Abstract
BACKGROUND
Whether in utero exposure to tobacco smoke increases a woman's risk of fetal loss later in life is unknown, though data on childhood exposure suggest an association may exist. This study evaluated the association between in utero exposure to tobacco smoke and fetal loss in the Norwegian Mother and Child Cohort Study (MoBa), which enrolled ∼40% of the pregnant women in Norway from 1999 to 2008.
METHODS
Information on exposure to tobacco smoke in utero, the woman's own smoking behavior during pregnancy and other factors was obtained by a questionnaire completed at ∼17 weeks of gestation. Subsequent late miscarriage (fetal death <20 weeks) and stillbirth (fetal death ≥20 weeks) were ascertained from the Norwegian Medical Birth Registry. This analysis included 76 357 pregnancies (MoBa data set version 4.301) delivered by the end of 2008; 59 late miscarriages and 270 stillbirths occurred. Cox proportional hazards models were fit for each outcome and for all fetal deaths combined.
RESULTS
The adjusted hazard ratio (HR) of late miscarriage was 1.23 [95% confidence interval (CI), 0.72–2.12] in women with exposure to maternal tobacco smoke in utero when compared with non-exposed women. The corresponding adjusted HR for stillbirths was 1.11 (95% CI, 0.85–1.44) and for all fetal deaths combined, it was 1.12 (95% CI, 0.89–1.43).
CONCLUSIONS
The relatively wide CI around the HR for miscarriage reflected the limited power to detect an association, due to enrollment around 17 weeks of gestation. However, for in utero exposure to tobacco smoke and risk of stillbirth later in life, where the study power was adequate, our data provided little support for an association.
Keywords: tobacco smoking, in utero exposure, miscarriage, pregnancy, stillbirth
Introduction
Smoking during pregnancy has been considered a cause of fetal growth restriction, low-birthweight, stillbirth, sudden infant death syndrome and reduced lung function in offspring (USDHHS, 2004; Hogberg and Cnattingius, 2007). Such exposure has also been consistently associated with neonatal death and respiratory diseases in offspring, though causality is not established (USDHHS, 2004). In utero exposure to tobacco smoke has been associated with childhood obesity and neurobehavioral disorders (Linnet et al., 2003; Oken et al., 2008), though recent studies among siblings indicate that these associations, when present, may be due to confounding (Lambe et al., 2006; Lundberg et al., 2009; Iliadou et al., 2010; Lindblad and Hjern, 2010). Questions regarding long-term effects of in utero exposure to tobacco smoke also apply to several outcomes among adults, which have been less studied. Among young adults, carotid wall thickening, high cholesterol, impaired glucose metabolism and type-2 diabetes have been reported in association with in utero smoking (Montgomery and Ekbom, 2002; Thomas et al., 2007; Geerts et al., 2008; Jaddoe et al., 2008). Previous studies suggest that men and women exposed to maternal tobacco smoking in utero may experience decreased fecundability later in life (Jensen et al., 2006; Ye et al., 2010); in women, early age at menopause has also been reported (Strohsnitter et al., 2008). A recent finding suggests that the number of oocytes available in adult life may be compromised by in utero exposure to tobacco smoking from the mother in the first trimester (Lutterodt et al., 2009), which may explain, in part, the previous findings on fecundability and menopause. Associations with other adverse reproductive end-points such as the risk of pregnancy loss in women who were exposed to their mother's tobacco smoking in utero have not yet been examined.
Childhood exposure to parental tobacco smoke, however, was recently associated with an increased risk of spontaneous miscarriage in women who conceived after use of assisted reproductive technology (ART) (Meeker et al., 2007). Although women who conceive by ART may be at a higher risk of stillbirths compared with fertile women (Wisborg et al., 2010), this study by Meeker et al. lacked power to assess the risk of stillbirths. In the present study, we evaluated the association between in utero exposure to maternal tobacco smoking and the daughter's risk of miscarriages and stillbirths later in life in a large prospective cohort.
Materials and Methods
This study is based on the Norwegian Mother and Child Cohort Study (MoBa), conducted by the Norwegian Institute of Public Health (Magnus et al., 2006). In brief, MoBa is a cohort based on 107 000 pregnancies recruited from 1999 to 2008. The majority of all pregnant women in Norway were invited to participate, and the participation rate was around 44%. Participants were recruited to the study through a postal invitation before a routine ultrasound examination offered to all pregnant women in Norway at 17–18 weeks of gestation (www.fhi.no/morogbarn). Some women in the cohort participated with more than one pregnancy. The present study is based on version 4.301 of the quality-assured data files released for research in December 2009. The study was approved by The Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate. Informed consent was obtained from each participant.
Exposure assessment
Exposure to tobacco smoke in utero was collected from a self-administered questionnaire filled out at enrollment (Q-1). The woman was asked: ‘Did your mother smoke when she was pregnant with you?’ For women with more than one pregnancy in the MoBa cohort, the consistency of answers across pregnancies was verified. In general, if the women gave two different answers in two consecutive pregnancies (e.g. yes/no; no/yes; no/don't know; yes/don't know), the answers were considered inconsistent and therefore excluded from the analysis. However, if the first answer was ‘Don't know’ and then she gave a different answer after that (yes or no), we used the latter under the assumption that the women had asked to her mother about her exposure in utero. Q-1 (and later questionnaires) had no questions on exposure to cigarette smoking during childhood.
Among the participants born in 1967 or later [when the Medical Birth Registry (MBR) of Norway was created (n = 62 099)], birthweight was available for 89.8% and was used as a surrogate to validate their reported in utero exposure to maternal tobacco smoke.
Outcomes
Fetal deaths were ascertained from the Norwegian MBR. Late miscarriages were defined as a fetal death that occurred before 20 weeks of gestation (140 days) and stillbirths as fetal deaths that occurred at ≥20 weeks of gestation (ACOG, 2009). Because the median gestational age (GA) at enrollment was 17.1 weeks (120 days), we anticipated a small number of late miscarriages. GA at the end of the pregnancy was calculated (in days) using the first day of the last menstrual period. For some women whose last menstrual period was not available, we used GA estimated from the pregnancy ultrasound. When the difference in GA estimated with the last menstrual period and ultrasound exceeded 2 weeks, the GA based on ultrasound was used.
Covariates
Information on women's age, pre-pregnancy body mass index (BMI, kg/m2), parity, education, income, the use of IVF, smoking during pregnancy, exposure to second-hand tobacco smoke (SHTS), binge drinking during pregnancy and partner's age was collected at enrollment by questionnaire. SHTS during pregnancy was defined as any passive exposure to tobacco smoke at home or work (yes/no). Binge drinking was defined as five or more alcohol units per occasion.
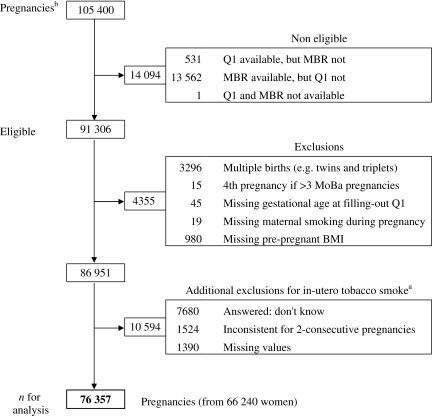
For the present analysis, we included all pregnancies with information on the first questionnaire (Q-1, completed around 17 weeks of gestation) and with corresponding outcome data in the MBR, which included deliveries through 2008 (n = 91 306). Pregnancies that ended in multiple births (e.g. twins and triplets) were excluded (n = 3296). For women with more than three pregnancies in MoBa, we excluded the fourth MoBa pregnancy (n = 15). Other observations (n = 1044) were excluded because of missing values in one the following variables: GA at Q-1, women's smoking during pregnancy and pre-pregnancy BMI. Additionally, observations were excluded from the main analysis (n = 10 594) if, for in utero tobacco smoking, there were missing values, unknown exposure or inconsistent answers (i.e. two different answers in two consecutive pregnancies: yes/no; no/yes; no/don't know; yes/don't know). Most of the inconsistencies (1446/1524) were present in women with only two pregnancies in MoBa. After all the exclusions, a total of 76 357 pregnancies from 66 240 women were available for the analysis (9111 women participated with two pregnancies and 503 with three pregnancies) (Fig. 1). We included more than one pregnancy per women in the analysis.
Figure 1.
Selection of sample for analysis of in utero exposure to maternal tobacco smokea and the daughter's risk of fetal loss later in life in the MoBa cohort. Abbreviations: BMI, body mass index; MBR, Medical Birth Registry; Q1, questionnaire-1. aAsked in the first MoBa questionnaire as: Did your mother smoke when she was pregnant with you? bBased on data set version 4.301.
Statistical analysis
Kaplan–Meier survival curves are presented for all fetal deaths according to woman's in utero exposure to her mother's tobacco smoke. Cox proportional hazard models were used to evaluate the women's risk of late miscarriages and stillbirths as separate outcomes, and after combining both in one overall model (all fetal deaths). The proportional hazard assumption was tested and corroborated for each model. Robust standard errors (SEs) were estimated to correct for the non-independence of the observations produced by women who participated with more than one pregnancy (Lin and Wei, 1989). GA (days) at filling-out the first questionnaire was entered into the model as the beginning of the risk period to correct for delayed entry (i.e. not every women was recruited at exactly the same GA). GA (days) at the end of the pregnancy was used as the time variable. Live births as well as medical abortions (n = 59) were censored at the GA when the pregnancy ended. For the analysis of late miscarriages, pregnancies that continued beyond GA day 140 were censored at that point. In addition, if Q-1 was completed at GA day 140 or later, the pregnancy was excluded from the analysis of late miscarriages (n = 11 743). For stillbirths, the beginning of risk period started at Day 140 of GA; thus, pregnancies that ended before Day 140 were excluded (n = 94).
Women who reported any miscarriage or stillbirth were more likely to participate in MoBa with more than one pregnancy (i.e. the cluster size was informative); therefore, we also fitted parametric shared frailty survival models (exponential and Weibull with gamma shared frailties) (Gutierrez, 2002). Because the results from the shared frailty models were comparable with those obtained from the Cox model with robust SEs, we present only the results obtained from the Cox models.
All models included in utero smoking as the main exposure and were adjusted for women's age as an a priori selected variable. Confounding was evaluated in models using the change in estimate method, starting with all variables and deleting one by one in a stepwise way (Greenland, 1989). Potential confounders entered in the full models were women's pre-pregnancy BMI, education, number of cigarettes smoked per day during pregnancy, SHTS at home or work, binge drinking and partner's age. Parity was not considered as a potentially confounding variable because its inclusion may cause over adjustment (Weinberg, 1993). Although none of the tested variables caused ≥10% change in the hazard ratio (HR) for in utero smoking, the final models were adjusted for pre-pregnancy BMI and number of cigarettes smoked per day during pregnancy in addition to age, because they were statistically significant predictors of the outcomes.
Interaction between the woman's in utero smoking exposure and her own smoking behavior during pregnancy was tested in the final models for stillbirths and for all fetal deaths combined. For late miscarriages, we did not have enough power to test this interaction; therefore, we restricted the analysis to only women who did not smoke during pregnancy. Because in utero exposure to maternal tobacco smoke has been associated with decreased fecundability, we tested the interaction between the woman's in utero smoking and her use of IVF in the final models for stillbirths and for all fetal deaths combined. Again, for miscarriages we restricted the analysis to only never users of IVF treatment as reported by the women. Interactions of in utero smoking with parity were also evaluated in all the final models. All analyses were done using Stata (Stata Statistical Software, release 10.1; StataCorp, College Station, TX, USA).
Results
Women exposed to their mother's tobacco smoke in utero differed from those with no exposure (Table I). Women who reported being exposed to tobacco smoke in utero were lighter at birth compared with those not exposed; this was observed among all births and among those born after 37 completed weeks of gestation. The adjusted birthweight difference (non-exposed-exposed, adjusted for GA, age and parity of the participant's mother) was 193 g for all births and 202 g for term births (data not shown). Exposed women were also younger, had less education, lower income, and were more likely to be overweight or obese and to have a young partner. Additionally, women with in utero exposure to tobacco smoke were more likely to smoke daily and to smoke 10 or more cigarettes per day during pregnancy; they also reported more exposure to SHTS at home or work (Table I).
Table I.
Characteristics of the women according to their in utero exposure to maternal tobacco smoke in the MoBa cohorta.
| Exposed to tobacco smoke in uterob |
||
|---|---|---|
| No [(n = 55 081) (%)] | Yes [(n = 21 276) (%)] | |
| Mean birth weight (SD)c | 3547 (493) | 3328 (495) |
| Age (year) | ||
| <20 | 1.1 | 2.1 |
| 20–24 | 10.8 | 13.8 |
| 25–29 | 35.2 | 36.0 |
| 30–34 | 37.6 | 35.5 |
| 35–39 | 13.5 | 11.7 |
| 40+ | 1.7 | 1.0 |
| Parity | ||
| 0 | 46.8 | 43.3 |
| 1 | 34.0 | 35.7 |
| 2 | 15.0 | 16.1 |
| 3+ | 4.3 | 4.9 |
| Pre-pregnant BMI (kg/m2) | ||
| ≤18.5 | 3.4 | 2.8 |
| 18.6 to <25.0 | 67.3 | 58.2 |
| 25.0 to <30.0 | 20.9 | 25.7 |
| 30.0+ | 8.4 | 13.3 |
| Education | ||
| <High School | 6.3 | 12.5 |
| High School | 25.5 | 34.8 |
| Some college | 42.2 | 36.0 |
| College + | 24.3 | 14.8 |
| Other | 1.3 | 1.6 |
| Missing | 0.4 | 0.3 |
| Gross income year (USD) | ||
| No income | 2.7 | 2.5 |
| <23 135 | 15.5 | 17.6 |
| 23 135–30 846 | 10.8 | 13.3 |
| 30 847–46 269 | 34.5 | 35.1 |
| 46 270–61 693 | 23.3 | 20.3 |
| 61 694–77 116 | 6.5 | 5.2 |
| >77 116 | 4.1 | 3.2 |
| Missing | 2.6 | 2.9 |
| Smoked during pregnancyd | ||
| No | 80.6 | 65.6 |
| Stopped while pregnant | 13.8 | 18.5 |
| Sometimes | 1.3 | 2.1 |
| Daily | 4.3 | 13.7 |
| Number of cigarettesd,e | ||
| <10 cigarettes/day | 3.8 | 8.9 |
| 10+ cigarettes/day | 1.8 | 7.0 |
| SHTS during pregnancy (home or work)d | ||
| No | 90.8 | 83.3 |
| Yes | 9.0 | 16.6 |
| Missing | 0.2 | 0.2 |
| Any tobacco exposure during pregnancyd | ||
| None | 75.5 | 59.0 |
| Stopped while pregnant | 11.8 | 15.1 |
| SHTS only | 7.1 | 10.0 |
| Active only | 3.7 | 9.3 |
| SHTS and active | 1.9 | 6.6 |
| Binge drink during pregnancyd | ||
| No drinker | 32.0 | 28.9 |
| Never | 58.0 | 60.0 |
| <1 month | 4.5 | 5.1 |
| 1–3 month | 0.4 | 0.4 |
| 1+ week | 0.2 | 0.2 |
| Missing | 5.0 | 5.4 |
| Partner's age (year) | ||
| <20 | 0.2 | 0.5 |
| 20–24 | 4.0 | 6.0 |
| 25–29 | 22.3 | 24.5 |
| 30–34 | 38.8 | 38.3 |
| 35–39 | 23.9 | 22.0 |
| 40+ | 10.4 | 8.3 |
| Missing | 0.4 | 0.5 |
| IVF treatment | ||
| No | 96.7 | 96.9 |
| Current pregnancy | 2.3 | 2.0 |
| In the past | 1.0 | 1.1 |
Abbreviations: BMI, body mass index; SD, standard deviation; SHTS, second-hand tobacco smoke; USD, US dollars.
P values from Pearson's χ2 or Fisher's exact test were <0.001 for all categorical variables listed in the table.
aBased on 76 357 pregnancies from 66 240 women.
bAsked in the first MoBa questionnaire as: Did your mother smoke when she was pregnant with you?
cBased on a subset of the participants who were born in 1967 or later and had available birthweight in the Norwegian MBR (n = 55 789; of whom 29.6 % were exposed). Among those born after 37 completed weeks of gestation (n = 51 751), the mean birthweights (SD) were: no—3573 (462); yes—3366 (457).
dReflects woman's exposure at ∼17 weeks of gestation from first MoBa questionnaire.
eFor the subset of women who smoked during pregnancy.
The proportion of women who were exposed to tobacco smoke in utero varied by year of birth, and higher percentages were observed among women born in 1971 or later when compared with those born before (Table II).
Table II.
Reported in utero exposure to maternal tobacco smoke by year of birth among women in the MoBa cohort, and proportion of women who smoked in Norway during the corresponding calendar years.
| Reproductive aged women (Norway) |
Pregnant women (MoBa) |
Post-partum period (Norway) |
|||
|---|---|---|---|---|---|
| Calendar year | % of women who smokeda | Birth yearb | % exposed in uteroc | Calendar year | % of women who smokedd |
| 1951–1965 | 37 | 1954–1965 | 20.0 | ||
| 1966–1970 | 47 | 1966–1970 | 26.4 | 1966–1970 | 38 |
| 1971–1975 | 41 | 1971–1975 | 28.1 | 1971–1975 | 37 |
| 1976–1980 | 41 | 1976–1980 | 27.9 | 1976–1980 | 31 |
| 1981–1990 | 41 | 1981–1992 | 32.8 | 1981–1991 | 29 |
aValue shown is an average calculated from data in Ronneberg et al. (1994). The age range is 21–45 years for all calendar years, except for 1976–1980 (age range, 26–40 years).
bDistribution of the women by categories of birth year: 3.7, 18.9, 38.7, 28.8 and 9.9%.
cAsked in the first MoBa questionnaire as: Did your mother smoke when she was pregnant with you?.
dThe values were obtained from Haug et al. (1992).
A total of 329 fetal deaths from 76 357 pregnancies were recorded. As expected, few of the deaths occurred before week 20 of gestation [59 (17.9%)] because of the study design. Most of the stillbirths occurred before week 40 [20–29.9 weeks, 105 (31.9%); 30–39.9 weeks, 113 (34.4%)] and only 52 (15.8%) occurred at 40 weeks or later. Overall, exposure to tobacco smoke in utero was relatively frequent (28%); pregnancies ending in fetal deaths reported slightly higher percentages of in utero smoking (31%) than pregnancies ending in live births (28%). The percentage exposed was comparable across miscarriages (32%) and stillbirths (31%).
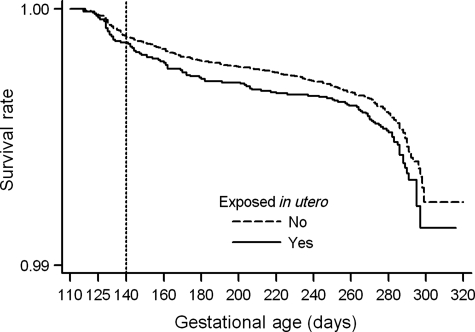
The risk of fetal death at any time during pregnancy was slightly higher in the group of women with in utero exposure to tobacco smoke (Fig. 2). After adjusting for women's characteristics, the HR of late miscarriage was 1.23 among women exposed to tobacco smoke in utero compared with the non-exposed (Table III). The adjusted HR for stillbirth was 1.11 and that for all fetal deaths combined was 1.12. The effect of adjustment for other covariates in the models for stillbirths and all fetal deaths was an attenuation of the HR. None of these results reached statistical significance. Limiting the analysis to the woman's first pregnancy in MoBa (n = 66 240), we observed slightly higher adjusted HRs for miscarriages (HR = 1.56; 95% CI, 0.87–2.78) and for all fetal deaths combined (HR = 1.16; 95% CI, 0.91–1.49), but a similar HR for stillbirths (HR = 1.10; 95% CI, 0.83–1.45) (data not shown).
Figure 2.
Kaplan–Meier survival curves for all fetal deaths by in utero exposure to tobacco smoke (Asked in the first MoBa questionnaire as: Did your mother smoke when she was pregnant with you?) in the MoBa cohort.
Table III.
HRs of fetal death by in utero exposure to maternal tobacco smokea in the women from the MoBa cohort.
| Cases n | All pregnancies (n = 76 357)b |
||
|---|---|---|---|
| HR | 95% CIc | ||
| Miscarriage (fetal death at <20 week)d | 59 | ||
| Unadjusted | 1.21 | 0.70–2.08 | |
| Age adjusted | 1.27 | 0.73–2.19 | |
| Fully adjustede | 1.23 | 0.72–2.12 | |
| Stratified by smoking during pregnancyf,g | |||
| No | 44 | 1.18 | 0.61–2.29 |
| Stillbirth (fetal death at ≥20 week)h | 268 | ||
| Unadjusted | 1.18 | 0.91–1.53 | |
| Age adjusted | 1.22 | 0.94–1.58 | |
| Fully adjustede | 1.11 | 0.85–1.44 | |
| Stratified by smoking during pregnancyf,g | |||
| No | 190 | 0.93 | 0.66–1.30 |
| Stopped while pregnant | 41 | 2.31 | 1.26–4.24 |
| Yes | 37 | 1.04 | 0.56–1.96 |
| All fetal deaths combined | 329 | ||
| Unadjusted | 1.18 | 0.93–1.49 | |
| Age adjusted | 1.22 | 0.96–1.54 | |
| Fully adjustede | 1.12 | 0.89–1.43 | |
| Stratified by smoking during pregnancyf,g | |||
| No | 236 | 0.96 | 0.71–1.30 |
| Stopped while pregnant | 52 | 1.82 | 1.05–3.15 |
| Yes | 41 | 1.27 | 0.69–2.32 |
aAsked in the first MoBa questionnaire as: Did your mother smoke when she was pregnant with you?
bFrom 66 240 women.
cRobust SEs were estimated taking into account the intragroup correlation (i.e. women with more than one pregnancy) in the Cox proportional hazard models.
dExcludes 11 743 pregnancies that entered to the risk set at or after 20 weeks of GA.
eAdjusted for maternal age (years), pre-pregnancy BMI (kg/m2) and number of cigarettes smoked at ∼17 weeks of gestation [no smoked (reference), stopped during pregnancy, <10 cigarettes/day and ≥ 10 cigarettes/day]. For the miscarriage model, frequency of smoking at ∼17 weeks of gestation [no smoked (reference), have stopped and yes] was used instead of number of cigarettes smoked. Global test for the proportional hazard assumption for all models gave P values >0.35.
fAs reported by the women at ∼17 weeks of gestation in the first MoBa questionnaire.
gStratified models were adjusted for woman's age (years) and pre-pregnancy BMI (kg/m2).
hExcludes 88 pregnancies that ended before 20 weeks of GA plus six pregnancies ending at 20 weeks.
When we restricted the analysis to women who did not smoke during pregnancy, the adjusted HR of late miscarriage with in utero exposure to tobacco smoke was little changed (Table III). In the models for stillbirths and all fetal deaths combined, statistically significant interactions between the woman's in utero exposure to tobacco smoke and her own smoking during pregnancy was observed (P for interaction ≤ 0.05). Thus, we fitted Cox models stratified by smoking during pregnancy. In the strata of women who did not smoke during pregnancy, the adjusted HRs of in utero smoking were below one for stillbirths (HR = 0.93) and for all fetal deaths combined (HR = 0.96). Among women who stopped smoking during pregnancy, statistically significant higher risks of stillbirths (adjusted HR = 2.31) and any fetal deaths (adjusted HR = 1.82) with in utero smoking exposure were observed. For the subset of women who continue smoking early in pregnancy the adjusted HR of stillbirth was 1.04 and for all fetal deaths was 1.27 (Table III).
Among women who reported never using IVF treatment in the past or for the current pregnancy, the adjusted HR of late miscarriage was 1.16 (95% CI, 0.67–2.01) for those with in utero exposure to tobacco smoke compared with non-exposed (data not shown). No statistically significant interactions between in utero smoking exposure and IVF treatment were observed in the models for stillbirths (P interaction = 0.26) or for all fetal deaths combined (P interaction = 0.50). No significant interaction was observed for in utero exposure and parity in any models (P interactions > 0.44).
Discussion
Overall, an association between in utero exposure to maternal tobacco smoke and the daughter's risk of fetal loss later in life was not supported in these data. A slightly increased risk of late miscarriages in women who reported being exposed to tobacco smoke in utero was observed but the estimate was imprecise. The findings for miscarriage and stillbirth were the same when the analysis was restricted to women who were non-smokers as adults (the majority).
We also observed that women who stopped smoking while pregnant had a statistically significant higher risk of stillbirths or any fetal deaths if they were exposed to tobacco smoke in utero. Compared with women who continued to smoke, women who stopped smoking during pregnancy were less likely to have second hand tobacco exposure, and more likely to binge drink during pregnancy, to be nulliparous, and to have higher education and income. In addition, these women smoked less before pregnancy (data not shown). Previous studies have also shown that women who quit smoking during pregnancy tend to have smoked less before pregnancy (<20 cigarettes/day), and are more likely have higher education and income (Hakansson et al., 1999; Solomon and Quinn, 2004). For each of the attributes associated with smoking cessation during pregnancy, we evaluated whether they modified the association of in utero smoking with fetal loss. None accounted for the effect modification. However, after excluding women who smoked 10+ cigarettes/day before pregnancy, the effect of modification observed by a woman's own smoking status during pregnancy was no longer there (data not shown). Perhaps the finding was due to an abrupt, large change in exposure to nicotine, or to an unmeasured factor correlated with smoking cessation in pregnancy.
To date, no previous results on in utero tobacco smoke and women's risk of fetal loss later in life have been reported. Women who smoke during pregnancy tend to continue smoking after the child is born (Weinberg et al., 1989; Tong et al., 2009), thus studies of childhood exposure to tobacco smoke might be expected to give results somewhat comparable to ours. Two studies, however, evaluated the association between childhood exposure to parental tobacco smoke and the risk of fetal loss. Childhood exposure to tobacco smoke from both parents was associated with an increased risk of spontaneous miscarriage [adjusted odds ratio (OR) = 1.75; 95% CI, 1.01–3.04] among women who conceived after the use of ART. This association, however, was not observed when only the mother was a smoker (adjusted OR = 0.98) (Meeker et al., 2007). Childhood exposure to tobacco smoke was not associated with increased risk of fetal deaths (miscarriages and stillbirths) in a retrospective study among women who visited a cancer hospital (adjusted OR = 1.07; 95% CI, 0.87–1.32) (Peppone et al., 2009). The results were similar to those we observed.
Most miscarriages occur early in pregnancy, by week 12 of GA (Wilcox et al., 1988; Goldhaber and Fireman, 1991) and a limitation of the present study is that we could include only late miscarriages. Subsequently, the power to evaluate the association of in utero smoking exposure with this outcome was limited. Additionally, as recently noted, women with previous stillbirths were underrepresented in the MoBa cohort (Nilsen et al., 2009), which may have lead us to an underestimation of the HRs for this outcome.
Smoking while pregnant increases fetal loss (CDC, 2002), thus subjects who were susceptible to smoking's adverse effects may not have survived to participate in the present study. The study population may have been resilient and thus shows no effect of in utero exposure. A possible exception may have been the subset exposed in utero who stopped smoking when pregnant. Some aspects of susceptibility such as threatened abortion may have triggered the cessation of smoking.
The intensity of in utero exposure to tobacco smoking was not ascertained in MoBa; therefore we could not evaluate a dose–response relationship. If women who experienced very intense exposure to maternal tobacco smoking in utero were underrepresented in our study population, we may have underestimated the HRs for late miscarriages and stillbirths. Another limitation was our inability to discriminate between in utero tobacco smoke and childhood exposure; as noted above it is likely that women whose mothers smoked while pregnant with them (i.e. exposed in utero) also experienced continuous exposure during childhood. Information on childhood exposure to tobacco smoke from both parents as well as in utero exposure to SHTS (e.g. from father) was not asked in MoBa. Thus, residual confounding by childhood exposure to tobacco smoke from parents cannot be ruled out. In addition, a considerable number of women who did not know their exposure status were excluded from the present analysis; the effect of this exclusion in our estimated HRs could be either an under- or overestimation. However, the percentage of women who did not know their exposure to tobacco smoking in utero did not vary among cases and non-cases; it was also comparable between those with a previous spontaneous miscarriage or stillbirth and those without a previous adverse outcome.
Random errors in the classification of the exposure might have occurred, causing an underestimation of the HRs. Previous studies have shown however that the reported exposure to maternal tobacco smoke in utero by the adult offspring is a valid and reliable measure (Simard et al., 2008; Cupul-Uicab et al., 2010). In addition, we were able to show evidence supporting the validity of the reported in utero exposure to tobacco smoke based on the birthweight of a subset of the MoBa participants. Maternal smoking during pregnancy has been associated with an average reduction of 149 g in birthweight (Kramer, 1987), and in the present study we observed a 202 g average reduction.
The validity of in utero exposure to tobacco smoke is also supported by data on the prevalence of smoking in Norway during the calendar years when the MoBa participants were born (Table II). Reproductive aged women had a higher prevalence of smoking compared with the mothers of the women in MoBa, which has two explanations. First, women enrolled in MoBa smoked less than the general population (Nilsen et al., 2009) and the same pattern may have held for their mothers. Second, pregnant women tend to quit smoking (in 1987, 16% of women in Norway who smoked daily stopped smoking before their first prenatal check-up (Haug et al., 1992)). After pregnancy the prevalence of smoking increases but not to pre-pregnancy levels (see last column in Table II) (Haug et al., 1998; Solomon and Quinn, 2004).
Information on important covariates was recorded early in pregnancy; usually women reported their in utero exposure to tobacco smoke before knowing their pregnancy outcome, therefore a serious recall bias is unlikely in the present study. The outcomes of interest were obtained from the MBR, which contains well-documented information on all deliveries in Norway since 1967 (Irgens, 2000). Therefore, the studied outcomes were not subject to recall bias.
Although women who participated in MoBa are not a representative sample of all pregnant women from Norway during the period of interest, previous analyses suggest that bias in the estimated parameters due to self-selection is negligible when evaluating exposure-outcome associations in the cohort (Nilsen et al., 2009).
In this large study of pregnant women from Norway, exposure to maternal tobacco smoke in utero was not associated with an increased risk of fetal losses in the daughters later in life. Our results, however, were suggestive of a slight increased risk of miscarriage in women who experience in utero tobacco smoke from their mothers, but our statistical power was limited for this outcome.
Author's roles
L.A.C.U. conducted the literature review, analyzed and interpreted the data, drafted and revised the paper. D.D.B. helped with the study design and gave critical comments on the paper. R.S. and K.H. participated in the planning and conduct of the Norwegian Mother and Child Cohort Study, helped with the study design and gave critical comments on the paper. P.S.C. assisted with the data analysis and gave critical comments on the paper. M.P.L. conceived the hypothesis, helped with the study design, supervised the analysis and interpretation of the data and supervised the drafting of the paper. All authors read and approved the final version of the paper.
Conflict of interest: none declared.
Funding
This study was supported, in part, by the Intramural Research Program, the National Institute of Environmental Health Sciences (NIEHS) and the National Institutes of Health (NIH). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, contract ES044008 with the National Institute of Environmental Health Sciences, NIH/NIEHS (grant no N01-ES-85433), NIH/NINDS (grant no.1 UO1 NS 047537-01) and the Norwegian Research Council/FUGE (grant no. 151918/S10).
References
- ACOG. ACOG Practice Bulletin No. 102: management of stillbirth. Obstet Gynecol. 2009;3:748–761. doi: 10.1097/AOG.0b013e31819e9ee2. [DOI] [PubMed] [Google Scholar]
- CDC. Women and smoking: a report of the Surgeon General. Executive summary. MMWR Recomm Rep. 2002;RR-12:1–13. : [PubMed] [Google Scholar]
- Cupul-Uicab LA, Ye X, Skjaerven R, Haug K, Longnecker MP. Reproducibility of reported in utero exposure to tobacco smoke. Ann Epidemiol. 2010 doi: 10.1016/j.annepidem.2010.10.008. . doi: 10.1016/j.annepidem.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts CC, Bots ML, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in young adult offspring: is early life exposure critical? The atherosclerosis risk in young adults study. Arterioscler Thromb Vasc Biol. 2008;12:2296–2302. doi: 10.1161/ATVBAHA.108.173229. doi:10.1161/ATVBAHA.108.173229. [DOI] [PubMed] [Google Scholar]
- Goldhaber MK, Fireman BH. The fetal life table revisited: spontaneous abortion rates in three Kaiser Permanente cohorts. Epidemiology. 1991;1:33–39. doi:10.1097/00001648-199101000-00006. [PubMed] [Google Scholar]
- Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;3:340–349. doi: 10.2105/ajph.79.3.340. doi:10.2105/AJPH.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez RG. Parametric frailty and shared frailty survival models. The Stata Journal. 2002;1:22–44. [Google Scholar]
- Hakansson A, Lendahls L, Petersson C. Which women stop smoking? A population-based study of 403 pregnant smokers. Acta Obstet Gynecol Scand. 1999;3:217–224. [PubMed] [Google Scholar]
- Haug K, Aaro LE, Fugelli P. Smoking habits in early pregnancy and attitudes towards smoking cessation among pregnant women and their partners. Fam Pract. 1992;4:494–499. doi: 10.1093/fampra/9.4.494. doi:10.1093/fampra/9.4.494. [DOI] [PubMed] [Google Scholar]
- Haug K, Irgens LM, Baste V, Markestad T, Skjaerven R, Schreuder P. Secular trends in breastfeeding and parental smoking. Acta Paediatr. 1998;10:1023–1027. doi: 10.1080/080352598750031310. doi:10.1080/080352598750031310. [DOI] [PubMed] [Google Scholar]
- Hogberg L, Cnattingius S. The influence of maternal smoking habits on the risk of subsequent stillbirth: is there a causal relation? BJOG. 2007;6:699–704. doi: 10.1111/j.1471-0528.2007.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliadou AN, Koupil I, Villamor E, Altman D, Hultman C, Langstrom N, Cnattingius S. Familial factors confound the association between maternal smoking during pregnancy and young adult offspring overweight. Int J Epidemiol. 2010;5:1193–1202. doi: 10.1093/ije/dyq064. [DOI] [PubMed] [Google Scholar]
- Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;6:435–439. [PubMed] [Google Scholar]
- Jaddoe VW, de Ridder MA, van den Elzen AP, Hofman A, Uiterwaal CS, Witteman JC. Maternal smoking in pregnancy is associated with cholesterol development in the offspring: a 27-years follow-up study. Atherosclerosis. 2008;1:42–48. doi: 10.1016/j.atherosclerosis.2007.01.032. doi:10.1016/j.atherosclerosis.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Joffe M, Scheike T, Skytthe A, Gaist D, Petersen I, Christensen K. Early exposure to smoking and future fecundity among Danish twins. Int J Androl. 2006;6:603–613. doi: 10.1111/j.1365-2605.2006.00701.x. doi:10.1111/j.1365-2605.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;5:663–737. [PMC free article] [PubMed] [Google Scholar]
- Lambe M, Hultman C, Torrang A, Maccabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;5:524–530. doi: 10.1097/01.ede.0000231561.49208.be. doi:10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;408:1074–1078. doi:10.2307/2290085. [Google Scholar]
- Lindblad F, Hjern A. ADHD after fetal exposure to maternal smoking. Nicotine Tob Res. 2010;4:408–415. doi: 10.1093/ntr/ntq017. doi:10.1093/ntr/ntq017. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;6:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. doi:10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Lundberg F, Cnattingius S, D'Onofrio B, Altman D, Lambe M, Hultman C, Iliadou A. Maternal smoking during pregnancy and intellectual performance in young adult Swedish male offspring. Paediatr Perinat Epidemiol. 2009;1:79–87. doi: 10.1111/j.1365-3016.2009.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterodt MC, Sorensen KP, Larsen KB, Skouby SO, Andersen CY, Byskov AG. The number of oogonia and somatic cells in the human female embryo and fetus in relation to whether or not exposed to maternal cigarette smoking. Hum Reprod. 2009;10:2558–2566. doi: 10.1093/humrep/dep226. doi:10.1093/humrep/dep226. [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;5:1146–1150. doi: 10.1093/ije/dyl170. doi:10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Missmer SA, Vitonis AF, Cramer DW, Hauser R. Risk of spontaneous abortion in women with childhood exposure to parental cigarette smoke. Am J Epidemiol. 2007;5:571–575. doi: 10.1093/aje/kwm128. doi:10.1093/aje/kwm128. [DOI] [PubMed] [Google Scholar]
- Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. BMJ. 2002;7328:26–27. doi: 10.1136/bmj.324.7328.26. doi:10.1136/bmj.324.7328.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;6:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. doi:10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond) 2008;2:201–210. doi: 10.1038/sj.ijo.0803760. doi:10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppone LJ, Piazza KM, Mahoney MC, Morrow GR, Mustian KM, Palesh OG, Hyland A. Associations between adult and childhood secondhand smoke exposures and fecundity and fetal loss among women who visited a cancer hospital. Tob Control. 2009;2:115–120. doi: 10.1136/tc.2008.027961. doi:10.1136/tc.2008.027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronneberg A, Lund KE, Hafstad A. Lifetime smoking habits among Norwegian men and women born between 1890 and 1974. Int J Epidemiol. 1994;2:267–276. doi: 10.1093/ije/23.2.267. doi:10.1093/ije/23.2.267. [DOI] [PubMed] [Google Scholar]
- Simard JF, Rosner BA, Michels KB. Exposure to cigarette smoke in utero: comparison of reports from mother and daughter. Epidemiology. 2008;4:628–633. doi: 10.1097/EDE.0b013e3181761cbd. doi:10.1097/EDE.0b013e3181761cbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L, Quinn V. Spontaneous quitting: self-initiated smoking cessation in early pregnancy. Nicotine Tob Res. 2004:S203–216. doi: 10.1080/14622200410001669132. doi:10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- Strohsnitter WC, Hatch EE, Hyer M, Troisi R, Kaufman RH, Robboy SJ, Palmer JR, Titus-Ernstoff L, Anderson D, Hoover RN, et al. The association between in utero cigarette smoke exposure and age at menopause. Am J Epidemiol. 2008;6:727–733. doi: 10.1093/aje/kwm351. [DOI] [PubMed] [Google Scholar]
- Thomas C, Hypponen E, Power C. Prenatal exposures and glucose metabolism in adulthood: are effects mediated through birth weight and adiposity? Diabetes Care. 2007;4:918–924. doi: 10.2337/dc06-1881. doi:10.2337/dc06-1881. [DOI] [PubMed] [Google Scholar]
- Tong VT, Jones JR, Dietz PM, D'Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;4:1–29. [PubMed] [Google Scholar]
- USDHHS. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA, U.S: Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol. 1993;1:1–8. doi: 10.1093/oxfordjournals.aje.a116591. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;5::1072–1078. doi: 10.1093/oxfordjournals.aje.a115211. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;4:189–194. doi: 10.1056/NEJM198807283190401. doi:10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Wisborg K, Ingerslev HJ, Henriksen TB. IVF and stillbirth: a prospective follow-up study. Hum Reprod. 2010;5:1312–1316. doi: 10.1093/humrep/deq023. [DOI] [PubMed] [Google Scholar]
- Ye X, Skjaerven R, Basso O, Baird DD, Eggesbo M, Cupul-Uicab LA, Haug K, Longnecker MP. In utero exposure to tobacco smoke and subsequent reduced fertility in females. Hum Reprod. 2010;11:2901–2906. doi: 10.1093/humrep/deq235. doi: 10.1093/humrep/deq1235. [DOI] [PMC free article] [PubMed] [Google Scholar]