Abstract
OBJECTIVE
To characterize the temporal progression of the monthly incidence of Clostridium difficile infections (CDIs) and to determine whether the incidence of CDI is related to the incidence of seasonal influenza.
DESIGN
A retrospective study of patients in the Nationwide Inpatient Sample during the period from 1998 through 2005.
METHODS
We identified all hospitalizations with a primary or secondary diagnosis of CDI with use of International Classification of Diseases, 9th Revision, Clinical Modification codes, and we did the same for influenza. The incidence of CDI was modeled as an autoregression about a linear trend. To investigate the association of CDI with influenza, we compared national and regional CDI and influenza series data and calculated cross-correlation functions with data that had been prewhitened (filtered to remove temporal patterns common to both series). To estimate the burden of seasonal CDI, we developed a proportional measure of seasonal CDI.
RESULTS
Time-series analysis of the monthly number of CDI cases reveals a distinct positive linear trend and a clear pattern of seasonal variation (R2 = 0.98). The cross-correlation functions indicate that influenza activity precedes CDI activity on both a national and regional basis. The average burden of seasonal (ie, winter) CDI is 23%.
CONCLUSIONS
The epidemiologic characteristics of CDI follow a pattern that is seasonal and associated with influenza, which is likely due to antimicrobial use during influenza seasons. Approximately 23% of average monthly CDI during the peak 3 winter months could be eliminated if CDI remained at summer levels.
Clostridium difficile is the most common cause of nosocomial infectious diarrhea in the United States, and several reports indicate that both the incidence and the severity of C. difficile infections (CDIs) are increasing,1–5 possibly because of the emergence of a more virulent strain.6,7 Multiple risk factors for CDI have been reported (eg, environmental exposure,8–12 older age,13 and underlying severity of disease14). However, prior antimicrobial use is the primary risk factor for CDI, and, historically, it has been unusual for patients to develop CDI without recent receipt of antimicrobial agents or exposure to a healthcare facility. Most classes of antimicrobial agents have been linked to CDI; however, the risk is particularly high with clindamycin, cephalosporins, and, more recently, fluoroquinolones.15–17
Because the use of antimicrobial agents varies by season, peaking in the winter months,18,19 the epidemiologic characteristics of CDI may also be seasonal. Furthermore, because antibiotic use increases during influenza seasons,20 we hypothesize that the seasonal variation of CDI may be related to influenza activity. The purpose of this study is to characterize the temporal progression of the monthly incidence of CDI and to determine whether the incidence of CDI is associated with the seasonal variation in the incidence of influenza.
METHODS
All data were extracted from the Nationwide Inpatient Sample, the largest all-payer database of national hospital discharges in the United States. It is maintained as part of the Healthcare Cost and Utilization Project by the Agency for Healthcare Research and Quality and contains data from a 20% stratified sample of nonfederal acute care hospitals.21 This sample includes academic medical centers, community hospitals, general hospitals, and specialty hospitals. It excludes long-term care facilities and rehabilitation hospitals.21 To adjust for yearly changes in the sample, we applied the weights provided by the Agency for Healthcare Research and Quality.22
We first identified all hospitalizations during the period from 1998 through 2005 during which a primary or secondary diagnosis of CDI was received. For case ascertainment, we used the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 008.45 (intestinal infection due to C. difficile). We then aggregated all cases of CDI by month to produce a national sample of cases of CDI over time. Cases of CDI were assigned to a calendar month on the basis of the date that the patient was admitted to the hospital. In a similar fashion, we identified all hospitalizations during the period from 1998 through 2005 during which a primary or secondary diagnosis of influenza was received. For case ascertainment, we used the ICD-9-CM codes 487.0 (influenza with pneumonia), 487.1 (influenza with other respiratory manifestations), and 487.8 (influenza with other manifestations). We then compiled monthly totals of cases of influenza in the same manner as for CDI.
To determine whether the epidemiologic characteristics of CDI follow a seasonal pattern, we modeled the monthly incidence of CDI as an autoregression. With a seasonal disease pattern, one would expect observations at the peak of the season to be highly correlated with those of the previous month, as well as with those of the months surrounding the peak the year before. Because the number of CDI cases tended to increase over time during the study period, we included a linear trend component (Figure 1). For the initial fit of the national CDI series, variables representing the incidence of CDI in all of the 25 previous months (ie, all possible monthly lags from 1 month through 25 months) were considered in the autoregression, and backward elimination was used to obtain the final model. The optimality of this final model was confirmed by comparing the Akaike information criterion for this fitted model with that of other viable candidates. After obtaining a suitable model for the national data, we then applied the same model structure to fit autoregressive models for monthly CDI cases in each of the 4 US census regions. The Durbin-Watson (DW) test was applied to the residuals of each fitted model to assess the adequacy of the fit.
FIGURE 1.
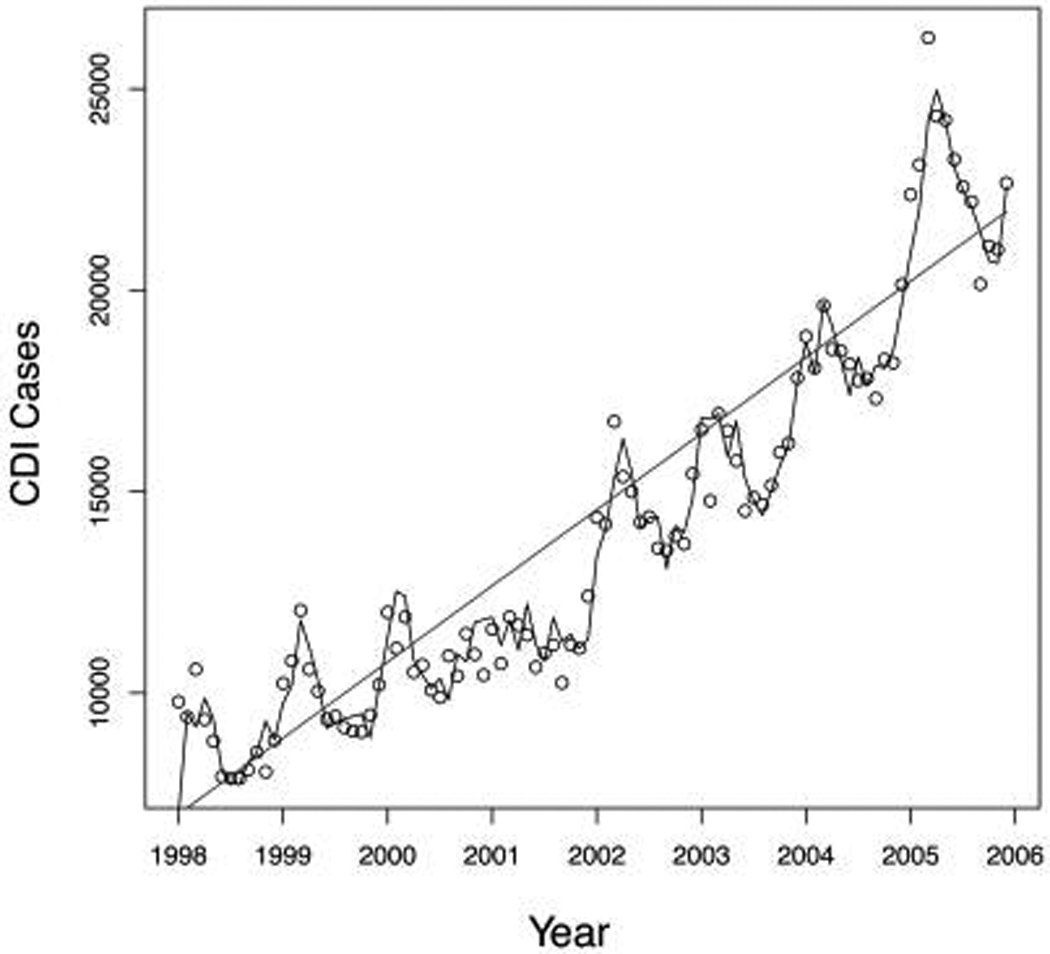
Cases of Clostridium difficile–associated disease in the United States, 1998–2005. Values are predicted on the basis of an autoregressive model (with a linear trend) based on discharge data from a 20% sample of nonfederal acute care hospitals. Circles, actual cases of C. difficile infection (CDI); solid line, predicted CDI cases from a model that uses the linear trend, as well as historical data from the previous 3 months (lags of 1, 2, and 3 months) and the previous year (lags of 12 and 13 months).
To investigate the association of CDI with influenza, we calculated a cross-correlation function between CDI and influenza. This cross-correlation function allows us to compare one time series (CDI) with another time series (influenza) to explore the temporal relationship between the 2 series. Specifically, correlations are computed between the CDI series and the influenza series with the CDI series lagging behind the influenza series by 1 month (CDI cases that had onset 1 month after the influenza peak), by 2 months, and so forth. We also examined the instantaneous cross-correlation. A high value for a cross-correlation function indicates that the 2 series are similar when 1 series is shifted by a specific amount of time (eg, 1 month).
Cross-correlations between time series can be spurious as a result of the effects of common temporal patterns. For example, CDI and influenza may seem to be correlated simply because they both follow seasonal cycles, tending to peak in the winter. To establish that the association between 2 series is not merely due to the nature of their temporal progressions, a process called prewhitening is often used. Informally speaking, prewhitening removes the temporal patterns from either (or both) of the series by the application of a common filter. Any correlation that persists is due to factors above and beyond shared temporal behaviors.
We prewhitened the series by differencing the CDI series (to remove the linear trend) and fitting a seasonal autoregressive model to the differenced series. The resulting autoregressive integrated model was used to filter both the CDI series and the influenza series. The filtered series are called the prewhitened series and serve as the new basis of comparison in computing cross-correlations.
To compare the timing of seasonal peaks of CDI incidence and influenza incidence for the national series, the month corresponding to the maximum number of cases for each type of infection was identified during the period from July of a chosen year through June of the next year. We used the exact Wilcoxon signed-rank test to investigate whether the timing of seasonal case peaks in CDI activity followed influenza activity.
Finally, to estimate the burden of seasonal CDI, we developed a proportional measure. The numerator is defined as the difference between the mean incidence of the 3 months centered at the CDI peak and the preceding summer CDI mean incidence, and the denominator is defined as the 3-month peak mean incidence. All analyses were performed using R, version 2.7.1 (R Foundation), or SAS, version 9.1.3 (SAS Institute). For the Wilcoxon signed-rank test, a P value of less than .05 was considered to indicate a statistically significant result. For partial tests on model parameters and goodness-of-fit tests, a more liberal level of significance (.1) was used.
RESULTS
Our time-series analysis of the monthly number of cases of CDI reveals a clear pattern of yearly seasonal variation at both national and regional levels. The final autoregressive model for national CDI incidence includes a positive linear trend (P < .001) to account for temporal case escalation, lags of 1 (P < .001), 2 (P = .09), and 3 months (P < .006) to account for recent activity, and lags of 12 and 13 months to account for yearly seasonal variation (both P < .001). Thus, variation in the incidence of CDI can be explained with the use of a simple and parsimonious model that accounts for growth over time, numbers of recent cases, and numbers of cases during the previous year. The DW test for autocorrelated residuals exhibits no evidence of lack of fit (P > .10). The total R2 value for the final model is 0.98 (Figure 1). We obtained similar results using the same lag structure for each of the 4 US census regions (all DW P values were greater than .20). The R2 values are 0.95, 0.96, 0.96, and 0.93 for the northeast, midwest, south, and west regions, respectively.
To prewhiten the series, we fit a seasonal autoregressive model with a period of 12 months to the differenced, detrended CDI series. The resulting autoregressive integrated model was used to filter both the CDI series and the influenza series. The cross-correlation function for the prewhitened series has a significant correlation only at lag 1, thus indicating that the number of cases of CDI tends to follow the number of cases of influenza by 1 month. We obtained similar results for the 4 US census regions: there was a statistically significant cross-correlation at the 1-month lag for 3 of the 4 regions. We also found that monthly seasonal peaks in influenza activity occurred mostly during January and February, while monthly peaks for CDI occurred mostly in March (Figure 2). At a national level, the peak in CDI incidence occurred during or after the monthly influenza peaks (P = .02, exact Wilcoxon signed-rank test).
FIGURE 2.
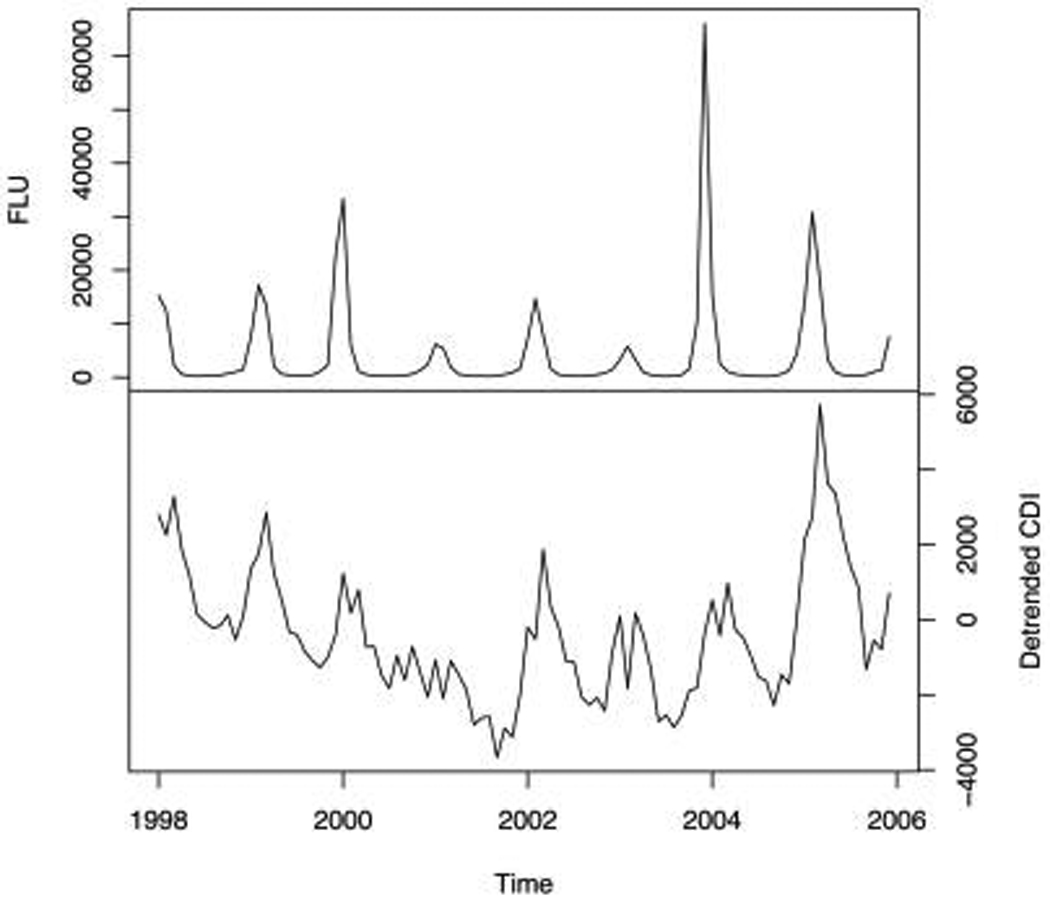
Cases of influenza and Clostridium difficile–associated disease in the United States, 1998–2005. Values are based on discharge data from a 20% sample of nonfederal acute care hospitals. Top, influenza series; bottom, linearly detrended C. difficile infection series.
Finally, the average burden of seasonal CDI is 23%, meaning that approximately 23% of average monthly CDI cases during the 3 peak months could be conceivably eliminated if we could keep CDI incidence at the minimal summer baseline level.
DISCUSSION
A seasonal pattern for the incidence of CDI has been previously described. However, it was determined on the basis of visual inspection; no formal time-series analyses were performed.1,23 Our analysis contributes to the literature by demonstrating a clear seasonal pattern to CDI by using the appropriate time-series methods for investigating autocorrelated data; we also demonstrate a strong seasonal association between influenza and CDI, and this association remains even after the series are prewhitened.
Influenza has occurred on a seasonal basis for hundreds of years, and it is a major cause of morbidity.24 In developed countries, mortality is at its highest during winter months, not only as a result of deaths from influenza and pneumonia but also as a result of deaths attributed to other diseases (eg, cardiovascular disease). In the 1930s, Collins25 proposed that because deaths due to influenza and deaths due to other apparently non–influenza-related diseases followed a similar temporal distribution, a causal relationship between influenza and other nonrespiratory causes of death might exist. More recently, a careful time-series analysis using 40 years of data on mortality from ischemic heart disease, cerebrovascular disease, and diabetes mellitus strongly suggested that the excess winter mortality due to these diseases is, in fact, attributable to influenza.26 Furthermore, vaccination against influenza is associated with significant reductions in the risk of hospitalizations for heart disease and cerebrovascular disease.27 Our results, to our knowledge, are the first to reveal a strong statistical association between influenza and CDI, with peaks in CDI activity occurring 1–2 months after seasonal influenza peaks. Instead of a direct causal pathway, as has been suggested for coronary artery disease,28–30 we suspect that seasonal influenza leads to an overall increase in the prescribing of antimicrobial agents in the community, which subsequently spurs an increase in CDI activity.
Although influenza can increase the likelihood of acquiring secondary bacterial illnesses, antimicrobial therapy has no efficacy in treating viral infections, and the administration of antimicrobial agents to prevent bacterial superinfections is not thought to be efficacious.20 Thus, a significant proportion of the increase in the use of antimicrobial agents during influenza season is undoubtedly unnecessary and needlessly contributes to antimicrobial resistance. In pediatric populations, influenza accounts for up to 10%–30% of excess antimicrobial use.31 In an adult population, the availability of rapid testing for influenza has been shown to lead to reductions in antibiotic use among hospitalized patients.32 This reduction in antibiotic use is most likely due to an increase in diagnostic certainty; that is, physicians are less likely to use antibiotics to treat a respiratory infection if they know that it is caused by influenza. This observation implies that hospitalized patients are subject to excess antimicrobial therapy during influenza seasons. However, other respiratory viruses are often cocirculating with the influenza virus during winter months (eg, respiratory syncytial virus), and antibiotics used to treat these other viral respiratory infections also likely contribute to the seasonality of CDI.
There are several limitations to this study. First, we use administrative data rather than clinical or microbiologic data for case ascertainment. However, the single ICD-9-CM code for CDI surveillance has demonstrated both a high sensitivity (78%) and a high specificity (99.7%).33 Other studies have shown that ICD-9-CM codes for influenza also have a reasonable sensitivity, specificity, and positive predictive value for detecting influenza.34 Second, as mentioned above, it is possible that viral infections other than influenza that also occur during winter months could have contributed to the seasonal pattern of CDI incidence. Third, ideally, we would have also considered seasonal antimicrobial use in our analysis, but the Nationwide Inpatient Sample does not include medication use data. Unfortunately, there are no readily available and nationwide data that aggregate the monthly receipt of antimicrobial agents by hospitalized patients. Fourth, some of the seasonality of CDI may be due to a form of surveillance bias. The incidence of other gastrointestinal infections peaks during winter months. For example, cases of rotavirus peak during winter months, presumably as a result of weather-related conditions (eg, humidity),35,36 which might also be the case with influenza.37 Given the seasonality of rotavirus and other viral causes of diarrhea, it is possible that more tests for CDI may be ordered during the winter, leading to an increase in false-positive results on CDI tests, possibly contributing to the observed seasonality of CDI. Finally, there may be some other unmeasured factors that account for or at least contribute to the seasonality of CDI, for example, overcrowding in hospitals or staff shortages in winter months that could enhance environmental risk factors for CDI. The presence of more C. difficile spores in the food supply during winter months might also help contribute to the seasonal pattern of CDI,38 especially if more people are receiving antibiotics during winter months.
In addition to causing significant morbidity and mortality, CDI is associated with excess lengths of hospital stays and a substantial increase in cost.39,40 In the United States alone, CDI management costs $3.2 billion annually.41 By demonstrating seasonal fluctuations in CDI activity and linking it to influenza activity, we provide yet another reason for preventing influenza and focusing on antimicrobial use during influenza seasons, a considerable proportion of which is likely unnecessary. Others have suggested that seasonal antimicrobial use may indicate and enable the identification of inappropriate patterns of prescribing antimicrobial agents.42 Therefore, focusing on seasonal patterns at an institutional level and linking inappropriate use to CDI may be helpful to antimicrobal stewardship initiatives, especially because the antimicrobial agent used to treat one patient may put other patients at risk for CDI through exposure to colonization pressure.43,44 Avoiding the administration of antibiotics to hospitalized patients with uncomplicated influenza and infections caused by other respiratory viruses that cocirculate with influenza may significantly decrease inappropriate seasonal antibiotic use and, indirectly, the incidence of CDI. The burden of excess CDI during winter months is nontrivial, as disease levels are, on average, 23% higher than in the summer. Thus, as CDI becomes more prevalent and possibly more difficult to treat,45,46 the goal of preventing CDI may provide yet one more reason to encourage the appropriate use of antimicrobial agents, especially during the respiratory virus season. Our results also indicate the importance of developing better diagnostic tests for viral respiratory infections. Finally, infection control researchers should consider seasonal trends when studying interventions to control CDI. For example, interventions implemented at certain times may seem to cause a decrease or increase in CDI rates that may actually have more to do with seasonal fluctuations of CDI rates than with the effects of the actual interventions designed to decrease CDI.
ACKNOWLEDGMENTS
Financial support. National Institutes of Health (research grant 1 K01 AI75089-01A1).
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
Presented in part: 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Annual Infectious Diseases Society of America meeting; Washington, DC; October 25–28, 2008; abstract K-502.
REFERENCES
- 1.Archibald LK, Banerjee SN, Jarvis WR. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987–2001. J Infect Dis. 2004;189:1585–1589. doi: 10.1086/383045. [DOI] [PubMed] [Google Scholar]
- 2.Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991–2003: a changing pattern of disease severity. CMAJ. 2004;171:466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infections in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12:409–415. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandler RE, Hedberg K, Cieslak PR. Clostridium difficile–associated disease in Oregon: increasing incidence and hospital-level risk factors. Infect Control Hosp Epidemiol. 2007;28:116–122. doi: 10.1086/511795. [DOI] [PubMed] [Google Scholar]
- 5.Elixhauser A, Jhung M. Clostridium difficile-associated disease in U.S. hospitals, 1993–2005. [Accessed January 30, 2009];Rockville, MD: Agency of Healthcare Research and Quality; HCUP Statistical Brief 50. 2008 April; http://www.hcup-us.ahrq.gov/reports/statbriefs/sb50.pdf. [PubMed]
- 6.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 7.McEllistrem MC, Carman RJ, Gerding DN, Genheimer CW, Zheng L. A hospital outbreak of Clostridium difficile disease associated with isolates carrying binary toxin genes. Clin Infect Dis. 2005;40:265–272. doi: 10.1086/427113. [DOI] [PubMed] [Google Scholar]
- 8.Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by hospitalized patients. J Infect Dis. 1992;166:561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 9.Kaatz GW, Gitlin SD, Schaberg DR, et al. Acquisition of Clostridium difficile from the hospital environment. Am J Epidemiol. 1988;127:1289–1294. doi: 10.1093/oxfordjournals.aje.a114921. [DOI] [PubMed] [Google Scholar]
- 10.Mayfield JL, Leet T, Miller J, Mundy LM. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis. 2000;31:995–1000. doi: 10.1086/318149. [DOI] [PubMed] [Google Scholar]
- 11.McFarland LV, Mulligan MME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 12.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile–associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45:1543–1549. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 13.Settle CD, Wilcox MH, Fawley WN, Corrado OJ, Hawley PM. Prospective study of the risk of Clostridium difficile diarrhoea in elderly patients following treatment with cefotaxime or piperacillin-tazobactam. Aliment Pharmacol Ther. 1998;12:1217–1223. doi: 10.1046/j.1365-2036.1998.00428.x. [DOI] [PubMed] [Google Scholar]
- 14.Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23:653–659. doi: 10.1086/501989. [DOI] [PubMed] [Google Scholar]
- 15.Gaynes R, Rimland D, Killum E, et al. Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use. Clin Infect Dis. 2004;38:640–645. doi: 10.1086/381551. [DOI] [PubMed] [Google Scholar]
- 16.Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 17.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile–associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. (published correction appears in N Engl J Med 2006;354:2200) [DOI] [PubMed] [Google Scholar]
- 18.Elseviers MM, Ferech M, Vander Stichele RH, Goossens H ESAC project group. Antibiotic use in ambulatory care in Europe (ESAC data 1997–2002): trends, regional differences and seasonal fluctuations. Pharmacoepidemiol Drug Saf. 2007;16:115–123. doi: 10.1002/pds.1244. [DOI] [PubMed] [Google Scholar]
- 19.Goossens H, Ferech M, Vander Stichele R, Elseviers M ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 20.Low D. Reducing antibiotic use in influenza: challenges and rewards. Clin Microbiol Infect. 2008;14:298–306. doi: 10.1111/j.1469-0691.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. HCUP NIS database documentation healthcare cost and utilization project (HCUP) [Accessed January 30, 2009];Rockville, MD: Agency for Healthcare Research and Quality; 2009 October; http://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp.
- 22.Agency for Healthcare Research and Quality. Nationwide Inpatient Sample Trends Supplemental (NIS-Trends) Files. [Accessed January 30, 2009];Rockville, MD: Agency for Healthcare Research and Quality; 2008 May; http://www.hcup-us.ahrq.gov/db/nation/nis/nistrends.jsp.
- 23.Jagai J, Naumova E. Clostridium difficile–associated disease in the elderly, United States. Emerg Infect Dis. 2009;15(2):343–344. doi: 10.3201/eid1502.080785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 25.Collins SD. Excess mortality from causes other than influenza and pneumonia during influenza epidemics. Public Health Rep. 1932;47:2159–2179. [Google Scholar]
- 26.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160(5):492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 27.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348:1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 28.Spodick DH, Flessas AP, Johnson MM. Association of acute respiratory symptoms with onset of acute myocardial infarction: prospective investigation of 150 consecutive patients and matched control patients. Am J Cardiol. 1984;53:481–482. doi: 10.1016/0002-9149(84)90016-x. [DOI] [PubMed] [Google Scholar]
- 29.Mattila KJ. Viral and bacterial infections in patients with acute myocardial infarction. J Intern Med. 1989;225:293–296. doi: 10.1111/j.1365-2796.1989.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 30.Meier CR, Jick SS, Derby LE, Vasilakis C, Jick H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet. 1998;351:1467–1471. doi: 10.1016/s0140-6736(97)11084-4. [DOI] [PubMed] [Google Scholar]
- 31.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr, Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 32.Falsey AR, Murata Y, Walsh EE. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167:354–360. doi: 10.1001/archinte.167.4.ioi60207. [DOI] [PubMed] [Google Scholar]
- 33.Dubberke ER, Reske KA, McDonald LC, Fraser VJ. ICD-9 codes and surveillance for Clostridium difficile-associated disease. Emerg Infect Dis. 2006;12:1576–1579. doi: 10.3201/eid1210.060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsden-Haug N, Foster VB, Gould PL, Elbert E, Wang H, Pavlin JA. Code-based syndromic surveillance for influenzalike illness by International Classification of Diseases, Ninth Revision. Emerg Infect Dis. 2007;13:207–216. doi: 10.3201/eid1302.060557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansari SA, Springthorpe VS, Sattar SA. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis. 1991;13(3):448–461. doi: 10.1093/clinids/13.3.448. [DOI] [PubMed] [Google Scholar]
- 36.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009;38:1487. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci U S A. 2009;106(9):3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Palacios A, Reid-Smith RJ, Staempfli HR, et al. Possible seasonality of Clostridium difficile in retail meat, Canada. Emerg Infect Dis. 2009;15(5):802–805. doi: 10.3201/eid1505.081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 40.Dubberke ER, Reske KA, Olsen MA, McDonald LC, Fraser VJ. Short- and long-term attributable costs of Clostridium difficile–associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46:497–504. doi: 10.1086/526530. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile–associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–1227. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 42.Abell S, Chapman S, Nadin L, Warren R. Seasonal variation in fluoroquinolone prescribing. J Antimicrob Chemother. 1999;43:315–316. doi: 10.1093/jac/43.2.315. [DOI] [PubMed] [Google Scholar]
- 43.Starr JM, Rogers TR, Impallomeni M. Hospital-acquired Clostridium difficile diarrhoea and herd immunity. Lancet. 1997;349:426–428. doi: 10.1016/S0140-6736(97)80053-0. [DOI] [PubMed] [Google Scholar]
- 44.Dubberke ER, Reske KA, Olsen MA, et al. Evaluation of Clostridium difficile–associated disease pressure as a risk factor for C difficile–associated disease. Arch Intern Med. 2007;167(10):1092–1097. doi: 10.1001/archinte.167.10.1092. [DOI] [PubMed] [Google Scholar]
- 45.Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586–1590. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 46.Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–1597. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]


