While expansion of natural Tregs requires OX40-OX40L interaction, adaptive Treg induction requires TGF-β and TCR signaling.
Keywords: T cells, autoimmunity, Foxp3, OX40-OX40L, TGF-β
Abstract
In our earlier work, we had shown that GM-CSF treatment of CBA/J mice can suppress ongoing thyroiditis by inducing tolerogenic CD8α− DCs, which helped expand and/or induce CD4+Foxp3+ Tregs. To identify the primary cell type that was affected by the GM-CSF treatment and understand the mechanism by which Tregs were induced, we compared the effect of GM-CSF on matured spDCs and BMDC precursors in vitro. Matured spDCs exposed to GM-CSF ex vivo induced only a modest increase in the percentage of Foxp3-expressing T cells in cocultures. In contrast, BM cells, when cultured in the presence of GM-CSF, gave rise to a population of CD11c+CD11bHiCD8α− DCs (BMDCs), which were able to expand Foxp3+ Tregs upon coculture with CD4+ T cells. This contact-dependent expansion occurred in the absence of TCR stimulation and was abrogated by OX40L blockage. Additionally, the BMDCs secreted high levels of TGF-β, which was required and sufficient for adaptive differentiation of T cells to Foxp3+ Tregs, only upon TCR stimulation. These results strongly suggest that the BMDCs differentiated by GM-CSF can expand nTregs and induce adaptive Tregs through different mechanisms.
Introduction
EAT is a chronic inflammatory autoimmune disease of the thyroid that serves as a murine model of Hashimoto's thyroiditis, a common human autoimmune disease. It can be readily induced in CBA/J mice by immunizing with mTg emulsified in CFA [1]. The disease pathology is characterized primarily by lymphocytic infiltration of mTg-specific CD4+ T cells [2], leading to the destruction of thyroid follicles [3, 4]. Earlier, we showed that administration of GM-CSF, a pleiotropic cytokine and potent DC growth factor, can prevent and suppress ongoing EAT [5]. GM-CSF-induced suppression of EAT was associated with a selective expansion of CD4+CD25+ T cells (Tregs) that suppressed mTg-specific responses in vitro [5]. Additionally, we demonstrated that the CD4+CD25+ Tregs suppressed EAT through increased production of IL-10 [6]. These observations have been substantiated by Kong and colleagues [7], who have shown that CD4+CD25+ T cells from Tg-tolerized mice can suppress mTg-specific responses in vitro.
In a more recent study, we showed that GM-CSF acted primarily on DCs and caused an expansion of CD8α− DCs [8]. Adoptive transfer of these “tolerogenic” DCs from GM-CSF-treated donor mice to recipient mice, followed by immunization with mTg, led to an expansion of Tregs in the draining LNs and prevented the development of EAT. Interestingly, ex vivo treatment of matured spDCs with GM-CSF, followed by adoptive transfer, did not replicate these results (unpublished data), suggesting that the tolerogenic effect of GM-CSF may primarily be upon DC precursors in the BM.
In our studies, the molecular interactions between the GM-CSF-induced tolerogenic DCs and T cells that lead to Foxp3+ Treg expansion have, however, remained elusive. On the basis of their origin, two different types of Foxp3+ Tregs have been defined [9]: Foxp3+ nTregs are generated in the thymus through MHCII-dependent TCR interactions [9–11]. However, other studies have shown that Foxp3+ Tregs can also be generated in the periphery [12, 13]. These peripherally generated Tregs are commonly termed adaptive Tregs (iTregs). Although peripheral homeostasis of nTregs is not clearly understood, cytokine TGF-β plays a key role in the conversion of TCR-activated, naïve T cells to Foxp3+ adaptive Tregs [14, 15], and DCs have been shown to generate adaptive Tregs in the presence of TGF-β through PDL1 cosignaling [16].
In this study, we wanted to verify our hypothesis that the capacity of tolerogenic DCs for Treg expansion/induction was imparted through the effect of GM-CSF on BM precursor cells rather than on differentiated lymphoid organ resident DCs. We also wanted to know whether the “GM-CSF”-educated DCs caused an increase in the numbers of Foxp3+ Tregs by expanding nTregs in the periphery and/or adaptively converting Teffs to Foxp3+ Tregs upon antigen presentation in a mTg-abundant milieu. Our results show that although GM-CSF may cause some phenotypic changes in the differentiated peripheral DCs, its predominant tolerogenic effect was through the mobilization of BM precursors to develop into tolerogenic DCs. Moreover, although these tolerogenic DCs could expand nTregs upon direct cell-to-cell contact in the absence of antigenic stimulation, they could facilitate adaptive conversion of CD4+CD25– T cells into Tregs through cytokine secretion only upon TCR activation.
MATERIALS AND METHODS
Animals
Six- to 8-week-old CBA/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). WT C57/B6 and MHCII−/− mice were purchased from Taconic Farms (Germantown, NY, USA). Mice were housed in the Biological Resources Laboratory Facility at the University of Illinois (Chicago, IL, USA) and provided food and water ad libitum. All animal experiments were approved by the University of Illinois at Chicago Animal Care and Use Committee.
GM-CSF, antibodies, and Tg
rGM-CSF, CFSE, and neutralizing antibodies to TGF-β were purchased from Invitrogen (Carlsbad, CA, USA). Pacific blue-conjugated anti-CD4, FITC-conjugated anti-CD62L, and mouse IgG1 isotype control were purchased from Caltag Laboratories (San Francisco, CA, USA/Invitrogen). PE-conjugated anti-H-2Kd (MHCII), anti-CD25, anti-CD80, anti-CD86, anti-CTLA4, and anti-IL-10, streptavidin, PE-conjugated anti I-Ab (MHCII), PE-Cy7-conjugated anti-GITR, FITC-conjugated anti-CD8α, and isotype control mAb were purchased from BD PharMingen (San Diego, CA, USA). Allophycocyanin-conjugated anti-CD11c, anti-CD11b, anti-Foxp3, and anti-IL-17; biotin-conjugated anti-PDL1, anti-PDL2, and anti-CD3; and PE-conjugated anti-OX40L and OX40 agonist (OX86) were purchased from eBioscience (San Diego, CA, USA). Mouse thyroids were obtained from Pel-Freeze (Rogers, AR, USA), and Tg was prepared as described earlier [17]. rTGF-β and neutralizing antibodies to OX40L and IL-2 were purchased from R&D Systems (Minneapolis, MN, USA). Antibodies for cytokine measurement using ELISA (capture and detection antibodies for IFN-γ, IL-1β, IL-12, IL-6, and TGF-β) were purchased from eBioscience. Antibodies for serum Ig measurement by ELISA were purchased from Zymed (San Francisco, CA, USA), Invitrogen (anti-mouse-IgG1, anti-mouse-IgM), Caltag Laboratories (anti-mouse-IgG2B), and BD PharMingen (anti-mouse-IgG2A).
Spleen, BMDCs, and T cell subpopulation isolation
BM cells were cultured in complete RPMI containing 10% heat-inactivated FBS in the presence of 20 ng/ml GM-CSF for 3 days. On Days 4 and 6, fresh medium containing 20 ng/ml GM-CSF was added. Nonadherent CD11c+ DCs were sorted using anti-CD11c-coated magnetic beads, according to the manufacturer's directions (Miltenyi Biotec, Auburn, CA, USA), on Day 8. CD11c+ spDCs, isolated using the same protocol, were kept in culture in the presence (G-spDC) or absence (C-spDC) of GM-CSF for 2 days. CD4+, CD4+25−, CD4+25+ T cell subpopulations were isolated using appropriate kits and Auto Macs, according to the manufacturer's directions (Miltenyi Biotec).
Priming mice with mTg and OVA
Groups of CBA/J mice were immunized (three mice/group for each experiment) s.c. with OVA (100 μg/mouse) or mTg (100 μg/mouse), emulsified in CFA on Days 1 and 10.
Treatment of mice with GM-CSF
Two groups of three mice each were treated with PBS (control) or 2 μg GM-CSF/mouse/day for 5 consecutive days from Days 1 to 5 and 12 to 16 and then killed and spleen isolated.
In vitro cocultures of DCs and T cells
Each in vitro experiment was conducted in triplicates with T cells, spDCs, and BMDCs, pooled from three mice. BMDCs (5×104) and CD11c+ spDCs were cultured with CD4+, CD4+CD25−, and CD4+CD25+ T cells at the ratio of 1:2 for 5 days. For cultures involving activation of CD4+ T cells, anti-CD3 (2 μg/ml) was added for 48 h, or mTg (100 μg/ml) was added for 5 days. For proliferation assays, T cell subpopulations were labeled with CFSE at 10 μM, according to the manufacturer's instruction (Invitrogen), before coculturing them with DCs. Some cultures were supplemented with TGF-β (3 ng/ml), 1-MT (200 μM), anti-TGF-β (30 μg/ml), anti-PDL2 (5 μg/ml), anti-CD80 (5 μg/ml), anti-OX40L (up to 10 μg/ml), and OX40 agonist (OX86; 5–10 μg/ml).
Isolation of in vitro-generated nTregs and iTregs
CD4+CD25+ cells (nTregs) were purified from 5-day BMDC-T cell cocultures using cell separation kits (Miltenyi Biotec). Adaptive CD4+GARP+ (iTregs) was generated from the CD4+CD25− T cells supplemented with supernatant from BM culture and anti-CD3. The iTregs were sorted from this culture using GARP-PE as a marker. Briefly, total cells were stained with fluorochrome-labeled anti-CD4 and anti-GARP and then subjected to sorting.
Suppression assay
CD4+CD25− Teffs were isolated from spleen of OVA-treated mice, stained with CFSE, and plated into flat-bottom 96-wells at 0.5 × 106 cells/well in the presence of OVA (100 μg/ml) and splenic APCs. Isolated CD4+CD25+ nTregs or CD4+GARP+ iTregs were then kept in coculture with different ratios of CD4+CD25− T cells isolated from OVA-primed mice.
TMRM and PI staining
Cells from the BMDC-CD4+ T cell cultures were stained with TMRM (100 nM) for 15 min at 37°C. Total cells were stained with fluorochrome-labeled anti-CD4 for the analysis of T cells before incubating them with TMRM and PI. For TMRM staining, cells were then washed with ice-cold PBS and subjected to FACS analysis. Loss of TMRM staining was used as a marker of apoptosis, as it determines mitochondrial depolarization. In our assay, percentage of CD4+ T cells from the coculture retaining the TMRM stain was determined from FACS analysis as a measure of live cells. We also performed PI staining of cells for the assessment of the number of nonvital cells.
LPS treatment of BMDCs
After 8 days of culture of BM cells in GM-CSF, CD11c+ DCs were isolated and treated with 1 μg/ml LPS (Sigma-Aldrich, St. Louis, MO, USA) for 24 h. Cells were then washed in PBS twice and subsequently used for coculture experiment.
FACS
Freshly isolated and ex vivo-cultured cells were washed with PBS-BSA-EDTA. For surface staining, cells were labeled with appropriate FITC-, PE-, allophycocyanin-, and PE-Cy-conjugated antibodies for 30 min. For cell proliferation assay, the cells were similarly stained with CFSE. For intracellular staining, surface-stained cells fixed and permeabilized, according to the manufacturer's instructions (eBioscience), and incubated with appropriate antibodies. Stained cells were washed three times and analyzed by Cyan flow cytometer.
RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen). First-strand cDNA was synthesized with Superscript 2 (Invitrogen). Gene-specific primers were used for semi-qPCR amplification (0.5 min at 94°C, 0.5 min at 55°C, and 0.5 min at 72°C for 33 cycles) to detect the relative amount of transcripts. The following primer sets were used to amplify the indicated products: HPRT-F, GTTGGATACAGGCCAGACTTTGTTG; HPRT-R, TACTAGGCAGATGGCCAGGACTA; IL-1β-F, AAGAGCTTCAGGCAGGCAGTATCA; IL-1β-R, TAATGGGAACGTCACACACCAGCA; IL-6-F, AACCGCTATGAAGTTCCTCTCTGC; IL-6-R, TAAGCCTCCGACTTGTGAAGTGGT; IL-10-F, TGCACTACCAAAGCCACAAAGCAG; IL-10-R, TGGCCTTGTAGACACCTTGGTCTT; IL-12-F, ACCTGCTGAAGACCACAGATGACA; IL-12-R, TAGCCAGGCAACTCTCGTTCTTGT; IFN-α-F, TGAAGGACAGGCAGGACTTTGGAT; IFN-α-R, TGGCAGCAAGTTGACTGAGGAAGA; IFN-β-F, TCCAGCTCCAAGAAAGGACGAACA; IFN-β-R, AGAAACACTGTCTGCTGGTGGAGT; IFN-γ-F, CTGCATCTTGGCTTTGCAGCTCTT; IFN-γ-R, TTCGCCTTGCTGTTGCTGAAGAAG; IDO-F, TGGGCCCATGACATACGAGAACAT; IDO-R, TGCGAGGTGGAACTTTCTCACAGA; TNF-α-F, TTCCGAATTCACTGGAGCCTCGAA; TNF-α-R, TGCACCTCAGGGAAGAATCTGGAA; TGF-β-F, TGATACGCCTGAGTGGCTGTCTTT; TGF-β-R, TGTACTGTGTGTCCAGGCTCCAAA; OX40L-F, ATGTCTGCCTGCAACTCTCTTCCT; OX40L-R, CTTTGAAAGCCAAAGAGGCCACCA; PDL1-F, ACTTGTACGTGGTGGAGTATGGCA; PDL1-R, TGGCTGGATCCACGGAAATTCTCT.
Real-time PCR
Proinflammatory cytokine levels were compared by qPCR analysis using an ABI 7500 Fast Real-Time PCR system from Applied Biosystems (Foster City, CA, USA) with SYBR Green Master Mix (Applied Biosystems) in the 96-well plate format. Primers were designed using the online software of Integrated DNA Technologies (Coralville, IA, USA), and all primers have approximately equal efficiency of amplification. All samples were analyzed in triplicates and averaged. The amount of PCR product amplified was calculated relative to a standard curve. All of the calculations were done using Microsoft Excel software. The sequences of the primers used are: IDO-RT-F, TCTGTGAGAAAGTTCCACCTCGCA; IDO-RT-R, TTCCACATTTGAGGGCTCTTCCGA; TGF-β-RT-F, GTGCGGCAGCTGTACATTGACTTT; TGF-β-RT-R, TGTACTGTGTGTCCAGGCTCCAAA; IL-10-RT-F, TGCACTACCAAAGCCACAAAGCAG; IL-10-RT-R, AGTAAGAGCAGGCAGCATAGCAGT; IFN-γ-RT-F, GGCCATCAGCAACAACATAAGCGT; IFN-γ-RT-R, TGGGTTGTTGACCTCAAACTTGGC; IFN-β-RT-F, TTGCCATCCAAGAGATGCTCCAGA; IFN-β-RT-R, AGAAACACTGTCTGCTGGTGGAGT; IFN-α-RT-F, TCTGTGCTTTCCTCGTGATGCTGA; IFN-α-RT-R, ATCCAAAGTCCTGCCTGTCCTTCA; IL-1β-RT-F, AAGGGCTGCTTCCAAACCTTTGAC; IL-1β-RT-R, ATACTGCCTGCCTGAAGCTCTTGT; IL-12-RT-F, TGATGATGACCCTGTGCCTTGGTA; IL-12-RT-R, ATTCTGAAGTGCTGCGTTGATGGC; TNF-α-RT-F, TCTCATGCACCACCATCAAGGACT; TNF-α-RT-R, ACCACTCTCCCTTTGCAGAACTCA; HPRT-RT-F, AGGAGTCCTGTTGATGTTGCCAGT; HPRT-RT-R, GGGACGCAGCAACTGACATTTCTA; IL-6-RT-F, ATCCAGTTGCCTTCTTGGGACTGA; IL-6-RT-R, TAAGCCTCCGACTTGTGAAGTGGT.
Adoptive transfer
Three groups of three mice each were given two treatments 10 days apart with mTg (100 μg/ml) emulsified in CFA. Ten days after the last treatment, these mice received via i.v. injection PBS, 1 × 106-purified CD11c+ DCs from untreated CBA/J mice, or 1 × 106 CD11c BMDCs purified from in vitro BM cultures. Three identical adoptive transfers were done for each group at 5-day intervals. Five days after the last transfer, mice were killed, and spleens were collected for analyzing Treg percentages.
Serological assays and cytokine measurements
Analysis of serum Ig levels after adoptive transfer was done by ELISA using standard methods. Cell-free supernatants collected from DC cultures were tested for cytokines by ELISA, as per the manufacturer's directions (eBioscience). Measurement for IFN-γ from supernatants of DC-T cell cocultures was also measured by ELISA using a Th1/Th2 measurement kit (eBioscience). The amount of cytokine was determined using an appropriate cytokine-specific standard curve.
Statistical analysis
Mean, sd, and statistical significance were calculated using the Microsoft Excel application software. Statistical significance was determined using the one-tailed Student's t test. A P value of ≤0.05 was considered significant.
Online Supplemental Material
Five supplemental figures are provided. Supplemental Fig. 1, A and B, demonstrates that CD4+ T cells in cocultures of spDCs and BMDCs exhibit similar viability and a Th1/Th2 cytokine profile in culture but show increased Tregs. Supplemental Fig. 1C also shows that the GM-CSF concentration does not affect Treg percentages in cocultures, with or without spleen-derived APCs. Supplemental Fig. 2A provides a supporting qRT-PCR-based estimation of the fold difference in cytokine transcript expression between BMDCs and spDCs. Supplemental Fig. 2B shows that iTregs can be induced in vitro with APCs and mTg in the presence of BM culture supernatant. Supplemental Fig. 3A shows expression of CD25 on nTregs and iTregs, respectively. Supplemental Fig. 3B shows that GARP can be used as a surrogate marker for Foxp3+ iTregs. Supplemental Fig. 4 provides a comparison of the mTg-specific antibody response between different adoptive transfer groups. Supplemental Fig. 5A shows that nTreg expansion by BMDCs is not inhibited by blockage of TGF-β and IL-6. Supplemental Fig. 5B shows a densitometric analysis of RT-PCR products of HPRT and PDL1 transcripts resolved on agarose gel to compare their levels between spDCs and BMDCs. Finally, Supplemental Fig. 5C shows that LPS stimulation can cause an increase in the expression of OX40L on BMDCs and subsequently, lead to increased percentage of Foxp3+ Tregs in BMDC cocultures.
RESULTS
DCs derived from GM-CSF-treated BMDC precursors can increase Foxp3+ Tregs in T cell cocultures
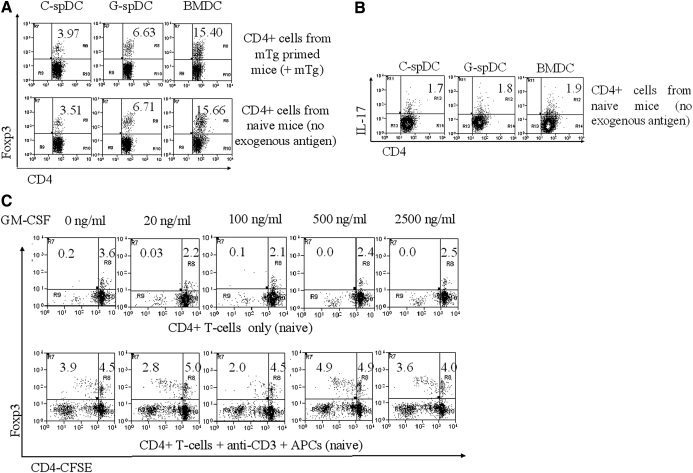
To test whether GM-CSF could differentially modulate differentiated spleen-derived CD11c+CD8α− (spDCs), we cultured DCs from 6- to 8-week-old naïve CBA/J mice in the presence (G-spDCs) or absence (C-spDCs) of GM-CSF for 48 h. CD4+ T cells from naïve or mTg-immunized mice were cocultured with G-spDCs, C-spDCs, or BMDCs, derived by culturing BM cells in the presence of GM-CSF for 7 days and analyzed for Foxp3+ Tregs (Fig. 1A). When cocultured for 5 days with CD4+ cells from mTg-immunized mice in the presence of mTg (100 μg/ml; Fig. 1A, upper panel), the G-spDCs showed only a modest increase (6.63±1.03%; P∼0.02) in the percentage of Foxp3+ cells compared with C-spDCs (3.97± 0.94%; Fig. 1A, upper panel). However, the BMDC-CD4+ T cell coculture showed a highly significant increase (15.40±0.48%) in the percentage of Foxp3+ T cells (four- to fivefold; P<0.001). This phenomenon was consistent (C-spDCs=3.51±0.88%; G-spDCs=6.71±1.5%; P∼0.2; BMDCs=15.66±0.96%; P<0.001), even when the CD4+ T cells derived from naïve mice were cocultured without the addition of any exogenous antigen (Fig. 1A, lower panel). These results indicated that the tolerogenic effect of GM-CSF is primarily mediated through its effects on BMDCs. Therefore, we focused our studies on understanding the mechanism of action of BMDCs in expanding/inducing Foxp3+ Tregs.
Figure 1. GM-CSF-derived BMDCs can increase the percentage of Foxp3+ Tregs in cocultures.
spDCs were isolated and cultured with or without GM-CSF for 48 h. BMDCs were generated in vitro with GM-CSF. C-spDCs, G-spDCs, and BMDCs were (A) cocultured with CD4+ T cells from mTg-primed mice in the presence of mTg (upper panel) or naïve mice without antigen (lower panel) and stained with FITC-labeled anti-CD4 and allophycocyanin-labeled anti-Foxp3 for FACS analysis. Each scatter plot is representative of five independent experiments gated over 3500 live CD4+ T cells. Each in vitro experiment was conducted with T cells, spDCs, and BMDCs pooled from three mice. (B) C-spDCs, G-spDCs, and BMDCs were cocultured with CD4+ T cells from naïve mice without antigen for 5 days and stained for the expression of CD4 and IL-17 by FACS. (C) CFSE-labeled CD4+ T cells were cultured in different concentrations of GM-CSF ranging from 0 to 2500 ng/ml in the presence and absence of anti-CD3/APCs for 4 days and analyzed for Foxp3 expression by FACS. The numbers indicate the percentage of double-positive (CD4+Foxp3+) T cells. The experiment was repeated three times with similar results.
To rule out any nonspecific effect, such as increased, naïve T cell differentiation by BMDCs, we set up cocultures of naïve T cells with C-spDCs, G-spDCs, and BMDCs without exogenous antigen and analyzed for IL-17-secreting Th17 cells. After 5 days of coculture, we did not see any difference in the percentage of IL-17+ T cells among any of these groups (Fig. 1B). In typical experiments, the percentage of Foxp3+ Tregs was reduced in the spDC cocultures over the course of 5 days from its initial level, and it increased in BMDC cocultures (Supplemental Fig. 1A). In contrast, the percentage of IL-17+ T cells remained similar for all of the groups.
We stained for IFN-γ and IL-4 to determine skewing, if any, toward a Th1 or Th2 phenotype. We found that the percentage of IFN-γ- or IL-4-producing T cells was similar between the groups (Supplemental Fig. 1B). We measured the percentage of necrotic and apoptotic cells by staining with PI and TMRM, respectively, and found that the Treg increase in cocultures could not be attributed to non-Treg death (Supplemental Fig. 1B). Based on these findings, we concluded that there was a real increase in the numbers of Foxp3 Tregs in BMDC cocultures.
In a recent study, it has been proposed that Tregs express a functional GM-CSFR α-chain CD116 and proliferate in response to GM-CSF [18]. To see if the tolerogenic effect of GM-CSF was a consequence of its direct action on T cells, we set up naïve CD4+ T cell cultures stained with CFSE, with or without APCs (splenocytes depleted of CD4+ T cells served as APCs) and treated them with varying concentrations of GM-CSF (Fig. 1C). In the absence of TCR stimulation and APCs, no increase in the CD4+ T cell proliferation was noted (Fig. 1C, upper panel) after 5 days of culture. Although there was proliferation of Foxp3+ and Foxp3− T cells in the presence of anti-CD3 and splenic APCs (Fig. 1C, lower panel), there was no significant difference in the percentages of Foxp3+ cells between GM-CSF-treated and untreated cultures. When we cocultured T cells with APCs in the absence of antigenic stimulus or in the presence of anti-CD3 alone or anti-CD3 along with anti-CD28 (Supplemental Fig. 1C), we found no correlation between GM-CSF concentration and increase of Tregs in vitro. These data supported the notion that GM-CSF does not directly cause selective expansion of Foxp3+ Tregs in the presence or absence of APCs and/or TCR activation.
The capacity of CD11c+ BMDCs to increase Foxp3+ Tregs in T cell cocultures is primarily contact-dependent
We tested to see if the “tolerogenic phenotype” was a characteristic of all BM-derived cells or that of GM-CSF-induced CD11c+ BMDCs alone. First, we followed the differentiation of BMDCs by scoring for the percentage of CD11c+ cells in BM cultures at Days 2 (∼5%), 4 (∼12%), 7 (∼51%), and 11 (∼95%; Fig. 2A, upper panel). The CD11c+ BMDC population developed almost exclusively from the CD11b+ precursors and was found to be CD8α− (Fig. 2, A and B, upper panel). Using BMDCs from each of these time-points, we set up T cell cocultures for 5 days without TCR stimulation and found a direct correlation between the percentages of Tregs and CD11c+ cells in BM cultures (Fig. 2A, lower panel). Further, we separated CD11c+ cells from CD11c− cells (Fig. 2B, lower panel) and cocultured them with naïve CD4+ T cells for 5 days without antigen. We found that cocultures with GM-CSF-derived CD11c+ BMDCs resulted in an increase of Foxp3+ cells, which were considerably more (17.4±1.0%) than that noted in the presence of GM-CSF-derived CD11c− cells (10.7±0.6%; P=0.004; Fig. 2B, lower panel). These data suggested that although other BM-derived cells may have the capacity to expand/induce Tregs in cocultures, CD11c+ BMDCs have a more potent ability to induce Tregs.
Figure 2. CD11c+ BMDCs increase the percentage Foxp3+ Tregs in cocultures primarily through a contact-dependent mechanism.
(A) BM cells were analyzed for the expression of CD11b and CD11c on Days 2, 4, 7, and 11 (upper panel). BM cells obtained from the respective days were cocultured with CD4+ T cells, and after 5 days, the cells were analyzed for Foxp3 expression by FACS (lower panel). (B) CD11c and CD8α expression on cells from GM-CSF-derived BMDCs (upper panel). CD11c+ and CD11c− cells from GM-CSF cultures were cocultured with CD4+ cells from naïve mice and percentage of CD4+Foxp3+ T cells from the coculture analyzed (lower panel). (C) Cocultures of BMDCs with CD4+ T cells, together or separated by transwell, were analyzed for Foxp3 expression without anti-CD3 (upper panel) or with anti-CD3 (lower panel). In transwell cocultures, CD4+ T cells were cultured in the bottom wells, and the BMDCs were cultured in the top wells. Numbers indicate the percentage of double-positive CD4+Foxp3+ T cells. Experiments A–C were repeated at least three times with similar results.
The BMDC-induced increase in the percentage of Tregs could be contact-dependent and/or cytokine-driven. To address this question, we set up cocultures of BMDCs with naïve T cells in direct contact or in transwells, where there is fluid exchange but no cell-to-cell contact. We found that the capacity of BMDCs to cause an increase in Tregs without antigenic stimulation was contact-dependent (12.6±0.7% Tregs), as BMDCs failed to exhibit this property when cultured in transwells (0.74±0.1% Tregs; Fig. 2C, upper panel). However, when the T cells were stimulated with anti-CD3 in the presence of BMDCs in transwell, it was able to cause an increase in Treg percentages (6.3±0.05% Tregs; P=0.002 vs. C-spDCs; Fig. 2C, lower panel), although not to the same extent as BMDCs (10.10% Tregs) when in contact with T cells. This also suggested that cytokines secreted by these BMDCs could facilitate induction of Tregs upon TCR stimulation.
Under antigenic stimulation, supernatants from BMDC cultures can adaptively convert Foxp3− T cells to Foxp3+ Tregs in a TGF-β-dependent manner
To understand how BMDC-secreted cytokines could facilitate an increase in Tregs upon anti-CD3 stimulation, we analyzed the transcript levels of various cytokines in Day 7 BMDCs by semi-qRT-PCR and compared them with C-spDCs and G-spDCs (Fig. 3A). We found reduced transcript levels of all tested, proinflammatory cytokines (IL-1β, IL-12, TNF-α, IFN-γ, and IL-6) in BMDCs, as compared with C-spDCs or G-spDCs. Interestingly, the level of transcript for IDO, which has been implicated in Treg generation [19], was increased in G-spDCs, and it was lower in BMDCs. In contrast, we observed elevated levels of TGF-β transcripts in BMDCs. Using real-time PCR, we quantified the TGF-β transcripts to be threefold higher in BMDCs than in spDCs (P<0.01), and IL-6 transcripts were reduced more than sixfold from that found in C-spDCs (P<0.001), as were other proinflammatory cytokines such as IL-12, IFN-β, and IFN-γ (P<0.001; Supplemental Fig. 2A). We also compared the levels of secreted TGF-β in the supernatants of these cultures by ELISA. Although we detected a wide range of TGF-β concentrations in different BMDC supernatants (150–350 pg/ml), we failed to detect any TGF-β in the spDC supernatants. On the contrary, although we estimated 50–75 pg/ml IL-1β and IL-12 in the spDC cultures, we could not detect any in the BMDC cultures (data not shown).
Figure 3. Contact-independent induction of adaptive Tregs in vitro by BM supernatant is TGF-β-dependent.
(A) RT-PCR analysis of cytokine transcripts from BMDC and spDC cultures. The two bands in each category of 1, 2, and 3 indicate the transcript levels after 31 and 33 PCR cycles. (B) CFSE-labeled CD4+CD25− T cell cultures, supplemented with TGF-β or BM culture supernatant (SUP; 1×), were stained with allophycocyanin-labeled anti-Foxp3 in the absence (upper panel) and presence (lower panel) of anti-CD3. (C) CFSE-labeled CD4+CD25− T cells were cultured with different concentrations of BM supernatant and anti-CD3. The induction of Foxp3+ in T cells in the presence of increasing concentrations of BM supernatant (upper panel) and its inhibition by different concentrations of anti-TGF-β (lower panel) are shown. Experiments B and C were repeated three times with similar results.
As TGF-β has been shown to induce iTregs [14, 15], we wondered whether the TGF-β, present in the BMDC supernatant, could be responsible for Treg differentiation and/or adaptive Treg generation in vitro. Therefore, we labeled CD4+CD25− cells from naive mice with CFSE and cultured them in the presence of BMDC supernatant or TGF-β, with and without TCR stimulation. In the absence of TCR stimulation (Fig. 3B, upper panel), neither BMDC supernatant nor TGF-β caused any appreciable conversion of Foxp3− T cells to Foxp3+ Tregs. However, upon anti-CD3 stimulation, there was significant adaptive conversion of Foxp3− T cells to Foxp3+ Tregs in the presence of BMDC supernatant (P<0.01), as well as TGF-β (P<0.01; Fig. 3B, lower panel). In the presence of different concentrations of BMDC supernatant and soluble anti-CD3, we found a dose-dependent conversion of Foxp3− cells to Foxp3+ Tregs (Fig. 3C, upper panel). Further, in the presence of anti-CD3 and 4× BM supernatant, a TGF-β-neutralizing antibody (anti-TGF-β) abrogated the adaptive conversion of Tregs, not only at the recommended dose of 50 μg/ml but also when used at a lower concentration of 12.5 μg/ml (P<0.01 for both doses), without affecting the proliferation of Foxp3− cells (Fig. 3C, lower panel). A mouse IgG1 isotype control antibody failed to demonstrate this inhibition at a concentration of 20 μg/ml. These data indicated that BMDC supernatant-mediated adaptive conversion of Foxp3− T cells to Foxp3+ Tregs was primarily through TGF-β.
We found that the BM supernatant could also be used to adaptively convert mTg-specific Tregs. When we cultured CD4+CD25− T cells isolated from mTg-immunized mice in the presence of splenic APCs and mTg, supplemented with 4× concentration of BM supernatant, ∼1.5% Foxp3 cells were detected after 72 h (Supplemental Fig. 2B). Although the percentage of Foxp3+ T cells was low, likely as a result of low frequency of mTg-specific T cells, nevertheless, these data suggest that it might be possible to induce antigen-specific Tregs.
BMDCs can selectively expand Foxp3+ Tregs
To understand whether the increase in the percentage of Tregs in cocultures of T cells with BMDC was a result of an expansion of the pre-existing Foxp3+ Tregs or an adaptive conversion of Foxp3− T cells to Foxp3+ cells, we stained total CD4+ T cells with CFSE and set up cocultures with BMDCs in the absence of antigenic stimulation. We found that in the presence of C-spDCs, neither the Foxp3+ nor the Foxp3− population showed appreciable proliferation (<1%; Fig. 4A). In contrast, when cultured with BMDCs, only the CD4+Foxp3+ population underwent robust expansion (11.4±0.7%; P=0.001). At least seven divisions were observed during a 5-day culture (Fig. 4A, lower panels). It is known that Tregs express the IL2-Rα (CD25), which is also a marker for T cell activation. Therefore, we asked if this selective expansion was dependent on the state of activation of the T cells. We found that a majority of the Foxp3+ Tregs after expansion was also CD25+, and only a small percentage of Foxp3− cells was CD25+ (Supplemental Fig. 3A). We found that the Foxp3− T cells failed to proliferate, irrespective of whether they were CD25+ or CD25− (Fig. 4B, top panel). In contrast, CD25+Foxp3+ and CD25−Foxp3+ cells proliferated (Fig. 4B, middle and bottom panels). However, we failed to observe any significant, adaptive conversion of naïve or Teffs to Tregs in BMDC cocultures (Fig. 4C). From this, we concluded that the ability of BMDCs to increase Tregs was primarily dependent on their ability to selectively expand pre-existing Foxp3+ T cells (nTregs) in a contact-dependent manner.
Figure 4. BMDCs can directly and selectively expand nTregs in T cell cocultures.
(A) spDCs and BMDCs were cocultured with CFSE-labeled CD4+ T cells from naïve mice and analyzed for proliferation and Foxp3 expression. Small panels on the right show the extent of CFSE dilution of Foxp3+ and Foxp3− cells in the original histograms. (B) The extent of CFSE dilutions in different T cell subpopulations is measured by gating on Foxp3+/− or CD25+/− T cells. The top panel shows the position of the gate, middle panel shows the gated population, and bottom panel shows the extent of CFSE dilution of the gated population. (C) CFSE-labeled CD4+CD25− T cells were cocultured with control (spDCs), with or without BM culture supernatant (BM sup), BM cells [BM (day 0)], or BMDCs, and analyzed for Foxp3 expression and CFSE dilution. (A–C) Each scatter plot is representative of five separate experiments.
The contact-mediated expansion of Foxp3+ cells in vitro is OX40L-dependent
We characterized the BMDCs for their expression of costimulatory molecules and compared them with G-spDCs and C-spDCs to gain some insight into the basis for their increased tolerogenic phenotype (Fig. 5A). The percentage of cells between C-spDCs and G-spDCs expressing CD80, CD86, MHCII, and PDL1 was comparable, and G-spDCs showed a higher percentage of PDL2 expression relative to the C-spDCs. The BMDCs, on the contrary, had higher percentages of CD80 and CD86 and lower percentage of PDL1 and PDL2 relative to G-spDCs. These data suggested that the differences in the expression of CD80/86 or PDL½ between G-spDCs and BMDCs could be contributing to the increased tolerogenic effect of BMDCs.
Figure 5. The selective expansion of Tregs in BMDC cocultures is OX40L-dependent.
(A) BMDCs and spDCs, cultured in the presence or absence of GM-CSF, were analyzed for the expression of CD80, CD86, MHCII, PDL1, and PDL2. Each scatter plot represents three independent experiments. (B) Coculture of BMDCs and CD4+ T cells, in the presence of various blocking and neutralizing antibodies to anti-inflammatory cytokines or cell surface molecules, was stained and analyzed for Foxp3 expression. (C) RT-PCR analysis of OX40L and PDL1 from BMDC and spDC cultures. The two bands in each category indicate the transcript levels at 31 and 33 PCR cycles. HPRT is shown as control. (D) Analysis of surface expression of OX40L in spDCs and BMDCs. (E) Coculture of BMDCs and CFSE-labeled CD4+ T cells in the presence of increasing concentrations of a neutralizing antibody to OX40L was analyzed for CFSE dilution and Foxp3 expression in T cells. (F) Cocultures of BMDCs and CD4+ T cells in the presence of anti-OX40L antibody, alone or in combination with OX40 agonist at two different concentrations (lo/low=5 μg/ml and hi/high=10 μg/ml). (D–F) Each scatter plot represents five separate experiments.
We used blocking antibodies and inhibitors to determine the relative importance of some of these molecules in Treg expansion (Fig. 5B). Our earlier study had shown that a preferential ligation of CD80 resulted in IL-10-dependent Treg induction [20]. Therefore, we blocked CD80 using an appropriate antibody, and this blockage appeared to show only a partial abrogation of Treg expansion (from 14.10% to 9.25%). The PDL1 expression was high in C-spDCs (95.1%) relative to BMDCs (64.6%), and therefore, we assumed that this molecule is unlikely to play a role in Treg induction by BMDCs. Although the percentage of cells expressing PDL2 was comparable in C-spDCs (39.0%) and BMDCs (42.2%), an earlier study had implicated PDL2 as a negative regulator of T cell activation [21]. Hence, we used a blocking antibody to PDL2 to further investigate its role in Treg expansion. Blocking PDL2 or addition of 1-MT, a negative regulator of indole deoxygenase and hence, tryptophan catabolism, failed to inhibit Treg induction.
As BMDCs selectively expanded Tregs in a contact-dependent manner, we asked whether it depended on the interaction of a Treg-specific molecule with the corresponding ligand on DCs. Tregs constitutively express OX40 on their surface [22, 23], and the OX40L is not constitutively expressed on DCs but can be induced [24]. Therefore, we analyzed the expression of OX40L transcripts in BMDCs and spDCs (Fig. 5C). We found that BMDCs expressed OX40L transcripts, and spDCs did not. Direct staining of OX40L on the surface of spDCs and BMDCs (Fig. 5D) confirmed that only a small fraction of spDCs expressed OX40L (4.5±0.7%), and a significantly higher percentage of BMDCs expressed OX40L (29.7±2.8%; P<0.01). Interestingly, the expression of OX40L in CD11c− cells in the BM cultures was also negligible (2.5±0.2%).
Based on the above findings, we used a blocking antibody against OX40L at three different concentrations (lo=2.5 μg/ml; mid=5 μg/ml; hi=10 μg/ml) to see if it could abrogate Treg expansion by BMDCs. Although the BMDC-positive control predictably drove the expansion of nTregs in cocultures (10.1±0.5% dividing cells, as measured by CFSE dilution), when supplemented by the OX40L-blocking antibody, the expansion was inhibited (lo=3.4±0.4%, med=2.3±0.2%; hi=1.1±0.1%) in a dose-dependent manner (P<0.01 in all cases; Fig. 5E), while leaving the percentage of nondividing nTregs unaffected (4.3–5.9%). Further, when we added back an OX40 agonist at two different concentrations (lo=5 μg/ml; hi=10 μg/ml) in combination with the OX40L-blocking antibody, we observed significant reversal of the inhibition of Treg expansion (Fig. 5F). Although the anti-OX40L reduced the proliferation of Tregs from 11.0 ± 0.5% in control to 2.1 ± 0.1%, increasing concentrations of the OX40 agonist (OX86) revived the proliferation from 3.4 ± 0.3% (lo, P<0.02, vs. anti-OX40L) to 8.2 ± 0.4% (hi, P<0.001, vs. anti-OX40L). These data strongly indicated that OX40-OX40L signaling is required for the expansion of nTregs by BMDCs.
Expansion of nTreg by BMDCs is TCR-independent but requires IL-2
Although we observed antigen-independent but OX40-OX40L interaction-dependent Treg expansion in BMDC cocultures, we did not know if this interaction required TCR activation. Additionally, as Foxp3+ Tregs are known to be dependent on IL-2 for survival in vitro, we wanted to determine the source of IL-2. First, we found that the BMDC-mediated Treg expansion in vitro could be abrogated by an anti-IL-2 antibody (Fig. 6A), which indicated that IL-2 was required (P<0.01). We then proceeded to sort for CD4+CD25+ T cells from naïve mice (which constituted the bulk of the nTregs) and set up cocultures with BMDCs, with and without IL-2 (Fig. 6B). Although the BMDCs predictably expanded Tregs in vitro from CD4+ cell cocultures (8.9±0.8% divided cells vs. 6.3±1.1% undivided cells), as measured by CFSE dilution, they could not efficiently expand the sorted CD4+CD25+ subset (8.2±0.5% divided cells vs. 63.8±4.5% undivided cells). However, when IL-2 was added to the cocultures, it not only increased proliferation of Tregs in CD4+ T cell cocultures (18.6±1.7% divided cells vs. 5.0±0.7% undivided cells; P<0.01 vs. splenic APCs with IL-2) but also restored efficient expansion of sorted CD4+CD25+ subsets (73.2±1.2% divided cells vs. 22.7±1.7% undivided cells; P<0.01 vs. splenic APCs with IL-2). We concluded that the CD4+CD25+ T cells were incapable of making IL-2, which is essential for their efficient expansion. The BMDCs were themselves not able to provide this required IL-2, as the CD4+CD25+ subset did not expand efficiently unless exogenous IL-2 was provided. However, in total CD4+ T cell cocultures, they were able to proliferate, as the IL-2 was most likely produced by the CD4+CD25− T cells. Interestingly, addition of exogenous IL-2 caused minor Treg expansion in splenic APC cocultures with CD4+ (2.4±0.1% divided cells vs. 9.0±0.8% undivided cells) and CD4+CD25+ T cells (9.0±0.8% divided cells vs. 67.3±2.8% undivided cells). We observed some loss of Foxp3 expression in the CD4+CD25+ T cell cocultures that were not supplemented with IL-2. These results showed that IL-2 was necessary for the maintenance of Foxp3+ status and expansion of Tregs in vitro.
Figure 6. BMDC-mediated Treg expansion is dependent on IL-2 but does not require TCR interaction.
(A) Abrogation of Treg proliferation by anti-IL-2. BMDCs were cocultured with CD4+ cells, without or with anti-IL-2. (B) Treg expansion is IL-2-dependent. BMDCs and control splenic APCs were cocultured with total CD4+ (upper panel) and sorted CD4+CD25+ (lower panel) T cells in the presence or absence of IL-2. On Day 4, cultures were analyzed for the CFSE dilution of Foxp3-expressing T cells by FACS. (C) Analysis of surface expression of CD11b, CD80, MHCII, and PDL2 on BMDCs of MHCII−/− mice. (D) BMDCs from C57/B6 mice can also expand Tregs. BMDCs from WT C57/B6 mice were used in cocultures with CD4+ T cells, also derived from WT C57/B6 mice. Splenic APCs were used as a negative control. (E) Treg expansion by BMDCs is TCR-independent. BMDCs from MHCII−/− mice were generated in vitro with GM-CSF, cocultured with CFSE-labeled CD4+ cells (upper panel) or CD4+CD25+ cells (lower panel) in the presence or absence of IL-2. In some cultures, anti-OX40L antibody was added as indicated. (A–E) Experiments were repeated three times with similar results.
We wanted to investigate the role of TCR in this BMDC-mediated Treg expansion. As TCR interactions of CD4+ T cells require presentation of antigen in the context of MHCII molecules, we decided to use BMDCs from MHCII-deficient mice. GM-CSF-cultured BMDCs from these mice were similar to BMDCs from CBA/J mice with respect to the expression of most surface molecules we tested (Fig. 6C), except for MHCII, which was not detected. At first, we set up cocultures of WT C57/B6 BMDCs with native CD4+ Tregs and found that they selectively expanded Tregs in vitro, just as we saw in the case of BMDCs from CBA/J mice (Fig. 6D). We then proceeded to set up BMDC cocultures with total CD4+ T cells or sorted CD4+CD25+ T cells from naïve WT C57/B6 mice (Fig. 6E). We found that MHCII−/− BMDCs failed to expand Tregs in CD4+ T cell (0.5±0.03% divided vs. 5.2±0.6% undivided) or CD4+CD25+ Treg cocultures (6.5±0.3% divided cells vs. 62.4±1.7% undivided cells). However, adding exogenous IL-2 restored Treg expansion in both cases (10.3±1.2% divided cells vs. 1.5±0.1% undivided cells in CD4+ cocultures and 79.8±4.2% divided cells vs. 13.31.2% undivided cells in CD4+CD25+ T cell cocultures; P<0.01 vs. BMDC cocultures without IL-2). This indicated that the production of IL-2 by the CD4+CD25− cells required MHCII-TCR interaction, but the Treg expansion itself did not. Furthermore, addition of anti-OX40L to the IL-2-supplemented cultures significantly reduced the proliferation of Tregs for total CD4+ cocultures (3.3±0.1% divided cells vs. 3.5±0.4% undivided cells; P<0.01 for dividing cells with respect to corresponding coculture without antibody) and CD4+CD25+ T cell cocultures (32.3±1.6% divided cells vs. 52.2±2.1% undivided cells; P=0.001 for dividing cells with respect to corresponding coculture without antibody). In contrast, we found little or no proliferation of Tregs when in culture with MHCII-deficient, splenic APCs. These data showed conclusively that the specific expansion of Tregs from a population of total CD4+ T cells in vitro by GM-CSF-cultured BMDCs is independent of TCR/antigen presentation but was dependent on the production of IL-2 by CD4+CD25− T cells present in the CD4 population, which in turn, required TCR/MHCII interaction.
In vitro-expanded nTregs can suppress Teff proliferation in vitro
We further characterized Tregs generated in vitro in the absence of TCR stimulation for their expression of key Treg-specific surface markers and cytokines. A majority of expanded nTregs (upper panels) and adaptive iTregs (lower panels) was CTLA4+ and GITR+ but surprisingly, IL-10− and TGF-β− (Fig. 7A). Although a majority of the expanded nTregs was positive for CD62L, the iTregs were mostly negative for this marker.
Figure 7. In vitro-expanded Tregs can suppress Teff proliferation.
(A) CD4+Foxp3+ T cells from cocultures of BMDC and total CD4+ T cells (nTregs, upper panel) or TCR-activated CD4+CD25− T cells (iTregs, lower panel), supplemented with BM culture supernatant, were stained for different cell surface markers. (B) Histograms show the proliferation of CFSE-labeled CD4+CD25− T cells from OVA-immunized mice in the presence of in vitro-generated CD4+CD25+ T cells (nTregs) and CD4+GARP+ T cells (iTregs) isolated from BMDC cocultures or TGF-β-supplemented cultures added in different ratios. The numbers in the gated population of the histograms indicate the percentage of cells proliferated. Results shown are representative of three independent experiments.
TGF-β-generated adaptive Tregs are known to be suppressive in vitro and in vivo [14, 15]. We wanted to see if the in vitro-expanded nTregs could also suppress Teff proliferation and compared them with the suppressive capacities of the iTregs. In the absence of TCR stimulation, the expanded nTregs were a major fraction of the CD25+ T cells and were therefore isolated on the basis of CD25 expression. The iTregs were not easily separable from Foxp3− T cells, as anti-CD3-activated Teffs express CD25, and the bulk of the CD25+ cells after anti-CD3 stimulation was Foxp3− (Supplemental Fig. 3A). Therefore, we used mouse GARP as a surrogate marker for activated Tregs [25]. We cultured naïve CD4+CD25− T cells in vitro and stimulated them with anti-CD3 and BM culture supernatant (4× concentrated), which induced them to become Foxp3+ Tregs (Supplemental Fig. 3B). We found that out of a total of 8.1% Foxp3+ Tregs, 5.0% was GARP+, and 1.3% was GARP+ but Foxp3−. We sorted for GARP+ cells and assumed that they represented the majority of Foxp3+ iTregs. Mice were immunized with 100 μg OVA to induce an antigen-specific Teff response, which we monitored through the emergence of serum antibodies to OVA. We then isolated CD4+CD25− T cells from these immunized animals, stained them with CFSE, and set up cocultures with splenic APCs in the presence of OVA, with or without nTregs (CD4+CD25+) or iTregs (CD4+GARP+), generated in vitro from naïve T cells. CD25− cells from OVA-treated mice (Fig. 7B) proliferated (19.7±2.1%) only in the presence of OVA. However, OVA-induced proliferation was suppressed when CD25+ nTregs were added at a 1:1 (5.4±0.4%; P<0.01 against OVA-only control and GARP+ iTregs at 1:1) or 1:2 (10.4±1.5%; not significant against iTregs at 1:2) ratio of Tregs:Teffs. At a 1:4 ratio, however, the proliferation was not inhibited (17.2±1.6%). GARP+ iTregs showed a lower capacity of suppression at 1:1 (11.9±0.8%; not significant against OVA-only control), and it failed to show suppression at higher ratios. These results indicated that Tregs, expanded or induced in vitro, can suppress Teff proliferation in an antigen-independent, bystander manner, although at different capacities.
The in vivo tolerogenic effect of GM-CSF is mediated by a special class of CD8α− DCs that differentiate from BM precursors
We have seen that BMDCs can expand Tregs in vitro. However, we did not know if these tolerogenic DCs are mobilized in vivo upon GM-CSF treatment. Therefore, we treated mice with GM-CSF to see if it led to an increase in CD11c+ CD11bHi cells in the spleen (Fig. 8A). Indeed, the percentage of CD11c+CD11bHi cells was much higher in GM-CSF-treated mice (0.5±0.04%) than PBS-treated controls (0.2±0.04%). When we gated on the double-positive population, we found that almost all of them (92–98%) were CD8α−. The percentage of Foxp3+ T cells was also higher in GM-CSF-treated mice (12.7±0.9%) than in PBS-treated controls (8.9±0.8%; P<0.01). These data suggest that GM-CSF most likely acted on BM precursors and mobilized the development of a class of DCs (CD11c+CD11bHiCD8α−), which then populated the lymphoid organs and might have contributed to the expansion of nTregs. Therefore, we wanted to see if the BMDCs could also expand Tregs in vivo.
Figure 8. GM-CSF treatment leads to the development of CD11b+CD11c+.
tolerogenic DCs in vivo. (A) Mice were treated with GM-CSF for 5 consecutive days for 2 weeks, and spleen cells were stained with FITC-labeled anti-CD11c, allophycocyanin-labeled anti-CD11b, FITC-labeled anti-CD4, and allophycocyanin-labeled anti-Foxp3 and analyzed by FACS. The middle panels indicate the percentage of double-positive CD11b+ CD11c+ cells that are CD8α−. The right panels indicate the percentage of CD4+Foxp3+ T cells from the control mice and GM-CSF-treated mice. Each scatter plot represents five different experiments. (B) Bar graph indicates the percentage of Foxp3+ Tregs in mice immunized with antigen (mTg+CFA), followed by adoptive transfer of buffer, spDCs, or BMDCs. Each column represents the mean ± sd of an experiment conducted with three animals in each group. ***, Statistically significant value.
We first immunized three groups of mice with mTg and confirmed a mTg-specific IgG response in the sera. Ten days after the last treatment, these mice were adoptively transferred i.v. with PBS (buffer), 1 × 106-purified CD11c+ DCs from untreated mice (spDC), or 1 × 106-purified CD11c+ BMDCs. A total of three identical adoptive tranfers, 5 days apart, was done for each group. Five days after the last transfer, mice were killed, and spleens were analyzed for Treg percentages. Relative to buffer (10.4±0.1% Tregs), the spDC treatment did not lead to an increased percentage of Tregs (10.1±0.5% Tregs), and the BMDCs did (14.4±0.3%; P<0.01 against both groups; Fig. 8B). We did not find any significant reduction of mTg-specific IgG responses in the sera of these mice upon adoptive transfer (Supplemental Fig. 4). An elevated, mTg-specific IgM level in the BMDC-transferred group indicated that a modulation of the Ig response may have just been initiated in these mice as a result of the increased percentage of Tregs. However, as our experiment was terminated on Day 35, we may not have allowed enough time for the IgG levels to be reduced significantly. These results indicated that BMDCs could also facilitate expansion of Tregs in vivo.
DISCUSSION
We have successfully used GM-CSF for the treatment of a range of autoimmune conditions in mice including EAT [5], experimental autoimmune myasthenia gravis [26], and type 1 diabetes [27] involving CBA/J, C57/B6, and NOD strains of mice, respectively. In all cases, we had observed a significant increase in the splenic Treg population. Results from adaptive transfer of DCs from—and naïve T cells from WT mice into—GM-CSF-treated and untreated scid mice led us to conclude that the increase in Tregs was mediated through CD8α− DCs from GM-CSF-treated and not untreated mice [8]. The present study was initiated to determine whether the GM-CSF effect was primarily on BM cells, which then migrated to the spleen or directly on spDCs. Although we found that G-spDCs could increase the Treg population, the BMDCs were more effective in increasing the proportion of Tregs in DC/T cell cocultures (Fig. 1). Furthermore, our data rule out any significant, direct effect of GM-CSF on T cells in the presence or absence of TCR stimulation. This conclusion is also supported by a recent study using T cells deficient in the common β-receptor for GM-CSF [28].
Results from our transwell experiments exposed two distinct mechanisms of action of BMDCs that cause Treg expansion (Fig. 2). The supernatant from BM cultures alone could induce Tregs in a cytokine-dependent manner but only upon TCR stimulation (Fig. 3). This effect was mediated primarily through the enhanced production of TGF-β by BMDCs relative to spDCs. It has been shown that Foxp3+ Tregs and IL-17+ Th17-type T cells arise from reciprocal developmental pathways, where in both, TGF-β plays a critical role [29]. However, in the copresence of IL-6, the activated T cells differentiate into Th17 cells. Our RT-PCR results clearly show that BMDCs produce far less IL-6 than spDCs (Fig. 3 and Supplemental Fig. 2A). Thus, a combination of high TGF-β and low IL-6 production by BMDCs likely favored the differentiation of TCR-stimulated CD4+ cells into Foxp3+ adaptive iTregs. The reason for the comparatively lower percentage of Foxp3+ Tregs in CD3-stimulated cocultures than antigen-free cultures is difficult to speculate. It is, however, possible that upon strong TCR stimulation, Teffs proliferated more robustly than Tregs, thereby contributing to lower Treg percentages.
BMDCs caused a significant increase in Foxp3+ nTregs in cocultures, primarily through proliferation, by a contact-dependent mechanism (Figs. 4–6), which did not require TCR stimulation. Based on these findings, we speculated that a ligand expressed on the BMDCs is binding to its cognate receptor on Tregs and causing T cell signaling. Therefore, we investigated the role of OX40, which is constitutively expressed on Tregs. OX40 and OX40L are members of the TNF superfamily with costimulatory function [30, 31]. Signaling through OX40 can promote survival and expansion of autoreactive Teffs, and the blocking OX40-OX40L pathway has been shown to diminish experimental autoimmune diseases including EAE [32], inflammatory bowel disease [33], and type 1 diabetes [34]. In contrast, in our current study, addition of increasing concentrations of neutralizing antibody to OX40L in BMDC/T cell cocultures correlated with decreased proliferation of Tregs. The apparent contradiction between the earlier studies and our current findings is most likely a result of experimental context. For example, in an interesting study, Ruby et al. [35] suggested that signaling through OX40 can result in divergent outcomes depending on the overall signaling context, including the cytokine milieu. Our observation is consistent with this notion, as OX40 stimulation, in the absence of cytokines, such as IL-6 and IFN-γ, likely resulted in the expansion of Tregs. However, we believe that the role of some of these cytokines is secondary to the contact-dependent mechanism, as BMDCs were capable of Treg expansion, even in the presence of blocking antibodies to TGF-β and IL-6 (Fig. 5B and Supplemental Fig. 5A). Interestingly, the expression of the OX40L transcript only in BMDCs and not in C-spDCs, as indicated by RT-PCR (Fig. 5C and Supplemental Fig. 5B), may partly explain why the nTreg expansion did not occur with C-spDCs. Incidentally, we found that LPS stimulation led to an increase in the expression of OX40L in BMDCs and correspondingly led to an increase in Treg percentages in coculture (Supplemental Fig. 5C). Although OX40L appears to be essential for the BMDC-mediated Treg expansion in vitro, we do not think it is sufficient, as addition of an OX40 agonist to cocultures of spleen-derived APCs and T cells did not lead to Treg expansion (data not shown). It is possible that in addition to the OX40-OX40L interaction, the Treg expansion may require other interactions as part of a more complex immunological synapse.
We found that BMDCs could not expand sorted CD4+CD25+ Tregs as efficiently as they could in total CD4+ cocultures but could do so upon IL-2 addition (Fig. 6B). This indicated that the required IL-2 was being produced by the CD4+CD25− T cells in the total CD4+ cocultures. Although IL-2 was necessary for Treg expansion, it was not sufficient, as spleen-derived APCs failed to expand Tregs efficiently from total CD4+ T cell cocultures or isolated CD4+CD25+ T cell cocultures, even in the presence of IL-2 (Fig. 6B). Using BMDCs from MHCII−/− mice in similar cocultures (Fig. 6E), we found evidence for the TCR requirement, as the Treg-specific expansion failed to occur, even in total CD4+ cocultures. However, addition of exogenous IL-2 restored the expansion to WT levels, showing that the involvement of TCR was most likely required only for the production of IL-2. Additionally, this expansion in the presence of IL-2 was reduced dramatically by the anti-OX40L antibody, showing that IL-2 was required for the maintenance of the Tregs, and the expansion was likely a TCR-independent phenomenon that required OX40-OX40L interaction. Taken together, these data suggest that the specific expansion of Tregs in CD4+ cocultures involves two separate mechanisms: the MHCII-TCR interaction (between BMDCs and CD4+CD25− cells), resulting in IL-2 production, and expansion of the CD4+CD25+ Tregs, which occurs through a TCR-independent interaction, which among other things, involves OX40-OX40L ligation. Our observation is similar to a recent study that showed that BMDCs, developed in vitro with Flt3L, can expand Tregs in vitro and in vivo in an IL-2-dependent but TCR-independent manner [36].
We have shown that in vitro-expanded nTregs and induced iTregs can suppress antigen-specific proliferation of Teffs (Fig. 7), albeit with different efficiencies. Our earlier findings of a critical role for IL-10 in GM-CSF-induced suppression of EAT and other autoimmune diseases [5, 8, 26] are consistent with findings from other studies that have also shown a role for IL-10 in the in vivo suppression of autoimmunity [37, 38], while other observations have shown no role for IL-10 or TGF-β in Treg-induced in vitro suppression [39–42]. In our suppression assays, it appears that the expanded nTregs possess better suppressive capacity than in vitro-induced iTregs. However, these findings are preliminary and would require additional studies before a firmer conclusion can be drawn. First, the nTregs and iTregs that we produced in vitro are from naïve mice. However, the Teff proliferation is OVA-dependent, as the cells were isolated from OVA-immunized mice. Therefore, in our assay, we could only measure bystander suppression. As mentioned before, the efficiency of in vitro conversion of Teff to Tregs using cells from immunized mice and exogenously added self-antigen was expectedly very low (Supplemental Fig. 2B). Therefore, this experiment ideally needs to be done with Tregs generated in vitro using CD4+ T cells from TCR transgenic mice with different antigen specificities. Second, we have not done a comprehensive analysis to determine the stability of Foxp3 expression in iTregs. It is possible that the iTregs transiently express Foxp3, which allows us to characterize them as Tregs, but without TGF-β, they lose their Foxp3 expression and hence, Treg function in the suppression assays, which typically last up to 5 days.
Although GM-CSF has been identified as a DC growth factor in vitro, and DCs have been routinely enriched from mouse BM cultures using GM-CSF [43, 44], GM-CSF null and GM-CSFR null mice have normal levels of DCs, indicating that GM-CSF may not be essential for DC development per se [45, 46]. In contrast, mice lacking Flt3L have impaired DC development [47]. Therefore, it has been proposed that although Flt3L is physiologically essential for the steady-state DC development from precursors, the GM-CSF may control the development of monocyte-derived “inflammatory” DCs characterized by an intermediate level of expression of CD11c, high expression of CD11b, surface MAC3 expression (a glycoprotein also found on activated macrophages), and the absence of CD4 or CD8 [48, 49]. Consistent with this concept, we observed an increase in the CD11b+CD11c+CD8α− DCs in the spleen of GM-CSF-treated mice, which also correlated with a higher percentage of Foxp3+ Tregs (Fig. 8A). Additionally, adoptive transfer of ex vivo-differentiated BMDCs into recipient mice treated with mTg led to an increase in Treg percentages (Fig. 8B). This increase could be a combination of TCR-independent nTreg expansion and TCR-dependent iTreg induction through mTg presentation. Therefore, in our animal models of GM-CSF-mediated suppression of autoimmunity, it is likely that GM-CSF treatment causes a faster turnover of myeloid differentiation in the BM that leads to an accumulation of semi-mature CD11b+CD11c+ DCs in the spleen, which then causes Treg expansion and differentiation. We do not yet know if this phenomenon is simply the result of increased GM-CSF-mediated differentiation in the BM and therefore, an aberration of “physiological DC homeostasis” or that there really exists a GM-CSF-directed (and Flt3L-independent) DC development pathway leading to increased tolerance to self-antigens.
Our observations have strong implications for the treatment of various autoimmune diseases. Deficiency of nTregs has been shown to contribute to a variety of autoimmune conditions [50, 51], and adoptive transfer of polyclonal or antigen-selected nTregs can be sufficient to overcome autoimmune and allergic conditions [52–54]. BMDCs provide a simple and efficient method of selectively expanding polyclonal Tregs ex vivo, which can then be adoptively transferred as a potential therapy for human autoimmune conditions.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant AI 058190 to B.S.P. This project was also supported by the University of Illinols at Chicago (UIC) Center for Clinical and Translational Science (CCTS), award number UL1RR029879 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the officical views of the National Center for Research Resources or the National Institutes of Health.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- 1-MT
- 1-methyl tryptophan
- BM
- bone marrow
- BMDC
- bone marrow-derived DC
- CD62L
- CD62 ligand
- C-spDC
- control spleen-derived DC
- EAT
- experimental autoimmune thyroiditis
- F
- forward
- Flt3L
- fms-like tyrosine kinase 3-ligand
- Foxp3
- forkhead box p3
- GARP
- glycoprotein A repetitions predominant
- GITR
- glucocorticoid-induced TNFR
- G-spDC
- GM-CSF spleen-derived DC
- HPRT
- hypoxanthine guanine phosphoribosyl transferase
- iTreg
- inducible regulatory T cell
- MHCII
- MHC class II
- mTg
- mouse thyroglobulin, nTreg, natural regulatory T cell
- OX40L
- OX40 ligand
- PDL
- programmed death ligand
- qPCR
- quantitative PCR
- R
- reverse
- RT-F/R
- real time-forward/reverse, spDC, spleen-derived DC
- Teff
- effector T cell
- Tg
- thyroglobulin
- TMRM
- tetramethyl rhodhamine methyl ester
- Treg
- regulatory T cell
AUTHORSHIP
Contributions: P.B. and A.G. designed research, performed research, and wrote the paper; B.B.G. and J.R.S. performed research; and B.S.P. designed research and wrote the paper.
DISCLOSURE
The authors declare no competing financial interests.
REFERENCES
- 1. Charreire J. (1989) Immune mechanisms in autoimmune thyroiditis. Adv. Immunol. 46, 263–334 [DOI] [PubMed] [Google Scholar]
- 2. Weetman A. P., McGregor A. M. (1994) Autoimmune thyroid disease: further developments in our understanding. Endocr. Rev. 15, 788–830 [DOI] [PubMed] [Google Scholar]
- 3. Sugihara S., Fujiwara H., Shearer G. M. (1993) Autoimmune thyroiditis induced in mice depleted of particular T cell subsets. Characterization of thyroiditis-inducing T cell lines and clones derived from thyroid lesions. J. Immunol. 150, 683–694 [PubMed] [Google Scholar]
- 4. Stafford E. A., Rose N. R. (2000) Newer insights into the pathogenesis of experimental autoimmune thyroiditis. Int. Rev. Immunol. 19, 501–533 [DOI] [PubMed] [Google Scholar]
- 5. Vasu C., Dogan R. N., Holterman M. J., Prabhakar B. S. (2003) Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor 3-ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J. Immunol. 170, 5511–5522 [DOI] [PubMed] [Google Scholar]
- 6. Gangi E., Vasu C., Cheatem D., Prabhakar B. S. (2005) IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J. Immunol. 174, 7006–7013 [DOI] [PubMed] [Google Scholar]
- 7. Morris G. P., Chen L., Kong Y. C. (2003) CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell. Immunol. 226, 20–29 [DOI] [PubMed] [Google Scholar]
- 8. Ganesh B. B., Cheatem D. M., Sheng J. R., Vasu C., Prabhakar B. S. (2009) GM-CSF-induced CD11c+CD8a– dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int. Immunol. 21, 269–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Curotto de Lafaille M. A., Lafaille J. J. (2009) Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30, 626–635 [DOI] [PubMed] [Google Scholar]
- 10. Apostolou I., Sarukhan A., Klein L., von Boehmer H. (2002) Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3, 756–763 [DOI] [PubMed] [Google Scholar]
- 11. Bensinger S. J., Bandeira A., Jordan M. S., Caton A. J., Laufer T. M. (2001) Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J. Exp. Med. 194, 427–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knoechel B., Lohr J., Kahn E., Bluestone J. A., Abbas A. K. (2005) Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 202, 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Apostolou I., von Boehmer H. (2004) In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 199, 1401–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen W., Jin W., Hardegen N., Lei K. J., Li L., Marinos N., McGrady G., Wahl S. M. (2003) Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fantini M. C., Becker C., Monteleone G., Pallone F., Galle P. R., Neurath M. F. (2004) Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25– T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172, 5149–5153 [DOI] [PubMed] [Google Scholar]
- 16. Wang L., Pino-Lagos K., de Vries V. C., Guleria I., Sayegh M. H., Noelle R. J. (2008) Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc. Natl. Acad. Sci. USA 105, 9331–9336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esquivel P. S., Rose N. R., Kong Y. C. (1977) Induction of autoimmunity in good and poor responder mice with mouse thyroglobulin and lipopolysaccharide. J. Exp. Med. 145, 1250–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kared H., Leforban B., Montandon R., Renand A., Layseca Espinosa E., Chatenoud L., Rosenstein Y., Schneider E., Dy M., Zavala F. (2008) Role of GM-CSF in tolerance induction by mobilized hematopoietic progenitors. Blood 112, 2575–2578 [DOI] [PubMed] [Google Scholar]
- 19. Curti A., Pandolfi S., Valzasina B., Aluigi M., Isidori A., Ferri E., Salvestrini V., Bonanno G., Rutella S., Durelli I., Horenstein A. L., Fiore F., Massaia M., Colombo M. P., Baccarani M., Lemoli R. M. (2007) Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25– into CD25+ T regulatory cells. Blood 109, 2871–2877 [DOI] [PubMed] [Google Scholar]
- 20. Perez N., Karumuthil-Melethil S., Li R., Prabhakar B. S., Holterman M. J., Vasu C. (2008) Preferential costimulation by CD80 results in IL-10-dependent TGF-β1(+) -adaptive regulatory T cell generation. J. Immunol. 180, 6566–6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y., Chung Y., Bishop C., Daugherty B., Chute H., Holst P., Kurahara C., Lott F., Sun N., Welcher A. A., Dong C. (2006) Regulation of T cell activation and tolerance by PDL2. Proc. Natl. Acad. Sci. USA 103, 11695–11700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. So T., Croft M. (2007) Cutting edge: OX40 inhibits TGF-β- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J. Immunol. 179, 1427–1430 [DOI] [PubMed] [Google Scholar]
- 23. Vu M. D., Xiao X., Gao W., Degauque N., Chen M., Kroemer A., Killeen N., Ishii N., Chang Li X. (2007) OX40 costimulation turns off Foxp3+ Tregs. Blood 110, 2501–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohshima Y., Tanaka Y., Tozawa H., Takahashi Y., Maliszewski C., Delespesse G. (1997) Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 159, 3838–3848 [PubMed] [Google Scholar]
- 25. Wang R., Wan Q., Kozhaya L., Fujii H., Unutmaz D. (2008) Identification of a regulatory T cell specific cell surface molecule that mediates suppressive signals and induces Foxp3 expression. PLoS ONE 3, e2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheng J. R., Li L., Ganesh B. B., Vasu C., Prabhakar B. S., Meriggioli M. N. (2006) Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J. Immunol. 177, 5296–5306 [DOI] [PubMed] [Google Scholar]
- 27. Cheatem D., Ganesh B. B., Gangi E., Vasu C., Prabhakar B. S. (2009) Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin. Immunol. 131, 260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou T., Caton A. J., Koretzky G. A., Kambayashi T. (2010) Dendritic cells induce regulatory T cell proliferation through antigen-dependent and -independent interactions. J. Immunol. 185, 2790–2799 [DOI] [PubMed] [Google Scholar]
- 29. Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., Weiner H. L., Kuchroo V. K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 [DOI] [PubMed] [Google Scholar]
- 30. Gramaglia I., Weinberg A. D., Lemon M., Croft M. (1998) Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 161, 6510–6517 [PubMed] [Google Scholar]
- 31. Godfrey W. R., Fagnoni F. F., Harara M. A., Buck D., Engleman E. G. (1994) Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J. Exp. Med. 180, 757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weinberg A. D., Bourdette D. N., Sullivan T. J., Lemon M., Wallin J. J., Maziarz R., Davey M., Palida F., Godfrey W., Engleman E., Fulton R. J., Offner H., Vandenbark A. A. (1996) Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat. Med. 2, 183–189 [DOI] [PubMed] [Google Scholar]
- 33. Higgins L. M., McDonald S. A., Whittle N., Crockett N., Shields J. G., MacDonald T. T. (1999) Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J. Immunol. 162, 486–493 [PubMed] [Google Scholar]
- 34. Pakala S. V., Bansal-Pakala P., Halteman B. S., Croft M. (2004) Prevention of diabetes in NOD mice at a late stage by targeting OX40/OX40 ligand interactions. Eur. J. Immunol. 34, 3039–3046 [DOI] [PubMed] [Google Scholar]
- 35. Ruby C. E., Yates M. A., Hirschhorn-Cymerman D., Chlebeck P., Wolchok J. D., Houghton A. N., Offner H., Weinberg A. D. (2009) Cutting edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J. Immunol. 183, 4853–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swee L. K., Bosco N., Malissen B., Ceredig R., Rolink A. (2009) Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood 113, 6277–6287 [DOI] [PubMed] [Google Scholar]
- 37. Hawrylowicz C. M., O'Garra A. (2005) Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5, 271–283 [DOI] [PubMed] [Google Scholar]
- 38. Annacker O., Asseman C., Read S., Powrie F. (2003) Interleukin-10 in the regulation of T cell-induced colitis. J. Autoimmun. 20, 277–279 [DOI] [PubMed] [Google Scholar]
- 39. Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. (1998) Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10, 1969–1980 [DOI] [PubMed] [Google Scholar]
- 40. Thornton A. M., Shevach E. M. (1998) CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188, 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dieckmann D., Plottner H., Berchtold S., Berger T., Schuler G. (2001) Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J. Exp. Med. 193, 1303–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jonuleit H., Schmitt E., Stassen M., Tuettenberg A., Knop J., Enk A. H. (2001) Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193, 1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lutz M. B., Kukutsch N., Ogilvie A. L., Rossner S., Koch F., Romani N., Schuler G. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 45. Vremec D., Lieschke G. J., Dunn A. R., Robb L., Metcalf D., Shortman K. (1997) The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 27, 40–44 [DOI] [PubMed] [Google Scholar]
- 46. Burnham K., Robb L., Scott C. L., O'Keeffe M., Shortman K. (2000) Effect of granulocyte-macrophage colony-stimulating factor on the generation of epidermal Langerhans cells. J. Interferon Cytokine Res. 20, 1071–1076 [DOI] [PubMed] [Google Scholar]
- 47. McKenna H. J., Stocking K. L., Miller R. E., Brasel K., De Smedt T., Maraskovsky E., Maliszewski C. R., Lynch D. H., Smith J., Pulendran B., Roux E. R., Teepe M., Lyman S. D., Peschon J. J. (2000) Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood 95, 3489–3497 [PubMed] [Google Scholar]
- 48. Naik S. H., Metcalf D., van Nieuwenhuijze A., Wicks I., Wu L., O'Keeffe M., Shortman K. (2006) Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat. Immunol. 7, 663–671 [DOI] [PubMed] [Google Scholar]
- 49. Shortman K., Naik S. H. (2007) Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7, 19–30 [DOI] [PubMed] [Google Scholar]
- 50. Sakaguchi S. (2004) Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 [DOI] [PubMed] [Google Scholar]
- 51. Sakaguchi S., Fukuma K., Kuribayashi K., Masuda T. (1985) Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J. Exp. Med. 161, 72–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zwar T. D., Read S., van Driel I. R., Gleeson P. A. (2006) CD4+CD25+ regulatory T cells inhibit the antigen-dependent expansion of self-reactive T cells in vivo. J. Immunol. 176, 1609–1617 [DOI] [PubMed] [Google Scholar]
- 53. Tang Q., Henriksen K. J., Bi M., Finger E. B., Szot G., Ye J., Masteller E. L., McDevitt H., Bonyhadi M., Bluestone J. A. (2004) In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199, 1455–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Joetham A., Takeda K., Okamoto M., Taube C., Matsuda H., Dakhama A., Gelfand E. W. (2009) Antigen specificity is not required for modulation of lung allergic responses by naturally occurring regulatory T cells. J. Immunol. 183, 1821–1827 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.