Unique adhesion-mediated FAK- and paxillin-dependent signaling events that control macrophage motility.
Keywords: integrin, invasion, polarization, elongation
Abstract
Macrophages function as key inflammatory mediators at sites of infection and tissue damage. Integrin and growth factor receptors facilitate recruitment of monocytes/macrophages to sites of inflammation in response to numerous extracellular stimuli. We have shown recently that FAK plays a role in regulating macrophage chemotaxis and invasion. As FAK is an established downstream mediator of integrin signaling, we sought to define the molecular circuitry involving FAK and the predominant β1 integrin heterodimers expressed in these cells—α4β1 and α5β1. We show that α4β1 and α5β1 integrins are required for efficient haptotactic and chemotactic invasion and that stimulation of these integrin receptors leads to the adoption of distinct morphologies associated with motility. FAK is required downstream of α5β1 for haptotaxis toward FN and chemotaxis toward M-CSF-1 and downstream of α4β1 for the adoption of a polarized phenotype. The scaffolding molecule paxillin functions independently of FAK to promote chemotaxis downstream of α4β1. These studies expand our understanding of β1 integrin signaling networks that regulate motility and invasion in macrophages and thus, provide important new insights into mechanisms by which macrophages perform their diverse functions.
Introduction
Macrophages are key mediators of innate immunity that can also contribute to a number of disease processes, including chronic inflammatory diseases such as Crohn's disease and cancer [1–4]. To fulfill these diverse functions, macrophages are recruited to sites of infection or injury through interactions between growth factor or chemokine receptors and their soluble ligands [5–7]. Adhesion signaling via integrin receptors and the surrounding ECM also plays an essential role in this process [8].
Upon integrin engagement, signaling is initiated through FAK and Src family kinases, as well as scaffolding molecules such as talin and paxillin [9]. The critical role played by these molecules is evident from defects attributed to their loss in genetic knockout cells and animals. For example, combined knockout of the three Src family kinases expressed in macrophages—Hck, Fgr, and Lyn—causes significant defects in macrophage spreading on FN and monocyte recruitment to sites of inflammation [10]. Loss of the FAK family member Pyk2 results in a failure of macrophages to polarize properly and migrate in response to chemokine stimulation [11]. Our group has shown that like Pyk2, loss of FAK expression in macrophages results in reduced chemotaxis and recruitment to sites of inflammation [12].
The Rho family GTPases link signals emanating from integrins, PTKs, and associated adaptor molecules to the actin cytoskeleton [13]. In macrophages, signaling through Rac½ is essential for cell migration. Knockout of these proteins results in motility defects [14], as does a combined loss of all three Vav isoforms, which are GEFs for Rac [15].
The ability of macrophages to reach sites of infection and tissue damage is essential to their function. Defining the molecular circuits through which integrins regulate macrophage motility is therefore important for gaining a better understanding of macrophage function. As macrophages express α4β1 and α5β1 integrins, we hypothesized that signaling through one or both of these receptors would promote FAK-dependent motility [16]. Using a myeloid-specific, conditional FAK knockout mouse model, we find that paxillin and FAK work in separate pathways in BMMs to control CSF-1-dependent invasion; FAK functions downstream of α5β1, and paxillin is downstream of α4β1. In addition, we show that stimulation of α5β1 and α4β1 leads to distinct, FAK-dependent changes in morphologies associated with motility. These data elucidate novel integrin signaling pathways, which together, control macrophage motility.
MATERIALS AND METHODS
Generation of myeloid-specific conditional FAK knockout mice
Conditional FAK knockout mice and control littermates were generated as described previously [12].
Antibodies and reagents
Immunoblot analyses were performed using the following antibodies: polyclonal FAK C-20 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), monoclonal paxillin (BD Biosciences, San Jose, CA, USA), and polyclonal ERK½ (Cell Signaling, Danvers, MA, USA). The following antibodies were used for integrin-blocking studies: monoclonal α4 integrin (Chemicon International, Billerica, MA, USA), α5 integrin (BD PharMingen, San Diego, CA, USA), and rat IgG isotype control (BD PharMingen). Murine M-CSF was purchased from PeproTech (Rocky Hill, NJ, USA). Soluble FN was purchased from Sigma Chemical Co. (St. Louis, MO, USA).
GST fragments
Bacterial expression vectors for GST FN CS-1 and GST FN 9–11 were kindly provided by Dr. David D. Schlaepfer (Moores UCSD Cancer Center, La Jolla, CA, USA).
Isolation of BMMs
BMMs were isolated from 6- to 10-week-old mice as described previously [12].
siRNA and transfection
siGenome Smartpool murine siPaxillin was purchased from Dharmacon (Lafayette, CO, USA). A nontargeting siControl oligonucleotide was purchased from Ambion (Austin, TX, USA). Cells were transfected using the Amaxa nucleofector and corresponding mouse macrophage nucleofector kit, as per the manufacturer's instructions (Lonza, Walkersville, MD, USA). For optimal protein knockdown, cells were transfected for 72 h using 300 nmol siRNA oligonucleotides. Knockdown of targeted proteins was assessed via immunoblot.
Invasion assays
Biocoat invasion chambers (24-well 8.0 μm growth factor-reduced matrigel matrix; BD Biosciences) were preincubated in CSF-1-free α-minimum essential medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% FBS (Invitrogen) for 2 h. Prior to cell inoculation, the media in the bottom chamber were replaced with media containing 20 μg/mL soluble FN (see Fig. 1) or 120 ng/mL CSF-1 (see Figs. 3 and 5 and Supplemental Fig. 1). BMMs were starved overnight for CSF-1 and plated (50,000 cells/well) into the top chamber of duplicate or triplicate wells. For blocking experiments using GST fusion proteins, CSF-1-starved cells were preincubated in suspension with 10 μg/mL GST fusion proteins for 30 min prior to plating in the top of invasion chambers in CSF-1-free media. For blocking experiments using antibodies, cells were suspended with 10 μg/mL blocking antibodies and plated immediately in invasion chambers. Antibodies (10 μg/mL) were also added to the bottom chamber for the duration of the experiment. For Src inhibition, cells were preincubated in CSF-1-free media containing ethanol (vehicle) or 10 μM SU6656 (Sigma Chemical Co.) for 30 min prior to inoculation into the invasion chamber. SU6656 was also added to the bottom chamber for the duration of the experiment. Following incubation for 24 h at 37°C, nonmigratory cells were removed from the topside of the invasion chamber membranes with cotton swabs. The undersides were then fixed, stained with Diff-Quik staining solutions (Fisher Scientific, Pittsburgh, PA, USA), mounted onto coverslips, and examined by microscopy to determine the total number of invading cells present on the entire membrane.
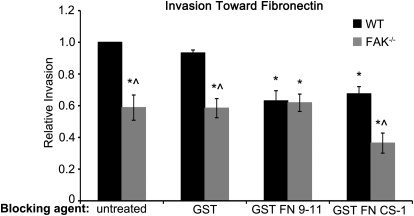
Figure 1. α5β1 and α4β1 control distinct haptotactic invasion pathways in macrophages.
Invasion of WT and FAK−/− BMMs toward FN was measured in matrigel-coated, modified Boyden chambers following pretreatment with the indicated GST proteins. Bars represent the average number of invaded cells divided by the number of WT BMMs that migrated under control (untreated) conditions. *Values significantly different from WT GST treatment conditions; ∧values significantly different from WT cells under the same treatment conditions; n = 8.
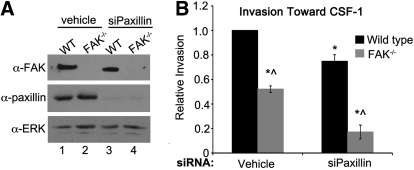
Figure 3. FAK and paxillin regulate CSF-1-dependent macrophage invasion through separate signaling pathways.
(A) Immunoblot analysis showing paxillin knockdown under control (Lanes 1 and 2) and siPaxillin-treated conditions (lanes 3 and 4) in WT and FAK−/− BMMs. (B) Relative invasion toward CSF-1 of WT and FAK−/− BMMs treated with vehicle or siRNAs targeting paxillin. *Values that are significantly different from vehicle-treated WT cells; ^values that are significantly different from WT cells under the same treatment conditions; n = 6.
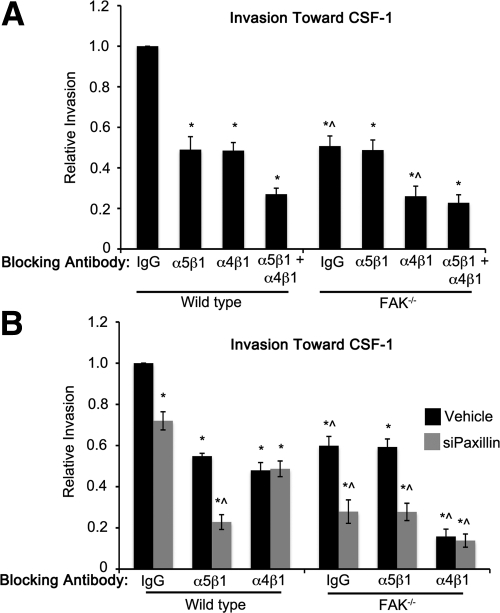
Figure 5. Chemotaxis of macrophages toward CSF-1 requires separate α5β1/FAK and α4β1/paxillin signaling pathways.
(A) Invasion of WT and FAK−/− BMMs toward CSF-1 was measured in the presence of the indicated function-blocking antibodies. Bars represent the average number of migrated cells divided by the number of WT BMMs that migrated under control (IgG-treated) conditions. (B) Invasion was measured as above in control (Vehicle, black bars) or siPaxillin-treated (gray bars) WT and FAK−/− BMMs. *Values significantly different from WT IgG control-treated cells; ^values significantly different from WT cells under the same conditions; n = 3–8.
Immunoblotting
Cells were rinsed in PBS and lysed in modified radioimmunoprecipitation assay buffer (50 mM Tris, 150 mM NaCl, 1% Igepal CA-630, and 0.5% deoxycholate) containing protease and phosphate inhibitors (100 μM leupeptin, 1 mM PMSF, 0.15 U/ml aprotinin, and 1 mM vanadate) as described previously [17]. Protein concentrations were determined using a bicinchoninic acid assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Immunoblotting was performed as described previously [12].
Polarization and elongation studies
For CSF-1 stimulation experiments, BMMs transfected with siRNA reagents for 48 h were plated on glass coverslips overnight in CSF-1-free media. Cells were then treated for 6 h with 120 ng/mL CSF-1 and fixed in 3% paraformaldehyde for 20 min at room temperature. For GST-FN fragment stimulation experiments, CSF-1-starved BMMs were plated overnight onto glass coverslips. The following day, cells were stimulated with GST, GST FN 9–11, or GST FN CS-1 for 5 min in CSF-1-free media and fixed as described above. Cells were then permeabilized in 0.4% Triton X-100, blocked in PBS containing 10% BSA, and incubated with Texas Red-conjugated phalloidin (Invitrogen), diluted in PBS containing 2% BSA for 1 h at room temperature. Coverslips were mounted in Prolong Gold with DAPI (Invitrogen). Cells were viewed using a Nikon Eclipse E800 fluorescence microscope and images captured with a charged-coupled device camera using Openlab software (Perkin Elmer, Waltham, MA, USA). Cells were designated as having a polarized phenotype if they exhibited a defined leading edge, coincident with the accumulation of F-actin, and a distinct lagging edge. Percent polarization was determined from 10 to 12 random fields/coverslip. Cellular elongation was determined by dividing the cell length by the cell width (elongation factor) using ImageJ software (National Institutes of Health, Bethesda, MD, USA). An elongation factor of 1 is indicative of a completely round cell; a value >1 represents an elongated cell.
Statistical methods
A two-sample t test, assuming unequal variance, was performed to determine statistical significance between conditions with a confidence level of ≥95%.
Online Supplemental material
Supplemental Fig. 1 depicts the relative invasion toward CSF-1 of WT and FAK−/− BMMs treated with vehicle or SU6656 to inhibit Src kinase activity. The data are representative of four independent experiments.
RESULTS AND DISCUSSION
α5β1 Integrins regulate macrophage haptotactic invasion via a FAK/Pyk2-dependent pathway that is distinct from α4β1 signaling
Previous work by our group [12] has established FAK as a key regulator of macrophage motility. FAK−/− BMMs isolated from myeloid-specific, conditional FAK knockout mice exhibit reduced chemotaxis toward CSF-1 compared with BMMs isolated from control mice (WT), and this coincides with a delay in recruitment of FAK−/− BMMs to sites of inflammation in vivo. In contrast to fibroblasts and endothelial cells, in which loss of FAK is accompanied by enhanced expression of Pyk2 [18, 19], Pyk2 levels are not altered in FAK−/− BMMs [12]. Despite its continued expression, however, the motility defects exhibited by FAK−/− macrophages show that endogenous Pyk2 is unable to fully compensate for the absence of FAK. Interestingly, although we reported that loss of Pyk2 in WT BMMs resulted in decreased invasion toward CSF-1, coordinated loss of FAK and Pyk2 had no greater impact on cell invasion than did loss of either molecule alone [12]. Moreover, in all cases, cell invasion was reduced by ∼50%. These data suggest that FAK and Pyk2 function within a single pathway to regulate macrophage motility, and a second pathway that is dependent on molecules other than FAK and Pyk2 also contributes to this process.
Macrophages express two β1 integrins that have been shown in fibroblasts and neuroblastoma cells to regulate cell motility—α4β1 and α5β1 [18, 20]. To determine which of these integrins is coupled to the FAK/Pyk2-dependent pathway, we took advantage of rGST-tagged FN fragments that bind exclusively to only α4β1 (GST FN CS-1) or α5β1 (GST FN 9–11) [18, 21]. When these proteins are preincubated with BMMs in suspension, they block signaling through the integrin, to which they bind and effectively funnel adhesion signals through the other β1 integrin [21]. WT and FAK−/− BMMs were preincubated with GST, GST FN 9–11, or GST FN CS-1 in suspension and then seeded in matrigel-coated, modified Boyden chambers to measure haptotactic invasion toward FN. Loss of FAK resulted in decreased (∼40%) invasion toward FN compared with WT BMMs under control conditions (Fig. 1; untreated and GST). Inhibition of α5β1 by GST FN 9–11 resulted in a similar 40% decrease in invasion of WT BMMs. However, it had no additional, inhibitory effect on haptotaxis by FAK−/− BMMs beyond that caused by loss of FAK alone. This result is consistent with FAK functioning downstream of α5β1 integrins to promote macrophage invasion. Blockade of α4β1 signaling by pretreatment with GST FN CS-1 had a similar inhibitory effect on WT BMMs, as did GST FN 9–11, indicating that α4β1 as well as α5β1 integrins contribute to this process. However, blockade of α4β1 by GST FN CS-1 resulted in an additional reduction in haptotaxis of FAK−/− BMMs above that caused by loss of FAK alone. Taken together, these data suggest that α5β1 and α4β1 control separate haptotactic invasion pathways in macrophages, one that uses FAK (α5β1) and a second that requires other factors independently of, or in addition to, FAK (α4β1).
FAK is required to induce cellular elongation in α5β1-stimulated macrophages and cell polarization in α4β1-stimulated cells
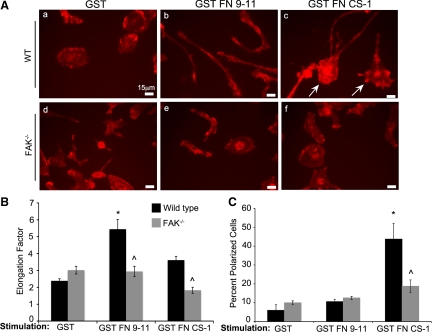
An essential element of cell migration and invasion is the ability to adopt a polarized phenotype with morphologically distinct leading and lagging edges. To determine the contributions of α5β1/FAK and/or α4β1 integrin signaling to these processes, we took advantage of the finding that the GST-tagged, recombinant FN fragments described above induce features of polarization when allowed to bind to their cognate receptors on adherent, CSF-1-starved BMMs. WT and FAK−/− BMMs were thus plated on FN-coated coverslips, starved overnight for CSF-1, and then stimulated with GST, GST FN 9–11, or GST FN CS-1 for 5 min in the continued absence of CSF-1. WT and FAK−/− cells treated with GST appeared rounded with no evidence of polarization (Fig. 2A , a and d), a morphology typical of CSF-1-starved cells (see Fig. 4A, a and d). Engagement of α5β1 integrins on WT BMMs with GST FN 9–11 resulted in a dramatic change in morphology, marked by an elongated phenotype with no clear distinction between front and rear ends (Fig. 2A, compare a and b, and B). This phenotype was FAK-dependent, as FAK−/− BMMs failed to elongate in response to α5β1 integrin stimulation (Fig. 2A, compare b and e, and B). A similar cell elongation phenotype has been reported for Vav1/2/3 and Rac½ knockout macrophages, suggesting that cellular elongation may be a consequence of decreased Rac activation [14, 15]. This would suggest that FAK activation downstream of α5β1 integrins may negatively regulate Rac in macrophages. Consistent with this hypothesis, we have observed elevated, protrusive activity coincident with increased basal Rac activity in FAK−/− BMMs compared with WT cells [12].
Figure 2. Stimulation of α4β1 and α5β1 integrins leads to FAK-dependent changes in macrophage morphology.
(A) Representative fields of CSF-1-starved WT and FAK−/− BMMs following 5 min stimulation with the indicated GST proteins. Cells were fixed and stained for phalloidin. Cell elongation (B) and polarization (C) were determined as described in Materials and Methods. *Values significantly different from WT cells treated with GST; ^values significantly different from WT cells under the same treatment conditions; n = 3–4.
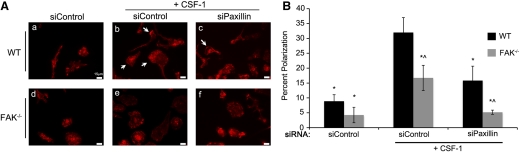
Figure 4. FAK and paxillin regulate CSF-1-dependent macrophage polarization through separate signaling pathways.
(A) Representative fields of CSF-1-starved WT and FAK−/− BMMs treated with siControl or siPaxillin and stained with phalloidin. (b, c, e, and f). Cells were stimulated with CSF-1 for 6 h. Arrows indicate areas of actin accumulation at the front of polarized cells. (B) Quantification of cell polarization. *Values that are significantly different from WT siControl-treated BMMs stimulated with CSF-1; ^values that are significantly different from WT cells under the same treatment conditions; n = 7.
In contrast to the elongated phenotype induced by α5β1 ligation, engagement of α4β1 integrin receptors by GST FN CS-1 induced a polarized phenotype in WT BMMs, characterized by morphologically distinct, actin-rich lamellipodia at the leading edge and clearly discernable, lagging-edge tails (Fig. 2A, c, and C). In the absence of FAK, cells failed to adopt this polarized phenotype in response to α4β1 integrin ligation (Fig. 2A, compare c and f, and C). The inability of FAK−/− BMMs to develop clearly defined leading and lagging edges under these conditions indicates that FAK works together with α4β1 to control BMM polarization.
Paxillin controls macrophage motility in a pathway that is separate from FAK
The asymmetric localization of active Rac in polarized endothelial cells, Jurkat T cells, and CHO cells expressing α4β1 has been attributed in part to α4β1/paxillin signaling [1, 22–24]. At the front end of the cell, paxillin is unable to interact with the cytoplasmic tail of α4, rendering it free to recruit the Rac GEF p21-activated kinase-interacting exchange factor [1, 22]. In contrast, paxillin and α4 readily associate with one another at the rear end of the cell, resulting in lower Rac activity as a result of recruitment of the Arf-GTPase-activating protein, GPCR kinase interactor 1, and inhibition of Arf6, which is thought to promote Rac activity when active [22]. As engagement of α4β1 resulted in polarization of WT BMMs, we hypothesized that α4β1 integrins and paxillin might function within a single pathway to control BMM migration. The role of FAK in this pathway was also of strong interest, as FAK was required for α4β1-dependent cell polarization (Fig. 2) and appeared to function in parallel with α4β1 in controlling haptotaxis toward FN (Fig. 1). To address these questions, paxillin was depleted from WT and FAK−/− BMMs by RNA interference, and the cells were then seeded in modified Boyden chambers coated with matrigel. In this case, CSF-1 rather than FN was added to the bottom of the chamber to measure the functional relationship between FAK and paxillin in controlling macrophage chemotaxis. Treatment of WT and FAK−/− BMMs with siPaxillin resulted in a nearly complete knockdown of paxillin (Fig. 3A, lanes 3 and 4). Invasion of the cells toward CSF-1 was impaired by ∼50% in the absence of FAK, as has been reported previously (Fig. 3B, first gray bar; [12]). Paxillin knockdown in WT BMMs resulted in a modest (25%) but significant decrease in invasion, indicating that paxillin indeed played a role in regulating invasion toward CSF-1. The combined loss of FAK and paxillin caused a much more significant reduction in invasion than did loss of either molecule alone (∼80%, compared with 50% and 25%, respectively), suggesting that FAK and paxillin contribute to CSF-1-dependent macrophage motility through separate pathways. This is supported further by the fact that CSF-1-dependent phosphorylation of paxillin is not reduced significantly in FAK−/− cells (data not shown). Interestingly, treatment of WT and FAK−/− BMMs with the small molecule Src inhibitor SU6656 showed that like paxillin, Src family kinases also appear to function independently of FAK to control BMM invasion toward CSF-1 (Supplemental Fig. 1). This suggests that Src family kinases rather than FAK may couple with paxillin to promote macrophage motility, particularly as paxillin is a substrate of Src [25, 26].
These results prompted us to investigate whether FAK and paxillin also function in separate pathways to control cell polarity in response to CSF-1. WT and FAK−/− BMMs were depleted for paxillin, as described above, starved overnight for CSF-1, and scored for the adoption of a polarized phenotype following CSF-1 stimulation. In the absence of CSF-1, the majority of cells had no clearly discernable leading and lagging edges (Fig. 4A, a and d). Treatment of BMMs with CSF-1 resulted in a polarized phenotype in ∼30% of WT cells (Fig. 4A, b, and B), similar to what was seen upon α4β1 engagement (Fig. 2). In the absence of FAK, this was only observed in ∼15% of the population (Fig. 4A, e, and B). Thus, FAK is not only important for BMM polarity in response to α4β1 integrin ligation but also following activation of the CSF-1R. Interestingly, BMMs lacking paxillin exhibited a similar reduction in the percentage of cells adopting the polarized phenotype as did FAK−/− cells (Fig. 4A, c), demonstrating that paxillin also plays a role in this process. BMMs lacking FAK and paxillin failed to demonstrate polarity in response to CSF-1 above the baseline levels seen in the absence of CSF-1 (Fig. 4A, f, and B). Thus, as was the case for chemotaxis, FAK and paxillin appear to contribute to CSF-1-induced cell polarization through separate pathways.
Having established the requirement for FAK and paxillin in CSF-1-dependent chemotaxis and polarity, we next used function-blocking antibodies to determine whether FAK and paxillin couple with α5β1 and α4β1 to control CSF-1 responses through similar pathways to those regulating haptotaxis toward FN. Chemotaxis of WT BMMs toward CSF-1 was reduced by ∼50% in the presence of α5β1 or α4β1 function-blocking antibodies, and treatment with both antibodies together resulted in a significantly greater inhibition (∼70% reduction; Fig. 5A). Consistent with FAK and α5β1 functioning in the same arm of the pathway, blockade of α5β1 in FAK−/− BMMs had no additional, deleterious effect on chemotaxis beyond the 45–50% reduction caused by the loss of FAK. In contrast, treatment of these cells with α4β1 function-blocking antibodies, alone or in a combination with the α5β1 antibodies, caused a further reduction in chemotaxis, similar to the level seen in WT cells undergoing combined α5β1 and α4β1 blockade. Together with the haptotactic data shown in Fig. 1, these data suggest that FAK α5β1 and α4β1 control two distinct arms of a generalized migration/invasion pathway.
Like α4β1, paxillin appeared to regulate macrophage chemotaxis through a pathway separate from FAK. We reasoned that if α4β1 and paxillin functioned within a single pathway to control these processes, then the decreased chemotaxis exhibited in the absence of paxillin (see Fig. 3) should be reduced further in WT cells by blockade of α5β1 but not α4β1 integrins. This was in fact found to be the case (Fig. 5B; WT grouping, gray bars). However, in FAK−/− cells, where the α5β1/FAK arm of the pathway is already inhibited, α5β1 blockade, in combination with paxillin knockdown, should have no more inhibitory effect on chemotaxis than does loss of paxillin alone. Again, this was exactly what was observed (FAK−/− grouping, gray bars). Furthermore, although inhibition of α4β1 reduced chemotaxis of FAK−/− cells significantly, as it did in Fig. 5A, the coincident loss of paxillin in these cells had no additional effect (Fig. 5B; compare black and gray bars, FAK−/− grouping). As was the case for haptotaxis, these data thus support the existence of two separate pathways controlling chemotaxis of BMMs toward CSF-1, one using α5β1/FAK and the second using α4β1/paxillin (Fig. 6). We suggest that these are the predominant pathways regulating CSF-1 chemotaxis in macrophages, as chemotaxis is inhibited by almost 90% under the most potent of conditions (loss of FAK, paxillin, and α4β1 function). However, additional FN-binding integrins, such as αvβ3, which are expressed on the surface of macrophages [27, 28], may also contribute independently of α5β1, α4β1, and/or the CSF-1R.
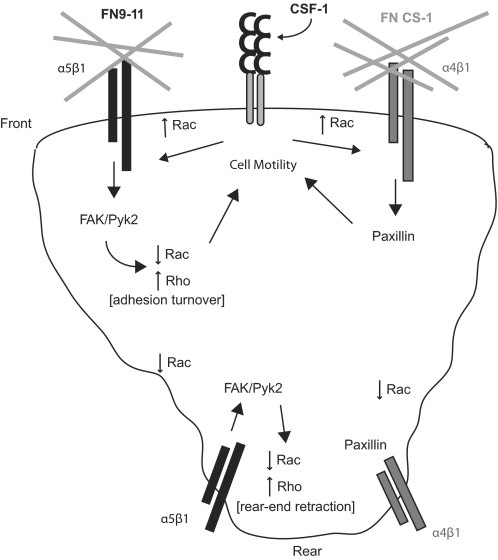
Figure 6. Regulation of macrophage polarity and motility.
We propose a model in which α5β1 couples with FAK and α4β1 with paxillin to regulate macrophage polarity and motility through modulation of Rac and Rho activity.
It would appear from our data that signaling through the α4β1 pathway induces cell polarization in macrophages through a process similar to that postulated for endothelial, CHO, and T cells [1, 22, 23]. We would argue that FAK is essential to this process, as FAK−/− BMMs failed to polarize upon engagement of α4β1 or treatment with CSF-1. We suggest that this may be a result of a failure of Rho to become activated at the rear of the cell, as FAK has been shown to promote Rho activation and tail-end retraction through direct interaction with RhoA GEFs [29, 30]. Interestingly, stimulation of α5β1 integrins in BMMs resulted in the adoption of an elongation phenotype similar to the phenotypes seen in Vav and Rac knockout macrophages [14, 15]. This suggests that α5β1 stimulation inhibits Rac activity in these cells. Moreover, as the FAK−/− cells failed to adopt this phenotype, FAK appears to be required for down-regulation of Rac under these conditions. This may be particularly relevant to the molecular dynamics at the rear of the cell, where a failure to activate Rho in the absence of FAK may lead to elevated Rac activity through a mechanism involving antagonism between Rac and Rho [31–33]. α4β1-Paxillin interactions may also contribute directly to lowering Rac levels in this region of the cell, as has been postulated for other cell types [1, 22, 23]. Whether FAK also contributes directly to the down-regulation of Rac in the rear of the cell remains to be determined.
α5β1/FAK signaling has been shown to be an important regulator of motility in MEFs, but unlike BMMs, paxillin appears to function downstream of α5β1 in these cells [18]. α4β1 is not normally expressed in MEFs, but it is able to promote cell migration upon forced expression. Under these conditions, FAK is not required for α4β1-mediated motility. FAK has also been shown to be required for α5β1- but not α4β1-mediated motility in late-stage neuroblastoma cells that endogenously express α5β1 and α4β1 integrins [20]. However, paxillin is not required for α4β1-dependent haptotaxis in these cells. Taken together, these data demonstrate a consistent role for α5β1/FAK coupling in the regulation of cell motility, irrespective of cell type. The contribution of FAK to α4β1-mediated motility is less clear; it is not required in MEFs and neuroblastoma cells but appears to be important for proper polarization in BMMs. The pathway(s) in which paxillin functions also appear to be cell-specific; in BMMs, paxillin is required downstream of α4β1, while paxillin is more closely linked to α5β1 in MEFs. In contrast, α4β1 and α5β1 seem to function independently of paxillin in advanced neuroblastoma cells.
It is perhaps not surprising that unique strategies are in place in macrophages to regulate motility, as these cells exhibit exquisite plasticity of shape and rapid migration in the course of performing their normal physiological functions [6, 34, 35]. As we gain more insight into the molecular regulation of these processes in macrophages, it may be possible to exploit differences between cell types to develop treatments with unique or preferential efficacy in macrophages.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R21 CA135532 and RO1 AI050733). We thank members of the laboratory and Drs. James E. Casanova, Kodi S. Ravichandran, Barry M. Gumbiner, Joanna B. Goldberg, Thomas J. Parsons, Jill K. Slack-Davis, and Miguel Vicente-Manzanares for their input and expertise.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- Arf
- ADP ribosylation factor
- BMM
- bone marrow-derived macrophage
- FN
- fibronectin
- GEF
- guanine nucleotide exchange factor
- GST FN 9–11
- GST fusion proteins encompassing the human alternatively spliced variable fibronectin type III repeats 9–11
- GST FN CS-1
- GST fusion protein encompassing the human alternatively spliced connecting segment-1 region of fibronectin
- MEF
- mouse embryonic fibroblast
- Pyk2
- proline-rich tyrosine kinase 2
- siControl
- nonspecific siRNA
- siPaxillin
- paxillin-targeted small interfering RNA
- siRNA
- small interfering RNA
AUTHORSHIP
M.Y.A. performed polarization studies, contributed to experimental design, and wrote the paper; K.S.T. performed invasion, elongation, and polarization assays and contributed to experimental design; K.A.O. contributed to invasion assays and developed the mouse model; A.H.B. contributed to experimental design and data analysis and wrote the paper.
REFERENCES
- 1. Kummer C., Ginsberg M. H. (2006) New approaches to blockade of α4-integrins, proven therapeutic targets in chronic inflammation. Biochem. Pharmacol. 72, 1460–1468 [DOI] [PubMed] [Google Scholar]
- 2. Rugtveit J., Brandtzaeg P., Halstensen T. S., Fausa O., Scott H. (1994) Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut 35, 669–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marshall D., Cameron J., Lightwood D., Lawson A. D. (2007) Blockade of colony stimulating factor-1 (CSF-I) leads to inhibition of DSS-induced colitis. Inflamm. Bowel Dis. 13, 219–224 [DOI] [PubMed] [Google Scholar]
- 4. Mantovani A., Sica A. (2010) Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. 22, 231–237 [DOI] [PubMed] [Google Scholar]
- 5. Webb S. E., Pollard J. W., Jones G. E. (1996) Direct observation and quantification of macrophage chemoattraction to the growth factor CSF-1. J. Cell Sci. 109, 793–803 [DOI] [PubMed] [Google Scholar]
- 6. Mantovani A., Sica A., Locati M. (2007) New vistas on macrophage differentiation and activation. Eur. J. Immunol. 37, 14–16 [DOI] [PubMed] [Google Scholar]
- 7. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 8. De Fougerolles A. R., Koteliansky V. E. (2002) Regulation of monocyte gene expression by the extracellular matrix and its functional implications. Immunol. Rev. 186, 208–220 [DOI] [PubMed] [Google Scholar]
- 9. Huveneers S., Danen E. H. (2009) Adhesion signaling—crosstalk between integrins, Src and Rho. J. Cell Sci. 122, 1059–1069 [DOI] [PubMed] [Google Scholar]
- 10. Meng F., Lowell C. A. (1998) A β 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 17, 4391–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D. P., Sheetz M. P., Schlessinger J. (2003) Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. USA 100, 10740–10745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Owen K. A., Pixley F. J., Thomas K. S., Vicente-Manzanares M., Ray B. J., Horwitz A. F., Parsons J. T., Beggs H. E., Stanley E. R., Bouton A. H. (2007) Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 179, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raftopoulou M., Hall A. (2004) Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23–32 [DOI] [PubMed] [Google Scholar]
- 14. Wheeler A. P., Wells C. M., Smith S. D., Vega F. M., Henderson R. B., Tybulewicz V. L., Ridley A. J. (2006) Rac1 and Rac2 regulate macrophage morphology but are not essential for migration. J. Cell Sci. 119, 2749–2757 [DOI] [PubMed] [Google Scholar]
- 15. Bhavsar P. J., Vigorito E., Turner M., Ridley A. J. (2009) Vav GEFs regulate macrophage morphology and adhesion-induced Rac and Rho activation. Exp. Cell Res. 315, 3345–3358 [DOI] [PubMed] [Google Scholar]
- 16. Shima M., Teitelbaum S. L., Holers V. M., Ruzicka C., Osmack P., Ross F. P. (1995) Macrophage-colony-stimulating factor regulates expression of the integrins α 4 β 1 and α 5 β 1 by murine bone marrow macrophages. Proc. Natl. Acad. Sci. USA 92, 5179–5183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanner S. B., Reynolds A. B., Parsons J. T. (1989) Immunoaffinity purification of tyrosine-phosphorylated cellular proteins. J. Immunol. Methods 120, 115–124 [DOI] [PubMed] [Google Scholar]
- 18. Hsia D. A., Lim S. T., Bernard-Trifilo J. A., Mitra S. K., Tanaka S., den Hertog J., Streblow D. N., Ilic D., Ginsberg M. H., Schlaepfer D. D. (2005) Integrin α4β1 promotes focal adhesion kinase-independent cell motility via α4 cytoplasmic domain-specific activation of c-Src. Mol. Cell. Biol. 25, 9700–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weis S. M., Lim S. T., Lutu-Fuga K. M., Barnes L. A., Chen X. L., Gothert J. R., Shen T. L., Guan J. L., Schlaepfer D. D., Cheresh D. A. (2008) Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J. Cell Biol. 181, 43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu L., Bernard-Trifilo J. A., Lim Y., Lim S. T., Mitra S. K., Uryu S., Chen M., Pallen C. J., Cheung N. K., Mikolon D., Mielgo A., Stupack D. G., Schlaepfer D. G. (2008) Distinct FAK-Src activation events promote α5β1 and α4β1 integrin-stimulated neuroblastoma cell motility. Oncogene 27, 1439–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C., Fields A. J., Kapteijn B. A., McDonald J. A. (1995) The role of α 4 β 1 integrin in cell motility and fibronectin matrix assembly. J. Cell Sci. 108, 821–829 [DOI] [PubMed] [Google Scholar]
- 22. Nishiya N., Kiosses W. B., Han J., Ginsberg M. H. (2005) An α4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 7, 343–352 [DOI] [PubMed] [Google Scholar]
- 23. Goldfinger L. E., Tzima E., Stockton R., Kiosses W. B., Kinbara K., Tkachenko E., Gutierrez E., Groisman A., Nguyen P., Chien S., Ginsberg M. H. (2008) Localized α4 integrin phosphorylation directs shear stress-induced endothelial cell alignment. Circ. Res. 103, 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldfinger L. E., Han J., Kiosses W. B., Howe A. K., Ginsberg M. H. (2003) Spatial restriction of α4 integrin phosphorylation regulates lamellipodial stability and α4β1-dependent cell migration. J. Cell Biol. 162, 731–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim L. C., Song L., Haura E. B. (2009) Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol. 6, 587–595 [DOI] [PubMed] [Google Scholar]
- 26. Rodina A., Schramm K., Musatkina E., Kreuser E. D., Tavitian A., Tatosyan A. (1999) Phosphorylation of p125FAK and paxillin focal adhesion proteins in src-transformed cells with different metastatic capacity. FEBS Lett. 455, 145–148 [DOI] [PubMed] [Google Scholar]
- 27. Antonov A. S., Kolodgie F. D., Munn D. H., Gerrity R. G. (2004) Regulation of macrophage foam cell formation by αVβ3 integrin: potential role in human atherosclerosis. Am. J. Pathol. 165, 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Charo I. F., Nannizzi L., Smith J. W., Cheresh D. A. (1990) The vitronectin receptor α v β 3 binds fibronectin and acts in concert with α 5 β 1 in promoting cellular attachment and spreading on fibronectin. J. Cell Biol. 111, 2795–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomar A., Schlaepfer D. D. (2009) Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr. Opin. Cell Biol. 21, 676–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwanicki M. P., Vomastek T., Tilghman R. W., Martin K. H., Banerjee J., Wedegaertner P. B., Parsons J. T. (2008) FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. J. Cell Sci. 121, 895–905 [DOI] [PubMed] [Google Scholar]
- 31. Ohta Y., Hartwig J. H., Stossel T. P. (2006) FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodeling. Nat. Cell Biol. 8, 803–814 [DOI] [PubMed] [Google Scholar]
- 32. Sanz-Moreno V., Gadea G., Ahn J., Paterson H., Marra P., Pinner S., Sahai E., Marshall C. J. (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135, 510–523 [DOI] [PubMed] [Google Scholar]
- 33. Nimnual A. S., Taylor L. J., Bar-Sagi D. (2003) Redox-dependent downregulation of Rho by Rac. Nat. Cell Biol. 5, 236–241 [DOI] [PubMed] [Google Scholar]
- 34. Hume D. A. (2006) The mononuclear phagocyte system. Curr. Opin. Immunol. 18, 49–53 [DOI] [PubMed] [Google Scholar]
- 35. Stout R. D., Watkins S. K., Suttles J. (2009) Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J. Leukoc. Biol. 86, 1105–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.