Abstract
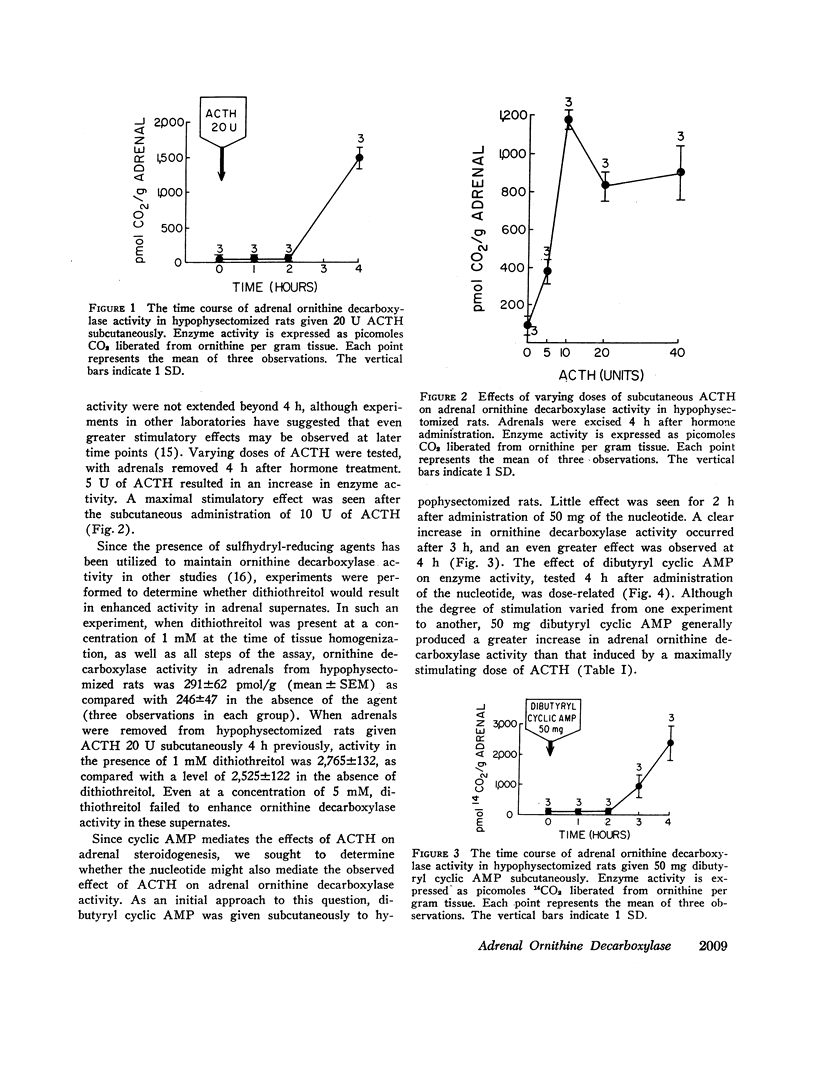
Adrenal ornithine decarboxylase activity was stimulated in a dose-related manner after administration of ACTH or dibutyryl (6N-2′-O-dibutyryl) cyclic AMP to hypophysectomized rats. Little effect was observed for 2 h, but striking increases in enzyme activity were observed 4 h after administration of these substances. Effects of ACTH and dibutyryl cyclic AMP were not secondary to stimulation of steroidogenesis, since hydrocortisone had no effect on adrenal ornithine decarboxylase although it did stimulate activity of the enzyme in the liver and kidney.
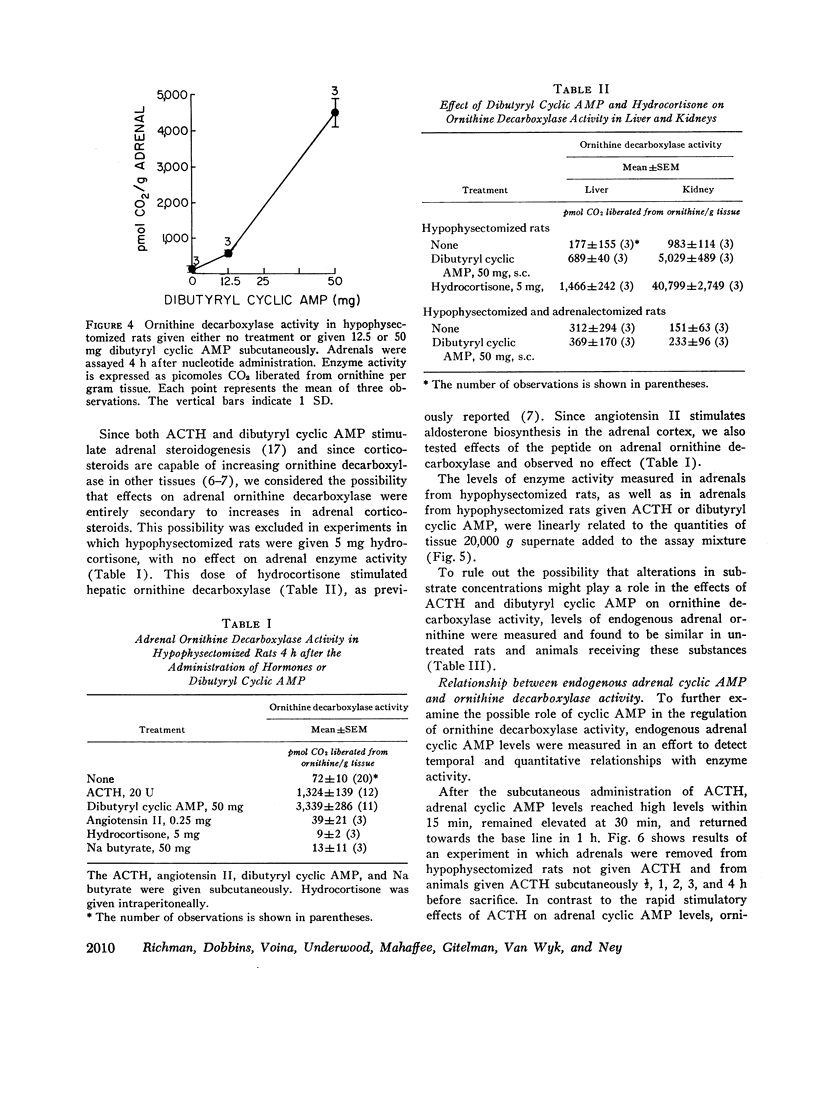
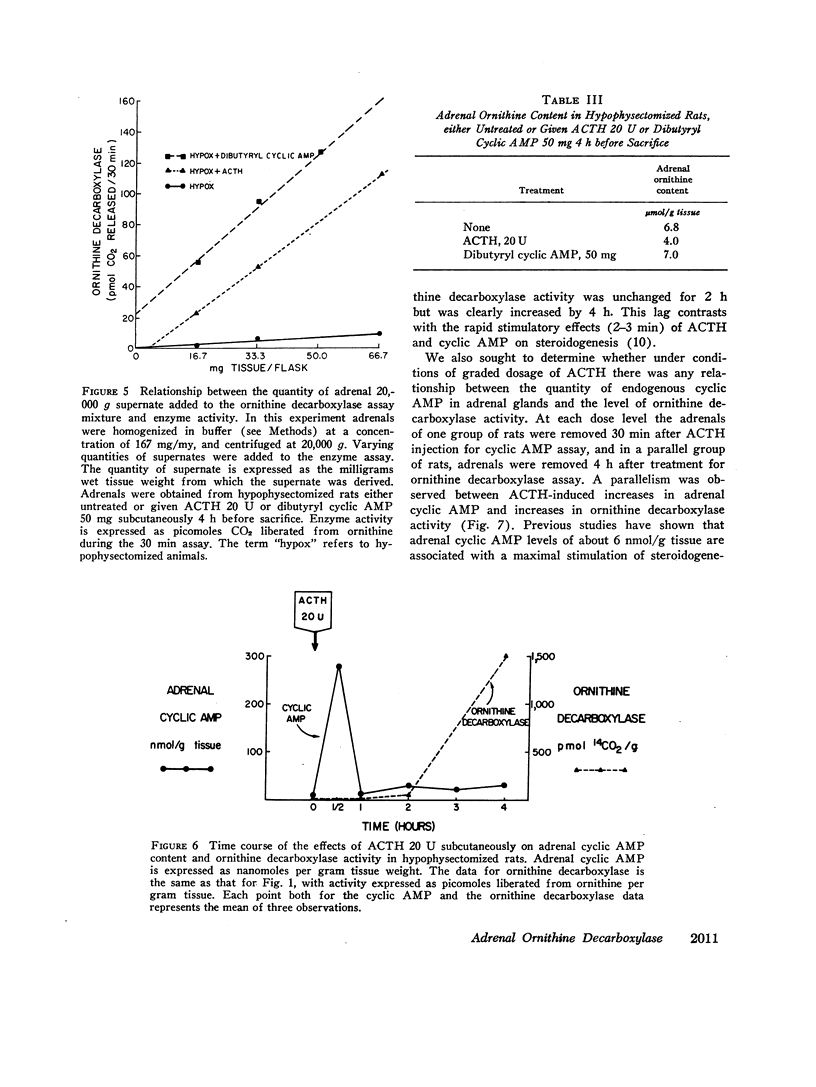
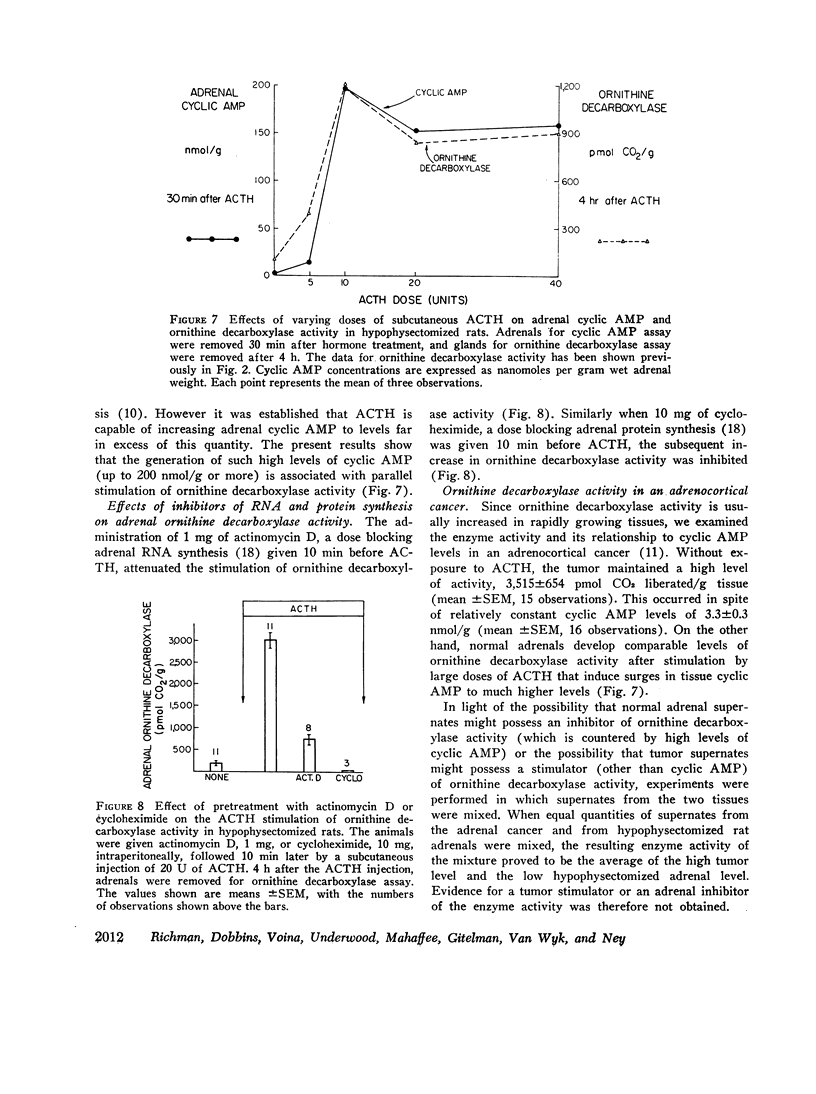
ACTH, given subcutaneously to hypophysectomized rats, induced striking increases in adrenal cyclic AMP levels within 15-30 min with a fall towards the base line in 1 h. Increases in ornithine decarboxylase activity lag several hours after this endogenous cyclic AMP peak, in contrast to the stimulatin of steroidogenesis by the nucleotide that requires only 2-3 min. After graded doses of ACTH, increases in adrenal cyclic AMP levels at 30 min were paralleled by proportional increases in adrenal ornithine decarboxylase activity 4 h after hormone treatment. Whereas maximal levels of adrenal steroidogenesis have been observed at tissue cyclic AMP levels of 6 nmol/g. ACTH is capable of inducing increases in nucleotide levels up to 200 nmol/g or more. These high tissue levels of cyclic AMP, although unneccessary for maximal steroidogenesis, appear to stimulate adrenal ornithine decarboxylase activity.
Several results in addition to the time lag in the stimulation of ornithine decarboxylase activity suggest a mechanism involving accumulation of the enzyme or some factor needed for its activity rather than direct activation of the enzyme by cyclic AMP. Thus, the addition of cyclic AMP directly to the ornithine decarboxylase assay mixture in vitro was without stimulatory effect. In addition, actinomycin D or cycloheximide in doses sufficient to block adrenal RNA and protein synthesis, respectively inhibited the stimulation of ornithine decarboxylase activity by ACTH in vivo.
An adrenocortical cancer was found to maintain ornithine decarboxylase activity at very high levels, but did so at much lower cyclic AMP levels than those of ACTH-stimulated adrenals.
It is concluded that ACTH stimulates adrenal ornithine decarboxylase activity and that this effect may be mediated by cyclic AMP. However, cyclic AMP be mediated by appear to be a determinant of the high level of enzyme activity found in adrenocortical cancer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bransome E. D., Jr Actinomycin D in vivo: paradoxical and nonspecific effects on adrenal cortex. Endocrinology. 1969 Dec;85(6):1114–1128. doi: 10.1210/endo-85-6-1114. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Interaction of the subunits of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of muscle. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2444–2447. doi: 10.1073/pnas.68.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exss R. E., Hill H. D., Summer G. K. Computer analysis of amino acid chromatograms. J Chromatogr. 1969 Jul 22;42(4):442–451. doi: 10.1016/s0021-9673(01)80652-x. [DOI] [PubMed] [Google Scholar]
- Garren L. D., Ney R. L., Davis W. W. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1443–1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. Role of the receptor in the mechanism of action of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):786–790. doi: 10.1073/pnas.68.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- IMURA H., MATSUKURA S., MATSUYAMA H., SETSUDA T., MIYAKE T. ADRENAL STEROIDOGENIC EFFECT OF ADENOSINE 3',5'-MONOPHOSPHATE AND ITS DERIVATIVES IN VIVO. Endocrinology. 1965 May;76:933–937. doi: 10.1210/endo-76-5-933. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kremzner L. T. Polyamines. Introductory remarks. Fed Proc. 1970 Jul-Aug;29(4):1560–1562. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney R. L. Effects of dibutyryl cyclic AMP on adrenal growth and steroidogenic capacity. Endocrinology. 1969 Jan;84(1):168–170. doi: 10.1210/endo-84-1-168. [DOI] [PubMed] [Google Scholar]
- Ney R. L., Hochella N. J., Grahame-Smith D. G., Dexter R. N., Butcher R. W. Abnormal regulation of adenosine 3',5'-monophosphate and corticosterone formation in an adrenocortical carcinoma. J Clin Invest. 1969 Sep;48(9):1733–1739. doi: 10.1172/JCI106139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J., Bader J., Johnson G. S., Pastan I. A mutation in a rous sarcoma virus gene that controls adenosine 3',5'-monophosphate levels and transformation. J Biol Chem. 1972 Mar 10;247(5):1632–1633. [PubMed] [Google Scholar]
- Panko W. B., Kenney F. T. Hormonal stimulation of hepatic ornithine decarboxylase. Biochem Biophys Res Commun. 1971 Apr 16;43(2):346–350. doi: 10.1016/0006-291x(71)90759-5. [DOI] [PubMed] [Google Scholar]
- Raina A., Jänne J. Polyamines and the accumulation of RNA in mammalian systems. Fed Proc. 1970 Jul-Aug;29(4):1568–1574. [PubMed] [Google Scholar]
- Richman R. A., Underwood L. E., Van Wyk J. J., Voina S. J. Synergistic effect of cortisol and growth hormone on hepatic ornithine decarboxylase activity. Proc Soc Exp Biol Med. 1971 Dec;138(3):880–884. doi: 10.3181/00379727-138-36011. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Russell D. H. Polyamine synthesis in rapidly growing tissues. Fed Proc. 1970 Jul-Aug;29(4):1575–1582. [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Walton G. M., Gill G. N., Abrass I. B., Garren L. D. Phosphorylation of ribosome-associated protein by an adenosine 3':5'-cyclic monophosphate-dependent protein kinase: location of the microsomal receptor and protein kinase. Proc Natl Acad Sci U S A. 1971 May;68(5):880–884. doi: 10.1073/pnas.68.5.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Pegg A. E., Lockwood D. H. Mechanisms and regulation of polyamine and putrescine biosynthesis in male genital glands and other tissues of mammals. Adv Enzyme Regul. 1969;7:291–323. doi: 10.1016/0065-2571(69)90024-7. [DOI] [PubMed] [Google Scholar]


