov;78(5):825–826. [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Characteristics of "big ACTH" in human plasma and pituitary extracts. J Clin Endocrinol Metab. 1973 Mar;36(3):415–423. doi: 10.1210/jcem-36-3-415. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Further studies on the nature of immunoreactive gastrin in human plasma. Gastroenterology. 1971 Feb;60(2):203–214. [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Purification of 131-I parathyroid hormone with microfine granules of precipitated silica. Nature. 1966 Oct 22;212(5060):357–358. doi: 10.1038/212357a0. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]
- Yalow T. S., Berson S. A. "Big, big insulin". Metabolism. 1973 May;22(5):703–713. doi: 10.1016/0026-0495(73)90242-4. [DOI] [PubMed] [Google Scholar]