Abstract
During cell division, interaction between kinetochores and dynamic spindle microtubules governs chromosome movements. The microtubule depolymerase mitotic centromere-associated kinesin (MCAK) is a key regulator of mitotic spindle assembly and dynamics. However, the regulatory mechanisms underlying its depolymerase activity during the cell cycle remain elusive. Here, we showed that PLK1 is a novel regulator of MCAK in mammalian cells. MCAK interacts with PLK1 in vitro and in vivo. The neck and motor domain of MCAK associates with the kinase domain of PLK1. MCAK is a novel substrate of PLK1, and the phosphorylation stimulates its microtubule depolymerization activity of MCAK in vivo. Overexpression of a polo-like kinase 1 phosphomimetic mutant MCAK causes a dramatic increase in misaligned chromosomes and in multipolar spindles in mitotic cells, whereas overexpression of a nonphosphorylatable MCAK mutant results in aberrant anaphase with sister chromatid bridges, suggesting that precise regulation of the MCAK activity by PLK1 phosphorylation is critical for proper microtubule dynamics and essential for the faithful chromosome segregation. We reasoned that dynamic regulation of MCAK phosphorylation by PLK1 is required to orchestrate faithful cell division, whereas the high levels of PLK1 and MCAK activities seen in cancer cells may account for a mechanism underlying the pathogenesis of genomic instability.
Keywords: Kinesin, Microtubules, Protein Kinases, Protein Phosphorylation, Protein-Protein Interactions, KIf2c, MCAK, PLK1, Spindle Checkpoint, Depolymerization
Introduction
During mitosis, proper bi-orientation of chromosomes is critical for the accurate segregation of chromosomes, which in turn is essential for maintaining genomic integrity. Microtubule (MT)2 dynamics, mediated by highly coordinated polymerization and depolymerization of MTs, governs both chromosome bi-orientation and segregation during cell division (1).
MT depolymerization is regulated by the Kin I family of ATPases (2). Unlike conventional kinesins that transport cargo in cells, Kin I kinesins lack motility but stimulate MT depolymerization by promoting catastrophe (3–5). The kinesin-13 family, consisting of Kif2a, Kif2b, and MCAK/Kif2c (mitotic centromere-associated kinesin), has a conserved motor domain in the middle of the protein (6) and is essential for the control of MT length in mitosis (7–9) and of MT dynamics in interphase (2, 10). Kif2a is localized to the spindle pole and contributes to MT flux through disassembling MTs, which is required for chromosome movement in anaphase (11). Kif2a also plays an essential role in bipolar spindle assembly (12). Kif2b, localized to centrosomes, spindle microtubules, midbody, and kinetochores, is critical for spindle assembly, chromosome movement, and cytokinesis (13). MCAK/kif2c, the best characterized member of the Kin I subfamily, localizes dynamically at various mitotic structures, such as inner centromeres, outer kinetochores, centrosomes, spindle MTs, MT tips, and the spindle midzone (8, 14–17). MCAK promotes catastrophe of both stable and dynamic MTs via depolymerizing MTs at both ends in vitro (3, 18, 19). Loss of MCAK function leads to extremely long spindle MTs, although overexpression of MCAK results in a short spindle in vivo (8, 20). Although the monomeric MCAK exhibits the depolymerization activity (21), the full-length MCAK exhibits a higher ATPase activity, MT plus-end binding activity, and lower tubulin heterodimer affinity (22). The structural-functional analysis indicated that the C-terminal domain of MCAK is required for its MT depolymerization activity in vitro (23), which may attribute to its activity to remove tubulin subunits (24). As a key MT depolymerase, MCAK contributes to faithful chromosome movement by promoting the turnover of spindle microtubules at the kinetochore (25). Depletion of MCAK leads to mal-oriented and misaligned chromosomes in achieving faithful metaphase alignment in addition to aberrant anaphase in mammalian cells (7, 11, 16, 26). On the other hand, hyperactive MCAK causes the formation of multipolar spindle (27, 28) and spindle assembly defects in mitosis (7, 20, 29). Interestingly, overexpression of MCAK has been observed in solid tumors such as gastric and breast cancers (30, 31), suggesting that aberrant regulation of MCAK may be involved in tumorigenesis. Previous studies have revealed that the localization and depolymerase activity of MCAK are regulated by Aurora A and Aurora B (15, 32–38). Tight spatiotemporal regulation of MCAK by Aurora B is critical for the timely correction of aberrant kinetochore-microtubule attachment by destabilizing MTs improperly attached to kinetochores (15, 16, 34–36). It has been shown that the activity of MCAK is negatively regulated by phosphorylation by Aurora B in centromere and by centrosome-associated Ca2+/calmodulin-dependent protein kinase IIγ at centrosome (28). It has also been recently reported that the activity of MCAK depolymerase was attenuated by CDK1 (cyclin-dependent kinase 1) phosphorylation at centrosomes (29). Although the MCAK activity was stimulated by the Inner Centromere Kin I Stimulator at inner centromere (39) and regulated by hSgo2 spatially (40), it was unclear whether the MCAK depolymerase activity is positively regulated by a protein kinase in mitosis.
Polo-like kinase 1 (PLK1), a critical mitotic kinase (41), is a key regulator of cell division in eukaryotic cells. PLK1 controls multiple events in mitosis such as mitotic entry, centrosome maturation, bipolar spindle formation, stable MT-kinetochore attachment, cohesion dissociation, chromosome alignment and segregation, and cytokinesis (42). PLK1 is overexpressed in many human tumors and is associated with tumorigenesis (43, 44). PLK1, first expressed in the S-phase (45, 46), localizes at centrosomes (47) and centromeres (48) from the G2 phase to mitosis. At centrosomes, PLK1 recruits γ-tubulin to promote MT nucleation. On the other hand, PLK1 promotes MT depolymerization by enhancing the localization of Kif2a to spindle MTs and spindle poles in addition to stimulating the depolymerase activity of Kif2a (49). As the kinesin-13 family consists of three members, it has remained elusive whether PLK1 regulates the other two members, MCAK and Kif2b.
Here, we show that PLK1 interacts with MCAK, both in vitro and in vivo. MCAK is a cognate substrate of PLK1. The PLK1-mediated phosphorylation promotes the depolymerase activity of MCAK in vivo. Overexpression of a phosphomimetic mutant or wild type MCAK leads to a significant increase in mitotic arrest with misaligned chromosomes and multipolar spindles. On the other hand, overexpression of a nonphosphorylatable MCAK results in anaphase disruption with lagging chromosomes and chromatid bridges. Our study suggests that accurate regulation of MCAK activity by PLK1 is essential for faithful chromosome segregation.
MATERIALS AND METHODS
Plasmid Construction
To generate GFP-tagged full-length human MCAK, PCR-amplified MCAK cDNA was cloned into the pEGFP-C2 vector (Clontech) with EcoRI and BamHI digestion. pFastBac MCAK WT-His (MCAK-wild type-His), pFastBac MCAK 6A-His, and pFastBac MCAK 6E-His were constructed by cloning full-length MCAK into pFastBac1 (Invitrogen) with SalI and XbaI digestion. The pFastBac1 was first inserted with a His tag by XhoI and HindIII.
All MCAK deletions were created by PCR and subcloning. The vectors used were as follows: pEGFP-C2 (Clontech), pET-28a (Novagen, WI), and pGEX-6P (Amersham Biosciences).
GFP-tagged nonphosphorylatable and phosphomimetic MCAK mutants were generated by PCR and confirmed by sequencing. The bacterial expression constructs of human PLK1 plasmids were created as described previously (50). All constructs were sequenced in full. The PLK1 construct for expression in insect cells was a generous gift from Ray Erickson (Harvard University).
Recombinant Protein Expression
GST-MCAK deletions and GST-PLK1 and GST-PLK1 deletions were all expressed in Rosetta (DE3) pLysS (Novagen). Briefly, 400 ml of LB media was inoculated with Rosetta pLysS transformed with the corresponding plasmid. The protein expression was induced by addition of 0.5 mm isopropyl 1-thio-β-d-galactopyranoside at 16 °C for 14–20 h. Bacterial cells were then collected, and the proteins were purified with glutathione-agarose chromatography in phosphate-buffered saline (PBS) containing protease inhibitor mixture (Sigma) and 0.5% Triton X-100. Recombinant MCAK full-length proteins were expressed and purified as described previously (7). MBP-MCAK (182–586 amino acids) expressed in Rosetta was also induced by addition of 0.5 mm isopropyl 1-thio-β-d-galactopyranoside at 16 °C for 14–20 h. Bacterial cells were then collected, and the maltose-binding protein-tagged proteins were purified by amylose resin (New England Biolabs). Proteins were eluted with 10 mm maltose in appropriate buffer for corresponding experiments.
Antibodies and siRNA
MCAK-related antibodies were described previously (51). Mouse monoclonal antibody to PLK1 was purchased from Invitrogen. Anti-FLAG antibody M2 was purchased from Sigma. Mouse monoclonal antibody to GFP was obtained from BD Biosciences. Mouse monoclonal antibody against α-tubulin (DM1A) was purchased from Sigma. MCAK siRNA targeting the 3′-noncoding sequence of the MCAK gene was purchased from Qiagen. PLK1 siRNA was purchased from Dharmacon.
In Vitro Pulldown Assay
GST fusion protein-bound Sepharose beads were incubated with 293T cell lysates containing ectopically expressed GFP-tagged MCAK or its deletion mutants or with purified His-tagged full-length MCAK expressed in Sf9. They were incubated in pulldown buffer (20 mm Tris-Cl, pH 7.4, 100 mm NaCl, 1 mm EGTA, 1 mm DTT) containing 0.25% Triton X-100, 1 mm PMSF, and protease inhibitor mixture for 3 h at 4 °C. The beads were then washed three times with the pulldown buffer containing 0.25% Triton X-100 and 1 mm PMSF and once with the pulldown buffer alone. The samples were boiled in the SDS sample buffer and separated on 10% SDS-PAGE. Proteins were then transferred onto nitrocellulose membrane for Western blotting with appropriate antibodies.
Cell Culture, Transfection, and Synchronization
HeLa and 293T cells (American Type Culture Collection, Manassas, VA) were maintained as subconfluent monolayers in Dulbecco's modified Eagle's medium (Invitrogen) with 10% FBS (Hyclone, Logan, UT) and 100 units/ml penicillin plus 100 μg/ml streptomycin at 37 °C with 10% CO2.
Cells were transfected with various plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's manuals. Cells were synchronized at G1/S with 2 mm thymidine for 16 h and then released into thymidine-free medium for 10 h. Cells were then incubated with 2 mm thymidine for 14 h, released into fresh media, and harvested 9 h post-release with enriched prometaphase cells. In some cases, 20 μm MG132 was added at 2 h prior to harvest to arrest cells at metaphase.
Immunoprecipitation
293T cells were grown to ∼40% confluency in DMEM and co-transfected with FLAG-PLK1 and GFP-MCAK using Ca3(PO4)2 methods. Cells were then harvested and solubilized by sonication on ice in lysis buffer (50 mm HEPES, pH 7.4, 100 mm NaCl, 2 mm EGTA, 1 mm MgCl2, 1 mm DTT) containing 0.25% Triton X-100, 1 mm PMSF, and protease inhibitor mixture (Sigma). The lysates were clarified by centrifugation at 16,000 × g for 20 min at 4 °C. FLAG-PLK1 was purified by incubation with the anti-FLAG M2 antibody-agarose beads (Sigma). Beads were washed three times with the lysis buffer containing 0.25% Triton X-100 and 1 mm PMSF and once with the lysis buffer alone. Beads were then boiled and loaded onto 10% SDS-PAGE for Western blotting analysis with anti-FLAG and GFP antibodies, respectively.
Phosphorylation of MCAK by PLK1
GST-tagged PLK1 was expressed in Sf9 cells and purified by glutathione-agarose beads. The kinase reactions were performed in 40 μl of 1× kinase buffer (25 mm HEPES, pH 7.2, 50 mm NaCl, 2 mm EGTA, 5 mm MgSO4, 1 mm DTT, 0.01% Brij35) containing 20 ng of eluted PLK1 kinase, 1 μg of GST-tagged or His-tagged substrates, 5 μCi of [γ-32P]ATP and 500 μm ATP. Reaction mixtures were incubated at 30 °C for 30 min, stopped by the SDS sample buffer, and separated by SDS-PAGE. The gel was stained with Coomassie Brilliant Blue and dried, and the 32P incorporation into MCAK proteins was quantified by PhosphorImager (Amersham Biosciences).
To identify the PLK1-mediated phosphorylation site of MCAK in mitosis, mitotic HeLa cells were collected 18 h after the treatment of 100 ng/ml nocodazole and divided into 2 aliquots. One aliquot was treated with PLK1 inhibitor 5 μm DAP81 as described previously (53), although another aliquot (control) was treated with an equal volume of DMSO for 15 min at 37 °C before the cellular proteins were solubilized in lysis buffer (50 mm HEPES, pH 7.4, 150 mm NaCl, 2 mm EGTA, 0.1% Triton X-100) plus protease inhibitor mixture (Sigma). Lysates were clarified by centrifugation at 16,000 × g for 10 min at 4 °C. MCAK proteins from DAP81-treated and control samples were immunoprecipitated with an anti-MCAK mouse antibody (Santa Cruz Biotechnology) bound to protein A/G beads (Thermo Fisher). Beads were washed five times with lysis buffer and then boiled in SDS-PAGE sample buffer for 2 min. The MCAK bands from both DAP81-treated and control samples were removed and subjected to trypsin in-gel digest followed by collection of tryptic peptides for mass spectrometric analyses as we described previously (54).
Immunofluorescence
Cells were seeded onto sterile, acid-treated 12-mm coverslips in 24-well plates (Corning Glass). At the time of immunofluorescence analysis, cells were washed once with pre-warm PHEM (60 mm PIPES, 25 mm HEPES, 10 mm EGTA, 2 mm MgCl2, 4 m glycerol, pH 6.9) and fixed in pre-cold methanol for 5min at −20 °C. In some cases, cells were washed once with pre-warm PHEM, permeabilized for 1 min with PHEM plus 0.1% Triton X-100, and then fixed with freshly prepared 3.7% paraformaldehyde in PHEM for 4 min at the room temperature. After washing three times with PBS containing 0.05% Tween 20 (PBST), cells were blocked with 1% BSA (Sigma) in PBST for 45 min at room temperature. Cells were then incubated with primary antibodies for 1 h at room temperature or for 12 h at 4 °C, followed by secondary antibodies for 1 h at the room temperature. DNA was stained with DAPI (Sigma). Images were collected using a DeltaVision wide field deconvolution microscope system as described previously (52).
Given the heterogeneous levels of exogenously expressed protein seen in transient transfected cells, we adapted an established protocol to quantify the specific depolymerase activities of GFP-MCAK mutants in transfected cells by measuring the pixels of microtubule intensity and GFP-MCAK from maximally projected stacks (15, 49). The function of MCAK depolymerase activity is judged by the ratio of measured microtubule intensity over GFP-MCAK protein intensity from the same cells. This protocol allows us to quantify microtubule integrity in response to different MCAK proteins (wild type and phospho-mimicking and nonphosphorylatable) in cells.
Specifically, GFP-MCAK (wild type and mutants) expressing cells counter-stained with tubulin antibody DM1A were imaged, and the images of individual cells were acquired as z-stacks from the top to the bottom at intervals of 0.25 μm and processed using DeltaVision Softworx software (Applied Precision Inc) as described previously (55). Given the heterogeneity of GFP-MCAK expression in transfected cells, we selected those cells containing equivalent levels of MCAK. The GFP-MCAK depolymerase activity was expressed by the microtubule intensity divided by MCAK intensity.
For quantifying samples from different transfectants, all transfections and subsequent immunocytochemistry were done in parallel, and images were acquired under a constant exposure for microtubule labeling without pixel saturation. Images for quantification were generated by projecting the sum of the optical sections using the maximum intensity method (55). The results were averaged from three independent experiments, and the highest mean value of microtubule immunofluorescence intensity over MCAK intensity was regarded as 1.
Live Cell Image
HeLa cells grown on coverslips were synchronized by 2 mm thymidine and transiently transfected with GFP-MCAK and its mutants. 24–36 h post-transfection, cells were imaged with a DeltaVision system (Applied Precision, Issaquah, WA).
RESULTS
PLK1 Interacts with MCAK in Vitro and in Vivo
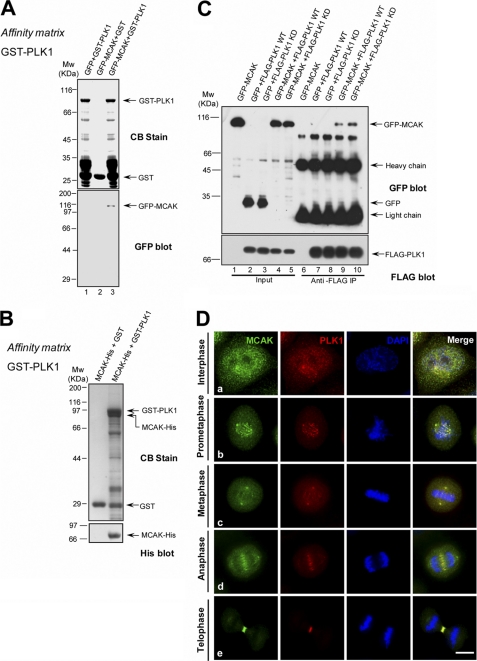
As PLK1 interacts with Kif2a and promotes its MT depolymerase activity (49), we examined whether PLK1 associates with Kif2c/MCAK. Recombinant GST-tagged PLK1 was purified and incubated with lysates of 293T cells transiently transfected with GFP-MCAK or GFP. GFP-MCAK, but not GFP, was pulled down by GST-PLK1 (Fig. 1A), indicating that PLK1 interacted MCAK specifically. To validate whether PLK1 interacts with MCAK directly in vitro, we used GST-PLK1 on glutathione-agarose beads to pull down MCAK-His expressed and purified from Sf9 cells. As shown in Fig. 1B, GST-PLK1, but not GST, pulled down His-MCAK.
FIGURE 1.
MCAK interacts with PLK1 in vitro and in vivo. A, GST-PLK1 on glutathione beads was incubated with lysates of 293T cells transiently transfected with GFP or GFP-MCAK. The beads were washed and analyzed by Western blotting with an anti-GFP antibody. CB, Coomassie Blue stain. B, GST-PLK1, but not GST, pulled down MCAK-His. GST-PLK1 on glutathione-conjugated beads was incubated with recombinant MCAK-His protein purified from Sf9 cells, followed by Western blot analysis using an anti-His antibody. C, co-immunoprecipitation of FLAG-PLK1 and GFP-MCAK. 293T cells were transiently transfected with GFP-MCAK plus FLAG-PLK1 WT (wild type) or FLAG-PLK1 kinase-dead (K82A). 36 h post-transfection, cells were lysed and incubated with the anti-FLAG antibody coupled to agarose beads. The beads were washed and analyzed by Western blotting with an anti-GFP mouse antibody and then probed with an anti-FLAG antibody. D, co-localization of endogenous MCAK and PLK1 during mitosis. HeLa cells were synchronized to mitosis by a thymidine block release, fixed, and then stained with an anti-MCAK rabbit antibody and an anti-PLK1 mouse antibody. DNA was stained with DAPI. MCAK is labeled in green and PLK1 is in red. Scale bar, 10 μm.
The interaction between PLK1 and MCAK in vivo was analyzed in an anti-FLAG immunoprecipitation assay. Anti-FLAG antibody beads were incubated with lysates of 293T cells transiently co-transfected with FLAG-PLK1 and GFP/GFP-MCAK and then washed, followed by Western blot analysis with an anti-GFP antibody. GFP-MCAK co-immunoprecipitated with FLAG-PLK1 (Fig. 1C). To test whether the PLK1 kinase activity is required for such an association, 293T cells were co-transfected to express GFP-MCAK and FLAG-PLK1 kinase-dead proteins. Cell lysates were generated and subjected to anti-FLAG immunoprecipitation. As shown in Fig. 1C, PLK1 kinase-dead pulled down MCAK more efficiently than PLK1 WT, indicating that the kinase activity is not essential for the PLK1-MCAK association.
Next, we examined the spatiotemporal pattern of PLK1 and MCAK distribution during the cell cycle. To this end, HeLa cells were fixed and stained with antibodies against MCAK and PLK1. In G2 and throughout mitosis, MCAK co-localized with PLK1 on controsomes (Fig. 1D, panel a). In prometaphase cells, both MCAK and PLK1 were readily seen at centromeres (Fig. 1D, panel b). At the prometaphase to metaphase transition, centromeric MCAK and PLK1 become liberated, whereas the levels of spindle-associated MCAK and PLK1 became increased (Fig. 1D, panel c). During the metaphase to anaphase transition, MCAK and PLK1 were relocated to the central spindle (Fig. 1D, panel d) and then concentrated at the Fleming body at telophase and cytokinesis (Fig. 1D, panel e). In addition, from anaphase A to telophase, the weak MCAK and PLK1 signal never completely disappeared from centromeres. Based on this cell cycle-dependent translocation of PLK1 and MCAK and spatiotemporal dynamics of PLK1 activity, we speculate that PLK1 may regulate MCAK activity and subsequent microtubule dynamics in mitosis.
Kinase Domain of PLK1 Associates with the Neck and Motor Domain of MCAK
To map the domain for the PLK1-MCAK interaction, a series of MCAK and PLK1 deletion mutants was constructed (Fig. 2, D and E). 293T cells were transiently transfected to express GFP-MCAK and its deletion mutants followed by a pulldown assay using GST-PLK1 beads. As shown in Fig. 2, A and B, GST-PLK1 pulled down MCAK full-length and MCAK(182–586) but not MCAK(1–181) or MCAK(587–725). The motor domain, MCAK(259–586), was also pulled down by GST-PLK1 but to less extent. The direct interaction between MCAK(182–586) and PLK1 was further validated using an in vitro binding assay with GST-PLK1 as an affinity matrix (Fig. 2C). Based on those analyses, we conclude that MCAK(182–586) contributes to the interaction of MCAK and PLK1.
FIGURE 2.
Neck and motor domain of MCAK binds to the kinase domain of PLK1. A and B, GST-PLK1 on glutathione beads was incubated with lysates of 293T cells expressing GFP-MCAK or GFP-MCAK deletion mutants, followed by Western blotting with an anti-GFP antibody. C, GST-PLK1, but not GST, interacts with MBP-MCAK(182–586). GST-PLK1 on glutathione beads was incubated with recombinant MBP-MCAK(182–586) purified from E. coli, followed by Western blot analysis with an anti-maltose-binding protein (MBP) antibody. CB, Coomassie Brilliant Blue. D, schematic representation and summary of the binding studies for a series of MCAK deletion mutants assayed in A–C. +, positive; +/−, weak positive; −, negative. Numbers indicate positions of the amino acid residues. E, schematic illustration of PLK1 functional domain and deletion mutants used in F. Numbers indicate the positions of the amino acid residues. F, GST-PLK1 deletion mutants interact with MCAK-His. GST fusion proteins containing N- and C-terminal PLK bound to glutathione beads were incubated with recombinant MCAK-His purified from Sf9 cells followed by Western blot analysis with an anti-His antibody.
We next determined the domain of PLK1 important for its interaction with MCAK. PLK1 contains a kinase domain at the N terminus and two polo-boxes domain at the C terminus. GST-PLK1(1–300) (GST-PLK1-N, kinase domain) and GST-PLK1(305–603) (GST-PLK1-C, polo-box domain) were constructed and used as affinity matrix to incubate with MCAK-His (Fig. 2E). MCAK was pulled down by full-length PLK1 and PLK1 kinase domain. In addition, MCAK interacts weakly with the PLK1 polo-box domain (Fig. 2F). The interaction between MCAK and the PLK1 kinase domain raised the possibility that MCAK may be a novel substrate of PLK1 in mammalian cells.
MCAK Is a Novel Substrate of PLK1
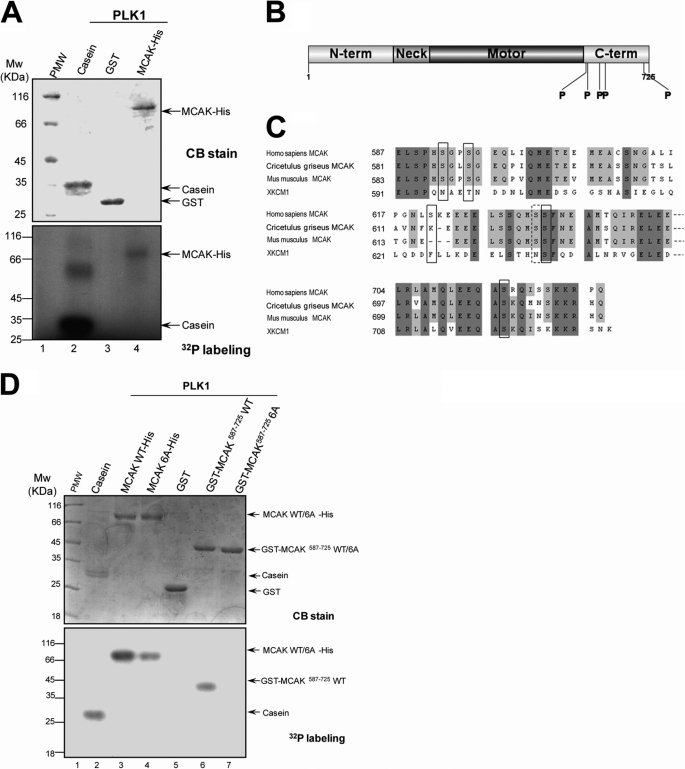
To examine whether MCAK is a cognate substrate of PLK1, in vitro phosphorylation reaction was performed on recombinant MCAK-His and its deletion mutants using recombinant GST-PLK1 purified from Sf9 cells in the presence of [γ-32P]ATP. The full-length of MCAK was phosphorylated by PLK1 in vitro judging by the incorporation of 32P (Fig. 3A). This phosphorylation is specific, as GST was not labeled in this assay. Analysis of MCAK deletion mutants indicated that MCAK(587–725) was strongly phosphorylated by PLK1.3 We conclude that MCAK is a novel substrate of PLK1.
FIGURE 3.
PLK1 kinase phosphorylates MCAK protein in vitro. A, bacterially recombinant MCAK-His and GST were incubated with insect cell recombinant PLK1 kinase in an in vitro phosphorylation reaction as described under “Materials and Methods.” Samples were separated by SDS-PAGE and stained with Coomassie Brilliant Blue (CB) to ensure equal amounts of MCAK-His and GST used in the reactions. The gels were then dried and exposed to an x-ray film. B, schematic illustration of sites of MCAK phosphorylated by PLK1. Recombinant MCAK-His and GST-MCAK(587–725) were phosphorylated by PLK1 in vitro in the absence of [γ-32P]ATP as described under “Materials and Methods,” and phosphorylation sites on MCAK were identified by mass spectrometry. Five phosphorylation sites (Ser592, Ser595, Ser621, Ser633, and Ser715) identified were shown with the boldface P. C, alignment of sequences around the phosphorylation sites for MCAK from human, Chinese hamster (CHO), mouse, and African Clawed Toad (Xenopus). Dark and light shading indicate the identical and conserved residues, respectively. The residues in the black squares indicate the phosphorylation sites. Although the residue Ser632 in the dotted square was not identified by mass spectrometry, it was also mutated in the following experiments as it is in the vicinity of the phosphorylation site Ser633. The numbers indicate the amino acid positions. D, recombinant MCAK-His (WT and its mutants) and GST-MCAK deletion mutants were phosphorylated by PLK1 in vitro as described in A.
To identify the phosphorylation sites on MCAK, recombinant MCAK-His and GST-MCAK(587–725) was phosphorylated by GST-PLK1 in vitro, and then the phosphorylation sites were determined by mass spectrometry. Five potential phosphorylation sites were identified at the C-terminal domain of MCAK (Ser592, Ser595, Ser621, Ser633, and Ser715) (Fig. 3B). Mass spectrometric analyses of endogenous MCAK from mitotic HeLa cells also support the in vitro phosphorylation site mapping.3 According to the alignment of the C-terminal domain of human MCAK and its orthologs from hamster, mouse, and Xenopus, three of the identified sites are conserved among vertebrates and one is conserved in mammals. Ser632 was not identified by mass spectrometry but is in the vicinity of the mapped Ser633. So Ser632 was also included for functional analyses (Fig. 3C). To validate that these six sites are indeed phosphorylated by PLK1, we performed in vitro phosphorylation assay on recombinant MCAK-His, GST-MCAK deletion mutants, and nonphosphorylatable MCAK mutants in which these six sites were replaced by six alanine residues (MCAK 6A). As shown in Fig. 3D (lower panel), incubation with [γ-32P]ATP with various recombinant MCAK proteins in the presence of polo-like kinase resulted in an efficient incorporation of 32P into recombinant MCAK-His and GST-MCAK(587–725) proteins, whereas the intensity of 32P labeling onto MCAK 6A-His was only one-third of that on MCAK-His (Fig. 3D, lane 4), suggesting that additional polo-like kinase sites beyond the C-terminal MCAK are likely phosphorylated by PLK1 in vitro. As expected, recombinant GST-MCAK(587–725) 6A protein was not labeled by 32P (Fig. 3D). The phosphorylation by PLK1 was specific for MCAK, as the GST tag protein was not labeled (Fig. 3D, lane 5). Based on in vitro phosphorylation (Fig. 3D) and mass spectrometric identification of endogenous MCAK protein from mitotic cells,3 we conclude that those C-terminal six serines (Ser592, Ser595, Ser621, Ser632, Ser633, and Ser715) of MCAK are cognate substrates of PLK1.
Phosphorylation of MCAK by PLK1 Stimulates Its Microtubule Depolymerase Activity
Because MCAK is an MT depolymerase and the six phosphoserine sites identified reside in the C-terminal domain that is responsible for the MT depolymerization activity (24), we speculated that the MT depolymerase activity of MCAK may be modulated by the PLK1 phosphorylation and that the MT dynamics in cells might be regulated by PLK1. To validate this hypothesis, we examined the MT density and length in mitotic cells depleted of PLK1. The α-tubulin immunofluorescence intensity in PLK1-depleted cells is dramatically increased compared with that of control cells,3 which is consistent with our earlier study (49). In addition, the MT length in PLK1-depleted mitotic cells was longer than that of control cells, suggesting that PLK1 promotes the MT depolymerization in vivo. Thus, we reasoned that MCAK is a novel effector of PLK1 in mitosis.
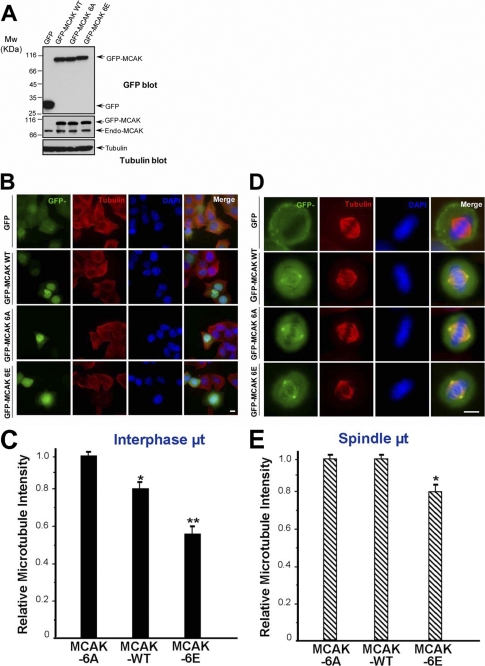
If PLK1 promotes MT depolymerization through stimulating the depolymerize activity of MCAK, the phosphomimetic mutant of MCAK should possess a higher depolymerase activity than the nonphosphorylatable MCAK. To evaluate this hypothesis, MCAK and its phosphorylation site mutants were expressed, and their effects on MT structure were then analyzed. The expression levels of GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E in HeLa cells were comparable, all at about two times higher than that of endogenous MCAK (Fig. 4A, endo-MCAK). Considering that the transfection efficiency was about 40% (data not shown), the expression levels of recombinant MCAK and its mutants were about 6–7 times higher than that of endogenous MCAK in positively transfected cells. Thus, in transfected cells, MT integrity is expected to be mainly controlled by the exogenously expressed MCAK. We then examined MT integrity in HeLa cells transiently transfected with GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E. Relative microtubule intensity in HeLa cells transfected with GFP-MCAK WT was 20% lower than that in GFP-MCAK 6A-transfected cells, whereas relative microtubule intensity in cells transfected with GFP-MCAK 6E was just 57% of that in GFP-MCAK 6A-transfected cells (Fig. 4, B and C). Next, we investigated the roles of MCAK and its phosphorylation site mutants on the mitotic spindle. The relative spindle MT density in GFP-MCAK 6E-transfected cells was 21% lower than that in GFP-MCAK 6A-transfected cells (Fig. 4, D and E). Thus, the phosphorylation of MCAK by PLK1 greatly enhances the depolymerization activity of MCAK.
FIGURE 4.
PLK1 phosphorylation stimulates the MT depolymerase activity of MCAK. A, expression levels of GFP-MCAK and its phosphorylation site mutants in HeLa cells. HeLa cells were transfected with GFP, GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E. At 36 h post-transfection, cells were harvested, boiled in the SDS-PAGE buffer, and analyzed by Western blotting with an anti-GFP antibody (top panel), anti-MCAK antibody (middle panel), or anti-tubulin antibody (as a loading control, bottom panel). B, in vivo analysis of GFP-MCAK and its phosphorylation site mutants in interphase cells. HeLa cells were transfected with GFP, GFP-MCAK WT, GFP-MCAK 6, or GFP-MCAK 6E. At 36 h post-transfection, cells were fixed with methanol and stained for α-tubulin (red) and DAPI (for DNA, blue), respectively. C, statistical analysis of the relative MT density in B as described under “Materials and Methods.” Data are presented as means ± S.E. and derived from 75 cells from three independent experiments. *, p < 0.05; **, p < 0.01. D, in vivo analysis of GFP-MCAK and its phosphorylation sites mutants in mitotic cells. HeLa cells were transfected with GFP, GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E and synchronized to metaphase. At 36 h post-transfection, cells were fixed and stained as described in B. E, statistical analysis of relative spindle MT density in D as described under “Materials and Methods.” Data are presented as means ± S.E. and derived from 33 cells from three independent experiments. *, p < 0.05.
To investigate the role of the phosphorylation by PLK1 on MT dynamics, we evaluated the kinetics of MT polymerization. Cells transfected with GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E were treated with 1 μg/ml nocodazole to completely depolymerize MTs. Cells were then fixed at the indicated times after release into fresh media, and MT polymerization kinetics was analyzed by measuring the relative microtubule intensity. The rate of MT repolymerization in cells transfected with GFP-MCAK 6E was much slower than that of GFP-MCAK 6A-expressing cells (at 10 min post release, 45% MT intensity in MCAK 6E cells compared with that in MCAK 6A cells; at 30 min post release, 52% MT intensity in MCAK 6E cells compared with that in MCAK 6A cells).3 We conclude that phosphorylation of MCAK by PLK1 inhibits MT regrowth.
Consistent with a previous report that overexpression of MCAK causes MT bundled to various extents (7), we observed that overexpression of GFP-MCAK 6E leads to MT bundled to a greater extent than that of GFP-MCAK WT. In contrast, overexpression of GFP-MCAK 6A resulted in a less extent of MT bundles than that of GFP-MCAK WT. This is true not only in interphase cells but also mitotic HeLa cells.3 The enhanced MT bundling activity is consistent with the increased MT depolymerization activity (7), indicating that phosphorylation of MCAK by PLK1 stimulates its depolymerase activity.
Regulated Phosphorylation of MCAK Is Essential for Faithful Chromosome Segregation
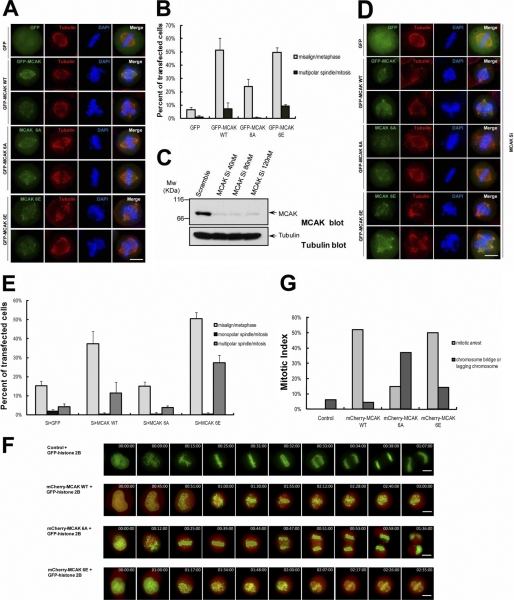
To explore the functional significance of phosphorylation on MCAK by PLK1, we analyzed the phenotype of HeLa cells overexpressing GFP-MCAK and its phosphorylation site mutants. HeLa cells were transfected with GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E and then synchronized to mitosis. Chromosome positioning at metaphase and spindle morphology in mitosis were examined by immunofluorescence staining. Overexpression of GFP-MCAK 6E, similar to GFP-MCAK WT, results in a significant increase of misaligned chromosomes in metaphase cells (50% cells) and a slight increase of multipolar spindles in mitotic cells (9% cells) (Fig. 5, A and B). In contrast, HeLa cells transfected with GFP-MCAK 6A exhibited fewer displaced chromosomes at metaphase (24% cells) and showed almost normal spindle phenotypes in mitosis.
FIGURE 5.
Perturbation of PLK1-mediated MCAK phosphorylation resulted in chromosome segregation errors. A, HeLa cells were transfected with GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E and synchronized to metaphase with thymidine and MG132 treatment. At 36 h post-transfection, cells were fixed and stained with an anti-α-tubulin antibody (red) and DAPI (blue) to determine the spindle morphology and chromosome position in metaphase. Scale bar, 10 μm. B, statistical analysis of misaligned chromosomes and multipolar spindles in A. Results are presented as means ± S.E. and from n = 150 cells from three independent experiments. C, suppression of MCAK with an siRNA targeted to 3′-UTR. HeLa cells were transfected with a scramble siRNA or MCAK siRNA and harvested at 48 h post-transfection. Cell extracts were probed with an anti-MCAK antibody (top panel) and anti-tubulin antibody (as a loading control, bottom panel). D, HeLa cells were transfected with 40 nm MCAK siRNA for 48 h and then transfected to express GFP, GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E for another 36 h. Cells were synchronized, fixed, and stained with an α-tubulin antibody (red) and DAPI (blue) as in A. Scale bar, 10 μm. E, statistical analysis of misaligned chromosomes and multipolar spindles in C. Results are presented as means ± S.E. and from n = 200 cells from three independent experiments. F, real time imaging of chromosome movement in cells expressing cherry-MCAK WT, cherry-MCAK 6A, and cherry-MCAK 6E. Cells were co-transfected with GFP-histone 2B plus cherry-MCAK WT or its phosphorylation site mutants together. At 36 h post-transfection, cells were observed with a DeltaVision system. G, statistical analysis of mitotic arrest and anaphase disruption in F. At least 20 cells were analyzed from three independent experiments for each construct.
To remove the interference of endogenous MCAK, we performed a rescue experiment using MCAK siRNA that only targets the endogenous MCAK with no effect on the exogenously expressed MCAK as the siRNA targets to the 3′-UTR. As shown in Fig. 5C, transfection of 40 nm MCAK siRNA resulted in an efficient knockdown of the endogenous MCAK at 48 h post-transfection. HeLa cells were transfected with GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E at 24 h post-transfected with MCAK siRNA. 36 h later, transfected cells were fixed and stained with an anti-α-tubulin antibody and DAPI. Similar to the overexpression assay (Fig. 5, A and B), add-back of GFP-MCAK 6E or GFP-MCAK WT led to a significant increase of misaligned chromosomes at metaphase and resulted in an increase in multipolar spindles in mitotic cells (relative to the GFP control) (Fig. 5, D and E). Expression of GFP-MCAK 6E led to 50% cells with misaligned chromosomes and 27% cells with multipolar spindles, respectively. On the other hand, expression of GFP-MCAK 6A only caused 15% cells with misaligned chromosomes and 3.7% cells with multipolar spindles, respectively (Fig. 5E). These phenotypes may be explained by the hyperactive microtubule depolymerase activity of MCAK 6E and the hypoactive activity of MCAK 6A.
To assess the effect of MCAK WT and its phosphorylation site mutants on mitotic progression, time-lapse imaging experiment was carried out. HeLa cells transfected with GFP-MCAK WT, GFP-MCAK 6A, or GFP-MCAK 6E were synchronized, and time-lapse imaging was conducted at 36 h post-transfection (Fig. 5, F and G). Consistent with results from Fig. 5, A, B, D, and E, overexpression of GFP-MCAK WT or GFP-MCAK 6E caused 50% cells arrested at prometaphase with at least one chromosome misaligned, although only 15% GFP-MCAK 6A-expressing cells were arrested at prometaphase with unaligned chromosomes. Surprisingly, overexpression of GFP-MCAK 6A resulted in a chromosome instability phenotype, as evidenced by a dramatic increase in cells exhibiting chromatid bridges in anaphase (about 37% cells). As it was reported that suppression of MCAK led to anaphase defects (7, 11, 16, 26), the phenotypes seen in MCAK 6A-expressing cells are likely due to a greatly reduced MT depolymerase activity of MCAK 6A mutants. Furthermore, MCAK 6A mutant protein is likely to act dominantly over the endogenous MCAK. Thus, we conclude that precise regulation of MCAK phosphorylation by PLK1 is critical for faithful mitotic progression.
Phosphorylation by PLK1 Orchestrates the Intramolecular Interaction of MCAK
The MCAK depolymerase activity is regulated by its C-terminal domain (22–24). We propose that the C-terminal domain and neck motor domain may act together to control the MCAK conformation and that the phosphorylation by PLK1 may regulate such a conformation change, which, in turn, modulates the depolymerase activity of MCAK. To test this hypothesis, we first examined whether there is an intramolecular interaction in MCAK. Recombinant GST-MCAK(1–181) and GST-MCAK(182–586) were incubated with purified MCAK-His, and GST pulldown assay was performed. MCAK-His associated with both MCAK(1–181) and MCAK(182–586) (Fig. 6A), indicating that the intramolecular interaction does exist in MCAK in addition to its dimerization that is mediated by the C-terminal coiled-coil domain from two MCAK molecules. On the other hand, neither MCAK(1–181) nor MCAK(182–586) formed a homodimer or a heterodimer (data not shown) indicating such intramolecular interaction is specifically mediated by the C-terminal domain with the N-terminal region and the neck motor region.
FIGURE 6.
PLK1 phosphorylation regulates MCAK intramolecular interaction. A, MCAK exhibits an intramolecular association in vitro. Recombinant GST-MCAK(1–181) and -(182–586) on glutathione beads were incubated with MCAK-His purified from Sf9 cells as described in Fig. 1B. Anti-MCAK antibody was used in Western blot analysis to determine the intramolecular interaction of MCAK. B, PLK1 phosphorylation promotes the intramolecular association of MCAK in vitro. Recombinant MCAK deletion mutant proteins (GST-MCAK(1–181) and GST-MCAK(182–586)) on glutathione-agarose beads were used as an affinity matrix to isolate MCAK WT-His and its phosphorylation sites mutants from Sf9 cells. Anti-MCAK antibody was used in Western blot analysis to determine the phospho-regulation of the intramolecular interaction of MCAK. C, statistical analysis of the relative binding activity in B. The ordinate indicates the binding ratio of the intensity of MCAK (WT, 6A, or 6E)-His to GST-MCAK (MCAK(1–181) or MCAK(182–586)); the abscissa indicates the corresponding binding assay shown in B.
Next, we determined whether phosphorylation by PLK1 regulates the intramolecular interaction of MCAK. Recombinant GST-MCAK(1–181) and GST-MCAK(182–586) were incubated with purified MCAK-His, MCAK 6A-His, and MCAK 6E-His, and protein complexes were pulled down by glutathione beads. MCAK 6E-His interacted stronger to GST-MCAK(182–586) than MCAK-His and MCAK 6A-His. MCAK 6E-His was also appreciably pulled down more efficiently by GST-MCAK(1–181) than MCAK-His and MCAK 6A-His. We used the MCAK C-terminal domain and its phosphorylation site mutants to pull down the N-terminal domain or the neck motor domain. However, the interaction was too weak to evaluate (data not shown). We conclude that the C-terminal domain of MCAK interacts with the N-terminal and neck motor domain of the molecule, which is modulated by PLK1 phosphorylation in mitosis.
DISCUSSION
Our studies revealed that PLK1 physically interacts with MCAK, both in vitro and in vivo, and that MCAK is a cognate substrate of PLK1 in mitosis. The PLK1-mediated phosphorylation promotes the MT depolymerization activity of MCAK in vivo. Importantly, the dynamic regulation of MCAK phosphorylation by PLK1 is essential for accurate chromosome segregation as perturbation of MCAK phosphorylation dynamics causes severe defects in chromosome segregation during mitosis. Our study provides novel insight into a better understanding of MCAK-PLK1 interaction in regulating MT dynamics and chromosomes stability during cell division.
MCAK is localized to various subcellular structures in mitotic cells, such as inner centromeres, outer kinetochores, centrosomes, spindle MTs, MT tips, and spindle midzone (8, 14–17). This diverse localization of MCAK in mitosis implies a complex regulation. Previous reports showed that MCAK activity is negatively regulated by Aurora B at centromeres (15, 34–38) and negatively controlled by Ca2+/calmodulin-dependent protein kinase IIγ and CDK1 at centrosomes (28, 29). However, no positive regulator of MCAK has been identified so far. We report here that PLK1 is a novel binding partner of MCAK (Figs. 1 and 2) and that phosphorylation of MCAK by PLK1 stimulates the depolymerization activity of MCAK (Fig. 4). Because most of the phosphorylation sites identified in this work on MCAK are conserved in human, mouse, Xenopus, and hamster, the regulation of MCAK by PLK1 is likely conserved during evolution (Fig. 3C).
Previous reports show that the C-terminal domain of MCAK controls its MT depolymerase activity in vitro (22, 23). Our study indicates that PLK1-mediated phosphorylation of MCAK in the C-terminal domain regulates its microtubule depolymerase activity. Overexpression of the phosphomimetic mutant of MCAK (MCAK 6E) in HeLa cells caused obviously reduced MT density relative to that of nonphosphorylatable MCAK mutant (MCAK 6A) (Fig. 4, B–E). In addition, phosphorylation of MCAK by PLK1 also controls the MT dynamics by attenuating MT regrowth rate and promotes the MT bundling activity of MCAK in vivo.3 Overexpression of MCAK results in bundling of spindle MTs and eventually depolymerization of MTs (7), suggesting that the MT bundling activity and MT depolymerization activity of MCAK may intimately related. Thus, the enhanced bundling activity of MCAK 6E is likely related to its increased microtubule depolymerase activity. Furthermore, HeLa cells with overexpressed MCAK 6E often displayed defects in spindle assembly (data not shown), which could also be explained by its hyperactive depolymerase activity (7, 29).
A previous study indicates that the C-terminal domain of MCAK controls its depolymerase activity (24). We found that PLK1 phosphorylates the C-terminal domain of MCAK and that this phosphorylation promotes its depolymerization activity. Intriguingly, our results revealed the existence of an intramolecular interaction in MCAK (Fig. 6A), and this interaction is regulated by PLK1 phosphorylation (Fig. 6B). Our study indicates that the regulation of MCAK conformation by PLK1 may be essential for the depolymerase function of MCAK C-terminal domain. Further investigations are warranted to elucidate the structure basis underlying this PLK1-regulated MCAK conformational change and the structural-functional relationship of MCAK depolymerase activity.
Overexpression of MCAK 6E or MCAK WT led to an increase in misaligned chromosomes at metaphase, although overexpression of MCAK 6A only caused a minimal increase (Fig. 5). Cells expressing MCAK WT or MCAK 6E, which has hyperdepolymerase activity, exhibited increased frequency of misaligned chromosomes (Fig. 5, A, B, D, and E) and displayed a mitotic arrest (Fig. 5, F and G) as a result of the abnormal spindle assembly (7, 20, 29)3 and improper spindle dynamics (20). In contrast, overexpression of MCAK 6A often resulted in anaphase disruption with lagging chromosomes (Fig. 5, F and G). Previous studies have revealed that lagging chromosomes are also observed in cells expressing motorless MCAK mutant and in cells depleted of MCAK (7, 11, 16, 26). Therefore, anaphase defects in MCAK 6A cells are likely due to the deficient depolymerase activity of the MCAK 6A mutant, suggesting that dynamic regulation of MCAK activity is essential to allow timely correction of aberrant microtubule-kinetochore attachments for subsequent anaphase onset. On the other hand, the hyperactivity of MCAK in cells resulted in the formation of multipolar spindles (27, 28). Consistent with this, in MCAK 6E- or MCAK WT-expressing cells, the frequency of multipolar spindle was significantly increased (Fig. 5, A, B, D, and E). Interestingly, MCAK has been observed to be overexpressed in human gastric cancer and breast cancer cells (30, 31), and the phosphorylation level of MCAK also significantly increases in the G2/M phase in these cells (31). Given the fact that PLK1 is typically overexpressed in many human tumors (43, 44), we speculate that the high level of MCAK and PLK1 in tumors may cooperate to promote genomic instability due to high frequency of chromosome mis-alignment and multipolar spindles due to a constitutive activation of MCAK. Thus, the precise regulation of MCAK activity by PLK1 may play a key role for the faithful chromosome alignment and segregation.
MCAK undergoes complex regulation in mitosis. Its activity is negatively regulated by Aurora B at centromeres at prometaphase (15, 16, 34, 36). At metaphase, when tension across kinetochores is established, the extent of Aurora B activity gradient across sister kinetochore pairs is limited; inhibition of MCAK by Aurora B is released, and active MCAK then acts to promote microtubule dynamics for correcting the mal-oriented chromosomes (26). However, no kinase that positively regulates MCAK in metaphase has been identified. We had speculated that MCAK is phosphorylated by PLK1 at metaphase at kinetochores, which is the main drive for this study. The identification of PLK1-mediated phosphorylation sites in MCAK in vitro and in vivo, from this study and by others (56), strongly supports this notion. The great excitement and challenges ahead are to illustrate the spatiotemporal dynamics of PLK1-mediated MCAK phosphorylation in mitosis by developing application of phosphorylation site-specific antibodies.
It is worth noting that PLK1-MCAK interaction may also orchestrate the anaphase events, as perturbation of MCAK phosphorylation (overexpression of MCAK 6A mutant) resulted in chromosome instability phenotypes such as chromatid bridges in anaphase cells (Fig. 5, F and G). In addition, it would be of great importance to delineate the spatiotemporal control of MCAK by different mitotic kinases such as CDK1, Aurora A, Aurora B, and PLK1 and to establish the functional relationships of these modifications in mitotic orchestration of MCAK activity in space and time (57).
Taken together, we propose that dynamic phospho-regulation of MCAK by PLK1 establishes accurate association between kinetochore and spindle microtubules and correcting kinetochore-microtubule attachment errors. It is likely that multiple mitotic kinase cascades interact to orchestrate faithful chromosome segregation in mitosis. The PLK1-MCAK interaction established here is a core of this dynamic molecular society that links cell cycle progression to chromosome stability during cell division.
Acknowledgments
We greatly appreciate the insect expressing GST-PLK1 from Dr. Ray Erickson (Harvard University). We thank members of our groups for insightful discussion and technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-56292 and CA132389 and NCRR Grant UL1 RR025008 from the Clinical and Translational Science Award Program, and NCRR Grant G12RR03034 (for use of facilities). This work was also supported by Chinese Natural Science Foundation Grants 30500183 and 30870990 (to X. D.) and 90508002 and 90913016 (to X. Y.), Chinese Academy of Science Grants KSCX1-YW-R-65, KSCX2-YW-H-10, and KSCX2-YW-R-195, Chinese 973 Project Grants 2006CB943603, 2007CB914503, and 2010CB912103, International Collaboration Grant 2009DFA31010 (to X. D.), Technology Grant 2006BAI08B01-07 (to X. D.). China National Key Projects for Infectious Disease Grant 2008ZX10002-021, a Georgia Cancer Coalition breast cancer research grant, Atlanta Clinical and Translational Science Award Chemical Biology Grant P20RR011104, and Anhui Province Key Project Grant 08040102005.
L. Zhang and X. Yao, unpublished observations.
- MT
- microtubule
- MCAK
- mitotic centromere-associated kinesin.
REFERENCES
- 1. Gadde S., Heald R. (2004) Curr. Biol. 14, R797–R805 [DOI] [PubMed] [Google Scholar]
- 2. Tournebize R., Popov A., Kinoshita K., Ashford A. J., Rybina S., Pozniakovsky A., Mayer T. U., Walczak C. E., Karsenti E., Hyman A. A. (2000) Nat. Cell Biol. 2, 13–19 [DOI] [PubMed] [Google Scholar]
- 3. Desai A., Verma S., Mitchison T. J., Walczak C. E. (1999) Cell 96, 69–78 [DOI] [PubMed] [Google Scholar]
- 4. Moores C. A., Yu M., Guo J., Beraud C., Sakowicz R., Milligan R. A. (2002) Mol. Cell 9, 903–909 [DOI] [PubMed] [Google Scholar]
- 5. Ogawa T., Nitta R., Okada Y., Hirokawa N. (2004) Cell 116, 591–602 [DOI] [PubMed] [Google Scholar]
- 6. Lawrence C. J., Dawe R. K., Christie K. R., Cleveland D. W., Dawson S. C., Endow S. A., Goldstein L. S., Goodson H. V., Hirokawa N., Howard J., Malmberg R. L., McIntosh J. R., Miki H., Mitchison T. J., Okada Y., Reddy A. S., Saxton W. M., Schliwa M., Scholey J. M., Vale R. D., Walczak C. E., Wordeman L. (2004) J. Cell Biol. 167, 19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maney T., Hunter A. W., Wagenbach M., Wordeman L. (1998) J. Cell Biol. 142, 787–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walczak C. E., Mitchison T. J., Desai A. (1996) Cell 84, 37–47 [DOI] [PubMed] [Google Scholar]
- 9. Cassimeris L., Morabito J. (2004) Mol. Biol. Cell 15, 1580–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mennella V., Rogers G. C., Rogers S. L., Buster D. W., Vale R. D., Sharp D. J. (2005) Nat. Cell Biol. 7, 235–245 [DOI] [PubMed] [Google Scholar]
- 11. Ganem N. J., Upton K., Compton D. A. (2005) Curr. Biol. 15, 1827–1832 [DOI] [PubMed] [Google Scholar]
- 12. Ganem N. J., Compton D. A. (2004) J. Cell Biol. 166, 473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manning A. L., Ganem N. J., Bakhoum S. F., Wagenbach M., Wordeman L., Compton D. A. (2007) Mol. Biol. Cell 18, 2970–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wordeman L., Mitchison T. J. (1995) J. Cell Biol. 128, 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews P. D., Ovechkina Y., Morrice N., Wagenbach M., Duncan K., Wordeman L., Swedlow J. R. (2004) Dev. Cell 6, 253–268 [DOI] [PubMed] [Google Scholar]
- 16. Kline-Smith S. L., Khodjakov A., Hergert P., Walczak C. E. (2004) Mol. Biol. Cell 15, 1146–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moore A. T., Rankin K. E., von Dassow G., Peris L., Wagenbach M., Ovechkina Y., Andrieux A., Job D., Wordeman L. (2005) J. Cell Biol. 169, 391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunter A. W., Caplow M., Coy D. L., Hancock W. O., Diez S., Wordeman L., Howard J. (2003) Mol. Cell 11, 445–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newton C. N., Wagenbach M., Ovechkina Y., Wordeman L., Wilson L. (2004) FEBS Lett. 572, 80–84 [DOI] [PubMed] [Google Scholar]
- 20. Kline-Smith S. L., Walczak C. E. (2002) Mol. Biol. Cell 13, 2718–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maney T., Wagenbach M., Wordeman L. (2001) J. Biol. Chem. 276, 34753–34758 [DOI] [PubMed] [Google Scholar]
- 22. Hertzer K. M., Ems-McClung S. C., Kline-Smith S. L., Lipkin T. G., Gilbert S. P., Walczak C. E. (2006) Mol. Biol. Cell 17, 700–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ems-McClung S. C., Hertzer K. M., Zhang X., Miller M. W., Walczak C. E. (2007) Mol. Biol. Cell 18, 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cooper J. R., Wagenbach M., Asbury C. L., Wordeman L. (2010) Nat. Struct. Mol. Biol. 17, 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wordeman L., Wagenbach M., von Dassow G. (2007) J. Cell Biol. 179, 869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bakhoum S. F., Thompson S. L., Manning A. L., Compton D. A. (2009) Nat. Cell Biol. 11, 27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmfeldt P., Stenmark S., Gullberg M. (2004) EMBO J. 23, 627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holmfeldt P., Zhang X., Stenmark S., Walczak C. E., Gullberg M. (2005) EMBO J. 24, 1256–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanhaji M., Friel C. T., Kreis N. N., Krämer A., Martin C., Howard J., Strebhardt K., Yuan J. (2010) Mol. Cell. Biol. 30, 2594–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakamura Y., Tanaka F., Haraguchi N., Mimori K., Matsumoto T., Inoue H., Yanaga K., Mori M. (2007) Br. J. Cancer 97, 543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimo A., Tanikawa C., Nishidate T., Lin M. L., Matsuda K., Park J. H., Ueki T., Ohta T., Hirata K., Fukuda M., Nakamura Y., Katagiri T. (2008) Cancer Sci. 99, 62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Luca M., Brunetto L., Asteriti I. A., Giubettini M., Lavia P., Guarguaglini G. (2008) Oncogene 27, 6539–6549 [DOI] [PubMed] [Google Scholar]
- 33. Zhang X., Ems-McClung S. C., Walczak C. E. (2008) Mol. Biol. Cell 19, 2752–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lan W., Zhang X., Kline-Smith S. L., Rosasco S. E., Barrett-Wilt G. A., Shabanowitz J., Hunt D. F., Walczak C. E., Stukenberg P. T. (2004) Curr. Biol. 14, 273–286 [DOI] [PubMed] [Google Scholar]
- 35. Ohi R., Sapra T., Howard J., Mitchison T. J. (2004) Mol. Biol. Cell 15, 2895–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knowlton A. L., Lan W., Stukenberg P. T. (2006) Curr. Biol. 16, 1705–1710 [DOI] [PubMed] [Google Scholar]
- 37. Zhang X., Lan W., Ems-McClung S. C., Stukenberg P. T., Walczak C. E. (2007) Mol. Biol. Cell 18, 3264–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sampath S. C., Ohi R., Leismann O., Salic A., Pozniakovski A., Funabiki H. (2004) Cell 118, 187–202 [DOI] [PubMed] [Google Scholar]
- 39. Ohi R., Coughlin M. L., Lane W. S., Mitchison T. J. (2003) Dev. Cell 5, 309–321 [DOI] [PubMed] [Google Scholar]
- 40. Huang H., Feng J., Famulski J., Rattner J. B., Liu S. T., Kao G. D., Muschel R., Chan G. K., Yen T. J. (2007) J. Cell Biol. 177, 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sunkel C. E., Glover D. M. (1988) J. Cell Sci. 89, 25–38 [DOI] [PubMed] [Google Scholar]
- 42. Petronczki M., Lénárt P., Peters J. M. (2008) Dev. Cell 14, 646–659 [DOI] [PubMed] [Google Scholar]
- 43. Eckerdt F., Yuan J., Strebhardt K. (2005) Oncogene 24, 267–276 [DOI] [PubMed] [Google Scholar]
- 44. Strebhardt K., Ullrich A. (2006) Nat. Rev. Cancer 6, 321–330 [DOI] [PubMed] [Google Scholar]
- 45. Hamanaka R., Maloid S., Smith M. R., O'Connell C. D., Longo D. L., Ferris D. K. (1994) Cell Growth & Differ. 5, 249–257 [PubMed] [Google Scholar]
- 46. Holtrich U., Wolf G., Bräuninger A., Karn T., Böhme B., Rübsamen-Waigmann H., Strebhardt K. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 1736–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Golsteyn R. M., Mundt K. E., Fry A. M., Nigg E. A. (1995) J. Cell Biol. 129, 1617–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kang Y. H., Park J. E., Yu L. R., Soung N. K., Yun S. M., Bang J. K., Seong Y. S., Yu H., Garfield S., Veenstra T. D., Lee K. S. (2006) Mol. Cell 24, 409–422 [DOI] [PubMed] [Google Scholar]
- 49. Jang C. Y., Coppinger J. A., Seki A., Yates J. R., 3rd, Fang G. (2009) J. Cell Sci. 122, 1334–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yuan K., Hu H., Guo Z., Fu G., Shaw A. P., Hu R., Yao X. (2007) J. Biol. Chem. 282, 27414–27423 [DOI] [PubMed] [Google Scholar]
- 51. Jiang K., Wang J., Liu J., Ward T., Wordeman L., Davidson A., Wang F., Yao X. (2009) EMBO Rep. 10, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu J., Wang Z., Jiang K., Zhang L., Zhao L., Hua S., Yan F., Yang Y., Wang D., Fu C., Ding X., Guo Z., Yao X. (2009) J. Biol. Chem. 284, 23059–23071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chu Y., Yao P. Y., Wang W., Wang D., Wang Z., Zhang L., Huang Y., Ke Y., Ding X., Yao X. (2010) J. Mol. Cell. Biol. doi 10.1093/jmcb/mjq037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han G., Ye M., Jiang X., Chen R., Ren J., Xue Y., Wang F., Song C., Yao X., Zou H. (2009) Anal. Chem. 81, 5794–5805 [DOI] [PubMed] [Google Scholar]
- 55. Yao X., Abrieu A., Zheng Y., Sullivan K. F., Cleveland D. W. (2000) Nat. Cell Biol. 2, 484–491 [DOI] [PubMed] [Google Scholar]
- 56. Santamaria A., Wang B., Elowe S., Malik R., Zhang F., Bauer M., Schmidt A., Sillje H. H., Koerner R., Nigg E. A. (2010) Mol. Cell. Proteomics doi 10.1074/mcp.M110.004457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ding X., Yan F., Yao P., Yang Z., Wan W., Wang X., Liu J., Gao X., Abrieu A., Zhu T., Zhang J., Dou Z., Yao X. (2010) Cell Res. 20, 1386–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]