Abstract
Rho family proteins regulate multiple cellular functions including motility and invasion through regulation of the actin cytoskeleton and gene expression. Activation of Rho proteins is controlled precisely by multiple regulators in a spatiotemporal manner. RhoA and/or RhoC are key players that regulate the metastatic activity of malignant tumor cells, and it is therefore of particular interest to understand how activation of these Rho proteins is controlled. We recently identified an upstream regulator of RhoA activation, p27RF-Rho (p27kip1 releasing factor from RhoA) that acts by freeing RhoA from inhibition by p27kip1. p27kip1 is a cell cycle regulator when it is localized to the nucleus, but it binds RhoA and inhibits activation of the latter when it is localized to the cytoplasm. Here, we show that a metastatic variant of mouse melanoma B16 cells (F10) exhibits greater expression of p27RF-Rho, RhoA, and RhoC than the nonmetastatic parental cells (F0). Injection of F10 cells into mouse tail vein resulted in the formation of metastatic lung colonies, whereas prior knockdown of expression of either one of the three proteins using specific shRNA sequences decreased metastasis markedly. p27RF-Rho regulated the activation of RhoA and RhoC and thereby modulated cellular adhesion and motility, in addition to pericellullar proteolysis. The Rho activities enhanced by p27RF-Rho had a marked effect upon efficiency of lodging of F10 cells in the lung, which represents an early step of metastasis. p27RF-Rho also regulated metastasis of human melanoma and fibrosarcoma cells. Thus, p27RF-Rho is a key upstream regulator of RhoA and RhoC that controls spreading of tumor cells.
Keywords: Cell Adhesion, Cell Migration, Cell Motility, Rho, Tumor Metastases
Introduction
Dissemination of tumor cells from a primary tumor mass and formation of metastatic colonies at secondary sites is the major cause of mortality of cancer patients (1, 2). The formation of metastases involves a series of discrete steps, including invasion of the extracellular matrix, intravasation into vascular structures, extravasation, and regrowth at the secondary site (1, 3). Although metastasis is a complex process, and the underlying cellular mechanism has been difficult to understand, recent studies have shown that the epithelial-mesenchymal transition program, which is employed during development, inflammation, and wound healing, is activated in tumor cells and confers upon the cells both invasive and metastatic ability (2, 4). Metastatic tumor cells are highly migratory and invasive, and these properties are regulated by members of the Rho family of GTPases (5–8).
The Rho family of GTPases comprises Rho, Rac, and Cdc42 subfamilies, which regulate different aspects of the dynamic reorganization of the actin cytoskeleton during cell movement (9, 10). Proteins of the Rac and Cdc42 subfamilies regulate polymerization of branching actin to form ruffling membranes and filopodia, respectively, whereas Rho subfamily proteins are known to regulate the formation of stress fibers, which bridge cell adhesion structures and generate contractile forces during movement. Members of the Rho subfamily include RhoA, RhoB, and RhoC, and in this study we use the term “Rho proteins” to represent these subfamily members. Although the genes encoding Rho proteins are rarely mutated in tumor cells, changes in their level of expression has been associated with invasive and metastatic tumors (11). In particular, RhoA and RhoC are frequently overexpressed in metastatic cancer cell lines and clinical specimens (9), whereas the expression of RhoB is occasionally decreased (9, 12). Moreover, analysis of cells generated from repeated selection of metastatic melanoma cells in mice revealed that increased expression of RhoC is associated strongly with the acquisition of metastatic ability (13). Activation of Rho proteins is mediated by Rho guanine nucleotide exchange factors in response to cell stimuli, and this process is antagonized by Rho protein GDP dissociation inhibitors. The latter bind and sequester the GDP-bound form of Rho proteins so as to prevent their activation at the inner surface of the plasma membrane (9, 10). The concerted action of these two types of regulator gives rise to dynamic changes in the actin cytoskeleton during cell movement (14, 15).
In addition to these regulators of Rho proteins, the cell cycle regulator p27kip1 has recently been demonstrated to inhibit RhoA activation when p27kip1 is localized to the cytoplasm (16, 17). Cytoplasmic p27kip1 binds GDP-RhoA and prevents the latter from binding Rho guanine nucleotide exchange factors (16, 18–20). We recently identified a new p27kip1-binding protein, p27RF-Rho, which prevents binding of p27kip1 to GDP-RhoA (21). Therefore, p27kip1-free GDP-RhoA, which can be activated by guanine nucleotide exchange factors, is expected to be generated focally at sites of p27RF-Rho localization. In the previous study we found that p27RF-Rho is expressed in invasive tumor cells and exhibits a punctuate distribution at the inner surface of the basal membrane attached to the extracellular matrix in cultured cells. Activation of Rho gives rise to formation of stress fibers and invadopodia-like protrusions that are associated with pericellular proteolysis (21). However, Rho proteins regulate diverse cellular functions that are dependent upon actin polymerization, such as cytokinesis and maintenance of cell polarity and adhesion, etc. Therefore, it is of interest to clarify whether p27RF-Rho regulates activation of Rho proteins during metastasis of tumor cells.
In the present study, we made use of nonmetastatic (F0) and metastatic (F10) variants of the B16 murine melanoma cell line and found that expression of p27RF-Rho, RhoA, and RhoC is greater in F10 than in F0 cells. We further show that p27RF-Rho contributes to the metastatic activity of F10 cells by regulating the activation of RhoA and RhoC. We also demonstrate the prometastatic function of p27RF-Rho in human tumor cells.
EXPERIMENTAL PROCEDURES
Cells and Antibodies
B16F0 and B16F10 cells were obtained from the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University. HT1080 and A375 cells were obtained from the American Type Culture Collection, and Mum2B cells were a generous gift from Dr. V. Quaranta (Vanderbilt University). B16, HT1080, and A375 cells were maintained in DMEM containing 10% FBS (Hyclone), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Mum2B was maintained in RPMI containing 10% FBS (Hyclone), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). A polyclonal anti-p27RF-Rho antibody was described previously (21). We used commercially available antibodies for RhoA (Cell Signaling), RhoC (Cell Signaling), and p27kip1 (Santa Cruz). Alexa Fluor phalloidin was purchased from Invitrogen.
Knockdown Experiments Using shRNA
The shRNA sequences used for knockdown were 5′-caccgatcccttcctgccctctttccgaagaaagagggcaggaagggatc-3′ and 5′-caccgccgagcccaactaccatagccgaagctatggtagttgggctcggc-3′ for p27RF-Rhos; 5′-caccggctgccatcaggaagaaactcgaaagtttcttcctgatggcagcc-3′ and 5′-caccgcttgctcatagtcttcagcacgaatgctgaagactatgagcaagc-3′ for RhoAs; and 5′-caccggtagtagccttgttatttgacgaatcaaataacaaggctactacc-3′ and 5′-caccggacatgaataaaggtcaaagagaactttgacctttattcatgtcc-3′ for RhoCs. shRNA-expressing lentiviral vectors were generated and used according to the manufacturer's instructions (Invitrogen).
The Pull-down Assay of Rho-GTPases
The pull-down assay of Rho-GTPases was performed as described previously (21). Briefly, the cells were washed with ice-cold PBS and scraped off the plates in cell lysis buffer (50 mm Tris-HCl, pH 7.4, 2 mm MgCl2, 1% Nonidet P-40, 10% glycerol, 100 mm NaCl, supplemented with 1 mm dithiothreitol, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 10 μg/ml pepstatin-A) on ice. The lysates were centrifuged for 5 min at 20,000 × g at 4 °C. A fraction of the cleared lysates was incubated with 15 μg of GST-Rhotekin-Rho-binding domain bound to glutathione-coupled Sepharose beads for 30 min at 4 °C. The pellet containing the beads was collected, washed three times with ice-cold cell lysis buffer, and subjected to SDS-PAGE followed by Western blot analysis using the indicated antibodies.
Fluorescent Gelatin Degradation Assay
Oregon Green-labeled gelatin was obtained from Invitrogen. The coverslips were coated with 10 μg/ml poly-l-lysine for 20 min at room temperature, washed with PBS, and incubated for 10 min at room temperature in 0.2% fluorescently labeled gelatin in 2% sucrose in PBS. The cells were fixed with 0.5% glutaraldehyde (Sigma) for 15 min. After three washes, the coverslips were incubated in 5 mg/ml sodium borohydride for 3 min, washed three times in PBS, and finally incubated in 2 ml of serum-free medium for 1 h. To assess the ability of cells to form invadopodia and degrade the gelatin, the cells were placed on Oregon Green-coated coverslips and incubated at 37 °C for 4 h.
Immunofluorescence Microscopy
The cells were fixed with 4% paraformaldehyde and permeabilized using 0.1% Triton X-100 in PBS for 10 min. After the cells were blocked in PBS containing 5% goat serum and 3% BSA, they were incubated with Alexa 568-conjugated phalloidin (Invitrogen). Images of cells were captured with IX70 equipped with a CCD camera (Olympus).
Adhesion and Spreading Assay
The cells were plated in dishes coated with fibronectin (1 μg/ml; Sigma) or vitronectin (1 μg/ml; Sigma) for 30 min, and the cells were detached using 0.25% trypsin, 1 mm EDTA containing PBS and counted using a Coulter counter (Beckman). Spreading of cells was observed under a microscope equipped with a CCD camera, and the adherent cell area was analyzed using Metamorph software. Spreading was presented as the total area of cells adhering to the matrix.
Migration Assay
Transwells with 8-μm-pore size filters (Costar) covered with fibronectin (5 μg/ml; Sigma) were inserted into 24-well plates. DMEM (500 μl) containing 10% FBS was added to the lower chamber, and 100 μl of a cell suspension (1 × 105 cells) was placed in the upper chamber. The plates were incubated at 37 °C in a 5% CO2 atmosphere for 9 h. The cells in the lower chamber were then stained with crystal violet and counted.
Metastasis Assays
1 × 105 cells were suspended in 200 μl of PBS and injected via the lateral tail veins of C57BL/6 mice (Clea Japan). 6–7-week-old mice were used for the experiments. Two weeks following injection, the mice were killed, the lungs were extirpated, and the black spherical B16F10 colonies were counted. Short term lung colonization assays used cells fluorescently labeled with CellTracker Green and CellTracker Orange (Invitrogen). p27RF-Rho-depleted and control cells (1 × 105 each for B16F0 and B16F10 and 2.5 × 105 each for A375, Mum2B, and HT1080) were injected into the tail veins of C57BL/6 mice (B16) or nude mice (A375, Mum2B, and HT1080), which were killed 1 or 24 h later. Fluorescently labeled cells in the lung were counted by confocal microscopy (Nikon).
RESULTS
Expression of Rho Proteins and p27RF-Rho in Variant B16 Melanoma Cell Lines
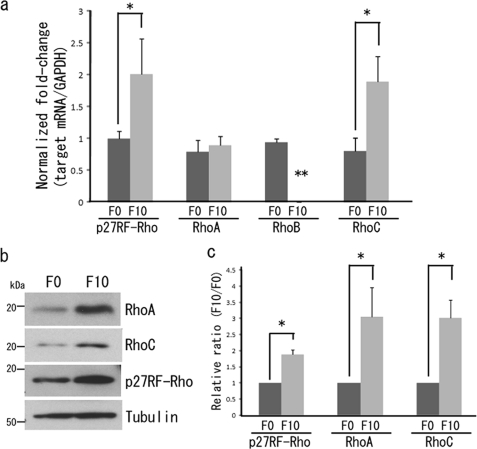
To evaluate a role for p27RF-Rho in the regulation of invasion and metastasis of tumor cells in mice, we made use of a nonmetastatic F0 and a highly metastatic F10 variant of the B16 mouse melanoma cell line. Quantitative analysis of mRNA levels by RT-PCR demonstrated that F10 cells expressed a greater level of both p27RF-Rho and RhoC than F0 cells (Fig. 1a). However, expression of RhoA mRNA was comparable between F0 and F10 cells, whereas expression of RhoB was suppressed in F10 cells.
FIGURE 1.
Analysis of Rho mRNA and protein in B16 melanoma cells. a, quantitative PCR analysis of p27RF-Rho, RhoA, RhoB, and RhoC in B16F0 and B16F10 cells. Gene expression was normalized to that of GAPDH and presented as the ratio of expression in B16F0 relative to B16F10 cells. The data are presented as the means ± standard deviation (n = 3). *, p < 0.05. **, not detected. b, protein levels of p27RF-Rho, RhoA, and RhoC in B16F0 and B16F10 cells. c, quantification of Fig. 1B. The data are presented as the means ± standard deviation (n = 3). *, p < 0.05.
Expression of Rho proteins was also examined by Western blot analysis (Fig. 1b). Consistent with the greater expression of the mRNAs encoding p27RF-Rho and RhoC, expression of the corresponding proteins was also greater in F10 cells. Despite the similarity in the level of expression of RhoA mRNA in the two variant cell lines, its protein was expressed more strongly in F10 than F0 cells (Fig. 1c). Post-transcriptional modifications may alter the stability of RhoA protein. For example, the stability of the RhoA protein is reported to be regulated by the ubiquitin-dependent degradation pathway (22, 23). We could not detect expression of RhoB protein in either cell line under the conditions used in our assay (not shown). Because RhoB is not likely a pro-metastatic regulator in the downstream of p27RF-Rho, we do not follow this protein further in this study.
p27RF-Rho Plays a Critical Role in F10 Cell Metastasis to the Lung
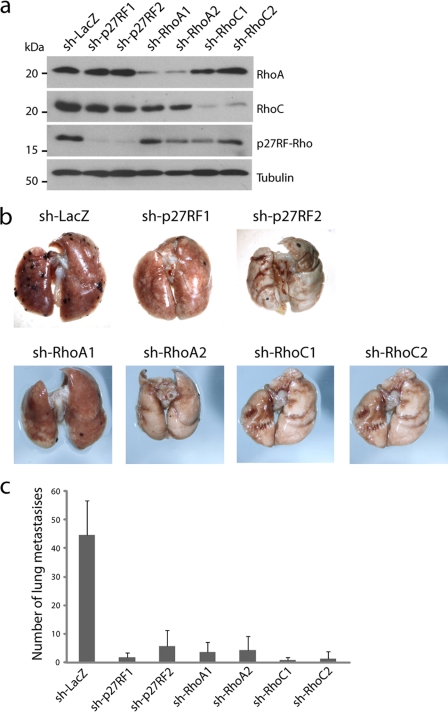
B16-F10 cells are known to form metastatic colonies in the lung when the cells are injected into the tail vein of mice. We also confirmed that our cells formed multiple metastatic nodules in the lung, and we further found that expression of a control shRNA (sh-LacZ) did not affect the metastatic activity of the cell (data not shown). Using this assay system, we evaluated whether the elevated expression of p27RF-Rho, RhoA, and RhoC plays a role in metastasis by knocking down expression of each protein. We prepared stable F10 transfectants in which the expression of either p27RF-Rho, RhoA, or RhoC was knocked down by expressing the appropriate shRNAs, and we further confirmed knockdown of the expression of the corresponding proteins (Fig. 2a). Lungs were dissected from mice 14 days after injection of the cells, and black nodules corresponding to the melanoma cells were visible on the lung surface (Fig. 2b). The number of the nodules was counted and is presented in Fig. 2c. Compared with control cells expressing sh-LacZ, knockdown of either RhoA or RhoC expression markedly reduced the number of metastatic colonies in the lung. p27RF-Rho knockdown cells expressing either of two different shRNAs (sh-p27RF1 and sh-p27RF2) also exhibited a reduced number of metastatic nodules, and this effect was comparable with that observed following knockdown of Rho protein expression. Thus, p27RF-Rho expression in F10 cells contributes to the metastatic activity as effectively as does expression of RhoA or RhoC. Constitutive knockdown of the expression of each protein had no apparent effect upon the growth properties of the cells in culture (data not shown). Nonmetastatic F0 cells did not form lung metastasis at all, and forced expression of p27RF-Rho as a V5-tagged form in the cells was not sufficient to convert them metastatically (data not shown).
FIGURE 2.
The p27RF-Rho-Rho axis is required for melanoma cell metastasis in vivo. a, the efficiency of knockdown of p27RF-Rho (sh-p27RF1 and sh-p27RF2), RhoA (sh-RhoA1, 2), or RhoC (sh-RhoC1, 2) in B16F10 cells was analyzed by Western blot analysis. b, appearance of murine lungs 14 days following injection of control or p27RF-Rho-, RhoA-, or RhoC-depleted cells into the tail veins of 7–8-week-old mice. The number of surface tumor nodules is shown in c. The data represent the means ± standard deviation (n = 7).
p27RF-Rho Regulates Activation of RhoA and RhoC in B16-F10 Cells
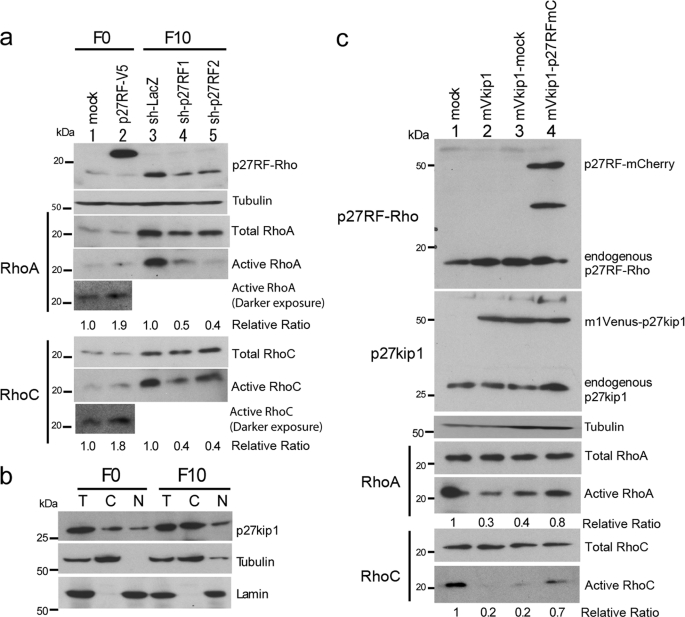
In the previous study, we demonstrated that p27RF-Rho releases inhibition of RhoA activation by p27kip1 without affecting the gene expression of RhoA (21). To confirm whether p27RF-Rho can regulate activation of endogenous RhoA and RhoC in B16 cells, we altered the level of expression of p27RF-Rho in the cells by expressing either additional protein or shRNAs targeting each gene (Fig. 3a). Active RhoA and RhoC proteins were pulled down from cell lysates prepared from the cells using the Rho-binding domain of Rhotekin and analyzed by Western blot. F0 cells expressed low levels of p27RF-Rho and Rho proteins. Forced expression of p27RF-Rho fused to a V5 tag (p27RF-V5, lane 2) did not affect the total protein level of RhoA and RhoC but resulted in a modest increase in the level of active RhoA and RhoC (Fig. 3a, lane 2). In contrast, F10 cells express greater levels of p27RF-Rho and active forms of RhoA and RhoC than F0 cells (Fig. 3a, lane 3). Knockdown of p27RF-Rho expression in F10 cells gave rise to a decrease in the level of the active forms RhoA and RhoC (Fig. 3a, lanes 4 and 5). Thus, p27RF-Rho regulates the activation of RhoA as we reported previously using human tumor cell lines (21), and it also regulates the activation of RhoC.
FIGURE 3.
p27RF-Rho modulates RhoA and RhoC activation via inhibition of the p27kip1 pathway. a, regulation of Rho proteins by p27RF-Rho. B16F0 cells expressing an empty vector (mock) or p27RF-Rho fused to a V5 tag (p27RF-V5, lanes 1 and 2) and B16F10 cells expressing a control shRNA (sh-LacZ, lane 3) or either one of two shRNA sequences targeted to the p27RF-Rho mRNA (sh-p27RF1 and sh-p27RF2, lanes 3–5) were used for the assay. The cell lysates were analyzed by Western blot using antibodies that recognize p27RF-Rho, RhoA, or RhoC. Active Rho proteins were pulled down using the Rhotekin fragment and analyzed similarly. The ratio of active to total protein is indicated below the blots (n = 3). b, subcellular localization of p27kip1 in B16F0 and B16F10 cells. After cells were lysed and separated into cytoplasmic and nuclear fractions, p27kip1 was detected by Western blot analysis. Tubulin and lamin B1 are markers for cytoplasmic and nuclear proteins, respectively. T, total lysate; C, cytoplasmic fraction; N, nuclear fraction. c, p27RF-Rho suppresses p27kip1 so as to inhibit activation of RhoA and RhoC. An empty vector (mock, lane 1), p27kip1 fused to an mVenus tag (mVkip1, lanes 2–4), and mVkip1 with additional empty vector (mVkip1-mock, lane 3) or mVkip1 with p27RF-Rho fused to an mCherry tag (mVkip1-p27RFmC, lane 4) were expressed in B16F10 cells. Total and active Rho proteins were analyzed as in a. The ratio of active to total protein is indicated below the blots (n = 3).
We next examined whether p27RF-Rho regulates the ability of p27kip1 to inhibit Rho proteins in B16 melanoma cells. F0 and F10 cells expressed p27kip1 at a similar level (Fig. 3b). Subcellular fractionation revealed that p27kip1 is more abundant in the cytoplasm of F10 cells than in the nucleus, whereas it is distributed almost equally in F0 cells. This is consistent with previous studies reporting that cytoplasmic p27kip1 is more abundant in malignant compared with nonmalignant cells (18, 24). To confirm that p27kip1 suppresses the activation of Rho proteins in B16 melanoma cells, we overexpressed p27kip1 fused to an mVenus tag (mVkip1) in F10 cells (Fig. 3c). Forced expression of mVkip1 resulted in a decreased level of active RhoA and RhoC (30 and 20%, respectively) compared with the levels detected in lysates prepared from mock-transfected control cells (Fig. 3c, lane 1 versus lane 2). Subsequent expression of p27RF-Rho fused to an mCherry tag (p27RFmCherry) in mVkip1-expressing cells increased the level of the activated forms of RhoA and RhoC (Fig. 3c, lane 3 versus lane 4). These results are consistent with the mechanism we reported previously, which suggests that p27RF-Rho enhances activation of RhoA by suppressing the inhibitory activity of p27kip1 (21). Thus, elevated expression of p27RF-Rho is important to increase the amount of active RhoA and RhoC by counteracting the increased levels of cytoplasmic p27kip1 in F10 cells.
p27RF-Rho Regulates the Formation of Invadopodia and Degradation of the Extracellular Matrix
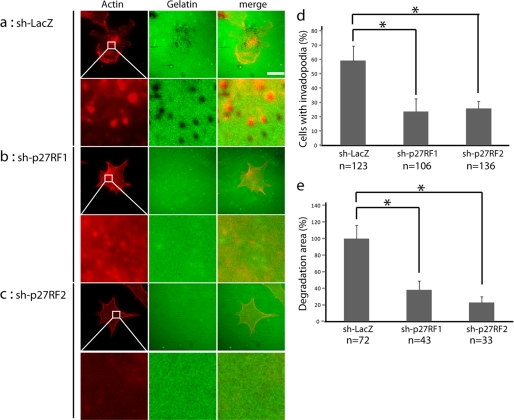
In a previous study, we observed that p27RF-Rho regulates the formation of invadopodia in HT1080 fibrosarcoma cells (21). To analyze whether p27RF-Rho also regulates the formation and proteolytic activity of invadopodia in F10 cells, we cultured the cells on a layer of gelatin labeled with Oregon Green for 4 h (Fig. 4a). Degradation of the gelatin beneath the cultured cells was visualized as a darker area within the fluorescent green background. The initial pattern of degradation appeared as punctate foci (Fig. 4a) that gradually expanded and fused with one another over time (not shown). These foci of degradation overlapped with the punctate pattern of the fluorescent signals corresponding to actin-based structures (red) that form invadopodia. Knockdown of the expression of p27RF-Rho in F10 cells reduced the extent of degradation of the gelatin by the cells as well as the number of punctate actin signals (Fig. 4, b and c). Regions of colocalization of the foci of degradation with the puncate actin signals were deemed to represent invadopodia. Counting of the number of control (sh-LacZ) and knockdown (sh-p27RF1 and sh-p27RF2) cells with invadopodia revealed that a smaller percentage of p27RF knockdown cells exhibited such structures (Fig. 4d). In addition, the total area per cell of gelatin degradation was also decreased in the knockdown cells relative to the control (Fig. 4e).
FIGURE 4.
p27RF-Rho regulates gelatin degradation activity in F10 cells. a, degradation of Oregon Green-labeled gelatin (middle column) and actin immunohistochemistry (red, left column) in control (sh-LacZ) and p27RF-Rho-depleted (sh-p27RF1 and sh-p27RF2) cells cultured on glass coverslips (scale bar, 20 μm). Higher magnification views of the boxed areas are shown underneath each image. b, quantification of cells with invadopodia. The cells were scored as invadopodia-positive if they contained punctate structures positive for both actin filaments and gelatin degradation. c, quantification of gelatin degradation area. The data are presented as the means ± standard errors of means (n = 5). The number of cells analyzed in each data set is indicated at the bottom of the data bars in the graphs. *, p < 0.05.
p27RF-Rho and Downstream Effectors Regulate Cell Adhesion and Migration of F10 Cells
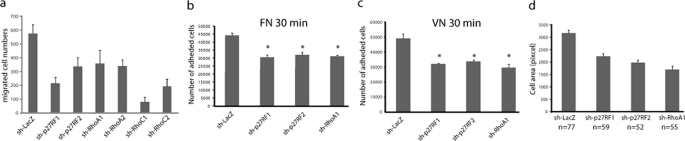
Rho proteins play central roles in the regulation of the dynamics of the actin cytoskeleton during cell adhesion, spreading, and migration (7, 25). We used the stable F10 knockdown cells (Fig. 2a) to examine cell motility in an assay chamber equipped with a fibronectin-coated filter (Fig. 5a). All of these cells exhibited significantly reduced cell motility compared with the control sh-LacZ-expressing cells. The p27RF-Rho or RhoA knockdown cells also exhibited reduced adhesion to fibronectin (Fig. 5b) or vitronectin (Fig. 5c). In addition, the knockdown cells exhibited reduced spreading (Fig. 5d) and exhibited a spherical morphology (supplemental Fig. S1), which is frequently observed in cells in which Rho proteins have been inactivated (7, 26).
FIGURE 5.
p27RF-Rho is required for cell migration, adhesion, and spreading. a, the number of migratory cells analyzed using a Transwell chamber equipped with a fibronectin-coated filter. The data are presented as the means ± standard deviation (n = 3). b and c, B16F10 cells depleted of p27RF-Rho or RhoA were plated on fibronectin (b) or vitronectin (c), and adhesion was quantified by counting cells 30 min after plating. The data are presented as the means ± standard deviation (n = 3). *, p < 0.05. d, quantification of cell spreading. The data are presented as the means ± standard errors of means (n = 3). The number of cells analyzed in each data set is indicated at the bottom of the graphs.
p27RF-Rho Regulates an Early Step of Experimental Metastasis to Lung
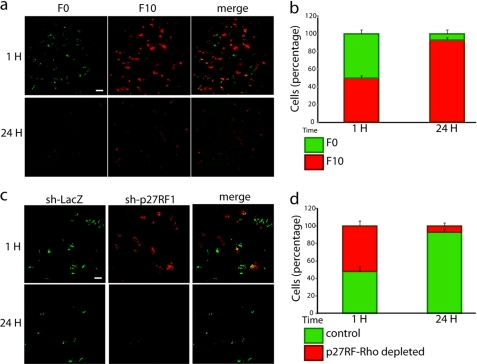
We have demonstrated that the expression of p27RF-Rho in F10 cells affects adhesion, migration, and invasion of the cells. These activities of tumor cells are particularly important for extravasation and the following invasion into the lung parenchyma in the experimental metastasis assay. Therefore, we analyzed the effect of p27RF-Rho expression in F10 cells at an early step so as to establish the initial extent of deposition of tumor cells in the lung in the experimental metastasis assay. We injected a mixture of F0 and F10 cells labeled with green fluorescent and red fluorescent protein cell trackers, respectively, into the tail vein of mice and analyzed the number of cells in the lung 1 and 24 h later (Fig. 6, a and c). A similar number of F0 and F10 cells were localized to the lung 1 h after injection. However, most F0 cells were cleared during the following 24 h, whereas a significant number of F10 cells were retained. Therefore, it is clear that F10 cells initially lodge in the lung more efficiently than F0 cells. Similar results have been reported using other B16 melanoma variant cells (26). Using the same assay system, we compared the ability of F10 cells expressing either control sh-LacZ or sh-p27RF1 to establish the initial deposition in lung tissue (Fig. 6, a and c). A similar number of control and p27RF-Rho knockdown cells reached the lung, whereas the p27RF-Rho knockdown cells were not retained in the lung 24 h later.
FIGURE 6.
p27RF-Rho is required for murine melanoma lung colonization in vivo. Localization of tumor cells to the lung 1 or 24 h following injection into the tail vein of mice is shown. a, localization of a mixture of B16F0 (labeled with CellTracker Green) and B16F10 cells (labeled with CellTracker Orange). c, localization of a mixture of control B16F10 cells (expressing sh-LacZ, labeled with CellTracker Green) and B16F10 cells depleted for p27RF-Rho (expressing sh-p27RF1, labeled with CellTracker Orange). b and d, the numbers of cells in a and c are presented in b and d, respectively.
p27RF-Rho Regulates the Metastatic Ability of Human Tumor Cells
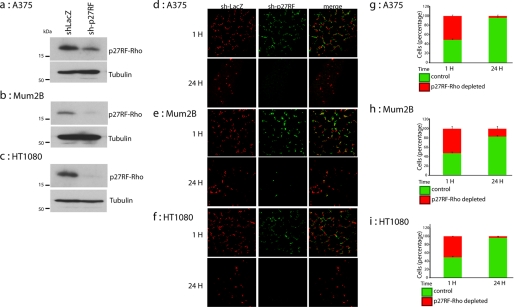
To extend our observations with mouse melanoma cells to human tumors, we chose human malignant melanomas (A375 and Mum2B) and human fibrosarcoma HT1080 cells. All of these tumor cells expressed p27RF-Rho, whose expression could be inhibited by expressing the appropriate shRNA (Fig. 7, a–c). A mixture of green fluorescent protein-labeled control cells expressing sh-lacZ and red fluorescent protein-labeled cells expressing sh-p27RF was injected into the tail vein of mice, and we analyzed the number of cells in the lung 1 and 24 h later (Fig. 7, d–f). The number of knockdown cells lodging in the lung 24 h later was decreased compared with the control cells for all three cell types (Fig. 7, g–i), indicating that p27RF-Rho expressed in human tumor cells plays a role similar to that observed in mouse melanoma cells.
FIGURE 7.
p27RF-Rho is required for malignant human tumor cells lung colonization in vivo. Knockdown efficiency of p27RF-Rho (sh-p27RF) in A375 (a), Mum2B (b), and HT1080 (c) cells was evaluated by Western blot analysis. Localization of a mixture of control A375, Mum2B, and HT1080 cells (expressing sh-LacZ, labeled with CellTracker Green) and corresponding cells depleted for p27RF-Rho (expressing sh-p27RF1, labeled with CellTracker Orange) is shown. The numbers of cells shown in d–f are presented in g–i. The data represent the means ± standard deviation (n = 4).
DISCUSSION
Rho signaling is known to regulate invasion and metastasis of tumor cells, and increased expression of RhoA or RhoC has been linked to acquisition of malignancy (13, 15, 27–29). We show here that metastatic F10 cells also exhibit greater expression of RhoA and RhoC than their nonmetastatic F0 counterparts. Although the enhanced expression of RhoC is regulated at the mRNA level, expression of RhoA is regulated at the post-translational level. Ubiquitin ligase smurf1, which regulates the stability of RhoA (30), may be involved in the regulation of RhoA expression. Expression of RhoB was decreased in F10 cells relative to its level of expression in F0 cells. RhoB is thought to have tumor suppressor activity, based on studies in other malignant melanoma cells (9, 12). Increased expression of the Rho proteins (RhoA and RhoC) in F10 compared with F0 cells does not seem to be the consequence of up-regulation of p27RF-Rho in the cells from the results presented in Fig. 3 (a and c). Expression of RhoB in F0 cells was not affected by forced expression of p27RF-Rho (data not shown).
Both RhoA and RhoC are indispensible for the metastatic activity of F10 cells, because knockdown of the expression of either one was sufficient to suppress formation of lung metastases (Fig. 2). Transmission of signals by RhoA and RhoC to their effectors, such as ROCK and mDia, requires their activation in addition to increased expression of the Rho proteins. The ratio of the expression of the cytoplasmic and nuclear forms of p27kip1 is increased in F10 cells (Fig. 3b), as is seen in other malignant tumors (31, 32). Whereas a lower level of p27kip1 in the nucleus is favorable for continuous cell cycle progression, cytoplasmic p27kip1 suppresses Rho signaling by binding to the GDP-bound form of RhoA and possibly RhoC. Indeed, increased expression of p27kip1 decreased the level of the activated forms of both RhoA and RhoC in F10 cells (Fig. 3c). Therefore, increased expression of p27RF-Rho in parallel with the increased expression of RhoA and RhoC in F10 cells appears to be a plausible mechanism by which Rho signaling enhances metastasis. Knockdown of p27RF-Rho expression suppressed the metastatic activity of F10 cells as effectively as knockdown of either RhoA or RhoC (Fig. 2) perhaps by decreasing the amount of active RhoA and RhoC in F10 cells (Fig. 3a). However, the expression of p27RF-Rho alone in F0 cells is not sufficient to convert the cells metastatically (data not shown). This is presumably because upon forced expression of p27RF-Rho in F0 cells, there was no corresponding increase in the expression levels of RhoA and RhoC (Fig. 3a), and therefore the total amount of the active Rho proteins may be insufficient to cause the cells to metastasize. In addition to above, it is also possible that additional uncharacterized factors are required to render F0 cells fully metastatic.
p27RF-Rho-depleted F10 cells injected into the mouse tail vein reached the lung within an hour with almost the same efficiency as the control F10 cells. However, unlike the parental F10 cells, the p27RF-Rho-depleted cells were not retained in the lung 24 h later and therefore failed to form metastatic colonies. Cells depleted of p27RF-Rho exhibited reduced adhesion, migration, and pericellular proteolysis. The effect of p27RF-Rho knockdown on an early step of the experimental metastasis assay is presumably a reflection of these cellular activities in the processes of extravasation from the capillaries and successive invasion into the lung parenchyma. Recently two modes of tumor cell invasion in a three-dimensional environment have been described, and tumor cells are thought to employ either of these modes in a flexible fashion through interaction with the ECM (33, 34).2 Tumor cell invasion can be regulated via a balance between the Rac/Cdc42- and Rho-dependent modes of migration (35, 36). During a mesenchymal mode of migration where Rac and CDC42 are active, cells exhibit an elongated morphology during invasion and employ proteases to degrade the surrounding ECM. In contrast, Rho regulates an amoeboid type of cell movement that enables cells to move through gaps between components of the ECM without degrading the structure itself (34). However, a recent study raised the possibility that even amoeboid-like invasion requires ECM degradation by proteases, based on an analysis using cross-linked collagen instead of artificially pepsinized collagen (37). B16F10 cells expressing p27RF-Rho exhibited a spherical morphology in a three-dimensional collagen/Matrigel mixture (supplemental Fig. S2A). Conversely, knockdown of p27RF-Rho expression increased the number of cells with an elongated morphology (supplemental Fig. S2B). Based on these observations, F10 cells may employ an amoeboid type of migration that requires proteolytic activity during invasion.
p27RF-Rho is nearly ubiquitously expressed in a variety of cell types (21). However, an Oncomine database search revealed that p27RF-Rho tends to be expressed at a greater level in myeloma and seminoma cells compared with that observed in the normal cellular counterparts of these tumors. These findings raise the possibility that genetic or epigenetic alterations of the p27RF-Rho gene may be linked to the development of tumors or other diseases. Single nucleotide polymorphisms have been identified within the coding regions of p27RF-Rho, including SNPs predicted to cause amino acid substitutions. The relevance of these single nucleotide polymorphisms to human disease remains to be determined. Therefore, p27RF-Rho expressed in human tumors is also expected to enhance Rho signaling and increase metastatic ability of the cells. Indeed, p27RF-Rho expressed in human melanoma and fibrosarcoma cells was a crucial regulator of an early step of experimental metastasis, as was observed in F10 cells (Fig. 7).
In conclusion, we have demonstrated that p27RF-Rho-mediated suppression of p27kip1 regulates the metastatic activity of mouse and human melanoma cells in addition to human fibrosarcoma cells. Rho proteins have been thought to be promising targets for the suppression of the invasive and metastatic properties of tumor cells. On the other hand, inhibition of Rho proteins in patients may cause severe side effects because of their ubiquitous roles in regulating diverse cellular functions. Thus, specific modulation of p27RF-Rho may represent a more targeted strategy for the development of anti-cancer therapeutics.
Supplementary Material
Acknowledgments
We thank Drs. M. Nagano and T. Sakamoto for useful discussion and C. Konish and T. Ando for excellent technical support.
Note Added in Proof
During the preparation of this publication, we found reports describing that p27RF-Rho is the protein having three different names and functions (38–40).
This work was supported by a grant from the Specific Coordination Fund for Promoting Science (to D. H.) and by a Grant-in-Aid for Scientific Research on Priority Areas, “Integrative Research toward the Conquest of Cancer,” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to M. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- ECM
- extracellular matrix.
REFERENCES
- 1. Gupta G. P., Massagué J. (2006) Cell 127, 679–695 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen D. X., Massagué J. (2007) Nat. Rev. Genet. 8, 341–352 [DOI] [PubMed] [Google Scholar]
- 3. Steeg P. S. (2006) Nat. Med. 12, 895–904 [DOI] [PubMed] [Google Scholar]
- 4. Berger J. C., Vander Griend D. J., Robinson V. L., Hickson J. A., Rinker-Schaeffer C. W. (2005) Cancer Biol. Ther. 4, 805–812 [DOI] [PubMed] [Google Scholar]
- 5. Wang W., Eddy R., Condeelis J. (2007) Nat. Rev. Cancer 7, 429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamaguchi H., Condeelis J. (2007) Biochim. Biophys. Acta 1773, 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narumiya S., Tanji M., Ishizaki T. (2009) Cancer Metastasis Rev. 28, 65–76 [DOI] [PubMed] [Google Scholar]
- 8. Olson M. F., Sahai E. (2009) Clin. Exp. Metastasis 26, 273–287 [DOI] [PubMed] [Google Scholar]
- 9. Ridley A. J. (2004) Breast Cancer Res. Treat 84, 13–19 [DOI] [PubMed] [Google Scholar]
- 10. Hall A. (2009) Cancer Metastasis Rev. 28, 5–14 [DOI] [PubMed] [Google Scholar]
- 11. Bellovin D. I., Simpson K. J., Danilov T., Maynard E., Rimm D. L., Oettgen P., Mercurio A. M. (2006) Oncogene 25, 6959–6967 [DOI] [PubMed] [Google Scholar]
- 12. Jiang K., Sun J., Cheng J., Djeu J. Y., Wei S., Sebti S. (2004) Mol. Cell. Biol. 24, 5565–5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clark E. A., Golub T. R., Lander E. S., Hynes R. O. (2000) Nature 406, 532–535 [DOI] [PubMed] [Google Scholar]
- 14. Bishop A. L., Hall A. (2000) Biochem. J. 348, 241–255 [PMC free article] [PubMed] [Google Scholar]
- 15. Sahai E., Marshall C. J. (2002) Nat. Rev. Cancer 2, 133–142 [DOI] [PubMed] [Google Scholar]
- 16. Besson A., Gurian-West M., Schmidt A., Hall A., Roberts J. M. (2004) Genes Dev. 18, 862–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McAllister S. S., Becker-Hapak M., Pintucci G., Pagano M., Dowdy S. F. (2003) Mol. Cell. Biol. 23, 216–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Besson A., Dowdy S. F., Roberts J. M. (2008) Dev. Cell 14, 159–169 [DOI] [PubMed] [Google Scholar]
- 19. Larrea M. D., Hong F., Wander S. A., da Silva T. G., Helfman D., Lannigan D., Smith J. A., Slingerland J. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9268–9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berton S., Belletti B., Wolf K., Canzonieri V., Lovat F., Vecchione A., Colombatti A., Friedl P., Baldassarre G. (2009) Mol. Cell. Biol. 29, 5031–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoshino D., Tomari T., Nagano M., Koshikawa N., Seiki M. (2009) J. Biol. Chem. 284, 27315–27326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bryan B., Cai Y., Wrighton K., Wu G., Feng X. H., Liu M. (2005) FEBS Lett. 579, 1015–1019 [DOI] [PubMed] [Google Scholar]
- 23. Wu S., Wolf D. A. (2009) Mol. Cell 35, 735–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Denicourt C., Saenz C. C., Datnow B., Cui X. S., Dowdy S. F. (2007) Cancer Res. 67, 9238–9243 [DOI] [PubMed] [Google Scholar]
- 25. Salsmann A., Schaffner-Reckinger E., Kieffer N. (2006) Eur. J. Cell Biol. 85, 249–254 [DOI] [PubMed] [Google Scholar]
- 26. Medjkane S., Perez-Sanchez C., Gaggioli C., Sahai E., Treisman R. (2009) Nat. Cell Biol. 11, 257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fritz G., Brachetti C., Bahlmann F., Schmidt M., Kaina B. (2002) Br. J. Cancer 87, 635–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Itoh K., Yoshioka K., Akedo H., Uehata M., Ishizaki T., Narumiya S. (1999) Nat. Med. 5, 221–225 [DOI] [PubMed] [Google Scholar]
- 29. Wang W., Goswami S., Lapidus K., Wells A. L., Wyckoff J. B., Sahai E., Singer R. H., Segall J. E., Condeelis J. S. (2004) Cancer Res. 64, 8585–8594 [DOI] [PubMed] [Google Scholar]
- 30. Sahai E., Garcia-Medina R., Pouysségur J., Vial E. (2007) J. Cell Biol. 176, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viglietto G., Motti M. L., Bruni P., Melillo R. M., D'Alessio A., Califano D., Vinci F., Chiappetta G., Tsichlis P., Bellacosa A., Fusco A., Santoro M. (2002) Nat. Med. 8, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 32. Shin I., Yakes F. M., Rojo F., Shin N. Y., Bakin A. V., Baselga J., Arteaga C. L. (2002) Nat. Med. 8, 1145–1152 [DOI] [PubMed] [Google Scholar]
- 33. Friedl P., Wolf K. (2008) Cancer Res. 68, 7247–7249 [DOI] [PubMed] [Google Scholar]
- 34. Sahai E. (2005) Curr. Opin. Genet. Dev. 15, 87–96 [DOI] [PubMed] [Google Scholar]
- 35. Sanz-Moreno V., Gadea G., Ahn J., Paterson H., Marra P., Pinner S., Sahai E., Marshall C. J. (2008) Cell 135, 510–523 [DOI] [PubMed] [Google Scholar]
- 36. Pinner S., Sahai E. (2008) Nat. Cell Biol. 10, 127–137 [DOI] [PubMed] [Google Scholar]
- 37. Sabeh F., Shimizu-Hirota R., Weiss S. J. (2009) J. Cell Biol. 185, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guillaumot P., Luquain C., Malek M., Huber A. L., Brugière S., Garin J., Grunwald D., Régnier D., Pétrilli V., Lefai E., Manié S. N. (2010) PLoS One 5, e10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nada S., Hondo A., Kasai A., Koike M., Saito K., Uchiyama Y., Okada M. (2009) EMBO J. 28, 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sancak Y., Bar-Peled L., Zoncu R., Markhard A. L., Nada S., Sabatini D. M. (2010) Cell 141, 290–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.