Abstract
Chronic infection with the intracellular protozoan parasite Toxoplasma gondii leads to tissue remodelling in the brain and a continuous requirement for peripheral leucocyte migration within the CNS (central nervous system). In the present study, we investigate the role of MMPs (matrix metalloproteinases) and their inhibitors in T-cell migration into the infected brain. Increased expression of two key molecules, MMP-8 and MMP-10, along with their inhibitor, TIMP-1 (tissue inhibitor of metalloproteinases-1), was observed in the CNS following infection. Analysis of infiltrating lymphocytes demonstrated MMP-8 and -10 production by CD4+ and CD8+ T-cells. In addition, infiltrating T-cells and CNS resident astrocytes increased their expression of TIMP-1 following infection. TIMP-1-deficient mice had a decrease in perivascular accumulation of lymphocyte populations, yet an increase in the proportion of CD4+ T-cells that had trafficked into the CNS. This was accompanied by a reduction in parasite burden in the brain. Taken together, these findings demonstrate a role for MMPs and TIMP-1 in the trafficking of lymphocytes into the CNS during chronic infection in the brain.
Keywords: astrocyte, cell migration, central nervous system (CNS), T-cell, tissue inhibitor of metalloproteinases-1 (TIMP-1), Toxoplasma gondii
Abbreviations: CNS, central nervous system; DAPI, 4′,6-diamidino-2-phenylindole; EAE, experimentally induced autoimmune encephalomyelitis; ECM, extracellular matrix; GFAP, glial fibrillary acidic protein; HFF, human foreskin fibroblast; IFN-γ, interferon-γ; IL, interleukin; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; mfi, mean fluorescent intensity; MMP, matrix metalloproteinase; PECAM-1, platelet/endothelial cell adhesion molecule-1; PFA, paraformaldehyde; RT–PCR, reverse transcription–PCR; sTAg, soluble Toxoplasma antigen; TIMP-1, tissue inhibitor of metalloproteinases-1; TNF, tumour necrosis factor; WT, wild-type
INTRODUCTION
Key mediators of tissue remodelling following brain injury or disease-mediated insult include the MMPs (matrix metalloproteinases). Increased expression of MMPs and proteolysis of ECM (extracellular matrix) and non-matrix substrates has been implicated in diverse processes during disease states such as cancer, and neurological and infectious pathologies (Ethell and Ethell, 2007). MMPs are inhibited systemically by the general protease inhibitor α2-macroglobulin, and at sites of their activity by local TIMPs (tissue inhibitors of metalloproteinases). Although these molecules have been implicated in a variety of cell processes including cell growth and arrest (Stetler-Stevenson, 2008), they are primarily associated with their ability to bind the active site of MMPs preventing their protease activity. Among these, the inducible inhibitor TIMP-1 can be produced in an autocrine fashion by cell populations producing MMPs. It is therefore critical in the regulation of cell migratory processes including tumour progression, metastasis and the immune response to sites of inflammation (Bloomston et al., 2002; Baratelli et al., 2004; Burrage et al., 2007; Ramer and Hinz, 2008).
In the CNS (central nervous system), spatial and cell-specific expression of MMPs/TIMPs is noted and is dependent on inflammatory signals (Pagenstecher et al., 1998; Crocker et al., 2006a, 2006b). The activity of MMP-2 and MMP-9 is of particular significance in the brain with expression associated with diverse CNS inflammatory conditions including infection with Mycobacterium tuberculosis (Harris et al., 2007), severity of EAE (experimentally induced autoimmune encephalomyelitis; Dubois et al., 1999) and focal ischaemia (Asahi et al., 2000) and their activity contributes to permeability of the blood–brain barrier (Thwaites et al., 2003). Possibly due to the vulnerability of the brain to inflammatory processes and uncontrolled protease activity, TIMP-1 is produced by both astrocytes and microglia under non-inflammatory conditions and during inflammation (Gardner and Ghorpade, 2003). The absence of TIMP-1 can reduce pathogen load but also lead to increased severity of CNS inflammation, pointing to a pivitol role of this molecule in the balance of immune responses in the brain (Toft-Hansen et al., 2004; Lee et al., 2005; Zhou et al., 2005; Crocker et al., 2006a; Thorne et al., 2009; Althoff et al., 2010).
Toxoplasma gondii is among the most successful of intracellular parasites, infecting virtually every warm-blooded animal including an estimated one-third of the global human population (Tenter et al., 2000; Dubey, 2008). Despite a robust pro-inflammatory response that effectively clears fast-replicating tachyzoites from the periphery, Toxoplasma converts to a slow-growing bradyzoite form that encysts in the brain parenchyma for the life of the host (Hunter et al., 1993). Although the symptoms of infection are largely subclinical in immune-competent individuals, acquired or latent infection in the context of immune compromise leads to focal intracerebral lesions caused by unchecked parasite re-activation and replication. Throughout chronic infection, parasite re-activation is suppressed by a well-orchestrated immune response characterized by IFN-γ (interferon-γ) producing CD4+ and CD8+ T lymphocytes (Gazzinelli et al., 1992). Recent in vivo observations of T-cell behaviour in Toxoplasma-infected brain tissue revealed that lymphocyte infiltration is accompanied by substantial tissue remodelling associated with migrating cells (Wilson et al., 2009). The mechanism by which peripheral immune cells migrate within inflamed tissue and specifically that of the brain remains unknown.
To address the factors involved in this cell trafficking, we investigated the role of MMP production during Toxoplasma infection. In the present paper, we demonstrate the up-regulation of MMP-8 and -10 in the brain that is accompanied by a striking increase in transcription of their inhibitor, TIMP-1. Using flow cytometry and immunohistochemistry to analyse the source of MMP production ex vivo we find that CD4+ and CD8+ T-cells produce MMP-8 and MMP-10, and that these populations also contribute to the induction of TIMP-1 during chronic brain infection. In addition, CNS-resident astrocytes produce TIMP-1 in response to direct infection by Toxoplasma tachyzoites. Finally, parasite burden in TIMP-1-deficient mice is significantly reduced, associated with efficient penetration of lymphocytes into the brain parenchyma. These results demonstrate the importance of the MMP/TIMP axis in the trafficking of infiltrating populations into sites of infection and what factors may contribute to the significant tissue remodelling that has been observed in the context of T. gondii infection of the CNS. Furthermore, regulation of metalloproteinases necessary for the access of immune populations to infected CNS tissues may be key to the balanced, non-pathological yet persistent immune response that is the hallmark of chronic infection with Toxoplasma.
MATERIALS AND METHODS
Parasite culture and infections
T. gondii parasites (Type II, Prugniaud strain) were cultured in HFF (human foreskin fibroblast) cells at 37°C, 5% CO2. For infection, parasites were purified by needle passage of infected HFFs, passed through a 5 μm filter to remove cellular debris, washed, centrifuged and resuspended in sterile PBS at 5×104 parasites/ml. C57Bl/6J, B6.129S4 and B6.129S4-Timp1tm1Pds/J (TIMP-1−/−) mice were obtained from the Jackson Laboratory and housed according to institutional protocol. Animals aged 8–12 weeks were infected by injecting 104 T. gondii tachyzoites in 200 μl sterile PBS intraperitoneally. Animals sham injected with 200 μl sterile PBS served as uninfected controls. All studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes for Health. The protocol was approved by the IACUC Committee of the University of California Riverside (IACUC number 2008002).
Quantitative RT–PCR (reverse transcription–PCR)
Immediately following killing, naïve or T. gondii-infected animals were transcardially perfused and whole brains were collected. Total RNA from 3 week infected and naïve mice was extracted using TRIzol®/chloroform phase separation (Invitrogen) according to the manufacturer's protocol. Pooled RNA from three individual mice was reverse transcribed using the oligo(dT) primer according to the First Strand cDNA Synthesis kit protocol (Fermentas Life Sciences), and the resulting cDNA was used as a template in an RT–PCR array of primers for ECM and ECM-associated molecules including metalloproteinases and their inhibitors (SA Biosciences). To focus on the kinetics of MMP-8, MMP-10 and TIMP-1 observed by array, kinetic analysis was conducted over eight time points (3, 7, 14, 21, 28, 35, 42 and 60 days post-infection) and compared with expression in uninfected brains. Real-time PCR amplification was performed using primer sequences as previously described (Hasebe et al., 2007) for MMP-10, forward (5′-CCTGTGTTGTCTGTCTCTCCA-3′), reverse (5′-CGTGCTGACTGAATCAAAGGA-3′); and designed for MMP-8, forward (5′-ACGGAGTGAGAGGTGTGGAT-3′), reverse (5′-TCTGCCTGGGAACTTATTGG-3′); and TIMP-1, forward (5′-ATCTGGCATCCTCTTGTTGC-3′), reverse (5′-CATTTCCCACAGCCTTGAAT-3′). DNA was amplified using a Bio-Rad iCycler in the presence of SYBR Green. Reaction conditions were as follows: denaturation at 95°C for 10 min, followed by 40 cycles consisting of 15 s denaturation at 95°C, 30 s annealing at 60°C and 30 s extension at 72°C. Melting curve analysis in 0.5°C increments from 95 to 60°C was conducted to verify primer specificity. Threshold values were acquired and analysed using Bio-Rad iQ5 2.0 optical-system software. Fold induction was calculated using the comparative CT method described by Livak and Schmittgen (2001). Parasite burden was measured by amplifying the T. gondii B1 gene using the primer sequences: forward (5′-TCCCCTCTGCTGGCGAAAAGT-3′), reverse (5′-AGCGTTCGTGGTCAACTATCGATTG-3′), followed by comparison with a DNA standard acquired from known numbers of purified parasites (Noor et al., 2010).
Immunohistochemistry
Brain, spleen and liver tissue were snap-frozen by immersion in isopentane chilled to −70°C, and immediately mounted in OCT medium (Sakura Finetek). Samples were stored at −80°C until sectioned by Microm OMV cryostat to 6 μm (for haematoxylin and eosin staining) or 10–15 μm (for immunofluorescence staining). Frozen tissue sections were fixed in 2% (w/v) PFA (paraformaldehyde) and permeabilized in 0.5% Triton X-100 in PBS prior to incubation with purified antibodies. Primary antibodies against metalloproteinases and TIMP-1 are as listed previously and were used at a concentration of 10 μg/ml. Purified rat-anti GFAP (10 μg/ml; Invitrogen), PECAM-1 (platelet/endothelial cell adhesion molecule-1;0 μg/ml; Abcam) or goat-anti-T. gondii (10 μg/ml; Abcam) were incubated with tissue samples for 2 h at room temperature or overnight at 4°C, and followed with appropriate secondary antibodies conjugated to Alexa Fluor® 488, Alexa Fluor® 568 or Alexa Fluor® 647 at 2 μg/ml (Invitrogen). Samples were mounted in Prolong Gold with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen) for nuclear counterstaining. Images were collected on a TCS/SP2 UV confocal microscope (Leica) and analysed using Improvision Volocity 5.0 (PerkinElmer).
Flow cytometry
Splenocytes were prepared from naïve and infected mice. Single-cell suspensions were generated by passing through a 40 μm filter mesh, and the resulting suspension washed and incubated with 0.86% NH4Cl in PBS to lyse erythrocytes. For isolation of BMNC (brain mononuclear cells), whole brain tissue was collected following transcardial perfusion with 40 ml of ice-cold PBS. Tissue was minced and then digested with collagenase/dispase at 37°C for 45 min, followed by DNase (Sigma–Aldrich) at 37°C for 45 min. Cell suspensions were purified using a density gradient composed of 60 and 30% solutions of Percoll (GE Healthcare). Following washing, cells were counted and resuspended in FACS buffer (1% BSA, 0.1 mM EDTA in PBS) for incubation with FcBlock and antibodies against cell surface markers. For tetramer staining, cells were incubated with SIINFEKL tetramer for 15 min at room temperature followed by 15 min at 4°C. For intracellular staining, cells were then fixed in 4% PFA (EMS) in PBS, permeabilized with 0.3% saponin in PBS, and incubated with purified rabbit anti-mouse MMP-8, MMP-10 or TIMP-1 (Abcam). Secondary antibodies to rabbit IgG conjugated to Alexa Fluor® 488 or Alexa Fluor® 647 (Invitrogen) were used for detection. A FACSCanto II (BD Biosciences) was used to collect fluorescence signal, and data were analysed using FlowJo version 8.8.6 (Tree Star).
Primary astrocyte cultures and ELISA
Cell culture supernatants were prepared from astrocytes isolated from mixed glial cultures according to previously published methods (Giulian and Baker, 1986). Briefly, whole brains were collected from 1–3 day postnatal C57Bl/6 mice, and regions caudal to the midbrain were discarded to exclude cerebellar tissue. The remaining forebrain tissues were strained, washed and plated for 12 days with medium changed every 3 days. On the 12th day, cultures were shaken at 37°C for 2 h at 240 rpm, and the supernatant aspirated to remove less adherent cells. The cultures were then subjected to an additional 18 h shaking at 37°C, after which the cells still adherent were enriched for astrocytes as confirmed by staining for GFAP (glial fibrillary acidic protein). Astrocytes were trypsinized, counted in the presence of Trypan Blue dye, and replated in 12-well plates at a density of 1×105 cells/cm2 and left to adhere overnight. Cultures were then stimulated with medium alone [DMEM (Dulbecco's modified Eagle's medium), 10% FCS, 2 mM glutamine, 1% non-essential amino acids, 10 mM Hepes, 100 IU (international units)/ml penicillin and 100 μg/ml streptomycin] or 25 μg/ml sTAg (soluble Toxoplasma antigen) or 20 units/ml recombinant mouse IFN-γ (R&D Systems) or with 100 ng/ml LPS (lipopolysaccharide) or were infected with the type I RH strain of T. gondii at an MOI (multiplicity of infection) of five parasites per cell. After 24 h, supernatants were collected and centrifuged, and diluted 1:100 for use in ELISA. ELISA was performed using the Quantikine Immunoassay kit for mouse TIMP-1 (R&D Systems) according to the manufacturer's protocol.
Cytokine measurement
Peripheral blood was collected from the tail vein of mice at days 7 and 14 post-infection. Serum was diluted 10-fold for pro-inflammatory CBA (cytokine bead assay; BD Pharmingen) according to the manufacturer's protocol. Samples were collected on a FACSCanto II flow cytometer (BD Biosciences), and concentrations of IFN-γ, IL-12p70 (interleukin-12p70), IL-6, MCP-1 (monocyte chemoattractant protein-1), TNF (tumour necrosis factor) and IL-10 were determined by comparison with a standard curve.
RESULTS
MMPs and TIMPs are up-regulated in the CNS following T. gondii infection
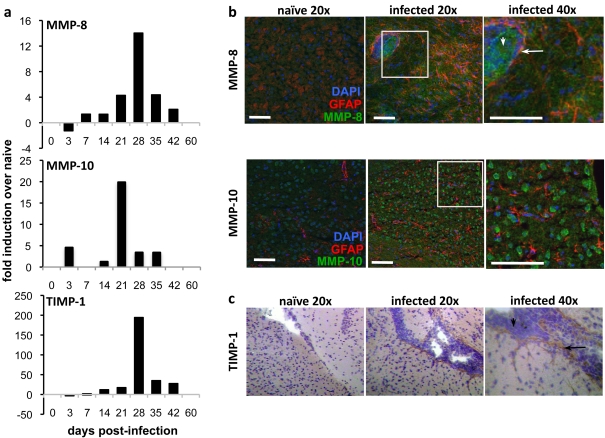
To investigate which brain ECM proteins are altered by T. gondii infection, whole brain mRNA was isolated from naïve and chronically infected mice. Quantitative RT–PCR was conducted using a commercial array of primers for ECM genes and associated molecules (SA Biosciences). Fold induction over naïve transcript levels of MMPs and their inhibitors at 21 days post-infection are listed in Table 1. Although post-infection up-regulation of the ubiquitous CNS gelatinases MMP-2 (>2.0-fold) and MMP-9 (>4.0-fold) was observed, the most notable increase in transcription was of MMP-8 (∼96-fold) and MMP-10 (∼20-fold). This was accompanied by a significant increase of their inhibitor TIMP-1 (∼165-fold). To determine the kinetics of these molecules following infection, brain tissue was collected at time points ranging from 3 days to 6 weeks post-infection for real-time RT–PCR using specifically designed primers (Figure 1a). An increase in MMP-8 transcript was detectable as early as day 7, a time point representing the peak of acute systemic infection and prior to infiltration of parasites and immune cells in the brain. This expression peaked at >14-fold over naïve at day 28 post-infection, and remained increased 2-fold over naïve at 6 weeks post-infection. Peak expression of MMP-8 occurs during chronic infection when the parasite exists predominantly as cysts in the brain and large numbers of lymphocytes are required to traffic into the CNS to maintain parasite latency. In contrast, MMP-10 increase was observed earlier beginning at day 3 and peaking abruptly at day 21 post-infection. Transcripts decreased rapidly to naïve levels by 6 weeks. The increase in TIMP-1 transcription was of greatest magnitude, beginning at day 7 and increasing to >190-fold over naïve by 4 weeks post-infection, with levels remaining >20-fold over naïve at 6 weeks. At late time points after infection (≥60 days) no change in RNA expression of MMP-8, MMP-10 or TIMP-1 could be detected in the brain over that of naïve (Figure 1a). These results suggest a need for MMP-8, MMP-10 and TIMP-1 during chronic infection, specifically during a period associated with lymphocyte influx into the brain.
Table 1. Brain infection with T. gondii induces up-regulation of genes for MMPs and their endogenous inhibitors.
cDNA generated from whole brain mRNA collected at day 21 post-infection was used as template in qRT–PCR reactions with an array of 80+ ECM-associated gene primers. Fold induction shown is compared with transcript from uninfected brain tissue. mRNA were pooled from three animals per time point.
| Gene product | Fold-induction |
|---|---|
| TIMP-1 | 164.78 |
| MMP-8 | 95.93 |
| MMP-10 | 19.86 |
| MMP-14 | 5.75 |
| MMP-9 | 4.02 |
| TIMP-2 | 3.56 |
| TIMP-3 | 3.44 |
| MMP-2 | 2.76 |
| MMP-7 | 2.34 |
| MMP-11 | 2.20 |
| MMP-15 | 1.96 |
| MMP-3 | 1.29 |
| MMP-13 | 1.07 |
Figure 1. Expression of MMP-8, MMP-10 and TIMP-1 in the T. gondii-infected brain.
(a) Quantitative real-time PCR of cDNA synthesized from mRNA harvested from forebrain tissue of C57BL/6 mice over a time course from 3 to 60 days post-infection. Induction is measured as fold change from naïve. (b) Immunohistochemistry (IHC) of cortical regions of infected mice collected from naïve and 4 weeks post-infection. Green MMP-8 or MMP-10; red, GFAP; blue, DAPI. Scale bar, 50 μm. (c) IHC of naïve and infected brain sections for TIMP-1 counterstained with haematoxylin. Arrows pointing left indicate vasculature and down arrows indicate infiltrating cells.
To assess the location and distribution of these molecules following infection immunohistochemistry was performed on brain sections from chronically infected mice at 4 weeks post-infection, and compared with uninfected tissue (Figure 1b). A strong MMP-10 signal was present on purkinje neurons in the naïve cerebellum (results not shown), consistent with previously published reports (Toft-Hansen et al., 2004; Cuadrado et al., 2009). However, the distribution of T. gondii in the CNS is not equal and parasites are found preferentially in the frontal cortex (Dellacasa-Lindberg et al., 2007; Hermes et al., 2008). In this area, no constitutive expression of MMP-8, MMP-10 or TIMP-1 is noted; however, following infection, expression of all three molecules is observed. Cytoplasmic MMP-8 is clearly expressed by cells entering from the vasculature with more diffuse staining within the brain parenchyma (Figure 1, upper panels). MMP-10 is similarly observed in the frontal cortex that is cytoplasmic in appearance. Neither MMP-8 nor MMP-10 co-localize with the astrocyte marker GFAP (Figure 1b, lower panels). Increased expression of TIMP-1 is apparent closely associated with blood vessels, pointing to expression by either astrocytes or cells in the vasculature (Figure 1c). These results demonstrate that MMP-8, MMP-10 and TIMP-1 are up-regulated in the brain following Toxoplasma infection at temporal and physical proximity to parasites and leucocyte infiltration.
MMPs and TIMP-1 are expressed by infiltrating lymphocytes in the infected brain
Significant populations of infiltrating immune cells, including CD4+ and CD8+ T-cells, macrophages and dendritic cells can be detected in the brain of an infected animal by 3 weeks post-infection. Our observation that MMPs and TIMP-1 peak at 21–28 days post-infection would coincide with the significant infection-induced presence of T-lymphocytes infiltrating the CNS. We therefore examined the production of MMPs and TIMP-1 from T-cell populations in the infected brain to assess whether their up-regulation could be attributed to immune cells that had extravasated into brain parenchymal tissue.
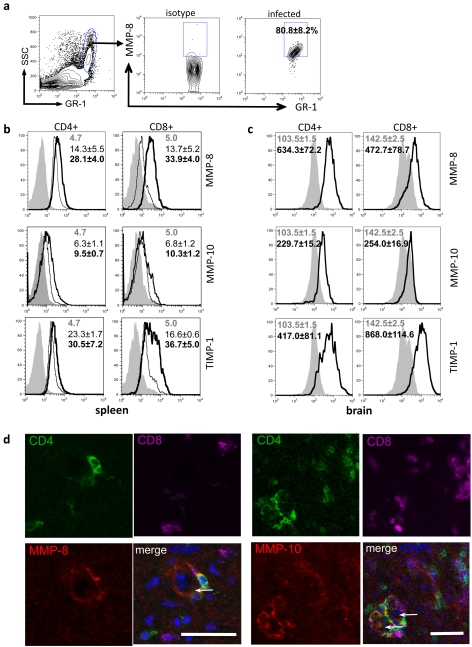
At 4 weeks post-infection, cells were analysed for surface markers and intracellular expression of MMP-8, MMP-10 and TIMP-1 from the spleen and brain. MMP-8 is typically associated with highly migratory and invasive cell types including neutrophils and melanoma cells (Giambernardi et al., 2001). Analysis of inflammatory monocyte populations (Gr1hi) found in the spleen and brain were almost uniformly expressing MMP-8 consistent with its expression by neutrophils during migration into sites of inflammation (Figure 2a). However, in addition to this classical expression, both CD4+ and CD8+ T-cells from the spleen produced MMP-8 following infection (Figure 2b). Thus, although splenocytes from naïve mice showed low-level expression of MMP-8 over isotype control values (∼5.0), the mfi (mean fluorescent intensity) of MMP-8 expression on CD4+ T-cells and CD8+ T-cells from infected spleens was 28.1±4.0 and 33.9±4.0 respectively (Figure 2b). In contrast, MMP-10 expression was only slightly up-regulated in splenocytes from infected mice over those of naïve cells (Figure 2b). Analysis of TIMP-1 expression in the spleen revealed constitutive expression by CD4+ T-cells; however, little to no increase in expression following infection (Figure 2b). A distinct pattern was observed in CD8+ T-cells with low constitutive expression in naive cells (mfi: 16.6±0.6) rising significantly (P = 0.02) to (36.7±5.0) following infection.
Figure 2. T-cell expression of MMPs and TIMP-1 in response to Toxoplasma infection.
(a) Expression of MMP-8 in a population of high SSC, GR-1hi cells from infected spleens. (b and c) Intracellular flow cytometry of MMP-8 and -10 and TIMP-1 in splenocytes and brain mononuclear cells at 4 weeks post-infection. Shaded area represents isotype control for MMPs and TIMP; fine line, uninfected; bold line, infected. Results are representative of three independent experiments; n = 3–4 animals per group. (d) Immunohistochemistry of infected brain slices of MMP expression and perivascular T-cells. Green, CD4; magenta, CD8; blue, DAPI; red, MMP-8 (left) and MMP-10 (right). Scale bar, 50 μm. Results are representative of three independent experiments; results are means±S.E.M. from n = 3 mice per group for each experiment. Arrows pointing left indicate vasculature.
In naïve mice, there are no T-cells in the brain; however, following Toxoplasma infection substantial populations of CD4+ and CD8+ T-cells migrate into the CNS. Both CD4+ and CD8+ T-cells that had migrated to this site had significant expression of MMP-8 over isotype control, in a similar pattern to cells in the spleen (Figure 2c). In contrast, despite seeing no change in MMP-10 expression between naïve and infected splenocytes, MMP-10 expression was uniquely up-regulated on CD4+ T-cells that were in the brain (mfi: isotype = 103.5±1.5; infected = 229.7±15.2) (Figure 2c). Lastly, consistent with the expression of TIMP-1 by CD4+ and CD8+ T-cells in the spleen both these populations express TIMP-1 in the infected brain (Figure 2c). To confirm the disparate production of MMPs by CD4+ and CD8+ T-cells immunohistochemistry was conducted on brains from infected mice. MMP co-localization was found primarily with CD4 expression (Figure 2d). In addition, it is apparent that the secretion of MMP-8 and MMP-10 is by T-cells associated with the vasculature with minimal co-localization observed in the brain parenchyma. Therefore vascular-associated T-helper and cytotoxic T-cell populations are a source of MMP-8 and TIMP-1 during chronic Toxoplasma infection, but only CD4+ helper T-cells contribute to MMP-10 production.
TIMP-1 is produced by CNS-resident cells in response to infection
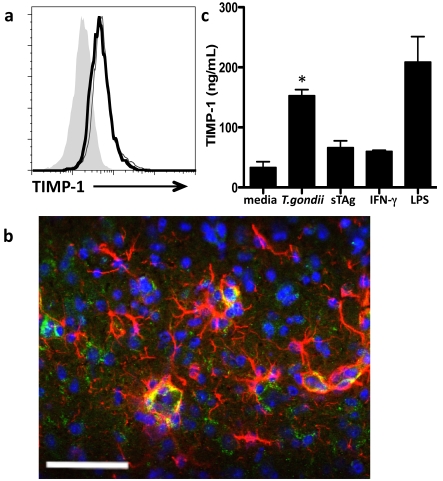
In addition to T-cell production of TIMP-1, it has been reported that microglia and astrocytes can be sources of this inhibitor during inflammation in the brain (Crocker et al., 2006a, 2006b; Dhar et al., 2006). To determine if CNS-resident cells contribute to TIMP-1 mediated control of MMPs during Toxoplasma infection microglial populations isolated from naive and infected whole brain tissue were compared by flow cytometry for TIMP-1 production. Modest production of TIMP-1 by microglia was observed in uninfected brains; however, there is no significant increase in production following infection (Figure 3a). Astrocyte expression in naïve brains was not observed; however, following infection there was significant, although not exclusive, co-localization of TIMP-1 with the astrocyte-specific structural protein GFAP (Figure 3b). To further investigate the infection-associated stimulus for astrocyte production of TIMP-1, primary astrocytes were infected or stimulated with parasite antigen and TIMP-1 production measured by ELISA. Unstimulated astrocytes demonstrated modest secretion of TIMP-1 (33.1±9.7 ng/ml), consistent with its role under normal physiological conditions (Dhar et al., 2006) (Figure 3c). Astrocytes exposed to IFN-γ, a cytokine that is not specific to but nonetheless necessary and prevalent in Toxoplasma infection, as well as those incubated with soluble parasite antigen (sTAg), demonstrate a trend for increased production of TIMP-1 compared with unstimulated cells. However, there was a significant (P = 0.02) increase in TIMP-1 production by astrocytes that had been directly infected with Toxoplasma, an increase of nearly 5-fold over unstimulated cells and similar to levels generated in response to the positive control LPS. These results demonstrate that although astrocytes boost production of TIMP-1 in response to both non-specific and parasite-specific inflammatory stimuli, up-regulation is greatest upon direct infection with T. gondii. These results reveal that TIMP-1 is produced by CNS-resident cells in response to direct parasite infection.
Figure 3. TIMP-1 is up-regulated by astrocytes in response to infection with T. gondii.
(a) Flow cytometry of microglia (CD45int, CD11b+) isolated from brains of uninfected mice (fine line) compared with those from mice at 4 weeks post-infection (bold line) and isotype control (grey shaded area). Median fluorescence intensity (mfi) of populations expressing TIMP-1 are compared. (b) Immunohistochemistry of chronically infected C57Bl/6 cortical tissue section demonstrates co-localization (yellow) of TIMP-1 with astrocytes. Green, TIMP-1; red, GFAP; blue, DAPI. Scale bar, 50 μm. (c) TIMP-1 production by primary astrocyte cultures incubated for 24 h in medium alone, infected with T. gondii, stimulated with Toxoplasma antigen (sTAg), or with IFN-γ. LPS served as a positive control. Results are from two replicates per sample, three to five bioreplicates per condition, and three independent experiments. *P<0.05.
TIMP-1 inhibits parasite clearance during Toxoplasma infection
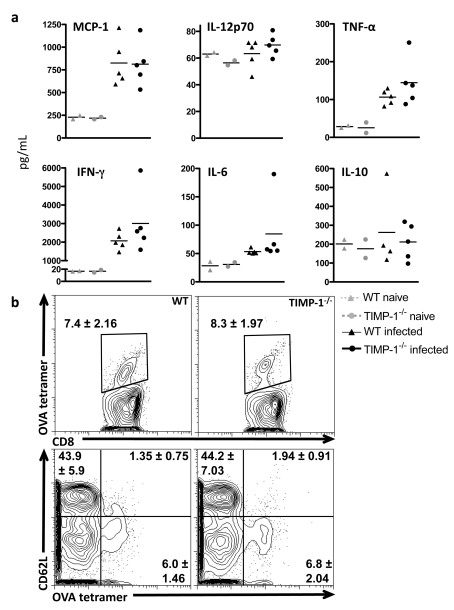
Having demonstrated the up-regulation of TIMP-1 production by infiltrating T-cells and CNS-resident astrocytes in response to infection, experiments were conducted to examine the significance of this expression during the maintenance of chronic immune responses in the brain. TIMP-1-deficient mice were infected and pathology, immune function and parasite burden compared with that of WT (wild-type) infected mice. No significant signs of systemic illness (hunching, fur ruffling) were observed in either group during the 6-week observation period following infection. Serum analysis of systemic cytokines during the acute phase of infection revealed no defects in pro- or anti-inflammatory responses (MCP-1, IL-12p70, IL-6, IFN-γ, TNF-α or IL-10) in TIMP-1−/− animals at day 7 or day 14 post-infection (Figure 4a and results not shown). Analysis of the early T-cell response generated by T. gondii infection revealed no significant difference between the proportion of antigen-specific CD8+ T-cells generated in the absence of TIMP-1 or in their activation status (Figure 4b).
Figure 4. Acute immune responses to Toxoplasma infection in the absence of TIMP-1.
(a) Cytometric bead analysis of pro-inflammatory cytokines in serum from peripheral blood collected 7 days post-infection from WT and TIMP-1−/− animals. Markers represent results from individual animals; bars represent mean levels per condition. (b) Tetramer staining of antigen-specific CD8+ T-cells and CD62L expression. Numbers represent proportion of cells in gate as a percentage of total live CD8+ T-cells means±S.E.M. from n = 3 mice/group.
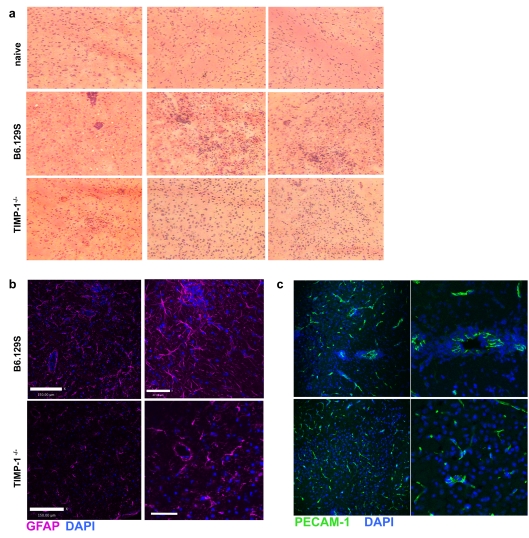
The production of MMPs by T-cells at the vasculature points to a role in metalloproteinase mediated entry of infection responding lymphocytes into the brain parenchyma. Thus, in the absence of MMP inhibition by TIMP-1, lymphocyte entry may be expected to increase. We examined histological sections of brain tissue from control and TIMP-1−/− animals for inflammation and cellular localization. As expected, naïve brains showed no cellular infiltrate or pathology. However, in control (WT) mice that had been infected there was increased cellular infiltrate and evidence of changes in tissue morphology (Figure 5a). In contrast, sections from TIMP-1−/− animals showed little to no morphological changes in tissue structure. Further analysis revealed increased GFAP expression in WT infected brains, with long elongated dendrites confirming astrocyte activation. In TIMP-1−/− brains there was no sign of activated astrocytes with less GFAP expression and no polarization of dendrites (Figure 5b). Associated with areas of activated astrocytes were large groups of cells. Astrocytes are key components of the blood–brain barrier and therefore these observations could represent areas of perivascular cuffing. To confirm this, we used antibodies to PECAM-1, present on the endothelial cells of blood vessels during inflammation. In WT mice, there were several areas in which the majority of PECAM staining had profound perivascular cuffing characteristic of inflamed brain tissue during T. gondii infection (Figure 5c). In contrast, brain inflammation in infected TIMP-1-deficient animals demonstrated reduced or absent perivascular cuffing of brain vessels with cells more commonly observed in the parenchyma (Figure 5c). This finding is indicative of increased access of infiltrating cells to the brain, perhaps via uninhibited metalloproteinase-mediated cleavage of basement membrane proteins in the perivascular space (Agrawal et al., 2006).
Figure 5. Histological analysis of TIMP-1 infected brains.
(a) Haematoxylin and eosin histology at ×10 magnification of naïve, infected WT and infected TIMP-1−/− mice at 4 weeks post-infection. (b and c) Immunohistochemistry of tissue in (a); (b) GFAP signal (magenta) demarcates glia limitans; DAPI nuclear counterstaining (blue) (c) PECAM-1 (green) and DAPI. Arrows indicate vasculature.
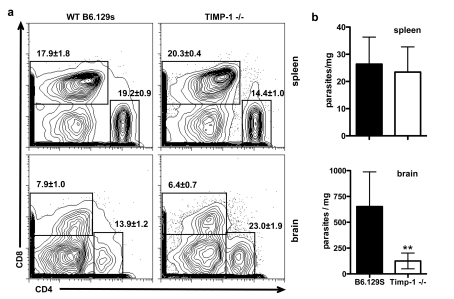
To quantify the ability of cells to access the brain in the absence of TIMP-1, we analysed the cellular infiltrate from spleen and infected brains using flow cytometry. Analysis of T-cell populations in the spleen revealed slight variations between WT and TIMP-1−/− mice, however, the ratio of CD4:CD8 T-cells remained close to 1 (Figure 6a). In contrast, although the proportion of CD8+ T-cells in infected brains was unchanged, there was a significant increase in the proportion of CD4+ T-cells in the absence of TIMP-1. Thus the ratio of CD4:CD8 changed from 1.75 in WT mice to 3.6 in the absence of TIMP-1 (Figure 6a).
Figure 6. Parasite burden and T-cell infiltration in the absence of TIMP-1.
(a) Flow cytometry of CD4 and CD8 T-cells in infected spleen and brain of WT and TIMP-1−/− mice. Numbers represent means±S.E.M. of total live cells. (b) Quantitative real-time PCR of DNA isolated from whole spleen and brain tissue of WT and TIMP-1−/− at 6 weeks post-infection amplified with primers for T. gondii. A number of parasites per milligram of tissue were determined by comparison with a purified parasite DNA standard. Results are from two independent experiments, three animals per condition, three replicates per sample.
Furthermore, measurement of parasite burden revealed no changes in peripheral burden in the absence of TIMP-1 (Figure 6b). However, in TIMP-1−/− mice parasite burden in the brain was reduced more than 4-fold compared with WT brains (Figure 6b). Taken together, these results suggest that activated CD4+ T-cells gain access to the CNS during chronic Toxoplasma infection in a manner that is regulated by TIMP-1.
DISCUSSION
Although the events of lymphocyte rolling and adhesion at the blood–brain barrier are well characterized, the molecules that facilitate entry into and migration within the brain parenchyma are not (Wilson et al., 2010). Here we describe T-cell production of key MMPs, in conjunction with their inhibitor TIMP-1, during Toxoplasma infection in the brain. Previous studies have shown MMP production is stimulated in the brain by several aspects of the immune response known to be present during T. gondii infection, including IL-1 (Szenasi et al., 2008); IL-23 (Langowski et al., 2006); TNF-α (Szenasi et al., 2008) and COX-2 (Peng et al., 2008); thus it is perhaps not surprising that we found increased expression of several MMPs and their inhibitors in the brain throughout the course of Toxoplasma infection. However, in contrast to the classic MMP signature of CNS inflammation, that of increased MMP-2, MMP-3 and MMP-9 expression (Candelario-Jalil et al., 2009), our studies demonstrate a dominant increase in MMP-8 and MMP-10.
MMP-8 expression has been associated with fast-responding invasive cells including neutrophils (Giambernardi et al., 2001); however, several imaging studies have demonstrated that T-cells within the CNS can be highly migratory (Kawakami et al., 2005; Kim et al., 2009; Wilson et al., 2009). Our observations of MMP-8 and -10 production by T-cells, localized predominantly to the vasculature, implies a role for metalloproteinases in tissue penetration during lymphocyte trafficking into the brain. MMP production by migrating T-cells has been described in the context of Type I diabetes (Savinov and Strongin, 2009) and leukaemia cell infiltrative capacity (Ivanoff et al., 1999), but this is the first indication that T-cells produce metalloproteinases to migrate into or within brain tissue. Our observation that T-cells within the spleen express very little MMP-8 and -10 compared with those in the brain suggests that there is a secondary signal at the site of inflammation that turns on MMP production. This may be at the site of the blood–brain barrier where we know several signalling events are required prior to extravasation into the CNS (Wilson et al., 2010).
Lymphocyte-associated protease activity could be associated with penetrating the blood–brain barrier, the induction of a cell-trafficking network, or with other migratory processes. However, although originally characterized for their collective ability to degrade all proteins of the ECM, it has been subsequently demonstrated that metalloproteinases, and their inhibitors have diverse substrates related to immunomodulatory function (for review see Stetler-Stevenson, 2008). MMPs have been shown to activate and degrade cytokines and chemokines (Webster and Crowe, 2006), for proteolytic cleavage of proteins to generate autoimmunogenic peptides in EAE (Benson et al., 2010; Shiryaev et al., 2009) and are frequently required for cleavage activation of the secreted proenzyme forms of other members of the metalloproteinase family. MMP-10 cleaves the zymogen precursor of MMP-8. Thus, although results find MMP-8 and MMP-10 concentrated at vascular areas, the implications for this study are that non-ECM substrates of MMP-8 and -10 as well as matrix proteins may be a target for metalloproteinase activity following T. gondii infection.
The differences observed between CD4+ and CD8+ T-cell expression of MMPs implies distinct mechanisms of cell entry into the brain for these lymphocytes. Evidence that these populations access the CNS differently is prevalent within the literature (Kawakami et al., 2005; Kivisakk et al., 2006; Ploix et al., 2010; Wilson et al., 2009, 2010) and although integrins, chemokines and activation status probably play dominant roles in this process, the MMP signature of lymphocytes may provide a further level of control. Thus the addition of TIMP-1 may inhibit pathological CD4+ T-cell entry into the brain during multiple sclerosis for example, while leaving effector CD8+ T-cell trafficking intact for responses to pathogens.
Control of Toxoplasma infection in the CNS is achieved through negative regulation of pro-inflammatory factors such that parasite re-activation is suppressed with limited immunopathology (Gazzinelli et al., 1992; Wilson et al., 2005; Stumhofer et al., 2006). Similarly, uncontrolled MMP production, while facilitating access of infiltrating leucocytes, results in extensive tissue damage (Cuzner and Opdenakker, 1999; Newman et al., 2001). The TIMP family of molecules provides broad inhibition of MMP activity. In the present paper, we demonstrate TIMP-1 production from invading T-cells and CNS-resident astrocytes in response to infection. Although microglia, constitutively express TIMP-1, infection-induced TIMP-1 was only apparent in astrocytes possibly reflecting their role as the first line of defense during T-cell infiltration at the blood–brain barrier (Boz et al., 2006; Dhar et al., 2006). The significance that direct infection by parasites, and not the pro-inflammatory cytokine IFN-γ or Toxoplasma antigen alone, induces TIMP-1 is supported by previous studies observing the down-regulation of MMP activity in human monocytes following infection with Toxoplasma tachyzoites (Buache et al., 2007). Regulation of MMP activity and TIMP-1 induction in astrocytes is through the NF-κB (nuclear factor κB) signalling pathway (Harris et al., 2007; Gomez-Nicola et al., 2010; Green et al., 2010), a pathway that is transiently inhibited by direct infection with Toxoplasma (Mason et al., 2004). Thus inhibition of MMP via increased expression of TIMP-1 may point to an evasive strategy by the parasite to limit access of immune cells or a host response to minimize immune-mediated pathology. In support of the former, despite the development of a normal systemic immune response and equivalent parasite burden in the periphery, TIMP-1−/− mice have a significant decrease in parasite burden in the brain compared to WT controls. This is not accompanied by any substantial pathology in the brain– indeed histology suggests less focal immune clusters and decreased astrocyte activation. Previous studies have demonstrated a role for TIMP-1 in limiting inflammation in the brain during models of CNS autoimmunity (Althoff et al., 2010; Toft-Hansen et al., 2004; Crocker et al., 2006a, 2006b; Thorne et al., 2009). However, in support of our studies, during viral and bacterial infection TIMP-1 is associated with inhibition of pathogen clearance without development of adverse pathology in the brain (Zhou et al., 2002, 2005; Lee et al., 2005).
Toxoplasma represents a life-long infection with no treatment at present that targets the removal of latent infection in the brain, making it a critical complication for the immune compromised. Mechanisms that increase parasite removal but do not lead to increased immune-mediated pathology would be a justified focus of research. In summary, these studies demonstrate Toxoplasma-induced up-regulation of MMP-8 and -10 and the inhibitor TIMP-1 by T-cells and CNS resident astrocytes. In the absence of TIMP-1 parasite removal is increased without adverse pathology in the infected brain.
ACKNOWLEDGEMENTS
We thank I. Ethell, N. Schiller and D. Carter for helpful discussions and technical assistance.
Footnotes
This work was supported in part by grants to E.H.W. from CRCC, the UCR Division of Biomedical Sciences and the UCR academic senate.
REFERENCES
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff GE, Wolfer DP, Timmesfeld N, Kanzler B, Schrewe H, Pagenstecher A. Long-term expression of tissue-inhibitor of matrix metalloproteinase-1 in the murine central nervous system does not alter the morphological and behavioral phenotype but alleviates the course of experimental allergic encephalomyelitis. Am J Pathol. 2010;177:840–853. doi: 10.2353/ajpath.2010.090918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Baratelli FE, Heuze-Vourc'h N, Krysan K, Dohadwala M, Riedl K, Sharma S, Dubinett SM. Prostaglandin E2-dependent enhancement of tissue inhibitors of metalloproteinases-1 production limits dendritic cell migration through extracellular matrix. J Immunol. 2004;173:5458–5466. doi: 10.4049/jimmunol.173.9.5458. [DOI] [PubMed] [Google Scholar]
- Benson HL, Mobashery S, Chang M, Kheradmand F, Hong JS, Smith GN, Shilling RA, Wilkes DS. Endogenous MMP2 and MMP9 regulate activation of CD4+ and CD8+ T cells. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0125OC. doi:10.1165/rcmb.2010-0125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomston M, Shafii A, Zervos EE, Rosemurgy AS. TIMP-1 overexpression in pancreatic cancer attenuates tumor growth, decreases implantation and metastasis, and inhibits angiogenesis. J Surg Res. 2002;102:39–44. doi: 10.1006/jsre.2001.6318. [DOI] [PubMed] [Google Scholar]
- Boz C, Ozmenoglu M, Velioglu S, Kilinc K, Orem A, Alioglu Z, Altunayoglu V. Matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase (TIMP-1) in patients with relapsing-remitting multiple sclerosis treated with interferon beta. Clin Neurol Neurosurg. 2006;108:124–128. doi: 10.1016/j.clineuro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Buache E, Garnotel R, Aubert D, Gillery P, Villena I. Reduced secretion and expression of gelatinase profile in Toxoplasma gondii-infected human monocytic cells. Biochem Biophys Res Commun. 2007;359:298–303. doi: 10.1016/j.bbrc.2007.05.089. [DOI] [PubMed] [Google Scholar]
- Burrage PS, Huntington JT, Sporn MB, Brinckerhoff CE. Regulation of matrix metalloproteinase gene expression by a retinoid X receptor-specific ligand. Arthritis Rheum. 2007;56:892–904. doi: 10.1002/art.22417. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker SJ, Milner R, Pham-Mitchell N, Campbell IL. Cell and agonist-specific regulation of genes for matrix metalloproteinases and their tissue inhibitors by primary glial cells. J Neurochem. 2006a;98:812–823. doi: 10.1111/j.1471-4159.2006.03927.x. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Whitmire JK, Frausto RF, Chertboonmuang P, Soloway PD, Whitton JL, Campbell IL. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. Am J Pathol. 2006b;169:2104–2116. doi: 10.2353/ajpath.2006.060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado E, Rosell A, Penalba A, Slevin M, Alvarez-Sabin J, Ortega-Aznar A, Montaner J. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res. 2009;8:3191–3197. doi: 10.1021/pr801012x. [DOI] [PubMed] [Google Scholar]
- Cuzner ML, Opdenakker G. Plasminogen activators and matrix metalloproteases, mediators of extracellular proteolysis in inflammatory demyelination of the central nervous system. J Neuroimmunol. 1999;94:1–14. doi: 10.1016/s0165-5728(98)00241-0. [DOI] [PubMed] [Google Scholar]
- Dellacasa-Lindberg I, Hitziger N, Barragan A. Localized recrudescence of Toxoplasma infections in the central nervous system of immunocompromised mice assessed by in vivo bioluminescence imaging. Microbes Infect. 2007;9:1291–1298. doi: 10.1016/j.micinf.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Dhar A, Gardner J, Borgmann K, Wu L, Ghorpade A. Novel role of TGF-beta in differential astrocyte-TIMP-1 regulation: implications for HIV-1-dementia and neuroinflammation. J Neurosci Res. 2006;83:1271–1280. doi: 10.1002/jnr.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP. The history of Toxoplasma gondii – the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van den Oord J, Sciot R, Meinhardt T, Hammerling G, Opdenakker G, Arnold B. Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest. 1999;104:1507–1515. doi: 10.1172/JCI6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85:2813–2823. doi: 10.1002/jnr.21273. [DOI] [PubMed] [Google Scholar]
- Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res. 2003;74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- Giambernardi TA, Sakaguchi AY, Gluhak J, Pavlin D, Troyer DA, Das G, Rodeck U, Klebe RJ. Neutrophil collagenase (MMP-8) is expressed during early development in neural crest cells as well as in adult melanoma cells. Matrix Biol. 2001;20:577–587. doi: 10.1016/s0945-053x(01)00166-4. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D, Valle-Argos B, Nieto-Sampedro M. Blockade of IL-15 activity inhibits microglial activation through the NFkappaB, p38, and ERK1/2 pathways, reducing cytokine and chemokine release. Glia. 2010;58:264–276. doi: 10.1002/glia.20920. [DOI] [PubMed] [Google Scholar]
- Green JA, Elkington PT, Pennington CJ, Roncaroli F, Dholakia S, Moores RC, Bullen A, Porter JC, Agranoff D, Edwards DR, Friedland JS. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase-1 and -3 expression and secretion via NF-kappaB- and activator protein-1-dependent monocyte networks. J Immunol. 2010;184:6492–6503. doi: 10.4049/jimmunol.0903811. [DOI] [PubMed] [Google Scholar]
- Harris JE, Nuttall RK, Elkington PT, Green JA, Horncastle DE, Graeber MB, Edwards DR, Friedland JS. Monocyte-astrocyte networks regulate matrix metalloproteinase gene expression and secretion in central nervous system tuberculosis in vitro and in vivo. J Immunol. 2007;178:1199–1207. doi: 10.4049/jimmunol.178.2.1199. [DOI] [PubMed] [Google Scholar]
- Hasebe Y, Egawa K, Shibanuma M, Nose K. Induction of matrix metalloproteinase gene expression in an endothelial cell line by direct interaction with malignant cells. Cancer Sci. 2007;98:58–67. doi: 10.1111/j.1349-7006.2006.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, Mayr T, Kirisits MJ, Wollmann R, Ferguson DJ, Roberts CW, Hwang JH, Trendler T, Kennan RP, Suzuki Y, Reardon C, Hickey WF, Chen L, McLeod R. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48. doi: 10.1186/1742-2094-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Abrams JS, Beaman MH, Remington JS. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: importance of T-cell-independent regulation of resistance to T. gondii. Infect Immun. 1993;61:4038–4044. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff A, Ivanoff J, Hultenby K, Sundqvist KG. Infiltrative capacity of T leukaemia cell lines: a distinct functional property coupled to expression of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinases-1 (TIMP-1). Clin Exp Metastasis. 1999;17:695–711. doi: 10.1023/a:1006749304315. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisakk P, Tucky B, Wei T, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid contains CD4+ memory T cells expressing gut- or skin-specific trafficking determinants: relevance for immunotherapy. BMC Immunol. 2006;7:14. doi: 10.1186/1471-2172-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Lee MM, Yoon BJ, Osiewicz K, Preston M, Bundy B, van Heeckeren AM, Werb Z, Soloway PD. Tissue inhibitor of metalloproteinase 1 regulates resistance to infection. Infect Immun. 2005;73:661–665. doi: 10.1128/IAI.73.1.661-665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression results using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mason NJ, Artis D, Hunter CA. New lessons from old pathogens: what parasitic infections have taught us about the role of nuclear factor-kappaB in the regulation of immunity. Immunol Rev. 2004;201:48–56. doi: 10.1111/j.0105-2896.2004.00189.x. [DOI] [PubMed] [Google Scholar]
- Newman TA, Woolley ST, Hughes PM, Sibson NR, Anthony DC, Perry VH. T-cell- and macrophage-mediated axon damage in the absence of a CNS-specific immune response: involvement of metalloproteinases. Brain. 2001;124:2203–2214. doi: 10.1093/brain/124.11.2203. [DOI] [PubMed] [Google Scholar]
- Noor S, Habashy AS, Nance JP, Clark RE, Nemati K, Carson MJ, Wilson EH. CCR7 dependent immunity during acute Toxoplasma gondii infection. Infect Immun. 2010;78:2257–2263. doi: 10.1128/IAI.01314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- Peng BW, Lin JY, Zhang T. Toxoplasma gondii induces prostaglandin E2 synthesis in macrophages via signal pathways for calcium-dependent arachidonic acid production and PKC-dependent induction of cyclooxygenase-2. Parasitol Res. 2008;102:1043–1050. doi: 10.1007/s00436-007-0873-4. [DOI] [PubMed] [Google Scholar]
- Ploix CC, Noor S, Crane J, Masek K, Carter W, Lo DD, Wilson EH, Carson MJ. CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.09.014. doi:10.1016/j.bbi.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst. 2008;100:59–69. doi: 10.1093/jnci/djm268. [DOI] [PubMed] [Google Scholar]
- Savinov AY, Strongin AY. Matrix metalloproteinases, T cell homing and beta-cell mass in type 1 diabetes. Vitam Horm. 2009;80:541–562. doi: 10.1016/S0083-6729(08)00618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev SA, Remacle AG, Savinov AY, Chernov AV, Cieplak P, Radichev IA, Williams R, Shiryaeva TN, Gawlik K, Postnova TI, Ratnikov BI, Eroshkin AM, Motamedchaboki K, Smith JW, Strongin AY. Inflammatory proprotein convertase-matrix metalloproteinase proteolytic pathway in antigen-presenting cells as a step to autoimmune multiple sclerosis. J Biol Chem. 2009;284:30615–30626. doi: 10.1074/jbc.M109.041244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Szenasi G, Vegh M, Szabo G, Kertesz S, Kapus G, Albert M, Greff Z, Ling I, Barkoczy J, Simig G, Spedding M, Harsing LG, Jr. 2,3-benzodiazepine-type AMPA receptor antagonists and their neuroprotective effects. Neurochem Int. 2008;52:166–183. doi: 10.1016/j.neuint.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne M, Moore CS, Robertson GS. Lack of TIMP-1 increases severity of experimental autoimmune encephalomyelitis: Effects of darbepoetin alfa on TIMP-1 null and wild-type mice. J Neuroimmunol. 2009;211:92–100. doi: 10.1016/j.jneuroim.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Thwaites GE, Simmons CP, Than Ha Quyen N, Thi Hong Chau T, Phuong Mai P, Thi Dung N, Hoan Phu N, White NP, Tinh Hien T, Farrar JJ. Pathophysiology and prognosis in Vietnamese adults with tuberculous meningitis. J Infect Dis. 2003;188:1105–1115. doi: 10.1086/378642. [DOI] [PubMed] [Google Scholar]
- Toft-Hansen H, Nuttall RK, Edwards DR, Owens T. Key metalloproteinases are expressed by specific cell types in experimental autoimmune encephalomyelitis. J Immunol. 2004;173:5209–5218. doi: 10.4049/jimmunol.173.8.5209. [DOI] [PubMed] [Google Scholar]
- Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol. 2006;80:1052–1066. doi: 10.1189/jlb.0306152. [DOI] [PubMed] [Google Scholar]
- Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, Haydon PG, Laufer TM, Weninger W, Hunter CA. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol. 2005;165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Invest. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Marten NW, Bergmann CC, Macklin WB, Hinton DR, Stohlman SA. Expression of matrix metalloproteinases and their tissue inhibitor during viral encephalitis. J Virol. 2005;79:4764–4773. doi: 10.1128/JVI.79.8.4764-4773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Stohlman SA, Atkinson R, Hinton DR, Marten NW. Matrix metalloproteinase expression correlates with virulence following neurotropic mouse hepatitis virus infection. J Virol. 2002;76:7374–7384. doi: 10.1128/JVI.76.15.7374-7384.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]