Abstract
Disturbed endochondral ossification in X-linked hypophosphatemia indicates an involvement of Pi in chondrogenesis. We studied the role of the sodium-dependent Pi cotransporters (NPT), which are a widely recognized regulator of cellular Pi homeostasis, and the downstream events in chondrogenesis using Hyp mice, the murine homolog of human X-linked hypophosphatemia. Hyp mice showed reduced apoptosis and mineralization in hypertrophic cartilage. Hyp chondrocytes in culture displayed decreased apoptosis and mineralization compared with WT chondrocytes, whereas glycosaminoglycan synthesis, an early event in chondrogenesis, was not altered. Expression of the type III NPT Pit-1 and Pi uptake were diminished, and intracellular ATP levels were also reduced in parallel with decreased caspase-9 and caspase-3 activity in Hyp chondrocytes. The competitive NPT inhibitor phosphonoformic acid and ATP synthesis inhibitor 3-bromopyruvate disturbed endochondral ossification with reduced apoptosis in vivo and suppressed apoptosis and mineralization in conjunction with reduced Pi uptake and ATP synthesis in WT chondrocytes. Overexpression of Pit-1 in Hyp chondrocytes reversed Pi uptake and ATP synthesis and restored apoptosis and mineralization. Our results suggest that cellular ATP synthesis consequent to Pi uptake via Pit-1 plays an important role in chondrocyte apoptosis and mineralization, and that chondrogenesis is ATP-dependent.
Keywords: Apoptosis, ATP, ATPases, Caspase, Differentiation, Pit-1, Chondrocyte
Introduction
Endochondral ossification is critical to the development and growth of mammals. The process begins with condensation of undifferentiated mesenchymal cells, and these cells differentiate into proliferating chondrocytes that express type II, IX, and XI collagen and sulfated glycosaminoglycans (GAG)2 (1). Proliferating chondrocytes further differentiate into hypertrophic chondrocytes expressing type X collagen, undergo apoptosis, mineralize, and are ultimately replaced by bone. Disturbance of the endochondral ossification leads to a variety of skeletal disorders.
The genetic disease X-linked hypophosphatemia (XLH) is the most common form of inherited rickets in humans and is related to the dominant disorder of Pi homeostasis (2). XLH has been shown to be caused by inactive mutations of the PHEX gene and characterized by hypophosphatemia secondary to renal Pi wasting, growth retardation due to disturbed endochondral ossification, osteomalacia resulting from reduced mineralization, and abnormally regulated vitamin D metabolism (3). Hyp mice also display similar biochemical and phenotypic abnormalities to human XLH, including hypophosphatemia, osteomalacia, and skeletal abnormalities. Hyp mice thus are a mouse homolog of human XLH (4). Previous studies reported that Hyp mice exhibited disorganized hypertrophic cartilage with reduced apoptotic chondrocytes and hypomineralization (5). We have reported previously that osteoclast number was decreased in Hyp mice compared with WT mice and that a high-Pi diet partially restored this, showing that Pi influences osteoclastogenesis and suggesting that this Pi effect on osteoclastogenesis may be associated with the pathogenesis of abnormal skeletogenesis in Hyp mice (6). However, it remains unclear whether disturbed Pi homeostasis influences endochondral ossification, leading to abnormal skeletogenesis in Hyp mice. In this context, it is noted that intracellular Pi levels decrease and extracellular Pi levels prominently increase from the proliferating to the hypertrophic zone during chondrogenesis, suggesting that cellular Pi levels are associated with chondrocyte differentiation (7–10).
Cellular Pi levels are controlled by the sodium-dependent Pi cotransporters (NPT) (11). Previous studies reported that the type III NPT Pit-1 was expressed in hypertrophic chondrocytes during endochondral ossification in mice (12) and that the expression of the type IIa NPT Npt2a and Pit-1 was also detected in chick chondrocytes (13). Moreover, it has been demonstrated that Pi modulates chondrocyte differentiation (14–19) and apoptosis (13, 20).
On the basis of these earlier results, we hypothesized that the NPT-Pi system plays a critical role in the regulation of chondrocyte differentiation. We found that Pit-1 expression in chondrocytes was decreased in Hyp mice compared with WT mice and that Pit-1 regulated apoptosis and mineralization in chondrocytes through modulating intracellular ATP synthesis and apoptotic signaling activity. On the other hand, Hyp chondrocytes showed no changes in GAG synthesis, which is an early event in chondrogenesis. Our findings suggest that ATP synthesis mediated by Pi influx via Pit-1 is critical in the regulation of late chondrogenesis, including apoptosis and mineralization, and that the differentiation of cartilage is an ATP-dependent event.
EXPERIMENTAL PROCEDURES
Animals
All mice used were of the C57BL/6J strain. Normal mice were purchased from Nihon-Dobutsu Inc. (Osaka, Japan). Hyp mice were initially obtained from The Jackson Laboratory (Bar Harbor, ME) and were produced by cross-mating homozygous Hyp females (Hyp/Hyp) with hemizygous Hyp males (Hyp/Y). All animal experiments were performed according to the guidelines of the Institutional Animal Care and Use Committee of the Osaka University Graduate School of Dentistry.
Isolation and Culture of Mouse Growth Plate Chondrocytes
Growth plate chondrocytes were isolated from the ribs of 4-week-old normal and Hyp mice by sequential digestion with 0.2% trypsin (Invitrogen) for 30 min and 0.2% collagenase (Wako Pure Chemical Industries Ltd., Osaka, Japan) for 3 h as reported previously (21). Isolated cells were plated onto 100-mm tissue culture dishes at a density of 1 × 106 cells in α-minimal essential medium (Sigma) supplemented with 10% FCS (Valley Biomedical, Inc., Winchester, VA), 2 mmol/liter l-glutamine, and 0.1 mg/ml kanamycin. Two days later, to induce chondrogenesis and cartilage nodule formation, the cells were plated at 3 × 105 cells/well onto 24-well plates or at 5 × 104 cells/well on 96-well plates coated with type I collagen (Nitta Gelatin Inc., Osaka, Japan) and cultured in differentiation medium consisting of DMEM (Sigma) supplemented with 10% FCS, 50 μg/ml ascorbic acid, and 100 ng/ml recombinant human bone morphogenetic protein-2 (Astellas Pharma Inc., Tokyo, Japan) for 7 days. From day 5 to day 7, to promote matrix mineralization, 5 mm β-glycerophosphate was added to the differentiation medium.
RT-PCR and Real-time PCR
Total RNA from chondrocytes was prepared using an RNeasy kit (Qiagen, Inc., Valencia, CA) and reverse-transcribed with SuperScript II reverse transcriptase (Invitrogen). The primer sequences for mouse Npt1, mouse Npt2a, GAPDH, and β-actin are available on request. PCR assays were performed using Taq DNA polymerase (New England Biolabs, Ipswich, MA) and dNTP mixture (Promega Corp., Madison, WI). Real-time PCR assays were performed using a LightCycler system (Roche Diagnostics) according to the manufacturer's instructions. Each reaction was carried out with Qiagen QuantiTect SYBR Green PCR Master Mix. The expression levels of mRNA are indicated as the relative expression normalized by GAPDH. The primer sequences are available upon request. Each procedure was repeated at least four times to assess reproducibility.
Measurement of Sodium-dependent Pi Uptake
Assay for sodium-dependent Pi uptake by growth plate chondrocytes was performed essentially as described (22). Briefly, confluent cells cultured in 24-well Costar microtiter dishes were incubated in 2 ml of uptake solution (150 mmol/liter NaCl, 1.0 mmol/liter CaCl2, 1.8 mmol/liter MgSO4, and 10 mmol/liter HEPES (pH 7.4)) at 37 °C for 5 min. Transport was then initiated by replacing the uptake solution with fresh uptake solution (2 ml) supplemented with 0.1 mmol/liter KH2PO4 and containing 3 μCi/ml KH232PO4 (MP Biomedicals, Inc., Irvine, CA). Cells were then incubated for 5 min at 37 °C, and the reaction was stopped by the addition of ice-cold uptake solution supplemented with 150 mmol/liter choline chloride substitution for NaCl. The same solution was then used to wash the cells three times (2 ml/wash) and dissolved in 0.2 n NaOH, and the 32P activity was counted on a scintillation counter. As a control, sodium-independent Pi transport was measured in the same way, except that NaCl was replaced by choline chloride in the uptake solution. Data are expressed as nanomoles of Pi/mg of cellular protein/5 min, and sodium-dependent Pi transport was calculated by subtracting sodium-independent Pi transport from total Pi transport.
Alcian Blue Staining
Cell layers in 24-well plates were fixed with 3.7% formaldehyde for 10 min and with 70% ethanol for 5 min at room temperature. After fixation, the cells were incubated with 5% acetic acid (pH 1.0, adjusted with HCl) for 5 min and stained with 1% Alcian blue dye (Wako Pure Chemical Industries Ltd.) in 5% acetic acid for 10 min. The Alcian blue staining was quantified using NIH Image 1.63 software.
Alizarin Red Staining
Cell layers in 24-well plates were fixed with 3.7% formaldehyde for 10 min and with 95% ethanol for 10 min at room temperature. After fixation, mineralized nodules were stained with 1% alizarin red S (Wako Pure Chemical Industries Ltd.) at pH 6.4 for 10 min at room temperature. The stained samples were washed three times with water and then air-dried. The alizarin red staining was quantified using NIH Image 1.63 software.
Treatment with NPT Inhibitor
In vitro, chondrocytes were treated with phosphonoformic acid (PFA or foscarnet, Sigma), which is a competitive inhibitor of NPT (23), at concentrations of 10−5 to 10−3 m in the differentiation medium from day 1 to day 7. In vivo, mice received PFA as described (24). PFA injection (intraperitoneal; 1000 mg/kg of body weight) was started at 21 days of age and injected daily for 10 days into C57BL/6J mice. Histological analysis was performed at 31 days of age. Control mice received vehicle PBS.
Knockdown of Npt2a and Pit-1 by siRNA
Chondrocytes were seeded at a density of 5 × 105 cells in 100-mm tissue culture dishes in α-minimal essential medium supplemented with 10% FCS and 2 mmol/liter l-glutamine. The sequences of Stealth RNAi duplex oligoribonucleotides for mouse Npt2a and mouse Pit-1 are available upon request. Npt2a-targeted, Pit-1-targeted, or negative control (medium GC, Invitrogen) Stealth RNAi duplex oligoribonucleotides were each added to 1 ml of serum-free Opti-MEM I reduced serum medium (Invitrogen) at a final concentration of 24 nm. In a separate tube, 20 μl of Lipofectamine RNAiMAX (Invitrogen) were diluted in 1 ml of serum-free Opti-MEM I reduced serum medium. After adding the siRNA solution to the Lipofectamine solution, the final transfection mixture was incubated for 20 min at room temperature. This transfection mixture was applied to the cells. After 48 h, RNA extraction was performed for RT-PCR, and transfected chondrocytes were plated onto 24- or 96-well plates to determine Pi transport, intracellular ATP levels, caspase activity, and apoptosis.
Pit-1 Overexpression
The cDNA was subcloned into the 5′-XhoI/BamHI-3′ site of pcDNA3.1/Zeo (Invitrogen). The cells were transfected using FuGENETM-6 (Roche Diagnostics) according to the manufacturer's protocol. After 48 h of transfection, RNA extraction was performed for RT-PCR, or transfected chondrocytes were replated onto 24- or 96-well plates to determine Pi transport, intracellular ATP levels, caspase activity, and apoptosis. The cDNA for mouse Pit-1 in the plasmid pBluescript was a generous gift of Dr. Kenichi Miyamoto (University of Tokushima Graduate School, Tokushima, Japan).
Histology and TUNEL Staining
Tibias were harvested, washed with PBS, fixed with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) overnight, decalcified in 4.13% EDTA at room temperature for 2 weeks, and embedded in paraffin. Four-μm thick sections were made and stained with hematoxylin and eosin. Apoptotic cells were identified using the DeadEnd fluorometric TUNEL system (Promega Corp.). After treatment with 10 mg/ml proteinase K for 10 min at room temperature, sections were incubated with rTdT incubation buffer for 1 h at 37 °C, rinsed, counterstained with 1 μg/ml DAPI (Vector Laboratories, Ltd., Burlingame, CA), and mounted with Fluoromount-G (Southern Biotechnology Associates, Inc., Birmingham, AL). The green TUNEL emission was analyzed under a fluorescein filter set to view the green fluorescence of fluorescein at 520 nm and blue DAPI at 460 nm.
Measurement of Apoptotic Cell Death
DNA fragmentation was measured using the cell death detection ELISAPLUS kit (Roche Diagnostics), which detects the cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) by photometric enzyme immunoassay. Briefly, after differentiation of chondrocytes in 96-well plates, cell lysates were used for the ELISA procedure following the manufacturer's protocol. DNA fragmentation was quantified at 405 nm. Results were normalized to cellular protein concentration.
Measurement of Caspase-9 and Caspase-3 Activity
Activity of caspase-3 and caspase-9 was measured using the Caspase-Glo 3/7 and Caspase-Glo 9 assay kit (Promega corp.) according to the manufacturer's instructions. Chondrocytes were cultured at a density of 5 × 104 cells/well for 5 days in 96-well plates in the differentiation medium and processed for caspase-9 and caspase-3 activity assays. The luminescence was measured using a TD-20/20 luminometer (Tuner Designs, Sunnyvale, CA). Results were normalized to cellular protein concentration.
Measurement of Intracellular ATP Levels
Intracellular ATP levels were measured using an ATP assay kit (Calbiochem). This assay utilizes luciferase to catalyze the formation of light from ATP and luciferin. Luminescence was measured using a TD-20/20 luminometer. Chondrocytes were cultured at a density of 5 × 104 cells/well for 24 h in 24-well plates and processed for ATP bioluminescence assays. Results were normalized to cellular protein concentration.
Treatment with ATP Synthesis Inhibitor
In vitro, 3-bromopyruvate (3-BrPA; Sigma), a strong alkylating agent that abolishes cell ATP production via the inhibition of both glycolysis and oxidative phosphorylation (25–27), was added at 10−6∼10−5 m in the differentiation medium from day 1 to day 7. In vivo, 3-BrPA (20 μg/kg of body weight) was injected intraperitoneally daily for 10 days into C57BL/6J mice. Control mice received vehicle PBS.
Statistical Analysis
Data are presented as the mean ± S.E. Raw data were analyzed by the Mann-Whitney U test or one-way analysis of variance, followed by a post hoc test (Fisher's projected least significant difference) (StatView, SAS Institute, Inc., Cary, NC) with a significance level of p < 0.05.
RESULTS
Reduced Apoptosis and Mineralization in Growth Plate Cartilage in Hyp Mice
It has been reported that apoptosis is a prerequisite to mineralization of chondrocytes (28). Previous studies, including ours, have reported that the growth plate cartilage in Hyp mice is hypomineralized (5, 6). We therefore examined apoptosis in the growth plate cartilage in Hyp mice compared with WT mice. Histological examination revealed that hypertrophic cartilage was elongated and disorganized in Hyp mice (Fig. 1A, left). In conjunction with this, TUNEL staining showed decreased apoptosis in hypertrophic cartilage in Hyp mice (Fig. 1, A (right) and B). Consistent with these in vivo results, chondrocytes isolated from Hyp mice (Hyp chondrocytes) in culture showed decreased apoptosis assessed by DNA fragmentation using a commercially available ELISA kit (Fig. 1C), and mineralization was determined by alizarin red staining (Fig. 1D, lower). Quantification of alizarin red staining is shown in Fig. 1F. However, GAG synthesis, which takes place at an early stage of chondrogenesis, was not altered in Hyp chondrocytes as determined by Alcian blue staining (Fig. 1D, upper) Alcian blue staining is quantified in Fig. 1E.
FIGURE 1.
Apoptosis and related events in Hyp chondrocytes. A, histological examination of chondrocyte apoptosis. Hematoxylin/eosin staining (left) and TUNEL staining (right) were performed using tibias of 4-week-old WT and Hyp mice. The hypertrophic zone is marked with dotted lines, and the scale bars indicate 200 μm. Representative pictures obtained of numerous sections of four mice from each group are shown. B, number of TUNEL-positive cells in the tibial growth plates of WT and Hyp mice. C, quantitative determination of chondrocyte apoptosis. Cells were cultured in the differentiation medium in 96-well plates for 7 days. The determination was conducted using the cell death detection ELISAPLUS kit after differentiation. Data are shown as apoptotic activity. D, histochemical staining of WT and Hyp chondrocytes. Cells were cultured for 7 days in the differentiation medium and stained with Alcian blue for GAG synthesis (upper) and with alizarin red S for mineralization (lower). E, quantification of Alcian blue staining. F, quantification of alizarin red staining. Results are expressed as the mean ± S.E. of four separate experiments. *, significantly different from WT chondrocytes (p < 0.05).
Cellular Events Involved in Reduced Apoptosis in Hyp Chondrocytes
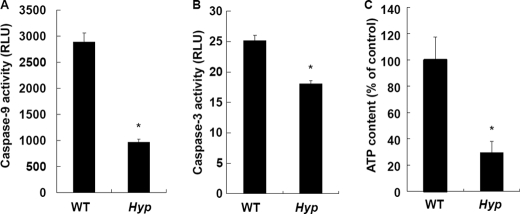
Because activation of caspase-9 and caspase-3 is an important step that leads to apoptosis, the activity of caspase-9 and caspase-3 was next determined in WT and Hyp chondrocytes in culture. The activity of caspase-9 (Fig. 2A) and caspase-3 (Fig. 2B) was significantly decreased in Hyp chondrocytes. ATP has been reported to be critical in the activation of caspase-9 and caspase-3 (29, 30). Accordingly, we determined intracellular ATP levels in WT and Hyp chondrocytes and found that intracellular ATP levels in Hyp chondrocytes were significantly reduced compared with WT chondrocytes (Fig. 2C). Collectively, these results suggest that decreased ATP levels impaired caspase signals following apoptosis in Hyp chondrocytes.
FIGURE 2.
Activity of apoptotic signaling pathways. A, caspase-9 activity in WT and Hyp chondrocytes. B, caspase-3 activity in WT and Hyp chondrocytes. Activity was measured using the Caspase-Glo 9 and Caspase-Glo 3/7 assay kits after differentiation. C, intracellular ATP levels in WT and Hyp chondrocytes. Cells were cultured at a density of 1 × 104 cells/well in 96-well plates for 24 h. ATP levels were measured using the ATP assay kit. *, significantly different from WT chondrocytes (p < 0.05). RLU, relative light units.
Disturbed Pi Homeostasis in Hyp Chondrocytes
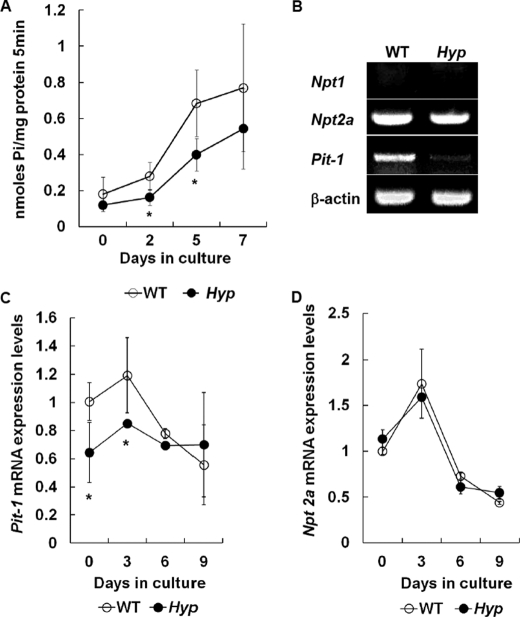
It has been described that Pi is a source of ATP (31). Accordingly, we next examined whether Pi uptake was changed in Hyp chondrocytes. As expected, we found that Pi uptake was significantly less in Hyp chondrocytes than in WT chondrocytes (Fig. 3A). Because cellular Pi uptake is under the control of NPT (11), NPT expression in Hyp chondrocytes was subsequently determined. RT-PCR showed that the type III NPT Pit-1 expression was decreased in Hyp chondrocytes (Fig. 3B), and real-time PCR demonstrated that Pit-1 expression was reduced at the early stages of chondrocyte culture (Fig. 3C). Consistent with our results, previous studies also reported that an increase in Pit-1 expression at an early stage was associated with late chondrocyte differentiation (16, 18). On the other hand, there was no difference in the type II Npt2a expression between WT and Hyp chondrocytes (Fig. 3, B and D). The type I Npt1 expression was not detected in WT and Hyp chondrocytes (Fig. 3B). These results suggest that Pi uptake via Pit-1 is specifically involved in the regulation of chondrogenesis, including apoptosis and mineralization.
FIGURE 3.
Characterization of Hyp chondrocytes. A, time course of Pi uptake in WT (○) and Hyp (●) chondrocytes. The cells were cultured for 7 days in the differentiation medium, and Pi uptake was determined as described under “Experimental Procedures.” B, expression of Npt1, Npt2a, and Pit-1 mRNAs in WT and Hyp chondrocytes. Total RNA isolated from chondrocytes cultured for 24 h was used for RT-PCR analysis using the primer pairs. β-Actin was amplified as a control. C, time-dependent expression of Pit-1 mRNA by real-time PCR. D, time-dependent expression of Npt2a mRNA by real-time PCR. The amount of Npt2a and Pit-1 of WT chondrocytes at day 0 was designated as 1.0 and normalized to GAPDH. Results are expressed as the mean ± S.E. of four separate experiments. *, significantly different from WT chondrocytes (p < 0.05).
Suppression of Chondrocyte Differentiation by NPT Inhibitor
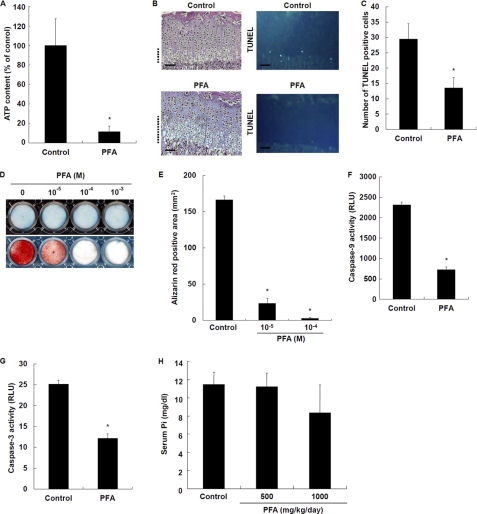
To verify whether a decrease in Pi uptake due to reduced Pit-1 expression is responsible for a reduction in ATP levels in Hyp chondrocytes, we determined the effects of PFA (foscarnet), which is a competitive inhibitor of Pi uptake via NPT (23), on intracellular ATP levels. PFA (10−5 to 10−3 m) reduced Pi uptake in chondrocytes in a dose-dependent manner (data not shown). PFA (10−5 m) profoundly reduced intracellular ATP levels (Fig. 4A). Of note, PFA treatment caused disorganization of growth plate cartilage (Fig. 4B, left) and significantly decreased the number of TUNEL-positive chondrocytes in hypertrophic cartilage (Fig. 4, B (right) and C) in a similar manner to that seen in Hyp mice. Consistent with these in vivo results, PFA markedly inhibited mineralization of chondrocytes in a dose-dependent manner (Fig. 4, D (lower) and E), whereas GAG synthesis was not affected by PFA treatment (Fig. 4D, upper). Furthermore, PFA also inhibited caspase-9 (Fig. 4F) and caspase-3 (Fig. 4G) activity. We determined serum Pi levels in PFA-treated mice. There was a trend of decreased serum Pi levels in PFA-treated mice, but it was not significantly different (Fig. 4H). The results are consistent with the notion that Pi uptake via Pit-1 is closely associated with late chondrogenesis, including apoptosis and mineralization, through reducing ATP synthesis. These results also suggest an important role for intracellular Pi over extracellular Pi in the regulation of apoptosis and ATP synthesis in chondrocytes.
FIGURE 4.
Effects of PFA on chondrocyte differentiation. A, intracellular ATP levels. Cells were cultured in the presence of 10−5 m PFA. ATP levels were measured using the ATP assay kit. B, histological examination of chondrocyte apoptosis. Hematoxylin/eosin staining (left) and TUNEL staining (right) were performed on tibial sections from 31-day-old control and PFA-treated mice. The hypertrophic zone is marked with dotted lines, and the scale bars indicate 200 μm. Representative pictures obtained of numerous sections of four mice from each group are shown. C, number of TUNEL-positive cells in the tibial growth plates of control and PFA-treated mice. D, histochemical staining of chondrocytes. Cells were cultured in the presence of 10−5 m PFA and stained with Alcian blue for GAG synthesis (upper) and alizarin red S for mineralization (lower). E, quantification of alizarin red staining. F, caspase-9 activity. G, caspase-3 activity. Cells were cultured in the presence of 10−5 m PFA. Activity was measured using the Caspase-Glo 9 and Caspase-Glo 3/7 assays. H, serum Pi levels. Results are expressed as the mean ± S.E. of four separate determinations. *, significantly different from control (p < 0.05). RLU, relative light units.
Suppression of Chondrocyte Differentiation by NPT siRNA
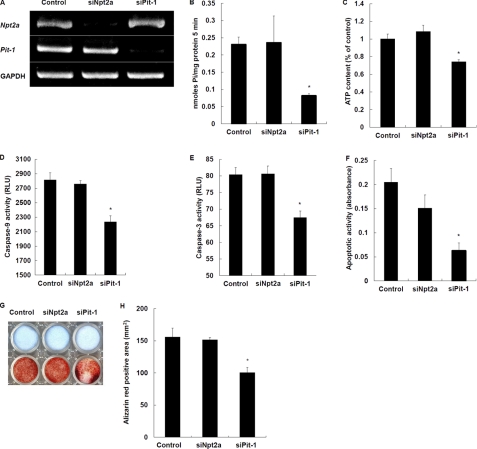
To further and more specifically verify the role of Pit-1 in chondrocyte differentiation, we performed knockdown experiments using siRNA for Pit-1. As control, Npt2a was also knocked down. Pit-1 and Npt2a siRNAs profoundly reduced Pit-1 and Npt2a mRNA levels in WT chondrocytes, respectively (Fig. 5A). Pit-1 knockdown by Pit-1 siRNA significantly decreased Pi uptake (Fig. 5B), intracellular ATP levels (Fig. 5C), and caspase-9 (Fig. 5D) and caspase-3 (Fig. 5E) activity. In parallel with these, apoptosis (Fig. 5F) and mineralization (Fig. 5, G and H) were also suppressed. In contrast, knockdown of Npt2a by Npt2a siRNA had no effects on apoptosis, mineralization, and other determinations (Fig. 5, B–H). These data suggest that Pit-1 specifically controls Pi uptake following cascades of ATP-dependent caspase signaling, apoptosis, and mineralization in chondrocytes.
FIGURE 5.
Npt2a and Pit-1 knockdown by siRNA in chondrocytes. A, negative control, Npt2a, or Pit-1 siRNA was transfected in chondrocytes, and the expression of NPT2a, Pit-1, or GAPDH was analyzed by RT-PCR. B, Pi uptake in negative control, Npt2a (siNpt2a), and Pit-1 (siPit-1) siRNA-transfected chondrocytes was determined in the presence of 3 μCi/ml KH232PO4. C, intracellular ATP levels in negative control, Npt2a, and Pit-1 siRNA-transfected chondrocytes. D, caspase-9 activity in negative control, Npt2a, and Pit-1 siRNA-transfected chondrocytes. E, caspase-3 activity in negative control, Npt2a, and Pit-1 siRNA-transfected chondrocytes. F, quantitative determination of chondrocyte apoptosis in negative control, Npt2a, and Pit-1 siRNA-transfected chondrocytes. G, histochemical staining of negative control, Npt2a, and Pit-1 siRNA-transfected chondrocytes. Cells were cultured and stained with Alcian blue for GAG synthesis (upper) and alizarin red S for mineralization (lower). H, quantification of alizarin red staining. We repeated the experiments twice using different preparations of primary chondrocytes and obtained identical results. Results are expressed as the mean ± S.E. of two separate determinations. *, significantly different from control (p < 0.05). RLU, relative light units.
Recovery of Differentiation in Hyp Chondrocytes by Pit-1 Overexpression
As an alternative approach to confirm a critical role of Pit-1 in apoptosis and mineralization in chondrocytes, we next examined the effects of Pit-1 overexpression on Hyp chondrocytes. Pit-1 overexpression significantly increased Pi uptake (Fig. 6A) and intracellular ATP levels (Fig. 6B) in Hyp chondrocytes. Furthermore, Pit-1 overexpression also stimulated caspase-9 (Fig. 6C) and caspase-3 (Fig. 6D) activity, apoptosis (Fig. 6E), and mineralization (Fig. 6, F and G). WT chondrocytes also showed significantly increased Pi uptake (Fig. 6A), intracellular ATP levels (Fig. 6B), caspase-9 (Fig. 6C) and caspase-3 (Fig. 6D) activity, apoptosis (Fig. 6E), and mineralization (Fig. 6, F and G) by Pit-1 overexpression. These results further suggest that Pit-1 is critical in the regulation of Pi uptake and following cascades of ATP-dependent caspase signaling, apoptosis, and mineralization in chondrocytes.
FIGURE 6.
Effects of Pit-1 overexpression in chondrocytes. A, empty vector (control) or Pit-1 was transfected in WT and Hyp chondrocytes. Pi uptake was determined in the presence of 3 μCi/ml KH232PO4. B, intracellular ATP levels in control or Pit-1-transfected WT and Hyp chondrocytes. C, caspase-9 activity in control and Pit-1-transfected WT and Hyp chondrocytes. D, caspase-3 activity in control and Pit-1-transfected WT and Hyp chondrocytes. E, quantitative determination of apoptosis in control and Pit-1-transfected WT and Hyp chondrocytes. F, histochemical staining of control and Pit-1-transfected WT and Hyp chondrocytes. Cells were cultured and stained with alizarin red S for mineralization. Pit-1 expression was confirmed by RT-PCR. G, quantification of alizarin red staining. We repeated the experiments twice using different preparations of primary chondrocytes and obtained identical results. Results are expressed as the mean ± S.E. of two separate determinations. *, significantly different from WT control (p < 0.05). +, significantly different from Hyp control (p < 0.05). RLU, relative light units.
Suppression of Chondrocyte Differentiation by ATP Synthesis Inhibitor
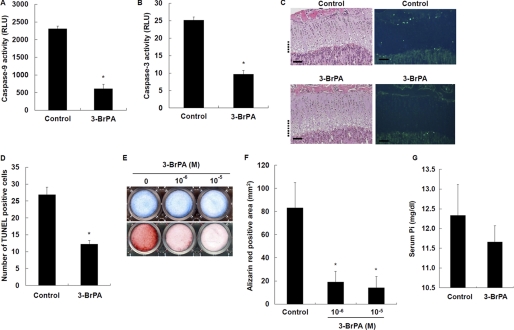
To further examine the role of intracellular ATP in chondrocyte differentiation, we studied the effects of the ATP synthesis inhibitor 3-BrPA. 3-BrPA (10−6 m) significantly reduced intracellular ATP levels in WT chondrocytes in culture (data not shown). Caspase-9 (Fig. 7A) and caspase-3 (Fig. 7B) activity was also significantly decreased in 3-BrPA-treated chondrocytes. More importantly, 3-BrPA treatment significantly decreased the number of TUNEL-positive chondrocytes in the hypertrophic zone in mice (Fig. 7, C and D). 3-BrPA inhibited chondrocyte mineralization in a dose-dependent manner (Fig. 7, E and F). However, GAG synthesis was not affected by 3-BrPA (Fig. 7E). The serum Pi levels in 3-BrPA-treated mice were not significantly different from those in control mice (Fig. 7G), suggesting an important role for intracellular Pi over extracellular Pi. These results suggest that ATP synthesis is important for chondrocytes to undergo apoptosis via caspase signaling and advance to mineralization.
FIGURE 7.
Effects of 3-BrPA on chondrocyte apoptosis and calcification. A, effects of 3-BrPA on caspase-9 activity. B, effects of 3-BrPA on caspase-3 activity. Cells were treated with 10−6 m 3-BrPA for 7 days and measured for caspase activity. C, histological examination of chondrocyte apoptosis. Hematoxylin/eosin staining (left) and TUNEL staining (right) were performed on tibial sections from 31-day-old control and 3-BrPA-treated mice. The hypertrophic zone is marked with dotted lines, and the scale bars indicate 200 μm. D, number of TUNEL-positive cells in tibial growth plates of control and 3-BrPA-treated mice. E, histochemical staining of chondrocytes. Cells were cultured for 7 days in the presence of 10−6 and 10−5 m 3-BrPA, and stained with Alcian blue for GAG synthesis (upper) and alizarin red S for mineralization (lower). F, quantification of alizarin red staining. G, serum Pi levels. Results are expressed as the mean ± S.E. of four separate experiments. *, significantly different from control (p < 0.05). RLU, relative light units.
DISCUSSION
In this study, we explored the role of the Pi-NPT system in chondrogenesis using Hyp mice compared with WT mice. We found that Hyp mice exhibited a widened and disorganized hypertrophic zone with reduced chondrocyte apoptosis compared with WT mice. In addition, PFA (a competitive inhibitor of Pit-1) or 3-BrPA (an ATP synthesis inhibitor) markedly caused elongation and disorganization of hypertrophic cartilage with reduced apoptosis in WT mice in a similar manner to that in Hyp mice. It is noted that the disorders in the hypertrophic zone were most severe in Hyp mice compared with PFA- or 3-BrPA-treated mice, despite the fact that the number of TUNEL-positive cells are comparable in these mice. We postulate that the disorders in Hyp mice are congenital and irreversible and thus most severe, whereas the disorders seen in PFA- and 3-BrPA-treated mice are due to transient exposure of these agents and reversible and thus less severe.
Consistent with these in vivo results, Hyp chondrocytes in culture exhibited decreased activity of apoptotic signaling, including caspase-9 and caspase-3, apoptosis, and mineralization following reduced Pi uptake and cellular ATP synthesis. Furthermore, PFA or 3-BrPA diminished caspase-9 and caspase-3 activity, apoptosis, and mineralization in conjunction with a reduction in Pi uptake and ATP synthesis in WT chondrocytes. Hyp primary chondrocytes displayed a decrease in Pit-1 (type III NPT) mRNA expression compared with WT chondrocytes, whereas there was no difference in type IIa NPT mRNA expression between WT and Hyp chondrocytes. WT and Hyp chondrocytes expressed no type I NPT mRNA. Meanwhile, GAG synthesis, which is an early event in chondrogenesis, was not reduced in Hyp chondrocytes, and PFA and 3-BrPA knockdown of Pit-1 failed to decrease GAG synthesis in WT chondrocytes. Pit-1 overexpression restored apoptosis and mineralization in Hyp chondrocytes. Taken together, these results suggest that Pi uptake via Pit-1 and consequent ATP synthesis are critical in the regulation of late chondrogenesis, including apoptosis and mineralization. These results also suggest that the disruption of cellular Pi homeostasis causes abnormal endochondral ossification due to a reduction of ATP synthesis in Hyp mice. In support of our study, Zalutskaya et al. (32) have recently described that Pi activates mitochondrial apoptotic pathways and promotes endochondral ossification.
ATP Synthesis and Chondrogenesis
A notable and novel finding obtained in this study is that 3-BrPA inhibits apoptosis and mineralization in growth plate hypertrophic cartilage in vivo and primary chondrocytes in vitro. 3-BrPA is an alkylating agent that decreases cellular ATP via inhibition of hexokinase in glycolysis and has been shown to promote cancer cell death through activation of the mitochondrial pathway of apoptosis or necrosis (33). Of note, the ATP-depleting effect of 3-BrPA is prominent only in tumor cells but is not apparent in nontransformed cells (34). Hence, it has been proposed that 3-BrPA could be an anticancer agent for a variety of cancers. In addition to these effects on cancers, our results show that 3-BrPA inhibits the differentiation of cartilage, suggesting that ATP generation is also necessary for nontransformed chondrocytes to differentiate and that chondrogenesis is thus an energy-dependent biological event.
Decreased Pit-1 Expression and Hyp Skeletal Phenotype
Decreased Pi uptake in Hyp chondrocytes is likely primarily due to reduced Pit-1 mRNA expression. Type IIa NPT expression was not diminished in Hyp chondrocytes, and type I NPT was not expressed in chondrocytes. Earlier reports described that disturbed endochondral ossification was not rescued by Pi supplementation in Hyp mice (35–37), suggesting that intrinsic factors are involved. Miao et al. (5) showed that reduced expression of PHEX and MMP-9 is associated with cartilage abnormalities in Hyp mice. Our results suggest that Pit-1 is one of these intrinsic factors responsible for the abnormal chondrogenesis seen in Hyp mice as well.
Regulation of Pit-1 Expression
The mechanism underlying down-regulation of Pit-1 expression in Hyp chondrocytes is unknown. Recent studies have reported that stanniocalcin (STC) 1 increases Pit-1 mRNA expression in osteoblasts (38), and STC1 and STC2 have been shown to regulate Pi uptake in chicken chondrocytes (39). STC1 stimulates renal Pi uptake and increases Pit-1 expression in osteoblasts (40), whereas STC2 inhibits Pit-1 expression and renal Pi uptake (38). Thus, STC1 and STC2 have an opposite action in the regulation of Pit-1 expression. Therefore, it is intriguing to examine whether STC1 or STC2 is involved in Pit-1 expression in chondrocytes. In preliminary experiments, we determined the expression of STC1 and STC2 mRNAs in WT and Hyp chondrocytes using RT-PCR and real-time PCR. The STC2 mRNA was expressed in both WT and Hyp chondrocytes at the same level (data not shown). However, the expression of STC1 mRNA was decreased in Hyp chondrocytes compared with WT chondrocytes (data not shown). These results suggest that STC1, but not STC2, regulates Pit-1 expression in chondrocytes.
Involvement of FGF23
FGF23 (fibroblast growth factor 23) is a hormone that regulates serum Pi levels (41). FGF23 requires Klotho for its signaling as the coreceptor in addition to the canonical FGFR1(IIIc) (42, 43). Mice transgenic for FGF23 display a reduction in Npt2a expression in the renal proximal tubules (44), indicating that FGF23 is a negative regulator of Npt2a expression, raising the possibility that Klotho-dependent FGF23 signaling regulates Pit-1 expression in chondrocytes as well. FGF23 expression is localized predominantly in osteoblasts, cementoblasts, and odontoblasts, with a sporadic expression in some chondrocytes, osteocytes, and cementocytes (45). However, we were not able to demonstrate FGF23 expression in primary mouse chondrocytes by RT-PCR. Further studies are needed to elucidate the relationship between FGF23 signaling and Pit-1 expression in cartilage.
In conclusion, we have found in this study that chondrogenesis is modulated by cellular Pi uptake via Pit-1 and cellular ATP synthesis and thus is a biological event that depends on mitochondrial energy generation. We believe that these findings should provide us with a novel concept and alternative approaches to study the cellular differentiation that occurs in physiological conditions and also to analyze the skeletal abnormalities seen in congenital hypophosphatemic disorders such as XLH.
Acknowledgments
We thank Drs. Kenichi Miyamoto and Hiroko Segawa (University of Tokushima Graduate School, Tokushima, Japan) for the kind gift of mouse Pit-1 cDNA.
This work was supported in part by the 21st Century COE Program entitled “Origination of Frontier BioDentistry” at Osaka University Graduate School of Dentistry, by the Ministry of Education, Culture, Sports, Science, and Technology, and by Grant-in-aid for Scientific Research A202290100 from the Japanese Society for the Promotion of Science (to T. Y.).
- GAG
- glycosaminoglycan(s)
- XLH
- X-linked hypophosphatemia
- NPT
- sodium-dependent Pi cotransporter(s)
- PFA
- phosphonoformic acid
- 3-BrPA
- 3-bromopyruvate
- STC
- stanniocalcin.
REFERENCES
- 1. Zuscik M. J., Hilton M. J., Zhang X., Chen D., O'Keefe R. J. (2008) J. Clin. Invest. 118, 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winters R. W., Graham J. B., Williams T. F., McFalls V. W., Burnett C. H. (1958) Medicine 37, 97–142 [DOI] [PubMed] [Google Scholar]
- 3. Holm I. A., Huang X., Kunkel L. M. (1997) Am. J. Hum. Genet. 60, 790–797 [PMC free article] [PubMed] [Google Scholar]
- 4. Eicher E. M., Southard J. L., Scriver C. R., Glorieux F. H. (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 4667–4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miao D., Bai X., Panda D. K., Karaplis A. C., Goltzman D., McKee M. D. (2004) Bone 34, 638–647 [DOI] [PubMed] [Google Scholar]
- 6. Hayashibara T., Hiraga T., Sugita A., Wang L., Hata K., Ooshima T., Yoneda T. (2007) J. Bone Miner. Res. 22, 1743–1751 [DOI] [PubMed] [Google Scholar]
- 7. Boyde A., Shapiro I. M. (1980) Histochemistry 69, 85–94 [DOI] [PubMed] [Google Scholar]
- 8. Kakuta S., Golub E. E., Shapiro I. M. (1985) Calcif. Tissue Int. 37, 293–299 [DOI] [PubMed] [Google Scholar]
- 9. Mwale F., Tchetina E., Wu C. W., Poole A. R. (2002) J. Bone Miner. Res. 17, 275–283 [DOI] [PubMed] [Google Scholar]
- 10. Shapiro I. M., Boyde A. (1984) Metab. Bone Dis. Relat. Res. 5, 317–326 [DOI] [PubMed] [Google Scholar]
- 11. Virkki L. V., Biber J., Murer H., Forster I. C. (2007) Am. J. Physiol. Renal. Physiol. 293, F643–F654 [DOI] [PubMed] [Google Scholar]
- 12. Palmer G., Zhao J., Bonjour J., Hofstetter W., Caverzasio J. (1999) Bone 24, 1–7 [DOI] [PubMed] [Google Scholar]
- 13. Mansfield K., Teixeira C. C., Adams C. S., Shapiro I. M. (2001) Bone 28, 1–8 [DOI] [PubMed] [Google Scholar]
- 14. Cecil D. L., Rose D. M., Terkeltaub R., Liu-Bryan R. (2005) Arthritis Rheum. 52, 144–154 [DOI] [PubMed] [Google Scholar]
- 15. Fujita T., Meguro T., Izumo N., Yasutomi C., Fukuyama R., Nakamuta H., Koida M. (2001) Jpn. J. Pharmacol. 85, 278–281 [DOI] [PubMed] [Google Scholar]
- 16. Guicheux J., Palmer G., Shukunami C., Hiraki Y., Bonjour J. P., Caverzasio J. (2000) Bone 27, 69–74 [DOI] [PubMed] [Google Scholar]
- 17. Montessuit C., Caverzasio J., Bonjour J. P. (1991) J. Biol. Chem. 266, 17791–17797 [PubMed] [Google Scholar]
- 18. Wang D., Canaff L., Davidson D., Corluka A., Liu H., Hendy G. N., Henderson J. E. (2001) J. Biol. Chem. 276, 33995–34005 [DOI] [PubMed] [Google Scholar]
- 19. Wu L. N., Guo Y., Genge B. R., Ishikawa Y., Wuthier R. E. (2002) J. Cell. Biochem. 86, 475–489 [DOI] [PubMed] [Google Scholar]
- 20. Magne D., Bluteau G., Faucheux C., Palmer G., Vignes-Colombeix C., Pilet P., Rouillon T., Caverzasio J., Weiss P., Daculsi G., Guicheux J. (2003) J. Bone Miner. Res. 18, 1430–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimomura Y., Yoneda T., Suzuki F. (1975) Calcif. Tissue Res. 19, 179–187 [DOI] [PubMed] [Google Scholar]
- 22. Rowe P. S., Ong A. C., Cockerill F. J., Goulding J. N., Hewison M. (1996) Bone 18, 159–169 [DOI] [PubMed] [Google Scholar]
- 23. Loghman-Adham M. (1996) Gen. Pharmacol. 27, 305–312 [DOI] [PubMed] [Google Scholar]
- 24. Swenson C. L., Weisbrode S. E., Nagode L. A., Hayes K. A., Steinmeyer C. L., Mathes L. E. (1991) Calcif. Tissue Int. 48, 353–361 [DOI] [PubMed] [Google Scholar]
- 25. Geschwind J. F., Ko Y. H., Torbenson M. S., Magee C., Pedersen P. L. (2002) Cancer Res. 62, 3909–3913 [PubMed] [Google Scholar]
- 26. Jones A. R., Gillan L., Milmlow D. (1995) Contraception 52, 317–320 [DOI] [PubMed] [Google Scholar]
- 27. Ko Y. H., Smith B. L., Wang Y., Pomper M. G., Rini D. A., Torbenson M. S., Hullihen J., Pedersen P. L. (2004) Biochem. Biophys. Res. Commun. 324, 269–275 [DOI] [PubMed] [Google Scholar]
- 28. Gibson G. (1998) Microsc. Res. Tech. 43, 191–204 [DOI] [PubMed] [Google Scholar]
- 29. Eguchi Y., Srinivasan A., Tomaselli K. J., Shimizu S., Tsujimoto Y. (1999) Cancer Res. 59, 2174–2181 [PubMed] [Google Scholar]
- 30. Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. (1997) Cell 91, 479–489 [DOI] [PubMed] [Google Scholar]
- 31. Rao N. N., Gómez-García M. R., Kornberg A. (2009) Annu. Rev. Biochem. 78, 605–647 [DOI] [PubMed] [Google Scholar]
- 32. Zalutskaya A. A., Cox M. K., Demay M. B. (2009) J. Cell. Biochem. 108, 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pelicano H., Martin D. S., Xu R. H., Huang P. (2006) Oncogene 25, 4633–4646 [DOI] [PubMed] [Google Scholar]
- 34. Xu R. H., Pelicano H., Zhou Y., Carew J. S., Feng L., Bhalla K. N., Keating M. J., Huang P. (2005) Cancer Res. 65, 613–621 [PubMed] [Google Scholar]
- 35. Ecarot B., Glorieux F. H., Desbarats M., Travers R., Labelle L. (1992) J. Bone Miner. Res. 7, 523–530 [DOI] [PubMed] [Google Scholar]
- 36. Tanaka H., Seino Y., Shima M., Yamaoka K., Yabuuchi H., Yoshikawa H., Masuhara K., Takaoka K., Ono K. (1988) Bone Miner. 4, 237–246 [PubMed] [Google Scholar]
- 37. Yoshikawa H., Masuhara K., Takaoka K., Ono K., Tanaka H., Seino Y. (1985) Bone 6, 235–239 [DOI] [PubMed] [Google Scholar]
- 38. Yoshiko Y., Candeliere G. A., Maéda N., Aubin J. E. (2007) Mol. Cell. Biol. 27, 4465–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu S., Yoshiko Y., De Luca F. (2006) J. Biol. Chem. 281, 5120–5127 [DOI] [PubMed] [Google Scholar]
- 40. Ishibashi K., Imai M. (2002) Am. J. Physiol. Renal Physiol. 282, F367–F375 [DOI] [PubMed] [Google Scholar]
- 41. Fukumoto S., Yamashita T. (2007) Bone 40, 1190–1195 [DOI] [PubMed] [Google Scholar]
- 42. Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K. P., Baum M. G., Schiavi S., Hu M. C., Moe O. W., Kuro-o M. (2006) J. Biol. Chem. 281, 6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., Fujita T., Fukumoto S., Yamashita T. (2006) Nature 444, 770–774 [DOI] [PubMed] [Google Scholar]
- 44. Shimada T., Urakawa I., Yamazaki Y., Hasegawa H., Hino R., Yoneya T., Takeuchi Y., Fujita T., Fukumoto S., Yamashita T. (2004) Biochem. Biophys. Res. Commun. 314, 409–414 [DOI] [PubMed] [Google Scholar]
- 45. Yoshiko Y., Wang H., Minamizaki T., Ijuin C., Yamamoto R., Suemune S., Kozai K., Tanne K., Aubin J. E., Maeda N. (2007) Bone 40, 1565–1573 [DOI] [PubMed] [Google Scholar]