θ-defensins account for a major portion of the antimicrobial activity in macaque neutrophil granules and for the superior activity of macaque preparations compared those from human PMNs.
Keywords: defensins, peptides, innate immunity
Abstract
Mammalian defensins are cationic, antimicrobial peptides that play a central role in innate immunity. The peptides are composed of three structural subfamilies: α-, β-, and θ-defensins. θ-Defensins are macrocyclic octadecapeptides expressed only in Old World monkeys and Orangutans and are produced by the pair-wise, head-to-tail splicing of nonapeptides derived from their respective precursors. The existence of three active θ-defensin genes predicts that six different RTDs (1–6) are produced in this species. In this study, we isolated and quantified RTDs 1–6 from the neutrophils of 10 rhesus monkeys. RTD-1 was the most abundant θ-defensin, constituting ∼50% of the RTD content; total RTD content varied by as much as threefold between animals. All peptides tested were microbicidal at ∼1 μM concentrations. The contribution of θ-defensins to macaque neutrophil antimicrobial activity was assessed by analyzing the microbicidal properties of neutrophil granule extracts after neutralizing θ-defensin content with a specific antibody. θ-Defensin neutralization markedly reduced microbicidal activities of the corresponding extracts. Macaque neutrophil granule extracts had significantly greater microbicidal activity than those of human neutrophils, which lack θ-defensins. Supplementation of human granule extracts with RTD-1 markedly increased the microbicidal activity of these preparations, further demonstrating a prominent microbicidal role for θ-defensins.
Introduction
The host defense properties of mammalian granulocytes rely, in part, on their ability to phagocytose pathogens and subject ingested microbes to the actions of antimicrobial proteins that are discharged from cytoplasmic granules into the phagosome. The azurophil granules of most mammals studied contain defensins, which are antimicrobial peptides consisting of the α-, β-, or θ-defensin subfamilies that are characterized by distinctive tridisulfide motifs [1, 2]. α- and β-defensins contain 29–45 aa. In contrast, θ-defensins are macrocylic octadecapeptides, the only known example of a cyclic polypeptide motif in the animal proteome.
Previously, we analyzed and compared the expression of defensins expressed in the neutrophils of humans [3, 4] and rhesus monkeys [5]. Four α-defensins, termed HNP 1–4 are expressed in human neutrophils, and these peptides have antimicrobial activities against bacteria [6], fungi [7, 8], and viruses [9–11] in vitro. Macaque neutrophils contain eight α-defensins (RMAD 1–8), four of which (RMAD 1–3 and 8) are highly similar to HNP 1–3 [5]. The granules of rhesus macaque neutrophils also contain θ-defensins, peptides that are derived from truncated α-defensin genes [12, 13]. The macrocyclic backbone is produced by pair-wise excision and head-to-tail splicing of two nine-residue segments derived from θ-defensin precursors [14]. The nonapeptides may be identical (homodimeric splicing) or derived from different precursors (heterodimeric splicing). Notably, θ-defensins are expressed in Old World monkeys such as macaques [12, 13] and baboons [15, 16] but not in humans, chimpanzees, gorillas, or New World monkeys. Phylogenetic studies reveal the loss of θ-defensins in the primate lineage to be the result of a mutation that introduced a stop codon in the signal-peptide coding region of exon 2 [17].
As human and macaque neutrophils differ in their defensin content, with humans lacking θ-defensins, we sought to determine the relative contributions of defensin subtypes to the microbicidal properties of neutrophil granule extracts. Here, we describe the isolation of three new, predicted θ-defensins and the determination of the relative abundance of RTDs 1–6 in macaque leukocytes and the relative microbicidal activities of five of six RTDs. The contribution of θ-defensins to the antimicrobial activities of neutrophil granule extracts was determined by determining the effect of specific inhibition of θ-defensins on the antimicrobial activities of macaque neutrophil granule extracts, determining the microbicidal activities of purified RTD-1 at concentrations equivalent to those contained in granule extracts, and comparing the microbicidal activities of macaque and human PMN granule extracts, the latter of which was tested with and without supplementation with θ-defensins.
MATERIALS AND METHODS
Peptides
RTDs 1–5 (see Fig. 1) were synthesized using Fmoc solid-phase synthesis, oxidized, cyclized, purified, and characterized as described previously [12, 18]. RMAD-4 was produced in Escherichia coli as described previously [19]. RMAD-1 was purified from rhesus buffy coat cells as described earlier [5].
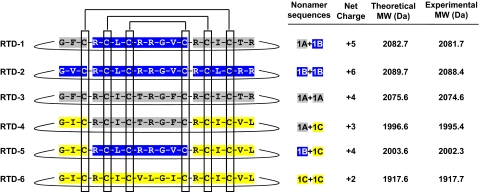
Figure 1. Derivation of RTDs 1–6.
Six θ-defensins may be derived from differential binary head-to-tail splicing of nona-peptides (1A–1C) derived from the corresponding pro-θ-defensin precursors. The covalent structures of the mature peptides are shown schematically with color coding of the respective nonapeptides. Also shown is the net charge of each θ-defensin, the theoretical mass, and that obtained experimentally by MALDI-MS of the peptide isolated from macaque leukocytes.
Antibodies
Anti-RTD-1 IgG was produced by immunizing a goat with a mixture of polymerized cyclic and aRTD-1 as described earlier [15]. Goat anti-RTD-1 IgG was IAP on a 5-ml column of Affigel 10 (Bio-Rad Laboratories, Inc., Hercules, CA, USA), derivatized with 3.0 mg aRTD-1 [12], as recommended by the manufacturer. Briefly, aRTD-1 was dissolved in 0.1 M sodium acetate, pH 6.5, and incubated with Affigel 10 slurry for 18 h at 8°C. Derivatized gel was transferred to a column, washed with 250 ml 0.1 M sodium acetate, pH 6.5, and quenched with 0.1 M glycine-HCl. The column was washed with 200 ml 20 mM Tris-HCl, 28 mM NaCl, pH 8.0 (IAP column buffer). The goat anti-RTD-1 antiserum (20 ml) was dialyzed in IAP column buffer, percolated over the aRTD-1-Affigel column, and washed sequentially with high salt buffer (20 mM Tris-HCl, 154 mM NaCl, pH 8.0) and IAP column buffer. Bound IgG was eluted in 10 ml 0.1 M glycine-HCl, pH 2.5, and dialyzed overnight in IAP column buffer. IAP anti-RTD-1 IgG was passed through a 0.22-μm filter and stored in 1 ml aliquots at −80°C. Goat pre-immune IgG was purified using DEAE-Affigel Blue (Bio-Rad Laboratories, Inc.), as described by the manufacturer, and dialyzed against IAP column buffer. IAP anti-HBD-4 antibody was purified from the antiserum of a goat immunized with rHBD-4 [20] (C-terminal 44 aa) using an Affigel 10 column derivatized with rHBD-4 peptide. Immunospecificity of IAP antibodies was confirmed by dot immunoblotting with the pre-immune goat IgG as a negative control.
Dot blot analysis
Synthetic RTDs 1–5, 300 ng each, were spotted in duplicate on a nitrocellulose membrane, air-dried overnight, and blocked using 5% horse serum in TTBS buffer (100 mM Tris-HCl, pH 7.5, 0.9% NaCl, and 0.1% Tween 20). The blot was probed with 1:10 diluted IAP anti-RTD-1 or IAP anti-HBD-4 IgGs. The blots were washed with TTBS, incubated with 1:250,000 HRP-labeled anti-goat IgG (Vector Laboratories, Burlingame, CA, USA), and developed using chemiluminescence per the manufacturer's instructions.
Leukocyte isolation
EDTA-anticoagulated whole blood was obtained from normal adult rhesus macaques housed at the California National Primate Research Center (Davis, CA, USA). For experiments using pooled cells, batches of peripheral blood leukocytes were prepared by dextran sedimentation of the erythrocytes as described previously [15]. Residual erythrocytes were removed by cold hypotonic lysis, and cells were snap-frozen as pellets containing 0.5–2.0 × 108 cells. Purity of leukocyte populations was determined by differential counts of cytospin preparations. In studies wherein the content of RTD-1 in monocytes was determined, monocytes were purified from mononuclear cell preparations using OptiPrep density gradient centrifugation (density=1.068 g/ml), and the resulting preparation contained ≤2% PMN.
Preparation and extraction of neutrophil granules
Freshly purified macaque neutrophils (∼90%) were suspended to 1 × 107 cells/ml in sterile ice-cold HBSS containing 2.5 mM MgCl2 and disrupted by nitrogen cavitation in a Parr bomb (Parr Instrument Co., Moline, IL, USA) at 4°C and 750 pounds/square inch. The cavitate was centrifuged at 700 g for 20 min at 4°C, and the granule-containing supernatant was collected and centrifuged at 27,000 g for 40 min at 4°C. The granule-rich pellets were stored at −80°C. Granule extract was prepared by adding 5 ml ice-cold 5% acetic acid to frozen pellets and stirring at 4°C for 18 h. The extract was clarified by centrifugation at 27,000 g for 20 min at 4°C, and the supernatant was lyophilized, dissolved in 0.1% acetic acid, diluted tenfold into IAP column buffer, clarified by centrifugation, and stored at −20°C. Quantitative HPLC analysis indicated that 2.7 μg rhesus macaque granule protein contained 0.1 μg RTD-1 (data not shown). Human neutrophil granule extracts were prepared as above using fresh blood from a healthy donor; collection of human blood was undertaken with the approval of the Institutional Review Board of University of Southern California (Los Angeles, CA, USA). Protein concentrations of the granule extracts were determined using the Bio-Rad protein dye reagent (Bio-Rad Laboratories, Inc.) using BSA as standard.
Extraction and quantification of θ-defensins
Frozen leukocyte cell pellets (0.5–2.0×108 cells) from 10 adult macaques (seven males, three females) were extracted individually with 60% aqueous ACN-1% TFA [15], clarified by centrifugation, lyophilized, resuspended in 4 ml 10% acetic acid, and reclarified by centrifugation. Extract from 1.0 × 107 cell equivalents (0.4–0.7 ml) was diluted to 2 ml with 0.1% TFA and applied to Strata-X polymeric SPE columns (60 mg/3 ml columns, Phenomenex, Torrance, CA, USA), preconditioned with 1 ml methanol, followed by 2 ml 0.1% TFA-water. Columns were washed with 1 ml 5% methanol, and bound material was eluted with 2 × 0.5 ml 20% aqueous ACN containing 0.1% TFA, followed by 0.5 mL (×2) elutions with 40% and 60% aqueous ACN-0.1% TFA. Eluant samples were mixed 1:1 with saturated α-cyano-4-hydroxycinnamic acid in 50% ACN-water with 0.1% TFA and spotted on a 96-well stainless-steel sample plate. Peptide masses were determined by MALDI-MS on a Microflex LRF mass spectroscope (Bruker Daltonics, Maynard, MA, USA).
θ-Defensins in Strata X SPE column eluants were quantified by RP-HPLC of 20 μl samples on a 2 × 150 mm Varian Polaris C-18 column using a Waters 2690 Alliance chromatography system with detection at 210 nm using a Waters 2487 dual-wavelength detector fitted with a nano-volume flow cell. RTDs 1–5, present in the 40% ACN + 0.1% TFA Strata X column eluant, were resolved using a 0–60% ACN/0.1% TFA (60 min) at 0.2 ml/min (RTD-1, -4, and -5) or a 0–60% ACN/0.1% phosphoric acid gradient (RTD-2 and -3). RTD-6, present in the 60% ACN + 0.1% TFA eluant of the Strata X column, was resolved using a 20–80% ACN + 0.1% TFA gradient in 60 min. Peptide identities were confirmed by co-elution (RTDs 1–5) with synthetic standards and by MALDI-MS (RTDs 1–6). Peptide quantities were determined using standard curves generated by injection of 6.2–100 ng synthetic peptide standards.
Microbicidal assays
The microbicidal activities of the θ-defensins (RTDs 1–5) and α-defensins (RMAD-1 and RMAD-4) were evaluated against Staphylococcus aureus 502a, E. coli ML 35, and Candida albicans 16820 using protocols described previously [12, 15, 17, 18]. Briefly, peptides were dissolved in 0.01% acetic acid and incubated at 0–10 μg/ml with ∼1 × 107 (see Figs. 3, 5, and 7) or 1 × 104 (see Figs. 4 and 6) CFUs/ml log-phase organisms for 2 h at 37°C in buffer plus 0.03% (w/v) trypticase soy broth (bacterial incubations) or Sabouraud dextrose broth (C. albicans incubations), and aliquots were plated on appropriate solid media. All microbicidal assays were performed at least twice, and the data shown are representative of replicate experiments.
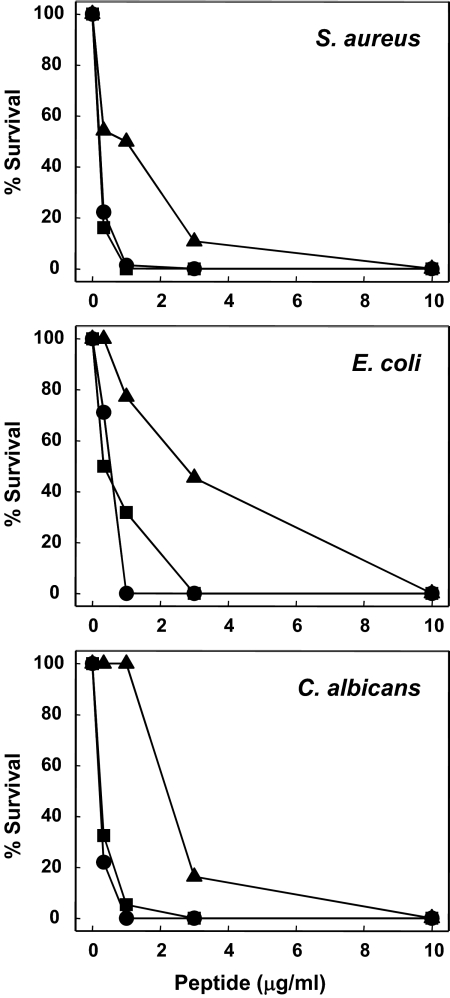
Figure 3. Microbicidal activities of RTD-4 and RTD-5.
The microbicidal properties of RTD-4 (▲) and RTD-5 (■) were compared with those of RTD-1 (●) in killing assays against S. aureus, E. coli, and C. albicans as described in Materials and Methods.
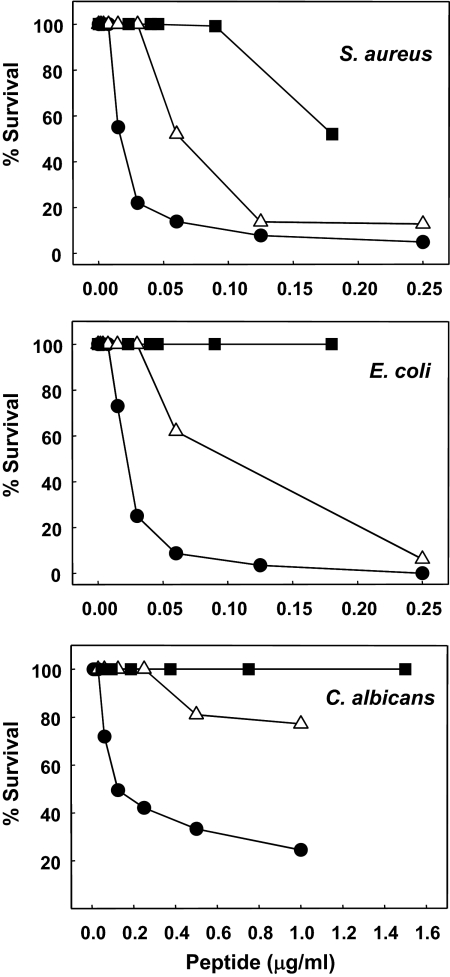
Figure 5. Comparative microbicidal potency of RTD-1 and rhesus macaque α-defensins, RMAD-1 and RMAD-4.
The antimicrobial activities of RTD-1 (●), RMAD-1 (■), and RMAD-4 (▵) were determined using S. aureus, E. coli, and C. albicans, as described in Materials and Methods.
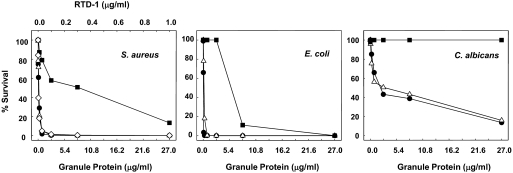
Figure 7. Antimicrobial potency of macaque and human neutrophil granule extracts.
The concentration-dependent killing of S. aureus, E. coli, and C. albicans by granule extracts from rhesus macaque (△) or human neutrophil (■) was evaluated in microbicidal assays. In parallel, incubations were performed using concentrations of RTD-1 (●) equivalent to those in the macaque granule preparations. Additionally, human PMN granule extract was supplemented with RTD-1 (◊) at the concentrations shown in a microbicidal assay against S. aureus (left panel).
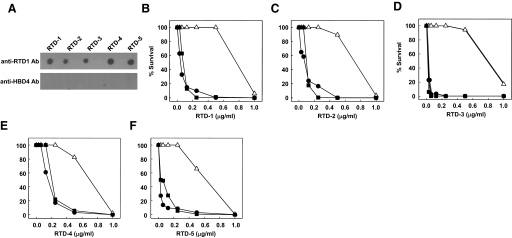
Figure 4. Immunoreactivity and neutralizing activity of anti-RTD-1 IgG.
(A) RTDs 1–5 were spotted on a nitrocellulose membrane, and the membrane was probed with IAP anti-RTD-1 IgG or IAP anti-HBD-4 IgG. (B–F) Log-phase S. aureus was incubated with 0–1.0 μg/ml peptide in buffer alone (●), with 50 μg/ml IAP anti-RTD-1 IgG (△), or with preimmune goat IgG (■). Bacterial survival was determined by colony counting and compared with 2 h buffer control incubations.
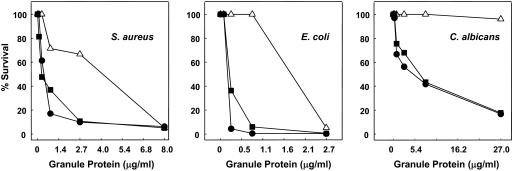
Figure 6. Neutralization of RTDs markedly reduces the microbicidal activities of macaque leukocyte granule extracts.
Log-phase suspensions of S. aureus, E. coli, and C. albicans were incubated with extracts of rhesus macaque granules preincubated in buffer alone (●), with 50 μg/ml IAP anti-RTD-1 IgG (△), or IAP anti-HBD-4 antibody (■). Microbial killing was quantified by colony counting.
For experiments in which the potential neutralizing effects of IgG preparations were evaluated, microorganisms were suspended in IAP buffer plus 0.03% (w/v) trypticase soy or Sabouraud broth. Each antibody preparation (5 μg) was preincubated with varied peptide or granule extract concentrations in 90 μl for 1 h at 22°C, after which, suspensions of test organisms were added in a 10-μl volume. The final antibody concentration (50 μg/ml) was shown to neutralize at least 0.5 μg/ml purified RTD-1 and was also neutralizing for RTDs 2–5, albeit with slightly different efficiencies (see Results). As negative controls, IAP anti-HBD-4 IgG or preimmune goat IgG was included in incubation mixtures.
RESULTS
Relative abundance of θ-defensins in rhesus macaque neutrophils
Rhesus macaques express three θ-defensin precursor genes, RTD1a, RTD1b, and RTD1c (Fig. 1) [12, 13]. Differential pairing of nonapeptides derived from the respective precursors theoretically produces six distinct θ-defensins, RTDs 1–6 (Fig. 1). To date, only RTDs 1–3 have been isolated from macaque tissues [13, 18]. RTDs 1–3 are products of homo- or heterodimeric splicing of nonapeptides derived from RTD1a and RTD1b precursors [18]. Lehrer and colleagues reported the sequence of a third θ-defensin precursor, RTD1c (termed demidefensin 3 in ref. [13]), from which another unique nonapeptide could be paired with itself or with nonapeptides derived from RTD1a and RTD1b. To analyze the activities of the predicted peptides (RTDs 4–6; Fig. 1) resulting from these nonapeptide pairings, we synthesized the peptides by solid-phase Fmoc chemical synthesis. The acyclic peptides were purified, folded/oxidized, and cyclized as described previously for RTDs 1–3 [12, 18]. RTD-4 and RTD-5 were ≥99% pure, as determined by analytical RP-HPLC, acid urea-PAGE, and MALDI-MS. The overall yield from 200 mg crude peptide was 1.2 mg for RTD-4 and 5.8 mg for RTD-5, yields that were considerably lower than from similar syntheses of RTD-1 [12, 18]. Several attempts to synthesize RTD-6 resulted in a very low yield of the correct peptide product as a result of its poor solubility in 6 M guanidine-HCl, DMSO, dichloromethane, ACN, and various alcohols. Although a small amount of linear RTD-6 was purified and folded/oxidized, the vast majority of the final product was lost during the cyclization step.
To analyze the expression of putative RTDs 4–6, rhesus macaque neutrophils were extracted with acidic aqueous ACN and fractionated by C18 RP-HPLC. Peptides with masses corresponding to RTDs 1–6 were detected in HPLC fractions by MALDI-MS. Reduction (DTT) and alkylation (iodoacetamide) [18] of putative RTDs 1–6 produced derivatives that were 348 Da greater than the respective native peptide masses, confirming the presence of six cysteine residues in each peptide (data not shown).
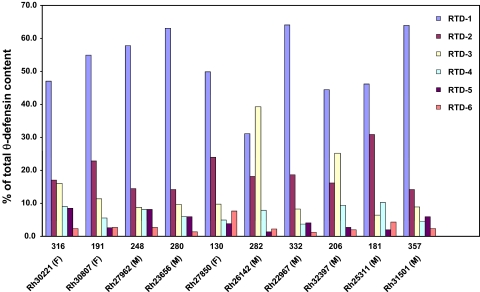
We next determined the relative quantities of RTDs 1–6 in buffy coat preparations from 10 adult monkeys. RTDs 1–5 were detected in the 40% ACN/0.1% TFA Strata X cartridge eluant, whereas RTD-6 eluted in the 60% ACN/0.1% TFA eluants. Peptides were quantified individually by analytical RP-HPLC, as described in Materials and Methods. RTDs 1–6 were identified in all 10 monkeys. RTD-1 was the predominant θ-defensin constituting 52.3 ± 10.6% of the total defensin content in neutrophils and was the most abundant of the RTDs in the leukocytes of all but one animal. The relative quantities of the other five θ-defensins were: RTD-2 (19.0±5.4%), RTD-3 (14.4±10.3%), RTD-4 (6.9±2.3%), RTD-5 (4.5±2.5%), and RTD-6 (2.9±1.9%; Fig. 2). The cellular θ-defensin content varied approximately threefold among 10 adults analyzed (Fig. 2).
Figure 2. Total and relative RTD content in rhesus macaque leukocytes.
The θ-defensins isolated from leukocytes of 10 adult animals were quantified individually, as described in Materials and Methods. The combined RTD content (μg/1×109 cells) is shown above the animal identifier (Rh number and gender). The percent of RTDs 1–6 isolated from each animal is represented in bar graphs.
In previous studies, we immunolocalized RTD-1 to the cytoplasmic granules of macaque neutrophils and monocytes [12], but the relative expression levels in the two cell types are unknown. We quantified the levels of RTD-1 in purified monocytes and neutrophils by Strata X SPE and analytical RP-HPLC as described above. The content of RTD-1/1 × 109 monocytes was 12.4 μg, whereas the same number of neutrophils contained ∼86.0 μg, indicating that neutrophils contain approximately sevenfold more RTD-1 than monocytes.
Microbicidal activities of RTD-4 and RTD-5
We showed previously that RTD-2 and RTD-3 have microbicidal activities in vitro that are comparable with those of RTD-1 [18]. To evaluate the microbicidal activities of newly isolated θ-defensins, RTD-4 and RTD-5, we tested the peptides for their ability to kill S. aureus, E. coli, and C. albicans and compared these activities with those of RTD-1. As shown in Fig. 3, the microbicidal activities of RTD-5 were comparable with those of RTD-1 against the three test organisms. However, RTD-4 was substantially less microbicidal than RTD-1 and RTD-5 against all three organisms. Inspection of the dose-response data indicates that RTD-1 and RTD-5 are at least threefold more potent than RTD-4.
RTD-1-neutralizing antibodies
IAP anti-RTD-1 IgG was evaluated for its reactivity with RTDs 1–5 by dot immunoblotting. As shown in Fig. 4A, the antibody detected RTDs 1–5 with approximately equal immunoreactivity. Reactivity with RTD-6 could not be tested because of the low quantities of this peptide recovered from leukocytes and in our synthetic preparation. Anti-RTD-1 antibody did not react with rhesus α-defensins RMAD-1, RMAD-4, or HBD-4 (data not shown). Moreover, IAP anti-HBD-4 IgG did not react with RTDs 1–5 (Fig. 4A). Similarly, purified, preimmune goat IgG did not detect any of the peptides in immunoblots (data not shown).
To determine whether IAP anti-RTD-1 IgG was neutralizing, we first tested the effect of the antibody on the staphylocidal activities of RTD-1. As shown in Fig. 4B, submicromolar concentrations of RTD-1 kill S. aureus 502a in a dose-dependent manner, as reported previously [18]. With addition of 50 μg/ml IAP anti-RTD-1 IgG, there was complete neutralization of RTD-1 killing at peptide concentrations of up to 0.5 μg/ml (Fig. 4B). As the anti-RTD-1 IgG also bound RTDs 2–5 in dot immunoblots (Fig. 4A), we tested for the neutralizing activity of the antibody against the other four θ-defensins. As shown in Fig. 4C–F, anti-RTD-1 IgG also neutralized the staphylocidal activities of RTDs 2–5 with efficiencies of 65–90% of that obtained in the neutralization of RTD-1. Neither the preimmune IgG (Fig. 4B–F) nor IAP anti-HBD-4 (data not shown) inhibited the staphylocidal activities of RTDs 1–5. Given the sequence and conformational similarities of RTDs 1–5, it is not surprising that the anti-RTD-1 IgG recognized and neutralized the five related molecules.
Contribution of θ-defensins to the microbicidal activities of neutrophil granules
The known antimicrobial constituents of neutrophil granules include defensins (α, β, and/or θ, depending on species), as well as azurocidin, lactoferrin, and serine proteases (cathepsin G, elastase, and proteinase 3). In contrast to human cells that lack θ-defensins, rhesus macaque neutrophil granules contain α- and θ-defensins. To evaluate the relative activities of α- and θ-defensins, we compared the microbicidal activities of RTD-1 with those of the most abundant macaque neutrophil α-defensins, RMAD-1 and RMAD-4 [5]. As shown in Fig. 5, RTD-1 was substantially more active against all three test organisms than either of the α-defensins tested. RMAD-1, which is 86% identical to HNP-1, had no activity at the concentrations tested against E. coli and C. albicans and was approximately tenfold-less active than RTD-1 against S. aureus. The activity of RMAD-4 was intermediate to that of RTD-1 and RMAD-1 against each test organism (Fig. 5).
To determine more directly the contribution of θ-defensin activities to the microbicidal properties of rhesus macaque granule proteins, we analyzed the effect of antibody-mediated RTD inhibition on the microbicidal activities of granule extracts. Killing of each test organism by granule extract occurred in a dose-dependent manner, however the microbicidal effect varied among the target microbes (Fig. 6). When the incubation mixtures were supplemented with IAP anti-RTD-1 IgG (final concentration, ∼50 μg/ml), there was a pronounced reduction in extract-mediated killing of all three organisms. Neither anti-HBD-4 antibody (Fig. 6) nor preimmune IgG (data not shown) inhibited the microbicidal activities of granule extracts. It is noteworthy that the addition of anti-RTD-1 IgG completely inhibited the killing of E. coli and C. albicans at granule protein concentrations that were otherwise highly effective in killing these targets (Fig. 6, middle and right panels). Neutralization of RTDs also reduced the staphylocidal activity of granule extract but to a lesser extent than observed with E. coli or C. albicans, demonstrating that granule constituents other than θ-defensins are effective in killing S. aureus (Fig. 6, left panel). Thus, θ-defensins are responsible for a substantial portion of the in vitro microbicidal activities of neutrophil granule constituents against three diverse targets.
Relative microbicidal activities of human and rhesus macaque neutrophil granule extracts
Humans, gorillas, chimpanzees, and New World monkeys do not express θ-defensins [17]. Therefore, a distinguishing feature of rhesus monkey and human neutrophils is their respective expression and absence of θ-defensins. As the specific inhibition of θ-defensins greatly reduced the microbicidal potency of macaque PMN granules, we hypothesized that granule extracts from human neutrophils lacking θ-defensins would be less active than those from macaques. As shown by the data in Fig. 7, granule extracts from macaque PMNs had much greater microbicidal activity than identically prepared samples from human neutrophils. Indeed, the concentration of human granule protein required to kill 80–90% of S. aureus and E. coli was ∼50- and ∼20-fold higher than that derived from macaque PMN granules. Remarkably, C. albicans was completely resistant to the highest concentration of human granule extract tested, whereas equivalent concentrations of macaque-derived extracts killed >80% of the input organisms. When human granule extracts were supplemented with RTD-1 to the level present in the macaque granule extract, the resulting preparation acquired a marked increase in staphylocidal activity, which approximated that observed in the macaque PMN granule extract as well as that of an equivalent amount of purified RTD-1 (Fig. 7, left panel).
DISCUSSION
Neutrophils play a major role in innate immunity by phagocytosing and killing microorganisms. The microbicidal mechanisms used by the neutrophil rely on ROS and oxygen-independent factors, the latter being composed of antimicrobial polypeptides packaged in cytoplasmic lysosome-like organelles of granulocytes [21, 22]. Indeed, biochemical and functional characterization of neutrophil granule components has provided fundamental insights into the identity of antimicrobial effector molecules produced by granulocytes. The granules of neutrophils are composed of three subpopulations (azurophil, specific, and gelatinase granules), which together, contain numerous antimicrobial substances. The relative roles of granule-derived microbicidal species have not been delineated with one exception, that being the demonstration that deletion of the cathelicidin gene renders mice susceptible to cutaneous infection with Group A streptococcus [23].
The θ-defensin cyclic structure is the only example of a macrocyclic polypeptide in animals. The cyclic conformation confers insensitivity to the inhibitory effects of physiologic salt and endows the peptide with remarkable protease stability [12]. Moreover, the θ-defensin biosynthetic pathway, entailing binary excision/splicing steps, is also novel and allows for the generation of structural and functional diversity. Differential combinations of substituent nonapeptides derived from three macaque θ-defensin precursors produce six unique RTDs. Similarly, nonapeptides derived from four θ-defensin precursors expressed in Olive baboons can be combined to produce 10 distinct θ-defensins in leukocytes of that species [15]. The functional diversity of these macrocyclic peptides has been reported by a number of laboratories. For example, in addition to the antibacterial and antifungal activities reported here and elsewhere [12, 16, 24, 25], a number of laboratories have demonstrated that θ-defensins inhibit uptake of HIV by target cells [26–28], reportedly via a lectin-dependent mechanism [29], and θ-defensins also kill vegetative and spore forms of Bacillus anthracis and neutralize the lethal toxin of this organism [30]. The killing of E. coli by θ-defensins is accompanied by the rapid entry of the peptide into the cytosol [24].
In the current study, we sought to determine the role of θ-defensins in the granule-associated antimicrobial armamentarium of macaque neutrophils. Earlier studies demonstrated that RTDs 1–3 have nearly equivalent microbicidal activities in vitro, but the existence of additional macaque θ-defensins (provisionally termed RTDs 4–6), which were predicted based on the identification of a third θ-defensin precursor cDNA [13], had not been demonstrated. The sequential use of Strata-X SPE of macaque neutrophil extracts and RP-HPLC enabled the isolation of RTDs 4–6 for the first time. Expression of the six θ-defensin in neutrophils of 10 adult macaques was analyzed. Although the relative ratios of the six θ-defensins varied little, the overall θ-defensin expression varied as much as threefold, revealing a surprising degree of animal-to-animal variability in RTD expression. Variations in gene copy numbers of several α- and β-defensin genes have been reported [31–34], and gene copy number has been shown to correlate with peptide levels [31]. It remains to be determined whether gene copy number polymorphisms are responsible for the variability in θ-defensin in rhesus macaques.
The microbicidal activities of synthetic versions of the newly isolated θ-defensins were determined in assays against three test organisms. The panel of test organisms was selected to provide representative models for studying the interactions of Gram+ and Gram− bacteria and yeast and as such, cannot be extrapolated directly to the complex microbe-host interactions that occur in vivo. Nevertheless, as many studies of phagocyte-mediated killing mechanisms have used analyses of the antimicrobial properties of granule constituents, we evaluated the microbicidal properties of purified θ-defensins, including those newly isolated, for their microbicidal properties in vitro. The activities of RTD-5 were similar to those of RTD-1 (Fig. 3), but RTD-4 was substantially less active than any θ-defensin evaluated to date. The inability to synthesize RTD-6 successfully and the small quantities of natural peptide obtained from neutrophil extracts precluded the functional characterization of this θ-defensin.
In addition to θ-defensins, eight myeloid α-defensins have been identified in rhesus macaques (RMAD 1–8) [5]. RTD-1 (which has antimicrobial activities that are similar to RTD-2, -3, and -5) had substantially greater microbicidal activity than the most-abundant macaque α-defensins, RMAD-1 and RMAD-4 (Fig. 5), suggesting that θ-defensins confer to macaque neutrophil potent microbicidal activities, resulting from the action of peptides that are absent in human cells. Consistent with this finding, granule extracts of human PMNs were markedly less active than those derived from macaque leukocytes, and the addition of RTD-1 to the human cell preparations dramatically augmented activity of the human extracts (Fig. 7). These data demonstrate an important difference in the portfolio of antimicrobial substances expressed in human and macaque leukocytes.
To further dissect the contribution of θ-defensins to the granule-derived antimicrobial activities in macaque PMNs, we used a RTD-neutralizing antibody that bound RTDs 1–5 and neutralized the microbicidal activities of RTDs 1–5, which together, account for >95% of the cellular θ-defensin content. The addition of anti-RTD-1 antibody to macaque PMN granule extracts produced a profound reduction in the microbicidal activity of these preparations (Fig. 6). This was most dramatic in experiments using C. albicans, where the addition of anti-RTD-1 IgG neutralized the candidacidal activity of the granule extract tested (Fig. 6). Of note is the fact that C. albicans was highly resistant to RMAD-1 and -4, which are highly expressed in these extracts (Fig. 5). Taken together, these results suggest that θ-defensins are responsible for a large portion of the microbicidal activity observed in rhesus macaque granule extracts.
The structural basis for the superior potency of θ-defensins, as compared with α-defensins, is not fully understood. However, in previous studies, we have demonstrated that the antibacterial activities of θ-defensins are salt-insensitive compared with those of α-defensins [12] and that θ-defensins are internalized rapidly by target bacteria [18]. θ-Defensins are extremely stable under the conditions used in these studies, raising the possibility that α-defensins might be less effective as a result of their inactivation. However, we have determined that α-defensins are also stable in vitro and in vivo. Thus, the intrinsic activity differences of α- and θ-defensins do not appear to be a function of differing peptide stabilities.
To our knowledge, this is the first report wherein a specific component of the granule protein complex has been antibody-neutralized to assess its role as a microbicide. Our results demonstrate that θ-defensins are significantly more microbicidal than α-defensins and play a prominent microbicidal role in the granules of rhesus macaque neutrophils, and the absence of θ-defensins in human cells correlates with the marked differences observed in the antimicrobial properties of human and macaque PMN granule extracts.
ACKNOWLEDGMENTS
This work was supported in part by National Institutes of Health awards AI078321 (P. Tongaonkar), AI059346 and DK044632 (A.J.O.), and AI22931, AI58129, and DE15517 (M.E.S.). We thank the California National Primate Research Center (Davis, CA, USA) for providing rhesus macaque blood samples used in these studies.
Footnotes
- ACN
- acetonitrile
- aRTD-1
- acyclic rhesus macaque θ-defensin-1
- HBD
- human β-defensin
- HNP
- human neutrophil peptide
- IAP
- immunoaffinity-purified
- MALDI-MS
- MALDI-TOF-mass spectroscopy
- RMAD
- rhesus myeloid α-defensin
- RTD
- rhesus macaque θ-defensin
- SPE
- solid-phase extraction
AUTHORSHIP
P. Tongaonkar collected, analyzed, and interpreted data, performed statistical analysis, and co-wrote the manuscript. P. Tran designed and performed research, contributed vital new reagents, collected, analyzed, and interpreted data, and co-wrote the manuscript. K.R. performed research and collected, analyzed, and interpreted data. J.S. contributed vital new reagents and collected data. G.Ö. contributed vital new reagents. D.T. designed and performed research, contributed vital new reagents and analytical tools, collected, analyzed, and interpreted data, and co-wrote the manuscript. A.J.O. contributed vital new reagents and analyzed and interpreted data. M.E.S. designed research, analyzed and interpreted data, and wrote the manuscript.
DISCLOSURE
M.E.S. has an equity interest in Resolve Therapeutics, LLC, an entity that seeks to commercialize θ-defensins as therapeutics.
REFERENCES
- 1. Selsted M. E., Ouellette A. J. (2005) Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557 [DOI] [PubMed] [Google Scholar]
- 2. Ganz T. (2003) Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 [DOI] [PubMed] [Google Scholar]
- 3. Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. (1985) Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Invest. 76, 1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. (1985) Primary structures of three human neutrophil defensins. J. Clin. Invest. 76, 1436–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang Y. Q., Yuan J., Miller C. J., Selsted M. E. (1999) Isolation, characterization, cDNA cloning, and antimicrobial properties of two distinct subfamilies of α-defensins from rhesus macaque leukocytes. Infect. Immun. 67, 6139–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ericksen B., Wu Z., Lu W., Lehrer R. I. (2005) Antibacterial activity and specificity of the six human {α}-defensins. Antimicrob. Agents Chemother. 49, 269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mambula S. S., Simons E. R., Hastey R., Selsted M. E., Levitz S. M. (2000) Human neutrophil-mediated nonoxidative antifungal activity against Cryptococcus neoformans. Infect. Immun. 68, 6257–6264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newman S. L., Gootee L., Gabay J. E., Selsted M. E. (2000) Identification of constituents of human neutrophil azurophil granules that mediate fungistasis against Histoplasma capsulatum. Infect. Immun. 68, 5668–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daher K. A., Selsted M. E., Lehrer R. I. (1986) Direct inactivation of viruses by human granulocyte defensins. J. Virol. 60, 1068–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bastian A., Schafer H. (2001) Human α-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 101, 157–161 [DOI] [PubMed] [Google Scholar]
- 11. Wu Z., Cocchi F., Gentles D., Ericksen B., Lubkowski J., Devico A., Lehrer R. I., Lu W. (2005) Human neutrophil α-defensin 4 inhibits HIV-1 infection in vitro. FEBS Lett. 579, 162–166 [DOI] [PubMed] [Google Scholar]
- 12. Tang Y. Q., Yuan J., Osapay G., Osapay K., Tran D., Miller C. J., Ouellette A. J., Selsted M. E. (1999) A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 286, 498–502 [DOI] [PubMed] [Google Scholar]
- 13. Leonova L., Kokryakov V. N., Aleshina G., Hong T., Nguyen T., Zhao C., Waring A. J., Lehrer R. I. (2001) Circular minidefensins and posttranslational generation of molecular diversity. J. Leukoc. Biol. 70, 461–464 [PubMed] [Google Scholar]
- 14. Selsted M. E. (2004) θ-Defensins: cyclic antimicrobial peptides produced by binary ligation of truncated α-defensins. Curr. Protein Pept. Sci. 5, 365–371 [DOI] [PubMed] [Google Scholar]
- 15. Garcia A. E., Osapay G., Tran P. A., Yuan J., Selsted M. E. (2008) Isolation, synthesis, and antimicrobial activities of naturally occurring θ-defensin isoforms from baboon leukocytes. Infect. Immun. 76, 5883–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stegemann C., Tsvetkova E. V., Aleshina G. M., Lehrer R. I., Kokryakov V. N., Hoffmann R. (2010) De novo sequencing of two new cyclic θ-defensins from baboon (Papio hamadryas) leukocytes by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 24, 599–604 [DOI] [PubMed] [Google Scholar]
- 17. Nguyen T. X., Cole A. M., Lehrer R. I. (2003) Evolution of primate θ-defensins: a serpentine path to a sweet tooth. Peptides 24, 1647–1654 [DOI] [PubMed] [Google Scholar]
- 18. Tran D., Tran P. A., Tang Y. Q., Yuan J., Cole T., Selsted M. E. (2002) Homodimeric θ-defensins from rhesus macaque leukocytes: isolation, synthesis, antimicrobial activities, and bacterial binding properties of the cyclic peptides. J. Biol. Chem. 277, 3079–3084 [DOI] [PubMed] [Google Scholar]
- 19. Tanabe H., Ouellette A. J., Cocco M. J., Robinson W. E., Jr. (2004) Differential effects on human immunodeficiency virus type 1 replication by α-defensins with comparable bactericidal activities. J. Virol. 78, 11622–11631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. García J. R., Krause A., Schulz S., Rodriguez-Jimenez F. J., Kluver E., Adermann K., Forssmann U., Frimpong-Boateng A., Bals R., Forssmann W. G. (2001) Human β-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 15, 1819–1821 [PubMed] [Google Scholar]
- 21. Nauseef W. M. (2007) How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 219, 88–102 [DOI] [PubMed] [Google Scholar]
- 22. Faurschou M., Borregaard N. (2003) Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 5, 1317–1327 [DOI] [PubMed] [Google Scholar]
- 23. Nizet V., Ohtake T., Lauth X., Trowbridge J., Rudisill J., Dorschner R. A., Pestonjamasp V., Piraino J., Huttner K., Gallo R. L. (2001) Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457 [DOI] [PubMed] [Google Scholar]
- 24. Tran D., Tran P., Roberts K., Osapay G., Schaal J., Ouellette A., Selsted M. E. (2008) Microbicidal properties and cytocidal selectivity of rhesus macaque θ defensins. Antimicrob. Agents Chemother. 52, 944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole A. M., Hong T., Boo L. M., Nguyen T., Zhao C., Bristol G., Zack J. A., Waring A. J., Yang O. O., Lehrer R. I. (2002) Retrocyclin: a primate peptide that protects cells from infection by T- and M-tropic strains of HIV-1. Proc. Natl. Acad. Sci. USA 99, 1813–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Münk C., Wei G., Yang O. O., Waring A. J., Wang W., Hong T., Lehrer R. I., Landau N. R., Cole A. M. (2003) The θ-defensin, retrocyclin, inhibits HIV-1 entry. AIDS Res. Hum. Retroviruses 19, 875–881 [DOI] [PubMed] [Google Scholar]
- 27. Cole A. L., Herasimtschuk A., Gupta P., Waring A. J., Lehrer R. I., Cole A. M. (2007) The retrocyclin analogue RC-101 prevents human immunodeficiency virus type 1 infection of a model human cervicovaginal tissue construct. Immunology 121, 140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seidel A., Ye Y., de Armas L. R., Soto M., Yarosh W., Marcsisin R. A., Tran D., Selsted M. E., Camerini D. (2010) Cyclic and acyclic defensins inhibit human immunodeficiency virus type-1 replication by different mechanisms. PLoS ONE 5, e9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang W., Cole A. M., Hong T., Waring A. J., Lehrer R. I. (2003) Retrocyclin, an antiretroviral θ-defensin, is a lectin. J. Immunol. 170, 4708–4716 [DOI] [PubMed] [Google Scholar]
- 30. Wang W., Mulakala C., Ward S. C., Jung G., Luong H., Pham D., Waring A. J., Kaznessis Y., Lu W., Bradley K. A., Lehrer R. I. (2006) Retrocyclins kill bacilli and germinating spores of Bacillus anthracis and inactivate anthrax lethal toxin. J. Biol. Chem. 281, 32755–32764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linzmeier R. M., Ganz T. (2005) Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in α- and β-defensin regions at 8p22–p23. Genomics 86, 423–430 [DOI] [PubMed] [Google Scholar]
- 32. Hollox E. J., Armour J. A., Barber J. C. (2003) Extensive normal copy number variation of a β-defensin antimicrobial-gene cluster. Am. J. Hum. Genet. 73, 591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aldred P. M., Hollox E. J., Armour J. A. (2005) Copy number polymorphism and expression level variation of the human α-defensin genes DEFA1 and DEFA3. Hum. Mol. Genet. 14, 2045–2052 [DOI] [PubMed] [Google Scholar]
- 34. Nuytten H., Wlodarska I., Nackaerts K., Vermeire S., Vermeesch J., Cassiman J. J., Cuppens H. (2009) Accurate determination of copy number variations (CNVs): application to the α- and β-defensin CNVs. J. Immunol. Methods 344, 35–44 [DOI] [PubMed] [Google Scholar]