Abstract
Many cognitive, emotional and behavioural traits, as well as psychiatric disorders are highly heritable. However, identifying the specific genes and mechanisms by which this heritability manifests has been elusive. One approach to make this problem more tractable has been to attempt to identify and quantify biological markers that are intermediate steps along the gene-to-behaviour path. The field of neuroimaging offers several anatomic and physiologic possibilities to quantify. Stability over time has been proposed as a desired feature for these intermediate phenotypes. However, in this paper we discuss the value of looking at trajectories of anatomic brain development (i.e. morphometric changes over time), as opposed to static measures, as a phenotype. Examples drawn from longitudinal anatomic magnetic resonance imaging studies of typical development, attention deficit/hyperactivity disorder, and childhood-onset schizophrenia are used to demonstrate the utility of trajectories of brain development as a phenotypic bridge between genes and behaviour in health and in illness.
The path from genes to behaviour is an elaborate and tortuous journey coursing through the most complex biological structure known, the human brain. One approach to elucidate this path has been to study biological measures at various stages along the way, sometimes referred to as intermediate phenotypes.
The rationale for this approach is that the intermediate phenotypes are more fundamental constituents of the behavioural phenomenon and are ‘closer’ to the gene effects, thereby more likely to demonstrate higher penetrance (Gottesman & Gould 2003). As sensible as this approach is, the benefits of utilizing intermediate phenotypes have not proved to be a panacea for simplifying the staggeringly complicated relationship between genes, brain and behaviour (Flint & Munafò 2007).
The field of imaging genetics encompasses various types of investigations linking neuroimaging outcome measures to genetic variation (Meyer-Lindenberg & Weinberger 2006). ‘Reverse genetics’ approaches attempt to identify the neural circuitry influence by a given gene variant by finding genetic associations with a clinical diagnosis and then exploring neuroimaging outcome measures thought to differ between the clinical group and controls within the normative population to determine if allelic variation occurring outside the presence of the disorder is associated with a similar phenotypic signature (Meyer-Lindenberg & Zink 2007). This allows the much greater pool of information from healthy control populations to be used to determine specific effects of particular polymorphisms and thus inform mechanisms underlying neuropsychiatric disorders.
In this paper we discuss a subset of imaging genetics targeting the trajectories of anatomic brain development (i.e. changes in the size/shape of brain structures with time) as the measures to link to genetic variation. Examples of the merits of trajectories as a phenotype are discussed in the context of typical development, attention deficit hyperactivity disorder (ADHD), and childhood-onset schizophrenia (COS).
Project design
Since 1989, the Child Psychiatry Branch of the National Institute of Mental Health has been conducting a longitudinal study in which participants return at approximately two-year intervals for brain imaging, psychological and behavioural testing, and genetics. Currently, the data set consists of approximately 4000 scans from 2000 subjects, about half of them typically developing and the other half from various clinical populations.
Trajectories of brain development in healthy children and adolescents
Singleton subjects for the typical development component of the project are recruited from the local community and twin subjects are recruited nationally. Subjects are screened via an initial telephone interview, mailed questionnaires, and subsequent in-person assessment which includes a clinical evaluation and neuropsychological testing. Exclusion criteria include having a psychiatric diagnosis or first-degree relatives with psychiatric diagnoses, head injury, or any known learning, developmental, or medical conditions likely to have affected brain development. Exclusion criteria related to pregnancy and birth events included gestational age of <30 weeks; very low birth weight (<3 lbs 4oz), any known exposure to psychotropic medications during pregnancy, and significant perinatal complications.
The merit of using trajectories, as opposed to static ‘final’ morphometry, has been demonsrated in a previous study by our group that examined the relationship between IQ and cortical thickness (Shaw et al 2006a). In this study of 307 unrelated children and adolescents the developmental trajectory of cortical thickness in the right superior frontal gyrus (and other brain regions), but not the cortical thickness itself, was different amongst groups with average intelligence (IQ range 83–108), high intelligence (IQ range 109–120), and superior intelligence (IQ range 121–149).
Trajectories have also been shown to be superior to static measures in discriminating male and female brains in our cohort (Lenroot et al 2007). In a study of 829 scans from 287 subjects aged 3 to 27 years, total cerebral volume peaked at age 10.5 in females and 14.5 in males (see Fig. 1). White matter increased across this age span in both males and females but with a steeper rate of increase in males during adolescence. Cortical and subcortical grey matter trajectories follow an inverted U-shaped path with peak sizes 1 to 2 years earlier in females.
FIG. 1.
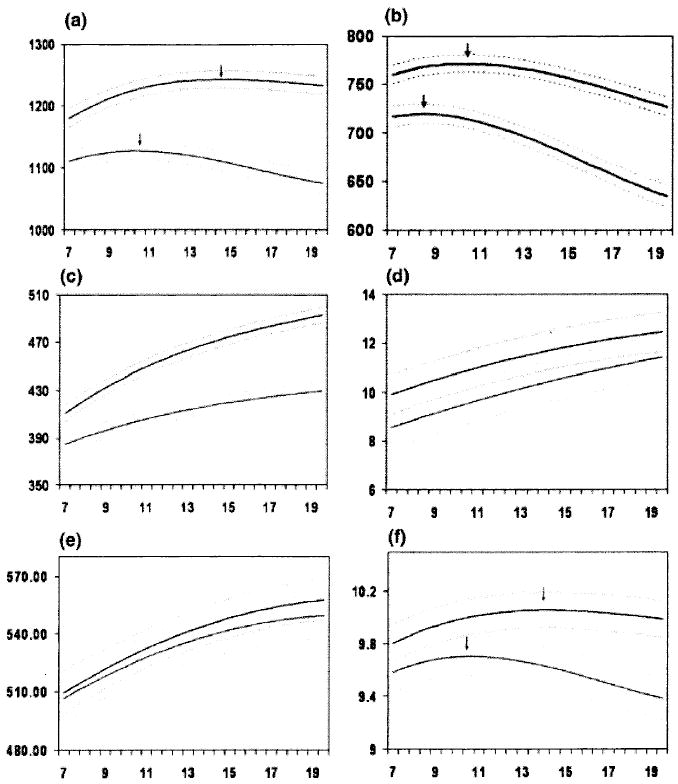
Mean volume by age in years for males (top lines, N = 475 scans) and females (bottom lines, N = 354 scans). Middle lines in each set of three lines represent mean values, and upper and lower lines represent upper and lower 95% confidence intervals. All curves differed significantly in height and shape with the exception of lateral ventricles, in which only height was different, and mid-sagittal area of the corpus callosum, in which neither height nor shape was different. (a) Total brain volume, (b) grey matter volume, (c) white matter volume, (d) lateral ventricle volume, (e) mid-sagittal area of the corpus callosum, and (f) caudate volume. (Adapted from Lenroot et al 2007.)
The inverted U shaped trajectories of grey matter volumes coinciding with improved cognitive abilities confound straightforward relationships between size and function. In a study of 13 subjects who had each been scanned 4 times at approximately 2 year intervals, the peak age of gray matter density along the inverted U-shaped trajectories of gray matter were used as a proxy for maturation on a voxel level of analysis throughout the cortex (Gogtay et al 2004). An animation of these changes is available at http://www.loni.ucla.edu/~thompson/DEVEL/dynamic.html. The peaks occurred earliest in the primary sensorimotor areas and latest in heteromodal association areas of the dorsolateral prefrontal cortex (DLPFC), inferior parietal and superior temporal gyrus. Implications of ongoing maturation throughout adolescence of the DLPFC (which is a key component of circuitry subserving control of impulses, judgment, organization, and long range planning) have prominently entered clinical, social, educational and judicial realms.
These examples from the typical development literature support the contention that trajectories of anatomic brain changes are often more powerful predictors of cognition and behaviour than static measures.
Trajectories of brain development in ADHD
Subjects meeting criteria for ADHD as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association 1994) are recruited from the surrounding community. Inclusion criteria are hyperactive, inattentive, and impulsive behaviours that cause impairment in at least two settings and a Conners’ Teacher Hyperactivity rating greater than 2 SD above age- and sex-specific means (Werry et al 1975)
In a study analysing 544 scans from 152 people with ADHD (age range, 5–18 years) and 139 age- and sex-matched controls (age range, 4.5–1,9 years) total cerebral and cerebellar volumes were smaller in ADHD and the difference persisted across this age span (Castellanos et al 2002). However, caudate volume was smaller in ADHD during childhood but the difference became statistically non-significant as caudate volumes decreased for both patients and controls during adolescence.
The relationship between trajectories of brain morphometry and clinical outcome in ADHD was examined in three studies. In the first, volumes of six cerebellar hemispheric lobes, the central white matter, and three vermal subdivisions were quantified on 229 scans from 36 subjects with ADHD and 36 controls. A measure of global clinical outcome and DSM-IV criteria were used to split the 36 ADHD subjects into groups of better or worse outcome (Mackie et al 2007). The superior cerebellar vermis was smaller in ADHD but did not show progressive loss with age and did not relate to clinical outcome. However, in the worse outcome group inferior–posterior cerebellar lobes became progressively smaller during adolescence than in the better outcome or control groups. An implication of this work is that the cerebellar hemispheres may constitute a more plastic, state-specific marker that could be targeted for clinical intervention.
In the second study relating anatomic trajectories to outcome in ADHD, cortical thickness was quantified on 636 scans from 163 children with ADHD (mean age at entry, 8.9 years) and 166 controls (Shaw et al 2006b). The ADHD group had thinner cortex globally, most pronounced in medial and superior prefrontal and precentral regions, with the worse outcome group having thinner left medial prefrontal cortex at baseline than the better outcome and control groups. Trajectories of right parietal cortex thickness converged with the control group in the better outcome but not worse outcome group. This normalization in patients with a better outcome may represent compensatory cortical change (Fig. 2).
FIG. 2.
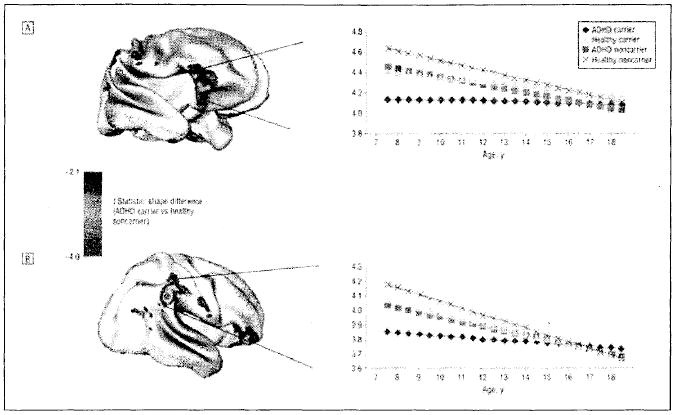
Brain maps of clusters where attention-deficit/hyperactivity disorder (ADHD) carriers of the seven-repeat allele of dopamine d4 receptor differ in trajectory of cortical growth and graphs illustrating trajectories for these clusters. (Adapted from Shaw et al 2007.)
In a follow up of the previous study, information regarding the type of dopamine D4 receptor polymorphism was combined with cortical thickness measures quantified on 442 scans from 105 subjects with ADHD and 103 controls (Shaw et al 2007). The DRD4 seven-repeat allele is associated with greater risk for ADHD. The group with the risk allele had thinner right orbitofrontal/inferior prefrontal and posterior parietal cortex, regions that were generally thinner in subjects with ADHD. However, ADHD subjects with the risk allele had a better clinical outcome and only this group demonstrated the previously mentioned normalization of the right parietal cortical region.
As with the studies of typical development, assessment of trajectories in brain morphometric studies of ADHD yields information linking genes to behaviour not discerned with static measures.
Trajectories of brain development in childhood-onset schizophrenia (COS)
COS is defined as onset of psychosis by the age of 13 and occurs in approximately 1 in 40000 people (Nicolson & Rapoport 1999). Although neurobiologically continuous with the adult onset form of schizophrenia(Nicolson & Rapoport 1999, Rapoport et al 2005), COS subjects have more pronounced delays in social, motor and language function (Alaghband-Rad et al 1995, Hollis 1995), a 10% rate of chromosomal abnormalities (Sporn et al 2004), higher familial rates of schizophrenia spectrum disorders (Nicolson et al 2003), and smooth pursuit eye movement abnormalities (Sporn et al 2005). These features suggest that genetic influences may be more salient in COS (Rapoport et al 2005).
As of August 2007 the NIMH Child Psychiatry Branch has acquired a sample of 95 well characterized cases of COS. The participants were recruited nationwide in a process involving over 1400 chart reviews and 230 in person screenings. The diagnosis was established by two psychiatrists using clinical and structured interviews of the patient and family and in-hospital observation during a 1–3 week medication-free period.
Several studies have identified anomalous trajectories of brain anatomy in this cohort. As in typical development grey matter volumes reduce during adolescence, although in COS the rate of reduction of gray matter volumes is significantly more pronounced during adolescence (Sporn et al 2003, Thompson et al 2001b) and progresses in a parieto-frontal direction. Across ages 7 to 26 the cortex is thinner in the COS group. However, with increasing age posterior (parietal) regions normalize while anterior regions (frontal and temporal) remain thinner merging with the pattern reported in adult onset schizophrenia studies (Greenstein et al 2006). A study examining 113 scans from 52 healthy siblings of this cohort revealed significant child and adolescent gray matter reductions in left prefrontal and bilateral temporal cortices and smaller deficits in the right prefrontal and inferior parietal cortices compared with the controls (Gogtay et al 2007). These differences normalized by age 20 years at which time there were statistically significant differences and the GM thickness correlated positively with the overall functioning of the healthy siblings (GAS scores). Similarly, thicker cortical GM in prefrontal and superior temporal cortices also correlates positively with the remission status at discharge 3–4 months after the MRI scans, suggesting that there may be a direct correlation between cortical thickness and functional outcome.
Gene variants have also been shown to influence trajectories in this COS cohort. GAD1 (2q31.1) encodes glutamic acid decarboxylase (GAD67), which expression studies show is decreased in dorsolateral prefrontal cortex in schizophrenic patients relative to controls. The effects of variants of GAD1 on grey and white matter trajectories were investigated in a family-based TDT and haplotype association analysis (Addington et al 2005). Three adjacent SNPs in the 5′ upstream region of GAD1 showed a positive pairwise association with illness and quantitative trait TDT analyses indicated several SNPs were associated with an increased rate of frontal grey matter loss.
In a subsequent study of a slightly extended sample (78 COS patients and 165 healthy controls) 56 markers (54 single-nucleotide polymorphisms and two microsatellites) spanning the NRG1 locus were genotyped (Addington et al 2007). The risk allele was associated with poorer premorbid social functioning and diagnostically specific different trajectories of grey and white matter lobar volumes (see Fig. 3). In the typically developing group the risk allele affected only frontal and temporal gray matter, whereas in the COS group the risk allele was associated with greater initial total gray and white volume but with an accelerated decline into adolescence.
FIG. 3.
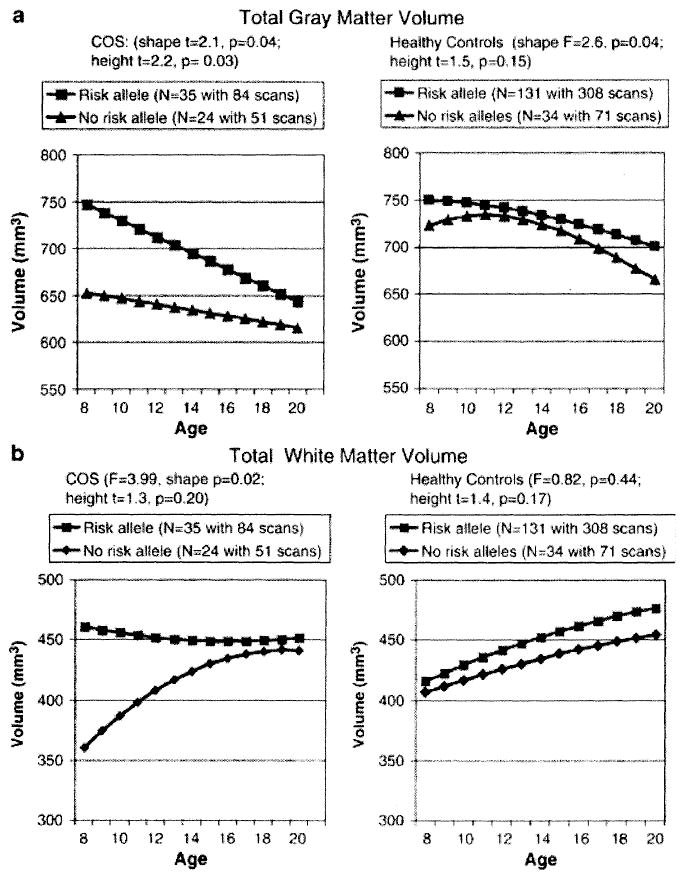
Effect of risk allele status at 420M9-1395 on brain MRI volume trajectories in COS and healthy controls for total (a) grey matter and (b) white matter. (Adapted from Addington et al 2005.)
We have started to explore these findings further using finer cortical thickness measures. Our initial results show that both COS probands (n = 61, 149 scans) and their healthy siblings (n = 24, 50 scans) who have the risk allele for GAD1 have steeper slopes for GM loss in prefrontal and parietal cortices compared to their counterparts without the risk allele suggesting the influence of risk genes (e.g. GAD) in a regionally specific manner.
Discussion
Development is the means by which genes express themselves in phenotypes, and there has been increasing recognition of the importance of understanding the factors regulating the developmental process itself in attempting to parse the factors associated with a healthy or pathological phenotype. Specific traits such as head circumference or height have characteristic patterns of healthy growth, and the detection of abnormalities in these patterns can be one of the first clues that development is going awry. The advent of non-invasive magnetic resonance imaging has made possible longitudinal measurements of the development of brain structure in children and adolescents and consequent characterization of the growth patterns of this previously elusive organ. The studies previously described have demonstrated the inverted U-shaped curves associated with normal brain development, the differences between male and female brain development, the relevance of trajectories as a measure of brain function such as IQ, and the specific impact of different neuropsychiatric disorders and associated genetic polymorphisms.
The sensitivity of growth patterns to such factors raises the question of whether the patterns themselves can serve as intermediate phenotypes in the search for specific genes associated with neurodevelopmental disorders such as schizophrenia. Such a goal presents significant challenges. The developmental course of a complex trait is the product of the interaction of multiple genetic and environmental factors over time. A fundamental step in assessing the usefulness of brain developmental trajectories as intermediate phenotypes is determining whether these trajectories are heritable (Gottesman & Gould 2003). Twin studies have shown that brain volumes are highly heritable (Baare et al 2001, Reveley et al 1984), and cortical thickness to be regionally so (Thompson et al 2001a). The potential interaction of age with heritability measures is supported by results from the large-scale paediatric twin study being carried out by our group, which has shown evidence of age-related changes in heritability of brain lobar volumes (Wallace et al 2006).
Evidence for the possibility that trajectories may prove to be heritable is supported by the well-documented findings of high heritability of growth patterns in other somatic traits such as height (Hauspie et al 1994). It has been observed in both humans and animal models that variance tends to decrease over development, which has been taken as evidence of the operation of canalization. Canalization refers to the frequently observed robustness of phenotypes against minor genetic or environmental perturbations, as in the example of compensatory growth (Flatt 2005, Schmalhausen 1949, Tanner 1963, Waddington 1942). Gene–envitonment interactions have been proposed as one path by which variance can be decreased over the course of development. Genetic determinants of plasticity in response to the environment may constrain structures to develop along a heritable trajectory from an undifferentiated beginning to a genetically determined mature state (Garlick 2002). Repetitive pattern of activity may also sculpt plastic developing structures. Zelditch and colleagues found that variance in murine skull morphometry decreased during early postnatal development. They hypothesized that high initial variance was due to the random stresses placed on skull tissues from relatively unorganized muscular activity early in life, and that variance decreased as patterns of activity took on the predictable characteristics of maturity (Zelditch et al 2004). Such a process could be relevant to activity-dependent changes in the cerebral cortex. The presence of canalization serves to stabilize developmental trajectories towards a specific heritable phenotypic target. Conversely, impairment of these buffering factors through pathology may be detectable as an increase in the variance seen in developing structures. Recent advances in statistical methodologies such as functional mapping (Wu & Lin 2006), which are designed to describe the genetic architecture of quantitative traits over development, show promise for disentangling these factors.
The ability to directly quantify aspects of brain structure and function has given us tools with which to form bridges between the genetic and environmental factors shaping biology and the resulting behaviours. Evidence from the first longitudinal structural brain imaging studies in children and adolescents who are developing normally or who have neurodevelopmental disorders has shown that trajectories can be sensitive to both genetic and functional factors. However, little is known about the heritability of other potential brain phenotypic measures such as the microstructural properties assessed with diffusion tensor imaging (DTI) or the functional BOLD responses that are the target of fMRI studies, and quantification of the heritability of the trajectories themselves awaits completion of longitudinal twin studies.
In contrast to the literature claiming stability over time as a desiderata (desired feature) for intermediate phenotypes (Almasy & Blangero 2001, Waldman 2005, Weinberger 1999), these examples illustrate the utility of trajectories of brain development as a phenotypic bridge between genes and behaviour in health and in illness.
References
- Addington AM, Gornick M, Duckworth J, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10:581–588. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- Addington AM, Gornick MC, Shaw P, et al. Neuregulin 1 (8p12) and childhood-onset schizophrenia: susceptibility haplotypes for diagnosis and brain developmental trajectories. Mol Psychiatry. 2007;12:195–205. doi: 10.1038/sj.mp.4001906. [DOI] [PubMed] [Google Scholar]
- Alaghband-Rad J, McKenna K, Gordon CT, et al. Childhood-onset schizophrenia: the severity of premorbid course. J Am Acad Child Adolesc Psychiatry. 1995;34:1273–1283. doi: 10.1097/00004583-199510000-00012. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. Am J Med Genet. 2001;105:42–44. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- Baare WF, Hulshoff Pol HE, Boomsma DI, et al. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Flatt T. The evolutionary genetics of canalization. Q Rev Biol. 2005;80:287–316. doi: 10.1086/432265. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick D. Understanding the nature of the general factor of intelligence: the role of individual differences in neural plasticity as an explanatory mechanism. Psychol Rev. 2002;109:116–136. doi: 10.1037/0033-295x.109.1.116. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;107:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenstein D, Lerch J, Shaw P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- Hauspie RC, Bergman P, Bielicki T, Susanne C. Genetic variance in the pattern of the growth curve for height: a longitudinal analysis of male twins. Ann Hum Biol. 1994;21:347–362. doi: 10.1080/03014469400003352. [DOI] [PubMed] [Google Scholar]
- Hollis C. Child and adolescent (juvenile onset) schizophrenia. A case control study of premorbid developmental impairments. Br J Psychiatry. 1995;166:489–495. doi: 10.1192/bjp.166.4.489. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, et al. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Zink CF. Imaging genetics for neuropsychiatric disorders. Child Adolesc Psychiatr Clin N Am. 2007;16:581–597. doi: 10.1016/j.chc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46:1418–1428. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Brookner FB, Lenane M, et al. Parental schizophrenia spectrum disorders in childhood-onset and adult-onset schizophrenia. Am J psychiatry. 2003;160:490–495. doi: 10.1176/appi.ajp.160.3.490. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Reveley AM, Reveley MA, Chitkara B, Clifford C. The genetic basis of cerebral ventricular volume. Psychiatry Res. 1984;13:261–266. doi: 10.1016/0165-1781(84)90041-6. [DOI] [PubMed] [Google Scholar]
- Schmalhausen II. The theory of stabilising selection. The Blakiston Co.; Philadelphia: 1949. Factors of evolution. [Google Scholar]
- Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006a;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006b;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gornick M, Lerch J, et al. Polymorphisms of the dopamine d4 receptor, clinical outcome, and cortical structure in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:921–931. doi: 10.1001/archpsyc.64.8.921. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Greenstein DK, Gogtay N, et al. Progressive brain volume loss during adolescence in childhood-onset schizophrenia. Am J Psychiatry. 2003;760:2181–2189. doi: 10.1176/appi.ajp.160.12.2181. [DOI] [PubMed] [Google Scholar]
- Sporn A, Addington A, Reiss AL, et al. 22q11 deletion syndrome in childhood onset schizophrenia: an update. Mol Psychiatry. 2004;9:225–226. doi: 10.1038/sj.mp.4001477. [DOI] [PubMed] [Google Scholar]
- Sporn A, Greenstein D, Gogtay N, et al. Childhood-onset schizophrenia: smooth pursuit eye-tracking dysfunction in family members. Schizophr Res. 2005;73:243–252. doi: 10.1016/j.schres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Tanner JM. The regulation of human growth. Child Dev. 1963;34:817–847. doi: 10.1111/j.1467-8624.1963.tb05970.x. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, et al. Genetic influences on brain structure. Nat Neurosci. 2001a;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001b;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- Waldman ID. Statistical approaches to complex phenotypes: evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1347–1356. doi: 10.1016/j.biopsych.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Schmitt JE, Lenroot RK, et al. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 2006;47:987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Schizophrenia: new phenes and new genes. Biol Psychiatry. 1999;46:3–7. [PubMed] [Google Scholar]
- Werry JS, Sprague RL, Cohen MN. Conners’ Teacher Rating Scale for use in drug studies with children—an empirical study. J Abnorm Child Psychol. 1975;3:217–229. doi: 10.1007/BF00916752. [DOI] [PubMed] [Google Scholar]
- Wu R, Lin M. Functional mapping—how to map and study the genetic architecture of dynamic complex traits. Nat Rev Genet. 2006;7:229–237. doi: 10.1038/nrg1804. [DOI] [PubMed] [Google Scholar]
- Zelditch ML, Lundrigan BL, Garland T., Jr Developmental regulation of skull morphology. I. Ontogenetic dynamics of variance. Evol Dev. 2004;6:194–206. doi: 10.1111/j.1525-142X.2004.04025.x. [DOI] [PubMed] [Google Scholar]


