Abstract
BACKGROUND
Sporadic anovulation among regularly menstruating women is not well understood. It is hypothesized that cholesterol abnormalities may lead to hormone imbalances and incident anovulation. The objective was to evaluate the association between lipoprotein cholesterol levels and endocrine and metabolic disturbances and incident anovulation among ovulatory and anovulatory women reporting regular menstruation.
METHODS
The BioCycle Study was a prospective cohort study conducted at the University at Buffalo from September 2005 to 2007, which followed 259 self-reported regularly menstruating women aged 18–44 years, for one or two complete menstrual cycles. Sporadic anovulation was assessed across two menstrual cycles.
RESULTS
Mean total and low-density lipoprotein cholesterol and triglycerides levels across the menstrual cycles were higher during anovulatory cycles (mean difference: 4.6 (P = 0.01), 3.0 (P = 0.06) and 6.4 (P = 0.0002) mg/dl, respectively, adjusted for age and BMI). When multiple total cholesterol (TC) measures prior to expected ovulation were considered, we observed a slight increased risk of anovulation associated with increased levels of TC (odds ratio per 5 mg/dl increase, 1.07; 95% confidence interval, 0.99, 1.16). Sporadic anovulation was associated with an increased LH:FSH ratio (P = 0.002), current acne (P = 0.02) and decreased sex hormone-binding globulin levels (P = 0.005).
CONCLUSIONS
These results do not support a strong association between lipoprotein cholesterol levels and sporadic anovulation. However, sporadic anovulation among regularly menstruating women is associated with endocrine disturbances which are typically observed in women with polycystic ovary syndrome.
Keywords: anovulation, lipoproteins, menstrual cycle, endocrine disturbances
Introduction
Ovulatory disorders are one of the leading causes of female infertility, affecting between 18 and 30% of infertile couples (Hull et al., 1985; Smith et al., 2003). Sporadic anovulatory cycles may occur among regularly menstruating women; however, the prevalence of eumenorrheic anovulation has not been well described (Malcolm and Cumming, 2003; Barbieri, 2004). Ovulation is the product of the intricate regulation of the hypothalamic–pituitary–ovarian axis by reproductive hormones. Reproductive hormones are derived from cholesterol; therefore, lipoprotein cholesterol abnormalities may lead to aberrant hormone production and anovulation, the so-called steroid hypothesis (Strauss, 2004).
There is some evidence of a more atherogenic lipid profile [increased levels of total cholesterol (TC), low-density lipoprotein (LDL) cholesterol and triglycerides, and decreased levels of high-density lipoprotein (HDL) cholesterol] among women with certain ovulatory disorders, specifically among women with polycystic ovary syndrome (PCOS) (Essah et al., 2007). In addition to anovulation (usually observed as irregular menstrual cycles), women with PCOS also display signs of androgen excess. Common endocrine abnormalities observed in women with PCOS include insulin resistance and elevated LH:FSH ratio (Azziz et al., 2009). It is possible that anovulation among regularly menstruating women may be associated with endocrine changes similar to those seen in women with PCOS.
The objective of this study was to (i) prospectively evaluate the relationship between serum lipoprotein cholesterol levels and incident anovulation among a group of regularly menstruating women and (ii) compare endocrine and metabolic parameters in ovulatory and anovulatory women with regular menstruation.
Materials and Methods
Study sample
The BioCycle Study was a prospective cohort of 259 women followed for one (n = 9) or two (n = 250) menstrual cycles (Wactawski-Wende et al., 2009). Participants were recruited from healthy premenopausal volunteers aged 18–44 years from the western New York region. Women were included if they had a self-reported cycle length between 21 and 35 days for each menstrual cycle during the past 6 months. Exclusion criteria included a history of gynecological problems, endometriosis or self-reported diagnosis of PCOS by a physician, use of oral contraceptives during the past 3 months, use of other medications including lipid-lowering drugs, pregnancy in the last 6 months, chronic disease or a self-reported BMI at screening <18 or >35 kg/m2. Full detail on inclusion and exclusion criteria have been reported elsewhere (Wactawski-Wende et al., 2009). The University at Buffalo Health Sciences Institutional Review Board approved the study, and all participants provided written informed consent. Under a reliance agreement, the National Institutes of Health depends on the designated IRB of the University at Buffalo for review, approval, and continuing oversight of its human subject research for the BioCycle Study.
Data collection
The study involved five to eight clinical visits per cycle (94% of all women completed at least seven visits per cycle) for up to two cycles. Visits were timed using fertility monitors (Clearblue® Easy Fertility Monitor, Inverness Medical, Waltham, MA, USA) so that biospecimen collection occurred during specific phases of the cycle, inclusive of peri-ovulation (Howards et al., 2009). Monitors measured estrone-3-glucuronide and LH in urine daily, starting on the sixth day following the start of the woman's menstrual cycle. Monitor indications of low, high and peak fertility were used to time mid-cycle visits. Other visits were then scheduled accordingly based on an algorithm that took each woman's self-reported cycle length into consideration.
Lipoprotein assessment
A complete lipid profile was performed for each participant from fasting serum samples collected at each clinic visit (Browne et al., 2008). The lipid profile included analysis of TC, HDL and triglycerides, measured using a Beckman LX20 automated chemistry analyzer. LDL was determined indirectly using the Friedewald formula (Friedewald et al., 1972). Across the study period, the coefficient of variation
(CV) was <5% for all lipid and lipoprotein assays. Baseline levels were assessed on the second day of menses during the first cycle.
Endocrine and metabolic assessment
LH, FSH, sex hormone-binding globulin (SHBG) and insulin were measured in fasting serum samples, using a solid-phase competitive chemiluminescent enzymatic immunoassay (Specialty Laboratories, Inc., Valencia, CA, USA) on the DPC Immulite®2000 analyzer (Siemens Medical Solutions Diagnostics, Deerfield, IL, USA). Fasting plasma glucose was assayed using a hexokinase-based methodology on a Beckman LX20 autoanalyzer. The CVs were <5% for LH and FSH, <3% for glucose and <10% for SHBG and insulin. Insulin resistance, as measured by the homeostasis model assessment of insulin resistance (HOMA-IR), was calculated based on the homeostasis model using the equation: fasting insulin (µU/ml) × fasting glucose (mmol/l)/22.5 (Matthews et al., 1985). Hirsutism was assessed at baseline via a body hair patterns questionnaire (a modification of the Ferriman–Gallwey hirsutism score) (Ferriman and Gallwey, 1961) and prevalence of current acne was assessed at baseline via questionnaire.
Classification of anovulation
Progesterone levels were measured in fasting serum samples collected at each clinical visit, using a solid-phase competitive chemiluminescent enzymatic immunoassay (Specialty Laboratories, Inc.) on the DPC Immulite®2000 analyzer (Siemens Medical Solutions Diagnostics) (CV < 14%). Menstrual cycles were classified as anovulatory if the peak progesterone concentration across the cycle was ≤5 ng/ml (n = 65, 13%) (Abdulla et al., 1983; Malcolm and Cumming, 2003). We also employed a more conservative classification of ovulation to minimize potential misclassification, in which cycles with progesterone concentrations ≤5 ng/ml, but with an observed serum LH peak on the mid- or late-luteal phase visit, were considered ovulatory (Gaskins et al., 2009). On the basis of the conservative approach, 42 of the 509 cycles (8.3%) in this study were classified as anovulatory. Overall, 35 (13.5%) women had at least one anovulatory cycle, 28 (10.8%) women had one anovulatory cycle and 7 (2.7%) women had two anovulatory cycles.
Covariate assessment
Participants were asked to complete questionnaires on demographics, lifestyle (smoking status), physical activity (Craig et al., 2003) and reproductive history. Physical and anthropometric measures were performed according to standardized protocols and included height, weight and waist and hip circumference (Lohman et al., 1988). Dietary intake was assessed up to four times per cycle by 24-h recall (University of Minnesota, Minneapolis, MN, USA), and used to calculate the average total fiber and energy intake across each cycle. Cycle length was defined as the number of days from the first day of bleeding until the day before the next onset of bleeding.
Statistical analysis
Descriptive statistics were computed for all study variables and compared between ovulatory women and women with at least one anovulatory cycle, using analysis of variance to test for differences in means and χ2 tests for differences in categorical variables. Mean baseline lipoprotein cholesterol levels, and levels across the cycle, were compared between ovulatory and anovulatory women, adjusted for age and BMI. Linear mixed models were used to calculate the predicted mean values and 95% confidence intervals (CIs) of age- and BMI-adjusted lipoprotein cholesterol levels and other endocrine markers. Geometric means are presented for non-normally distributed variables.
Generalized linear-mixed models with random intercepts were used to model the association between baseline lipoprotein cholesterol levels (TC, HDL, LDL and triglycerides) and the probability of incident anovulation across two cycles (Diggle et al., 2002). Odds ratios (ORs) and CIs were calculated to represent a 5-mg/dl change in lipoprotein levels adjusted for age, age at menarche, BMI, average fiber intake and average total energy intake. We compared the results after additional adjustment for the LH:FSH ratio, HOMA-IR, insulin, SHBG, hirsutism score and current acne, to determine whether associations between baseline lipoprotein cholesterol levels and anovulation could be related to underlying endocrine disturbances. To investigate these associations further, we evaluated whether these endocrine parameters themselves were significant predictors of sporadic anovulation when adjusting for lipoprotein cholesterol levels. We additionally assessed the impact of including multiple lipoprotein cholesterol measurements up to the time of expected ovulation (up to four per woman) to increase our statistical power to detect effects. Stabilized inverse probability weights were used to adjust for time-dependent confounding owing to changing hormone levels across the cycle (Robins et al., 2000; Cole and Hernan, 2008). Statistical significance was defined as P<0.05 for a two-tailed test. SAS version 9.1 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Women in the BioCycle Study were on average 27.3 years of age (range 18–44) and consisted mainly of single, nulliparous, normal weight, and white women with some post-secondary education. Women with at least one anovulatory cycle (13.5%) were on average younger, single, nulliparous and with fewer years since menarche (Table I). Neither age at menarche (P= 0.37), BMI (P = 0.25) or waist-to-hip ratio (P = 0.79) differed between women with ovulatory and anovulatory cycles.
Table I.
Baseline demographic characteristics and lipoprotein cholesterol levels of women, by ovulatory status.
| Overall | Ovulatory | Anovulatorya | P-valueb | |
|---|---|---|---|---|
| n = 259 | n = 224 | n = 35 | ||
| Demographics | ||||
| Age, years: mean ± SD | 27.3 ± 8.2 | 28.0 ± 8.3 | 22.5 ± 5.6 | <0.01 |
| BMI, kg/m2: mean ± SD | 24.1 ± 3.9 | 24.2 ± 3.9 | 23.4 ± 3.8 | 0.25 |
| Waist-to-hip ratio: mean ± SD | 0.75 ± 0.05 | 0.75 ± 0.06 | 0.76 ± 0.03 | 0.79 |
| Cycle 1 length, days: mean ± SD | 28.9 ± 4.6 | 29.0 ± 4.5 | 28.7 ± 5.3 | 0.73 |
| Cycle 2 length, days: mean ± SD | 28.7 ± 3.5 | 28.8 ± 3.2 | 28.2 ± 5.3 | 0.47 |
| Physical activity: n (%) | 0.86 | |||
| Low | 25 (9.7) | 22 (9.8) | 3 (8.6) | |
| Moderate | 92 (35.5) | 81 (36.2) | 11 (31.4) | |
| High | 142 (54.8) | 121 (54.0) | 21 (60.0) | |
| Race: n (%) | 0.85 | |||
| White | 154 (59.5) | 134 (59.8) | 20 (57.1) | |
| Black | 51 (19.7) | 43 (19.2) | 8 (22.9) | |
| Other | 54 (20.9) | 47 (21.0) | 7 (20.0) | |
| ≤High school education: n (%) | 33 (12.7) | 29 (13.0) | 4 (11.4) | 1.00 |
| Current smoker: n (%) | 10 (3.9) | 9 (4.0) | 1 (2.9) | 1.00 |
| Married: n (%) | 66 (25.5) | 65 (29.0) | 1 (2.9) | <0.01 |
| Nulliparous: n (%) | 187 (73.9) | 154 (70.0) | 33 (100.0) | <0.01 |
| Past OC use: n (%) | 140 (54.9) | 127 (57.2) | 13 (39.4) | 0.06 |
| Age at menarche, years: mean ± SD | 12.5 ± 1.2 | 12.4 ± 1.2 | 12.6 ± 1.4 | 0.37 |
| Years since menarche: mean ± SD | 14.9 ± 8.3 | 15.7 ± 8.3 | 9.6 ± 6.4 | <0.01 |
| Total energy intake, kcals: mean ± SD | 1603.5 ± 397.7 | 1597.5 ± 397.0 | 1641.5 ± 405.8 | 0.54 |
| Total fiber intake, g/day: mean ± SD | 13.6 ± 6.0 | 13.4 ± 5.7 | 15.1 ± 7.1 | 0.11 |
| Baseline lipoprotein cholesterol levels (mg/dl)c | ||||
| TC: mean ± SD | 163.4 ± 29.0 | 162.8 ± 1.9 | 167.3 ± 4.9 | 0.39 |
| HDL cholesterol: mean ± SD | 50.1 ± 11.5 | 50.0 ± 0.8 | 50.6 ± 2.0 | 0.79 |
| LDL cholesterol: mean ± SD | 101.5 ± 25.7 | 101.1 ± 1.7 | 104.0 ± 4.3 | 0.54 |
| Triglycerides: mean ± SD | 59.2 ± 27.9 | 58.5 ± 1.8 | 64.0 ± 4.7 | 0.28 |
ANOVA, analysis of variance; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; OC, oral contraceptives; TC, total cholesterol.
aAt least one anovulatory cycle. Twenty-eight women in the study had only one anovulatory cycle, and seven women in the study had two anovulatory cycles. There were a total of 42 anovulatory cycles out of a total 509 in the BioCycle Study.
bP-value for continuous variables calculated using ANOVA, and for categorical variables using Fisher's exact test.
cBaseline levels were measured at the first visit of the first cycle. Values for ovulatory and anovulatory cycles are predicted means after adjusting for age and BMI.
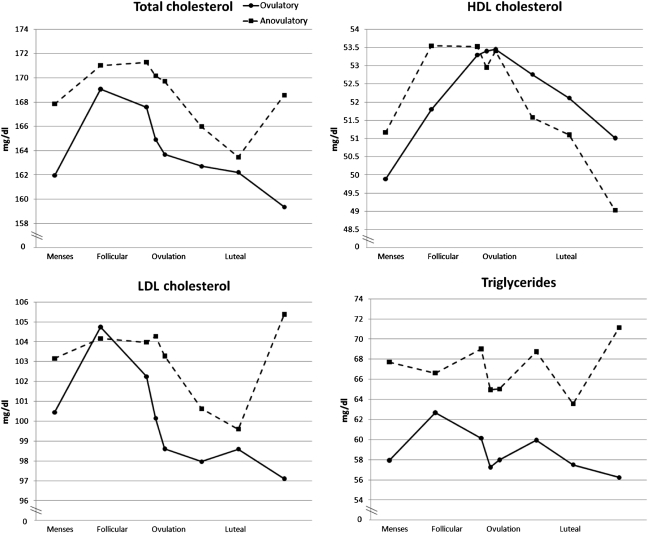
After adjustment for age and BMI, we observed that ovulatory women had lower baseline TC, LDL and triglyceride levels than anovulatory women, although these differences were not statistically significant (Table I). However, when we considered multiple measurements across the cycle, we detected higher levels of TC (mean difference 4.6 mg/dl, P = 0.01), LDL (mean difference 3.0 mg/dl, P = 0.06) and triglycerides (mean difference 6.4 mg/dl, P = 0.0002) among anovulatory cycles when compared with ovulatory cycles (Fig. 1) with more prominent changes in TC and LDL observed during the luteal phase. No differences were observed with HDL (mean difference −0.2 mg/dl, P = 0.8).
Figure 1.
Predicted mean lipoprotein cholesterol levels across the menstrual cycle adjusted for age and BMI, by anovulatory status (n = 259). HDL, high-density lipoprotein; LDL, low-density lipoprotein.
To assess the relationship between cholesterol and subsequent ovulation, we used two different measures of cholesterol: (i) baseline levels and (ii) four measures preceding ovulation (during menses, mid-follicular phase, late follicular phase and LH/FSH surge). Baseline lipoprotein cholesterol levels were not associated with risk of subsequent anovulation for any of the lipoprotein cholesterol parameters (Table II). However, we noted a slight increased risk of anovulation associated with increased levels of TC when multiple measures of TC up to the time of ovulation were used as the exposure. For every 5 mg/dl increase in TC, the odds of subsequent anovulation increased by 1.07 (OR 1.07; 95% CI 0.99–1.15; P = 0.09). To assess for confounding by indicators of androgen excess, additional models including LH:FSH ratio, HOMA-IR, insulin, SHBG, hirsutism score and current acne were created. The results did not differ (data not shown).
Table II.
Results of inverse probability weighted generalized linear mixed effects models of the association between lipoprotein cholesterol levels preceding predicted ovulation and incident anovulation in women.
| Baseline lipoprotein cholesterol levels and anovulationa |
Levels of lipoprotein cholesterol up to predicted time of ovulation and anovulationb |
||||
|---|---|---|---|---|---|
| ORc | 95% CI | P-value | OR | 95% CI | P-value |
| TC per 5 mg/dl | |||||
| 1.03 | (0.95, 1.11) | 0.51 | 1.07 | (0.99, 1.15) | 0.09 |
| HDL cholesterol per 5 mg/dl | |||||
| 1.01 | (0.85, 1.19) | 0.92 | 1.02 | (0.86, 1.20) | 0.86 |
| LDL cholesterol per 5 mg/dl | |||||
| 1.02 | (0.94, 1.11) | 0.66 | 1.05 | (0.96, 1.14) | 0.28 |
| Triglycerides per 5 mg/dl | |||||
| 1.05 | (0.97, 1.13) | 0.23 | 1.04 | (0.99, 1.09) | 0.16 |
OR, odds ratio; CI: confidence interval; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; TC, total cholesterol.
aBaseline levels of lipoprotein cholesterol levels were measured on Day 2 of menses during the first cycle under study. These models evaluate the association between baseline lipoprotein cholesterol and anovulation using generalized linear mixed models.
bLevels of lipoprotein cholesterol were allowed to vary up to the time of predicted ovulation, and included up to four measurements per woman per cycle. These models evaluate the association between lipoprotein levels preceding ovulation and anovulation in that cycle using inverse probability weighted generalized linear mixed models.
cModel is adjusted for age, age at menarche, BMI, fiber intake and total energy. In inverse probability weighted models for the association of lipoprotein cholesterol levels up to the predicted time of ovulation, models also adjusted for levels of other reproductive hormones that are changing up to the time of predicted ovulation. Models that only include baseline measurements are assumed to not be affected by time-varying confounding by other hormones.
The anovulatory women in our study displayed several characteristics of endocrine and metabolic disturbances after adjusting for age and BMI (Table III). Specifically, anovulatory women exhibited higher LH:FSH ratios (mean difference day 2, 0.26, P = 0.0002) and lower SHBG levels (mean difference, −9.88 nmol/l, P = 0.0003). In our assessment of hyperinsulinemia, we observed no differences in glucose (mean difference, −0.92 mg/dl, P = 0.38) or HOMA-IR (mean difference, 0.19, P = 0.17); however, insulin levels tended to be higher among women with at least one anovulatory cycle (mean difference, 1.10 uIU/ml, P = 0.06). Phenotypically, self-reported hirsutism score assessments based on a modification of the Ferriman–Gallwey scale with five sites (Ferriman and Gallwey, 1961) were similar between anovulatory and ovulatory women (mean difference, 0.13, P = 0.78). The prevalence of acne tended to be higher among women with at least one anovulatory cycle (mean difference, 15.4, P = 0.08).
Table III.
Markers of endocrine function by ovulatory status.
| Overalla | Ovulatory | Anovulatoryb | P-valuec | |
|---|---|---|---|---|
| n = 259 | n = 224 | n = 35 | ||
| Hirsutism score | 3.03 ± 2.58 | 3.01 ± 2.58 | 3.14 ± 2.66 | 0.78 |
| Currently have acne: n (%)d | 72 (30.5) | 58 (28.4) | 14 (43.8) | 0.08 |
| LH:FSH ratio | ||||
| Day 2 | 0.61 ± 0.44 | 0.61 (0.57, 0.65) | 0.87 (0.73, 1.02) | <0.01 |
| Day 7 | 0.71 ± 0.47 | 0.71 (0.67, 0.75) | 0.89 (0.76, 1.03) | 0.01 |
| Average of Day 2 and 7 | 0.70 ± 0.45 | 0.67 (0.64, 0.71) | 0.93 (0.80, 1.07) | <0.01 |
| SHBG (nmol/l): Day 2 | 44.50 ± 29.05 | 45.06 (42.54, 47.73) | 35.18 (30.33, 40.82) | <0.01 |
| Glucose (mg/dl): Day 2 | 87.00 + 7.00 | 87.31 (86.57, 88.05) | 86.39 (84.49, 88.30) | 0.38 |
| Insulin (uIU/ml): Day 2 | 6.00 + 4.50 | 5.79 (5.42, 6.18) | 6.89 (5.82, 8.15) | 0.06 |
| HOMA-IR: Day 2 | 1.32 + 0.56 | 1.29 (1.21, 1.38) | 1.48 (1.24, 1.76) | 0.17 |
ANOVA, analysis of variance; CI, confidence interval; HOMA-IR, homeostasis model assessment of insulin resistance; SHBG, sex hormone-binding globulin.
aValues are mean ± SD for overall population. Values for ovulatory and anovulatory women are predicted means (95% CI) after adjusting for age and BMI. LH:FSH ratio, SHBG, Insulin and HOMA-IR are geometric means.
bAt least one anovulatory cycle. Twenty-eight women in the study had only one anovulatory cycle, and seven women in the study had two anovulatory cycles. There were a total of 42 anovulatory cycles out of a total 509 in the BioCycle Study.
cP-value for continuous variables calculated using ANOVA, for acne calculated using a χ2 test.
dAcne: five or more pimples, pustules or nodules on the face (except nose) during the last 3 months.
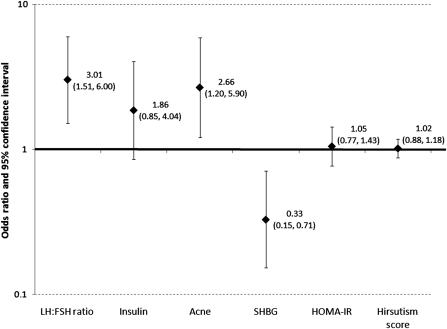
Finally, several endocrine and metabolic disturbances were associated with risk of incident anovulation. In particular, increased LH:FSH ratios and decreased SHBG levels on Day 2 were predictive of anovulation in a given cycle. The presence of current acne was also predictive of anovulation. However, insulin levels, hirsutism score and HOMA-IR were not predictive of anovulation. ORs and 95% CI reflecting the association between these covariates and the occurrence of anovulation are displayed in Fig. 2.
Figure 2.
ORs and 95% CIs for associations between increases in endocrine factors and risk of incident anovulation by cycle, adjusted for TC, age, age at menarche, BMI, fiber intake and total energy. HOMA-IR, homeostasis model assessment of insulin resistance; SHBG, sex hormone-binding globulin; TC, total cholesterol.
All analyses were completed using two classifications for anovulation. Results using the less conservative definition of anovulation (peak progesterone across the cycle ≤5 ng/ml regardless of LH concentration, n = 65) yielded similar results to those presented using the more conservative definition for anovulation (data not shown).
Discussion
In this prospective cohort of self-reported regularly menstruating women, we observed poorer lipid profiles during anovulatory cycles; however, there were no statistically significant associations between baseline lipoprotein cholesterol levels and risk of incident anovulation. It does not appear as though higher lipoprotein cholesterol levels predict anovulation. Although we cannot completely rule out the steroid hypothesis, the slight increased risk of anovulation associated with increased levels of TC observed when using multiple measures of TC raises the possibility of an underlying weak association that may be detected in a larger study with greater study power, or in women who are more likely to be anovulatory. The women who experienced at least one anovulatory cycle displayed several endocrine or metabolic disturbances. Furthermore, we observed that several of these endocrine characteristics—increased Day 2 LH:FSH ratio, presence of acne and decreased levels of SHBG—were predictive of sporadic anovulatory cycles. These results together suggest that an endocrinologic disturbance may lead to, or be the result of, sporadic anovulation.
As there is a lack of comparable research on this topic in normal, healthy women, we compared our results with prior studies of women diagnosed with ovulatory disorders, such as PCOS (Zawadski and Dunaif, 1992; Rotterdam, 2004; Azziz et al., 2009). Like our group of women with anovulatory cycles, despite regular menstrual cycles, women with PCOS have a poorer lipid profile (Rajkhowa et al., 1997; Pirwany et al., 2001; Yilmaz et al., 2005; Macut et al., 2008; Valkenburg et al., 2008; Moran and Teede, 2009; Akram et al., 2010). However, we did not observe lipid abnormalities as severe as those often reported for women with a PCOS diagnosis (Macut et al., 2008). This is likely a consequence of our strict study inclusion/exclusion criteria which purposefully excluded women at high risk or diagnosed with PCOS. However, we did not observe the differences in HDL cholesterol previously reported among women with a PCOS diagnosis compared with women without a PCOS diagnosis (Rajkhowa et al., 1997; Yilmaz et al., 2005; Macut et al., 2008; Valkenburg et al., 2008). This could in part be related to the fact that full metabolic disturbances may not be present in women with only sporadic anovulation (Rizzo et al., 2009).
Biologically, an association between lipid levels and ovulation might be expected because of the role of cholesterol in steroid biosynthesis (Strauss, 2004). Furthermore, in studies where women with PCOS were treated with cholesterol-lowering medications, not only did their cholesterol levels decline, but also their testosterone levels, LH:FSH levels, and hirsutism scores declined (Duleba et al., 2006; Banaszewska et al., 2007; Kodaman and Duleba, 2008). The results of the current study do not rule out the steroid hypothesis; rather, they suggest that an underlying association may exist (albeit weak), the detection of which will require greater study power. It is possible that perhaps the poorer lipid profiles observed among women with PCOS are a consequence, rather than a cause, of endocrine disturbance and anovulation. The observed associations between lipoprotein cholesterol levels and anovulation might be explained by changing androgen levels, for which we unfortunately did not capture data. Alternatively, the observed associations between lipoprotein cholesterol levels and anovulation may not be the result of the steroid biosynthesis pathway, but some heretofore unrecognized biological mechanism.
In this study, women with at least one anovulatory cycle demonstrated higher levels of several non-specific markers for androgen bioactivity, including higher LH:FSH ratios (Homburg, 2002) and higher follicular phase LH levels (Balen et al., 1995), all consistent with, but not diagnostic of, androgen excess. Increased LH:FSH ratios are indicative of hypothalamic–pituitary–ovarian dysfunction, characteristic of women with PCOS (Azziz et al., 2009). We also observed lower SHBG levels among the anovulatory women, likely secondary to hyperinsulinemia and associated with excess androgen activity (Pugeat et al., 1996). Insulin levels were increased to a small degree among anovulatory women, an observation consistent with the insulin resistance that also frequently accompanies PCOS (Dunaif, 2006). These endocrine parameters were found to be significant predictors of sporadic anovulation, an apparently reversible condition that may be more common among certain groups of eumenorrheic women than originally thought (De Souza et al., 1998). Other investigators, however, suggest that anovulation occurs among eumenorrheic women only on a very infrequent basis (Malcolm and Cumming, 2003; Chatterton et al., 2005). This is the first study, to our knowledge, to prospectively identify endocrine markers of sporadic anovulation in regularly menstruating women.
Our study offers several advantages over previous studies of lipids and anovulation, in particular the prospective evaluation of the association between baseline lipoprotein cholesterol levels and incident anovulation. By preserving temporality, we were able to further evaluate whether the detected associations were a result of the biological effects of lipoprotein cholesterol on anovulation, rather than being symptomatic of an underlying condition leading to both ovulatory dysfunction and a more atherogenic lipid profile. In addition, we were able to evaluate the possibility of confounding by underlying endocrine disturbances by adjusting for several markers of endocrine function. This study is further distinguished from previous research by its strict inclusion/exclusion criteria, which ensured a sample of eumenorrheic women without a prior clinical diagnosis of PCOS.
While the current study allowed us to expand upon previous studies in this area, we were limited by several factors. Importantly, we were unable to directly measure androgen levels, and had to rely on several non-specific markers to assess androgen activity. In addition, daily measures of progesterone and transvaginal ultrasounds (the gold standard) were not available to assess ovulation. However, multiple well-timed serum hormone measurements (on average, 2, 7 and 13 days before, and 2, 5, 8 and 12 days after the mid-cycle LH surge), along with the use of fertility monitors measuring LH daily in urine, were used to aid in classifying ovulatory cycles. We also employed a conservative definition for anovulation to avoid potential misclassification. Despite these measures, misclassification of anovulation is possible. The small number of anovulatory cycles limits the statistical power available to detect subtle effects, if such an effect exists.
In conclusion, we detected higher TC (P = 0.01), LDL (P = 0.06) and triglyceride (P = 0.0002) levels in regularly menstruating women with anovulatory cycles when multiple measurements across the cycle were considered; however, baseline lipoprotein cholesterol levels were not significantly associated with incident anovulation. Only when multiple measures of TC were included did we observe a weak and marginally significant association with risk for anovulation. While these results do not rule out the steroid hypothesis, they do not support a strong association between lipoprotein cholesterol levels and anovulation. Regularly menstruating women with at least one anovulatory cycle tended to exhibit endocrine and metabolic disturbances similar to, but less severe than, women with PCOS. Collectively, these findings further underscore the possibility of a gradient of severity of endocrine and metabolic disturbances proportional to the degree of ovulatory dysfunction. These data are preliminary and more research is needed to identify the pathophysiology of incident anovulation among eumenorrheic women, particularly given the absence of measured androgens here.
Authors’ roles
Dr S.L.M., Dr E.F.S. and Dr J.W.-W. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: E.F.S., J.W.-W. Acquisition of data: E.F.S., J.W.-W., K.H. Analysis and interpretation of data: S.L.M., E.F.S., A.M.S.-R., A.J.G., A.Z.S., J.L.D., A.F.O., M.L.H., K.H., J.W.-W., M.T., M.S.B. Drafting of the manuscript: S.L.M., E.F.S., M.S.B., A.Z.S., A.J.G. Critical revision of the manuscript for important intellectual content: E.F.S., A.M.S.-R., M.S.B., A.J.G., M.T., A.Z.S., J.L.D., J.W.-W. Statistical analysis: S.L.M., E.F.S. Study supervision: E.F.S., A.M.S.-R., J.W.-W.
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Acknowledgements
We are indebted to all the investigators and staff at the Epidemiology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the University at Buffalo for their respective roles in the study, their dedication and effort, and assistance in study implementation. We thank the BioCycle Working Group for their helpful suggestions. In addition, we would like to recognize the BioCycle participants for their extraordinary commitment to the study.
References
- Abdulla U, Diver MJ, Hipkin LJ, Davis JC. Plasma progesterone levels as an index of ovulation. Br J Obstet Gynaecol. 1983;90:543–548. doi: 10.1111/j.1471-0528.1983.tb08965.x. [DOI] [PubMed] [Google Scholar]
- Akram T, Hasan S, Imran M, Karim A, Arslan M. Association of polycystic ovary syndrome with cardiovascular risk factors. Gynecol Endocrinol. 2010;26:47–53. doi: 10.3109/09513590903159565. doi:10.3109/09513590903159565. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. doi:10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107–2111. doi: 10.1093/oxfordjournals.humrep.a136243. [DOI] [PubMed] [Google Scholar]
- Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: prospective, randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:456–461. doi: 10.1210/jc.2006-1988. doi:10.1210/jc.2006-1988. [DOI] [PubMed] [Google Scholar]
- Barbieri RL. Female infertility. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management, 5th edn. Philadelpha, PA: Elsevier; 2004. pp. 633–668. [Google Scholar]
- Browne RW, Bloom MS, Schisterman EF, Hovey K, Trevisan M, Wu C, Liu A, Wactawski-Wende J. Analytical and biological variation of biomarkers of oxidative stress during the menstrual cycle. Biomarkers. 2008;13:160–183. doi: 10.1080/13547500701775563. doi:10.1080/13547500701775563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Mateo ET, Hou N, Rademaker AW, Acharya S, Jordan VC, Morrow M. Characteristics of salivary profiles of oestradiol and progesterone in premenopausal women. J Endocrinol. 2005;186:77–84. doi: 10.1677/joe.1.06025. doi:10.1677/joe.1.06025. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. doi:10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. doi:10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- De Souza MJ, Miller BE, Loucks AB, Luciano AA, Pescatello LS, Campbell CG, Lasley BL. High frequency of luteal phase deficiency and anovulation in recreational women runners: blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83:4220–4232. doi: 10.1210/jcem.83.12.5334. doi:10.1210/jc.83.12.4220. [DOI] [PubMed] [Google Scholar]
- Diggle P, Heagerty P, Liang K-Y, Zeger SL. Analysis of Longitudinal Data, 2nd edn. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Duleba AJ, Banaszewska B, Spaczynski RZ, Pawelczyk L. Simvastatin improves biochemical parameters in women with polycystic ovary syndrome: results of a prospective, randomized trial. Fertil Steril. 2006;85:996–1001. doi: 10.1016/j.fertnstert.2005.09.030. doi:10.1016/j.fertnstert.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Dunaif A. Insulin resistance in women with polycystic ovary syndrome. Fertil Steril. 2006;86(Suppl 1):S13–S14. doi: 10.1016/j.fertnstert.2006.04.011. doi:10.1016/j.fertnstert.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol. 2007;50:205–225. doi: 10.1097/GRF.0b013e31802f3547. doi:10.1097/GRF.0b013e31802f3547. [DOI] [PubMed] [Google Scholar]
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–1447. doi: 10.1210/jcem-21-11-1440. doi:10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, Howards PP, Perkins NJ, Yeung E, Schisterman EF, et al. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am J Clin Nutr. 2009;90:1061–1069. doi: 10.3945/ajcn.2009.27990. doi:10.3945/ajcn.2009.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homburg R. What is polycystic ovarian syndrome? A proposal for a consensus on the definition and diagnosis of polycystic ovarian syndrome. Hum Reprod. 2002;17:2495–2499. doi: 10.1093/humrep/17.10.2495. doi:10.1093/humrep/17.10.2495. [DOI] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–112. doi: 10.1093/aje/kwn287. doi:10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. doi:10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodaman PH, Duleba AJ. Statins in the treatment of polycystic ovary syndrome. Semin Reprod Med. 2008;26:127–138. doi: 10.1055/s-2007-992933. doi:10.1055/s-2007-992933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- Macut D, Panidis D, Glisic B, Spanos N, Petakov M, Bjekic J, Stanojlovic O, Rousso D, Kourtis A, Bozic I, et al. Lipid and lipoprotein profile in women with polycystic ovary syndrome. Can J Physiol Pharmacol. 2008;86:199–204. doi: 10.1139/Y08-014. doi:10.1139/Y08-014. [DOI] [PubMed] [Google Scholar]
- Malcolm CE, Cumming DC. Does anovulation exist in eumenorrheic women? Obstet Gynecol. 2003;102:317–318. doi: 10.1016/s0029-7844(03)00527-1. doi:10.1016/S0029-7844(03)00527-1. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. doi:10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Moran L, Teede H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update. 2009;15:477–488. doi: 10.1093/humupd/dmp008. doi:10.1093/humupd/dmp008. [DOI] [PubMed] [Google Scholar]
- Pirwany IR, Fleming R, Greer IA, Packard CJ, Sattar N. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clin Endocrinol (Oxf) 2001;54:447–453. doi: 10.1046/j.1365-2265.2001.01228.x. doi:10.1046/j.1365-2265.2001.01228.x. [DOI] [PubMed] [Google Scholar]
- Pugeat M, Crave JC, Tourniaire J, Forest MG. Clinical utility of sex hormone-binding globulin measurement. Horm Res. 1996;45:148–155. doi: 10.1159/000184778. doi:10.1159/000184778. [DOI] [PubMed] [Google Scholar]
- Rajkhowa M, Neary RH, Kumpatla P, Game FL, Jones PW, Obhrai MS, Clayton RN. Altered composition of high density lipoproteins in women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:3389–3394. doi: 10.1210/jcem.82.10.4318. doi:10.1210/jc.82.10.3389. [DOI] [PubMed] [Google Scholar]
- Rizzo M, Berneis K, Hersberger M, Pepe I, Di Fede G, Rini GB, Spinas GA, Carmina E. Milder forms of atherogenic dyslipidemia in ovulatory versus anovulatory polycystic ovary syndrome phenotype. Hum Reprod. 2009;24:2286–2292. doi: 10.1093/humrep/dep121. doi:10.1093/humrep/dep121. [DOI] [PubMed] [Google Scholar]
- Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. doi:10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA. 2003;290:1767–1770. doi: 10.1001/jama.290.13.1767. doi:10.1001/jama.290.13.1767. [DOI] [PubMed] [Google Scholar]
- Strauss JF., III . The synthesis and metabolism of steroid hormones. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management, 5th edn. Philadelphia, PA: Elsevier,; 2004. pp. 125–154. [Google Scholar]
- Valkenburg O, Steegers-Theunissen RP, Smedts HP, Dallinga-Thie GM, Fauser BC, Westerveld EH, Laven JS. A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case–control study. J Clin Endocrinol Metab. 2008;93:470–476. doi: 10.1210/jc.2007-1756. doi:10.1210/jc.2007-1756. [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M for the BioCycle Study Group. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatr Perinat Epidemiol. 2009;23:171–184. doi: 10.1111/j.1365-3016.2008.00985.x. doi:10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M, Biri A, Bukan N, Karakoc A, Sancak B, Toruner F, Pasaoglu H. Levels of lipoprotein and homocysteine in non-obese and obese patients with polycystic ovary syndrome. Gynecol Endocrinol. 2005;20:258–263. doi: 10.1080/09513590400027265. doi:10.1080/09513590400027265. [DOI] [PubMed] [Google Scholar]
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine F, editors. Polycystic Ovary Syndrome. Boston: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]