Mu opioid receptor activation induces the expression of chemokine CCL2 through a pro-inflammatory, PKCζ-dependent, NF-κB pathway.
Keywords: inflammation, DAMGO, CCL2
Abstract
Opioid receptor agonists induce broad immunomodulatory activity, which substantially alters host defense and the inflammatory response. Previous studies have shown that the MOR selective agonist DAMGO has the capacity to increase the expression of the proinflammatory chemokines CCL2, CCL5, and CXCL10 in human PBMCs. NF-κB is a transcription factor that plays a pivotal role in innate and adaptive immune responses. We report that NF-κB is a vital player in the DAMGO-induced, MOR-mediated regulation of chemokine expression. Results show that NF-κB inhibitors prevent the induction of CCL2 expression in response to DAMGO administration and that the NF-κB subunit, p65, is phosphorylated at serine residues 311 and 536 in response to MOR activation. Furthermore, we demonstrate that PKCζ is phosphorylated following DAMGO-induced MOR activation, and this kinase is essential for NF-κB activation as well as CCL2 expression and transcriptional activity. Finally, ChIP analysis shows that DAMGO administration induces binding of p65 to the enhancer region of the CCL2 promoter. These data are consistent with the notion that MOR activation promotes a proinflammatory response, which involves NF-κB activation. Our results also suggest a significant and novel role for PKCζ as an essential participant in the MOR-mediated regulation of proinflammatory chemokine expression.
Introduction
Opioid receptors are expressed in the CNS and on cells of the immune system, and the MOR has been identified in monocyte/macrophage and T cell populations [1–3]. μ-Opioid agonists, including morphine, are known to modulate cellular and humoral acquired immune responses in vivo and in vitro [4, 5]. However, a more detailed analysis has shown that μ-opioids are capable of up-regulating the production of certain proinflammatory cytokines [6, 7]. We have previously reported that the MOR-selective agonist DAMGO can elevate the expression of the chemokines CCL2, CXCL10, and CCL5 in resting and activated PBMCs at the mRNA and protein levels [7]. Our studies have shown that the induction of chemokine production by DAMGO is apparent within the first 2 h, and this is then followed by a second phase of expression at 24–48 h [7, 8]. Moreover, we have found that the second phase of this response is mediated, at least in part, by the coincident induction of TGF-β expression [8]. However, the precise biochemical basis for the direct μ-opioid induction of chemokine expression has remained undefined.
NF-κB is a DNA-binding factor that plays an essential role in the activation of several inflammatory mediators, such as TNF-α [9], CXCL8 [10, 11], IL-1β [12], and IL-6 [13], as well as two chemokines, CCL2 and CCL5, which we find are up-regulated significantly by DAMGO [14–16]. The promoter region of CCL2 is well described [17, 18] and has been shown to contain putative consensus-binding sites for a variety of transcription factors including NF-κB [19], and NF-κB consensus sequences in the proximal promoter region (∼90 bp upstream of the transcriptional start site) and two NF-κB-binding sequences in the distal enhancer region (−2612 and −2603 bp) have been indentified and found to be required for maximal transcriptional activity in response to stimulation with LPS or TNF-α [20–22]. Although several studies have demonstrated opioid modulation of NF-κB activation [23, 24], it is important to point out that the influence of MOR on the activation of this transcription factor appears to vary based on a variety of parameters, including the cell type and stimulus. At this time, it is not clear whether activation of MOR at physiological agonist concentrations results in a direct activation of NF-κB.
The NF-κB family of transcription factors regulates the transcription of an exceptionally large number of genes, particularly those involved in innate, adaptive, and inflammatory responses [25, 26]. The canonical activation pathway is typically induced by activation of the IKK complex. IKK activation results in the phosphorylation of IκBα at serine residues 32 and 36 [27, 28]. IκB phosphorylation is a prerequisite for the subsequent polyubiquitination and degradation of IκB by the proteosome [28]. Proteolysis of IκB frees the prototypical NF-κB complex, which is typically a 50/p65 heterodimer. Activated NF-κB complexes then translocate to the nucleus, bind DNA, and initiate transcription of NF-κB target genes [29, 30]. Transcription of target genes is controlled by various NF-κB post-transcriptional modifications, as well as through the recruitment of transcriptional coactivators and corepressors [31]. p65 is the NF-κB subunit primarily responsible for transactivation, and the transcriptional activation activity of p65 requires a phosphorylation event. Identification of the kinase(s), which carry out this phosphorylation event, is critical for an understanding of the molecular basis for the activation of this transcription factor.
There is increasing evidence that PKCζ, a member of the atypical subfamily of PKC enzymes, is a critical component of a number of intracellular pathways induced by various stimuli. It is apparent that PKCζ is required for induction of NF-κB activation in response to IL-1 and TNF-α [32, 33]. Serine 311 lies in the TAD of p65 and is inducibly phosphorylated by the atypical PKCζ in response to TNF stimulation [34]. PKCζ-deficient fibroblasts show IKK activation and normal nuclear translocation of p65 but reduced DNA-binding activity supporting a role for this serine residue in transcriptional activity [32]. Further experiments indicate the serine 311 phosphorylation promotes the interaction of p65 with CBP and the recruitment of CBP and RNA Polymerase II to the IL-6 promoter [34].
We hypothesized that the direct early/immediate induction of CCL2 is dependent on NF-κB activation. We report results here from experiments that show that CCL2 mRNA and protein levels are elevated (three- to fourfold) as early as 4 h following DAMGO treatment of PBMCs. Using primary human leukocytes as well as HEK-293 cells transduced to express MOR, we demonstrate that pretreatment with the NF-κB inhibitors HNE, BAY 11-7082, or MG132 significantly reduces DAMGO-induced CCL2 expression. Furthermore, our results show that the activation of NF-κB and the induction of CCL2 expression are dependent on the activation of PKCζ.
MATERIALS AND METHODS
PBMC isolation and culture
PBMCs were obtained from whole blood of normal donors as described [8]. Cell cultures were maintained in RPMI-1640 medium, supplemented with 10% heat-inactivated (56°C, 30 min), endotoxin-free FBS (Hyclone, Logan, UT, USA), 10 μg/ml gentamicin (Life Technologies, Rockville, MD, USA), and 1 mM L-glutamine and maintained at 37°C, 5% CO2.
Cell culture treatments
Cells were treated with DAMGO (Multiple Peptide Systems, San Diego, CA, USA), diluted in PBS, and administered at the designated concentrations. For certain experiments, PBMCs were pretreated with the inhibitors HNE (Alexis Biochemicals, San Diego, CA, USA), BAY 11-7082 (Alexis Biochemicals), PKCζ PSI (Calbiochem, Gibbstown, NJ, USA), and MG132 (Biomol Research Laboratories, Plymouth Meeting, PA, USA) at the designated concentration, 30–60 min prior to DAMGO administration.
Preparation of cytosolic and nuclear fractions
Nuclear extracts were prepared from 5.5 × 107 PBMCs isolated from blood donors using the Panomics nuclear extraction kit following the manufacturer's protocol (Panomics, Fremont, CA, USA). PBMCs were seeded at 1.1 × 107 cells/well into a six-well cell culture plate. PBMCs were left untreated or treated with 100 nM DAMGO for the designated time.
TranSignal protein/DNA array
TranSignal protein/DNA Array I was used according to the manufacturer's protocol (Panomics). Briefly, 15 μg nuclear extract was incubated with the TranSignal probe mix. DNA/protein complexes were washed, and the DNA was separated from the protein and hybridized on the membranes at 42°C overnight in a hybridization oven. Following hybridization, the arrays were washed sequentially with Hybridization Wash I (2×SSC/0.5% SDS) and Hybridization Wash II (0.1×SSC/0.5% SDS). Arrays were then blocked with blocking buffer and detected using streptavidin-HRP and the detection buffer. Signal was detected through the use of ECL-Hyperfilm (GE Healthcare, Piscataway, NJ, USA), and semiquantitative analysis was performed based on the number of pixels present and the mean intensity of the spots in the untreated verus treated samples. All results were normalized to hybridization control spots (provided in the array) to ensure that results were not biased by loading viability. The results shown are representative of three independent donors.
ELISA: chemokine analysis
The concentrations of chemokines present in culture supernatants were determined by ELISA. Matching CCL2 capture and biotinylated detection antibodies were used for sandwich ELISA analysis (R&D Systems, Minneapolis, MN, USA). Briefly, capture antibody was diluted in capture buffer (0.1 M sodium carbonate, pH 9.5), transferred to an ELISA plate, and allowed to incubate overnight at room temperature. Plates were washed with wash buffer (0.05% Tween 20 in PBS) and blocked with 1% BSA, 5% sucrose, and 0.05% NaN3. Biotinylated detection antibodies and HRP-linked strepavidin (R&D Systems) were used to detect the chemokines. Tetramethylbenzidine/hydrogen peroxide was used as the substrate solution, and OD was determined at 450 nm using a Multiskan RC ELISA reader (ThermoFisher, Waltham, MA, USA). Data are represented as the mean of at least three donors and expressed as mean protein concentration ± sd.
Real-time PCR
CCL2 mRNA transcript levels were measured using real-time PCR. Cells were cultured in the presence or absence of HNE, BAY 11-7082, MG132, or a PKCζ PSI for 1 h, followed by treatment for 4 h with the indicated concentration of DAMGO, as reported previously [8]. Target gene cDNA concentrations were determined based on the cross-point and compared with β-actin to determine the concentration ratio, which was then correlated with the untreated sample to establish the normalized ratio. The results are presented as a collective analysis of five independent donors.
Western blot analysis
Whole cell lysates were prepared using RIPA buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris). Protease inhibitor cocktail Set II (EMD Chemicals, Gibbstown, NJ, USA) and phosphotase inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) were added to RIPA buffer immediately prior to use. Whole cell lysates (15–30 μg) were run on a 4–12% gradient gel (Invitrogen, Carlsbad, CA, USA) with 1× SDS MOPS running buffer (Invitrogen). The gels were then transferred onto a nitrocellulose membrane (ThermoFisher), and membranes were blocked for 30 min; primary antibody was added and incubated overnight at 4°C. Antibodies used include: anti-NF-κB p65, anti-phospho-p65 (Ser311), anti-GAPDH, and anti-PKCζ/λ from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and anti-phospho-p65 (Ser536) and anti-phospho-PKCζ/λ (Thr410/403; Cell Signaling Technology, Danvers, MA, USA). The following day, membranes were washed, and secondary antibodies were added. Anti-mouse IgG-HRP and anti-rabbit IgG-HRP were obtained from Cell Signaling Technology and Santa Cruz Biotechnology, respectively. Membranes were then washed and developed with SuperSignal West Pico chemiluminescent reagent (ThermoFisher) or ECL Plus (GE Healthcare), according to the manufacturer's protocol. Chemiluminescent signals were recorded using autoradiography film (Lab Scientific, Livingston, NJ, USA) and developed.
ViraPower™ lentiviral expression system
The ViraPower lentiviral expression system (Invitrogen) was used to generate a lentiviral system for protein expression. The MOR coding sequence was first amplified using the expression vector pcDNA3-MOR-FLAG as a template. The primers used for subcloning MOR were as follows: forward 5′-CGGTACCCCATGAAGACGATCATCGCCC-3′ and reverse 5′-CGATATCCGTAGGGCAACGGAGCAGTTTCTGC-3′. The DNA sequences generated for cloning include a 5′ FLAG tag and bacterial-derived signal sequence for membrane trafficking. This fragment was then subcloned into the intermediate Gateway® vector, pENTR4. Positive clones were verified by restriction digest and sequencing. A sequence-specific LR recombination between the pENTR4 entry clone and the destination vector pLenti4/V5-DEST (Invitrogen), using LR Clonase™ II enzyme mix (Invitrogen), was then carried out, and positive clones were purified using Qiagen (Valencia, CA, USA) mini preps and verified by DNA sequencing. Expression of MOR was verified by flow cytometry.
The human 293FT producer cell line (Invitrogen) was used to produce lentivirus. pLenti4/V5-DEST-MOR was transfected along with ViraPower™ packaging mix using Lipofectamine 2000 (Invitrogen), as described by the manufacturer. The virus was then concentrated using ultracentrifugation, and the virus was resuspended in 1 ml PBS containing 0.1% BSA and stored at −80°C for further use. The human fibrosarcoma line HT1080 (American Type Culture Collection, Manassas, VA, USA) was used to titer the lentivirus following ∼14 days of selection with 150 μg/ml Zeocin. Crystal violet (1%) was used to detect blue-stained colonies, which could then be counted and used to determine the titer based on the lentiviral dilutions.
Lentivirus transduction
HEK-293 was seeded into 10 cm cell culture plates and grown to 30–50% confluency for transduction. MOR lentivirus at a MOI of ∼0.025 in R10 medium was added, and the following day, the medium containing the virus was removed and replaced with fresh medium. Every 3–4 days, medium was changed and replaced with new medium containing 150 μg/ml Zeocin until antibiotic-resistant colonies could be identified (2–3 weeks). Colonies were then expanded, and expression was verified by flow cytometry, Western blot analysis, and calcium mobilization analysis.
SEAP assays
HEK-293 cells stably transduced with MOR lentivirus were used for SEAP reporter analysis. The SEAP NF-κB reporter vector pNF-κB-SEAP (Clontech, Palo Alto, CA, USA) was used to measure activation of the NF-κB signal transduction pathway. HEK-MOR cells were transfected with the SEAP construct using Lipofectamine 2000 (Invitrogen). In designated experiments, cells were pretreated with a PKCζ PSI, and after 45 min, cells were then treated with DAMGO at the designated concentrations. Following treatment, supernatants were collected at 0.5, 1, 2, and 4 h time-points. The Great EscAPe SEAP Chemiluminescence Kit 2.0 (Clontech) was used for SEAP detection. SEAP signal was read with a FB 12 (V 2.3) single-well luminometer (Berthold Detection Systems, Oak Ridge, TN, USA) with a 2-s delay and 10-s measurement. Data are represented as the mean of four samples and expressed as mean relative light units ± sd.
Chromatin preparation
Chromatin for ChIP analysis was prepared using the EZ-Zyme chromatin prep kit (Millipore, Billerica, MA, USA). PBMCs were seeded at 1.0 × 108 cells in 150 cm tissue-culture plates containing 40 ml medium for each chromatin preparation. Cells were treated with 100 nM DAMGO, and ChIP assays were performed over a 2-h period. Following DAMGO treatment, PBMCs were treated with 1% paraformaldehyde, and 1.25 M glycine was then added to quench unreacted formaldehyde. Cells were then collected, and cell pellets were snap-frozen in a dry-ice/alcohol bath and stored at −80°C until ready for isolation of nuclei. Cell pellets were resuspended in EZ-Zyme lysis buffer containing the Protease Inhibitor Cocktail II (Millipore) and subjected to three freeze-thaw cycles in a dry-ice/alcohol bath. EZ-Zyme enzymatic cocktail (Millipore; 1 U) in EZ-Zyme digestion buffer was added to the nuclear extracts and incubated in a 37°C water bath for 10 min. Reactions were stopped and supernatants containing cleaved chromatin stored at −80°C for further use.
ChIP assay and PCR analysis
ChIP assays were performed using the EZ-Magna ChIP A kit (Millipore), according to the manufacturer's protocol. Briefly, 0.5 μg antibodies were added to 15–30 μg of the chromatin mixture with 20 μl protein A magnetic beads and incubated overnight at 4°C with continuous rotation for mixing. The following day, Protein A bead-antibody/chromatin complexes were washed. To reverse paraformaldehyde cross-links, samples were incubated at 62°C for 3 h in ChIP elution buffer with Proteinase K and placed at 95°C for 10 min. DNA samples were then purified using spin filters and eluted into 50 μl 0.5 mM Tris (pH 8.0). PCR analysis was performed with Accuprime Pfx Supermix (Invitrogen) using 2–6 μl PCR product. PCR primers located in the enhancer region of CCL2 are as follows: sense 5′-CAAGGTTTGTGCCAGAGCCTAACC-3′; antisense 5′-GGGAAGGTGAAGGGTATGAATCAG-3′. GAPDH primers included: sense 5′-TACTAGCGGTTTTACGGGCG-3′; antisense 5′-TCGAACAGGAGGAGCAGAGAGCGA-3′. PCR conditions were 95°C for 10 min, followed by ∼35 cycles of 94°C for 30 s, 58°C for 40 s, and 72°C for 40 s, followed by a final extension of 72°C for 10 min and cooling to 4°C. PCR products were then analyzed by agarose gel electrophoresis.
Statistical analysis
The data are presented as the mean ± sd. The assessment of significant difference was made using ANOVA, and the treatment comparisons with a P value ≤0.05 were considered to be statistically different and therefore, significant.
RESULTS
Activation of MOR induces NF-κB, and this transcription factor is required for induction of CCL2 expression
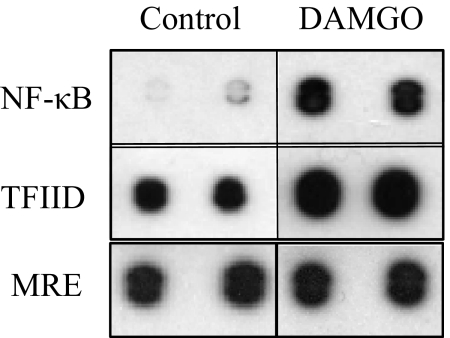
We have previously demonstrated that DAMGO administration to PBMCs induces expression of the chemokine CCL2 [7, 8], and to understand the molecular mechanism, experiments were carried out to determine the transcription factors involved in this pathway. We used a protein/DNA array to examine the transcription factors that showed a twofold or more increase in DNA binding in response to DAMGO administration, as compared with the controls. PBMCs were treated with 100 nM DAMGO, and transcription factor activity analysis was carried out. The protein/DNA array revealed a 6.4-fold increase in NF-κB binding to its consensus-binding sequence following DAMGO administration, as compared with the untreated cells (Fig. 1). The general transcription factor, TFIID, which can bind DNA in a sequence-specific manner and is part of the RNA Polymerase II preinitiation complex, also showed 2.4-fold increased association with its consensus sequence in response to DAMGO treatment (Fig. 1), suggesting initiation of transcription. Most of the transcription factors in this analysis failed to exhibit a change following DAMGO administration, including the metal response factor, which is well expressed in these cells.
Figure 1. MOR activation induces transcription factor/DNA interactions in PBMCs.
Human PBMCs were treated with 100 nM DAMGO for 45 min, and nuclear extracts were prepared for use with the TranSignal protein/DNA Array I. Transcription factors were allowed to bind to their DNA consensus sequences, and protein/DNA complexes were then separated, and the DNA sequences were hybridized to a membrane spotted with the complementary consensus sequences for detection by chemiluminescence. Results are representative of three independent donors. MRE, Metal responsive element.
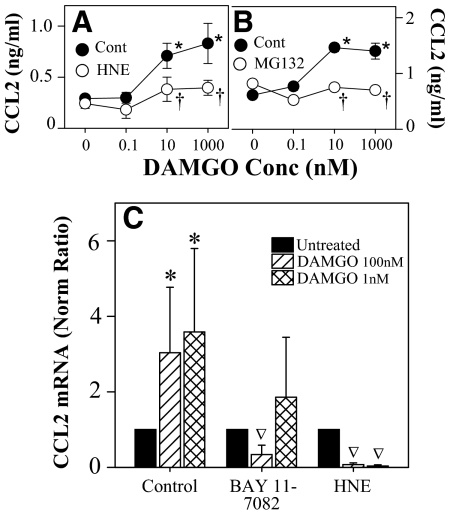
Because of the importance of NF-κB in the inflammatory response, we were particularly interested in investigating the potential role of this transcription factor in the μ-opioid induction of CCL2 expression. PBMCs were treated with DAMGO following pretreatment with the NF-κB inhibitors HNE, BAY 11-7082, and MG132. HNE and BAY 11-7082 were used, as they block the NF-κB signaling pathway by inhibiting the phosphorylation of IκB, and MG132 is a proteasome inhibitor that will inhibit the degradation of phosphorylated IκB. These inhibitors allow us to explore the role of NF-κB in the regulation of CCL2 expression. As previously reported, we show that chemokine expression in the supernatants of these cells was increased approximately threefold following 4 h of DAMGO treatment. However, pretreatment with HNE or MG132 resulted in a significant inhibition of the DAMGO-induced increase in CCL2 protein levels 4 h after treatment (Fig. 2A and B). To determine the role of NF-κB in the regulation of CCL2 mRNA transcription, cells were treated with HNE and BAY 11-7082 for 45 min prior to DAMGO treatment and were analyzed for CCL2 mRNA expression by RT-PCR. The results (Fig. 2C) show that DAMGO administration resulted in the expected increase in CCL2 levels of mRNA, but pretreatment with HNE or BAY 11-7082 resulted in a complete abrogation of the DAMGO-induced up-regulation of CCL2. Taken together, these results suggest that the NF-κB signaling pathway plays a significant role in the DAMGO induction of CCL2 expression.
Figure 2. NF-κB inhibitors block the MOR induction of CCL2 expression.
PBMCs were pretreated with 50 μM HNE (A) or MG132 (B) for 45 min prior to DAMGO administration at the designated concentrations. Supernatants were harvested at 4 h, and chemokine levels were determined by ELISA. Values represent the mean (±sd) of triplicate cultures, and results are representative of five donors. *P < 0.05 versus no DAMGO; †P < 0.05 versus no inhibitor. (C) PBMCs were pretreated with 50 μM HNE or 10 μM Bay 11-7082 for 45 min, followed by DAMGO administration, RNA was isolated, and real-time RT-PCR analysis was performed for CCL2. cDNA concentrations were determined and compared with the housekeeping gene β-actin to determine the concentration ratio, which was then compared with the untreated sample to establish the normalized ratio. The results show the mean values (±sd) of determinations from five different donors; *P < 0.05 compared with the control; ∇P < 0.05 compared with the DAMGO without inhibitor.
To understand the molecular mechanisms that regulate the MOR induction of chemokine expression, a cell line model system was developed to permit more extensive analysis of these regulatory effects. We used a lentiviral expression system to create stably transduced HEK-293 cells (designated HEK-MOR cells). Flow cytometry, Western blot analysis, and calcium mobilization assays were used to verify expression of functionally active MOR by these cells and the ability of DAMGO to induce CCL2 expression (data not shown).
MOR induction of the NF-κB activation pathway
To understand the biochemical basis for the activation of the NF-κB pathway in the induction of chemokine expression, we first chose to investigate the post-translational regulation of the p65 subunit of NF-κB. Cells were treated with 100 nM DAMGO over a period of 2 h, and we examined the levels of p65 and phospho-p65 (Ser536 and Ser311) expression. The results (Fig. 3A) from Western blot analysis show an increase in p65 phosphorylation at serine residue 536 in response to DAMGO administration in HEK-MOR cells (which appears as early as 30 min following treatment). We also detected increased phosphorylation at serine residue 311 as early as 30–60 min following DAMGO treatment, which reaches a peak at ∼120 min (Fig. 3A). In additional experiments, we consistently observed phosphorylation of p65 at Ser536 and Ser311 in response to 100 nM DAMGO administration as early as 10–30 min using primary cells (data not shown).
Figure 3. NF-κB and PKCζ are phosphorylated in response to activation of MOR and are blocked by MG132 and a PKCζ PSI.
HEK-293-MOR cells were pretreated for 45 min with 50 μM MG132, 50 μg/ml CHX, or 25 μM PKCζ PSI, followed by 100 nM DAMGO treatment. At the appropriate time following DAMGO treatment, whole cell lysates were prepared and subjected to Western blot analysis of total p65, phospho (P)-p65 (Ser536 and Ser311), and GAPDH (A) or phospho-PKCζ, total PKCζ, or GAPDH (B). This experiment is representative of three independent experiments.
Additional experiments were carried out with the proteasome inhibitor MG132 to further characterize the phosphorylation of p65. Cells were pretreated with MG132 for 45 min prior to DAMGO administration, and the phosphorylation status of p65 was determined. Our results showed a significant inhibition of p65 phosphorylation at both serine residues in the presence of MG132 (Fig. 3A). These results suggest that 26S-mediated proteasomal degradation is required for phosphorylation of p65 at both of these residues. Furthermore, pretreatment with CHX resulted in increased phosphorylation of p65 (we observed a fivefold increase at serine 536 and a threefold increase in phosphorylation at serine 311 following DAMGO treatment) compared with DAMGO treatment alone (Fig. 3A). We hypothesized that this is a result of the effect of CHX on the synthesis of IκB. As CHX prevents new protein synthesis, and IκB is a known NF-κB target gene, it is possible that the absence of newly synthesized IκB results in extended NF-κB activation, as it cannot effectively shut off the signal after termination of the stimulus.
Role of PKCζ in the induction of NF-κB following MOR activation
Currently, only one kinase known to phosphorylate p65 at serine residue 311 is known, and that is PKCζ, a member of the atypical subfamily of PKC enzymes. Therefore, we wanted to explore the possibility that PKCζ may play a role in the MOR-induced phosphorylation of p65. HEK-MOR cells were pretreated with a PKCζ PSI for 45 min prior to DAMGO administration, and the phosphorylation of p65 was examined. Our results (Fig. 3A) show that phosphorylation of p65 at S536 is particularly apparent, and S311 is less strongly phosphorylated in response to DAMGO. As expected, the phosphorylation at S311 in response to DAMGO is inhibited in the presence of the PKCζ inhibitor. Moreover, phosphorylation of p65 S536 is also inhibited by the PKCζ PSI, suggesting that this kinase has an apparent role in the phosphorylation of this residue as well.
Activation of PKCζ following MOR activation
We wished to explore the possibility that MOR may directly induce the activation and phorphorylation of PKCζ. This kinase is activated, in part, through a phosphorylation event at residue T410 [35, 36]. HEK-293-MOR cells were pretreated a PKCζ PSI or MG132 followed by 100 nM DAMGO administration. Western blot analysis of PKCζ and phosphorylated PKCζ (T410) expression showed that PKCζ T410 was phosphorylated in response to DAMGO treatment (Fig. 3B). We detected a threefold increase in PKCζ phosphorylation at 30 min following DAMGO administration, and this reached a sixfold elevation at 60 and 120 min for the T410 phosphorylation (Fig. 3B). Additional studies show that PKCζ is also phosphorylated in response to DAMGO treatment in PBMCs (data not shown). Data collected from studies with PKCζ PSI pretreatment, as expected, show (Fig. 3B) that this inhibitor completely abrogated this DAMGO-induced PKCζ phosphorylation. Furthermore, the presence of the proteosome inhibitor MG132 did not have a detectable effect on the level of PKCζ phosphorylation (Fig. 3B). This suggests that MOR-induced PKCζ phosphorylation and activation are upstream of NF-κB signaling.
Role of PKCζ in the induction of CCL2 following MOR activation
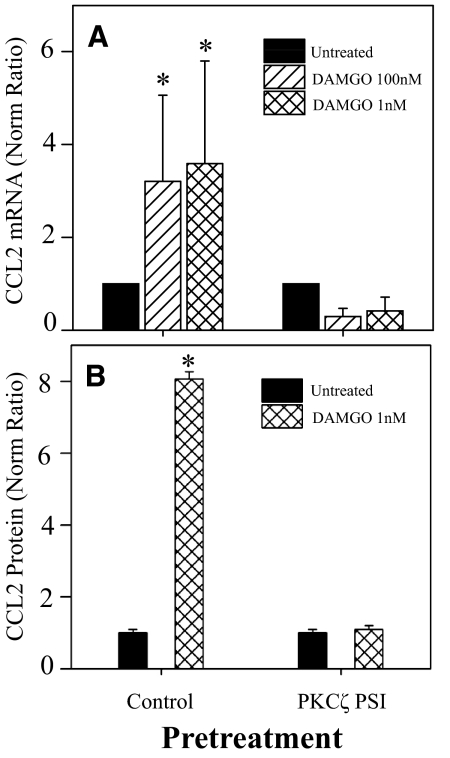
To determine the role of PKCζ in the DAMGO induction of CCL2 expression, PBMCs were pretreated with PKCζ PSI, and the DAMGO-induced expression of CCL2 was determined. In the presence of the PKCζ PSI, the inhibitor competes for binding to the kinase region of PKCζ, and PKCζ substrates do not have access to this region, which prevents activation of PKCζ. The data show (Fig. 4A) that PKCζ PSI pretreatment significantly attenuated the induction of CCL2 mRNA expression following 1 or 100 nM DAMGO administration. Similar results were observed following PKCζ PSI pretreatment at the CCL2 protein level (Fig. 4B).
Figure 4. Treatment with PKCζ PSI blocks the MOR induction of CCL2 mRNA and protein expression.
PBMCs were pretreated with 25 μM PKCζ PSI for 45 min, followed by DAMGO treatment, mRNA was isolated at 4 h, supernatants were collected at 18 h, and CCL2 mRNA expression was determined by real-time RT-PCR (A), or protein was determined by ELISA (B). cDNA concentrations were determined and compared with the housekeeping gene β-actin to determine the concentration ratio, which was then compared with the untreated sample to establish the normalized ratio. The results show the mean values of determinations from five different donors ± sd; *P < 0.05 compared with the untreated sample from each group normalized to the control, nontreated cell populations.
MOR-induced activation of NF-κB transcriptional activity
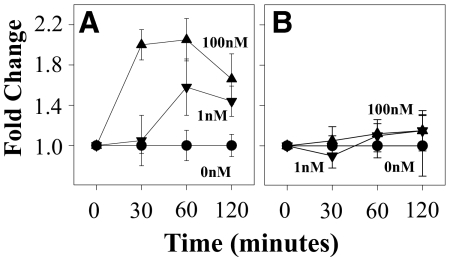
To directly assess the ability of MOR activation to induce NF-κB transcriptional activity, we carried out experiments using HEK-MOR cells transfected with the phosphorylated NF-κB-SEAP reporter plasmid. This molecular construct contains the SEAP reporter gene [37, 38] and is designed for monitoring the activation of NF-κB [39–41]. HEK-MOR cells were treated with 100 nM DAMGO, and at the designated times, supernatants were removed for the SEAP assay to detect the binding of transcription factors to the κ enhancer, which initiates transcription of SEAP. We found increased SEAP enzymatic activity as early as 30 min following DAMGO administration and reached a peak at 60 min (Fig. 5A). To determine the role of PKCζ, we also pretreated cells with the PKCζ PSI 45 min prior to DAMGO treatment. We found that the PKCζ PSI significantly inhibited the DAMGO-induced increase in NF-κB transcriptional activity (Fig. 5B). These results suggest that PKCζ is an essential regulator of the DAMGO-induced, NF-κB-dependent signal transduction pathway. These results are also consistent with previous results, indicating that PKCζ functions upstream of the NF-κB signaling pathway.
Figure 5. Activation of NF-κB transcriptional activity following MOR activation.
HEK-293-MOR cells were transiently transfected with the phosphorylated NF-κB-SEAP construct prior to NF-κB SEAP reporter analysis in the absence (A) or presence of the PKCζ PSI (B), which was added at 25 μM for 45 min prior to DAMGO treatment at 0, 1, or 100 nM, and cell supernatants were collected at the designated time-points for SEAP assay. Values are a mean (±sd) of four replicates and are representative of three independent experiments.
ChIP analysis demonstrates MOR-induced binding of NF-κB to the CCL2 promoter in vivo
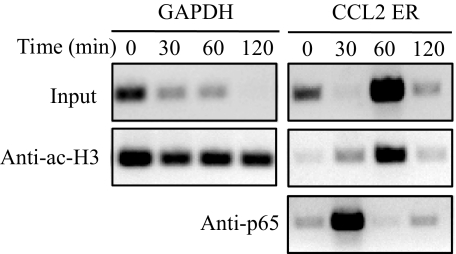
Studies up to this point have examined DAMGO-induced NF-κB activation and regulation of the CCL2 promoter activity. We wanted to examine whether the NF-κB subunit, p65, is actually induced to bind to the CCL2 promoter in vivo in response to MOR activation in PBMCs. Cells were treated with 100 nM DAMGO, and ChIP analysis was carried out to examine the interaction of p65 with the CCL2 promoter. Chromatin preparations were obtained at various times following MOR activation using anti-acetyl-histone H3 and anti-p65 antibodies. There are two NF-κB-binding sites located within the distal 5′ region of the CCL2 gene that are important for the inducible expression of CCL2 [22]. Based on these studies and our preliminary experiments, we designed PCR primers to span both of the NF-κB-binding elements in the CCL2 enhancer region for PCR analysis following ChIP. The results show that p65 bound the CCL2 enhancer very strongly, 30 min following DAMGO treatment (Fig. 6). Additionally, our results showed an increase in the CCL2 PCR product following DAMGO administration when immunoprecipitated with an anti-acetyl H3 antibody (Fig. 6). Acetylation of histone H3 is commonly observed in genes that are being actively transcribed into RNA; these results suggest that the NF-κB enhancer region is undergoing remodeling of the nucleosome structure to an open conformation that is more accessible to transcription complexes [42]. Therefore, the conformational change of the DNA organization results in increased amplification of the input DNA. These results are consistent with published data that suggest that NF-κB binds to the distal regulatory region of the CCL2 promoter [43]. As a control, we also observed (Fig. 6) that when immunoprecipitated with the anti-acetyl histone H3 antibody, PCR analysis showed equals amounts of GAPDH, which was used at each time-point, as our loading control. Together, we conclude that p65 recruited to the CCL2 promoter in vivo in response to DAMGO administration.
Figure 6. MOR-induced binding of NF-κB to the enhancer region of the CCL2 promoter in vivo.
Chromatin was prepared from human PBMCs and digested into one to two nucleosome fragments that were used for ChIP analysis. Chromatin (15–30 μg) was incubated with protein A magnetic beads and an anti-acetyl-H3 (Anti-ac-H3) antibody or an anti-p65 antibody overnight. Protein/DNA complexes were then washed and separated. Purified DNA was then subjected to PCR analysis for GAPDH or the CCL2 enhancer region (ER).
DISCUSSION
We have previously reported that activation of MOR induces the expression of several proinflammatory chemokines, including CCL2 and CCL5 [7], and this effect can be reversed in the presence of the MOR antagonist H-d-Phe-c[Cys-Tyr-d-Trp-Arg-Thr-Pen]-Thr-NH2 [7]. Our results have suggested that the production of these chemokines proceeds in two phases: an early phase that is apparent as early as 2–4 h and a second late phase that peaks at ∼48 h. Our earlier results have shown that the late phase of the CCL5 response is dependent on the intervening induction of TGF-β [8]. The results reported here show that the early induction of CCL2 expression following MOR activation is dependent on the rapid activation of NF-κB. We believe that this is the first report showing that MOR induction of this highly proinflammatory transcription factor NF-κB is responsible for a significant part of the inflammatory response and specifically, the regulation of CCL2 expression.
Based on the data presented here, we propose that MOR activation initiates a downstream signal transduction pathway(s) that lead to the activation of PKCζ and this is most likely through the G protein βγ subunit, PI3K, and PDK-1. Stimulation of MOR has been shown to activate PI3K and indirectly induce the activation of Akt, a serine/threonine kinase [44]. The activation of Akt can be mediated by PDK-1, and PKCζ is a known substrate for PDK-1 [35, 45]. Active PKCζ can activate the IKK complex through phosphorylation of IKKβ [46], and activation of the IKK complex by PKCζ and other kinases results in phosphorylation of IκB at serine residues 32 and 36 [28]. This phosphorylation is a prerequisite for the subsequent polyubiquitination by a ubiquitin ligase belonging to the Skp-1/Cul/F box family [47]. The ubiquitin-marked IκB proteins are rapidly degraded by the proteosome, thereby freeing the NF-κB complex of p65/p50, and PKCζ and other kinases can then phosphorylate p65 at serines 311 and 536 [30]. Additional post-translational modifications, as well as the unmasking of the nuclear localization signal on p65, allow nuclear translocation. Activated NF-κB complexes then translocate and enter the nucleus, bind DNA, and initiate transcription of NF-κB target genes including CCL2.
Proteasome inhibitors are well-known inhibitors of NF-κB activity and are considered potential therapeutic agents for treatment of inflammatory diseases. However, a recent report by Nakayama et al. [48] suggests that the proteasome inhibitor MG132 is able to induce CCL2 expression at the level of transcription in rat mesangial cells. Further analysis of this effect revealed that MG132 rapidly induced JNK activation and expression of c-jun and AP-1 activity. Transcriptional activation by MG132 was not only observed for CCL2 but also for other AP-1-dependent genes, including stromelysin and MAPK phosphatase 1 [48]. Nevertheless, in light of these results, we have used additional NF-κB inhibitors (HNE and BAY 11-7082) in our studies to address the role of this transcription factor in the MOR-induced expression of these chemokines.
The induction of NF-κB following MOR activation may provide a partial explanation for previous reports, showing that μ-opioids induce the expression of proinflammatory cytokines that are known to be regulated by this transcription factor [9–16]. Apte et al. [49] have shown that endorphins and enkephalins induce an increase in the production of IL-1. Roy et al. [50] have shown that morphine administration, at relatively low concentrations, can synergize with LPS and augment the expression of IL-6 and TNF-α. A more recent report from Peng et al. [51] has shown that macrophages from morphine-treated mice exhibit augmented IL-12 and TNF-α responses in vitro. However, it should be pointed out that μ-opioids have also been reported to inhibit the production of these and other proinflammatory cytokines. For example, Wang et al. [52] show that morphine-treated mice exhibit significantly reduced levels of IL-1, IL-6, and TNF-α in the BAL fluid. The molecular basis for the opposing results obtained from these studies is not clear at this time.
Previous studies have shown that MOR activation by DAMGO treatment is able to increase NF-κB DNA-binding activity in primary rat cortical neurons [53]. Liu and Wong [23] showed that MOR is capable of initiating NF-κB activition through the phosphorylation of IKK as well as p65 in the human neuroblastoma SH-SY5Y cells. Roy et al. [50] demonstrated that morphine treatment differentially modulates LPS-inducible gene expression through the regulation of NF-κB in murine macrophages. In this case, nanomolar concentrations of morphine resulted in an increase in NF-κB DNA-binding activity, and morphine treatments at micromolar concentrations led to a significant decrease in NF-κB activation [50]. Although the effects can be diverse and often cell type-dependent, NF-κB can be a critical component in opioid function and receptor gene expression [24]. Moreover, NF-κB has recently been implicated in the transcriptional regulation of MOR [54], δ-opioid receptor [55], as well as κ-opioid receptor [56] expression.
Recent studies investigating the mechanism of TNF-α-induced CCL2 gene expression in murine fibroblasts have suggested communication between the distal and proximal regions of the CCL2 promoter control histone acetylation and the transcriptional regulation of CCL2 [57]. In the inactive state, only the distal region of the CCL2 promoter appeared to be accessible to transcription factors. Following activation, NF-κB binds to the distal regulatory region of the CCL2 promoter, and this leads to the recruitment of the transcriptional coactivators CBP and p300 [43]. Recruitment of CBP and p300 leads to histone modifications that allow binding of Sp1 to the CCL2 proximal promoter region [43]. However, our examination of transcription factor binding using the transcription factor-binding array analysis reported here failed to show a significant change in Sp1 binding following MOR activation in PBMCs. Nevertheless, we cannot rule out the possibility that other transcriptional regulatory factors, in addition to NF-κB, may also play a role in the MOR induction of chemokine expression.
In this report, we show that NF-κB inhibitors can prevent the MOR-induced activation of CCL2 and that the NF-κB subunit, p65, is phosphorylated at serine residues 311 and 536 in response to opioid receptor activation. Phosphorylation of p65 at Ser-536, a site located in the TAD, can be carried out by the kinases IKKα/β and ribosomal S6 kinse 1 [58–61]. This site is phosphorylated in vivo in response to a variety of stimuli, including TNF-α, LPS, T cell costimulation, lymphotoxin B, or phorbol ester/ionomycin [61–63]. Despite its wide occurrence, the function of serine 536 phosphorylation for p65 activity is largely uncertain. In contrast, serine 311 lies in the TAD of p65 and is inducibly phosphorylated by PKCζ, in response to TNF-α stimulation [34]. For PKCζ to interact with p65 in cytokine-activated cells, IκB is first degraded by the proteosome to allow access to this serine residue. Additionally, PKCζ-deficient fibroblasts show IKK activation and normal nuclear translocation of p65 but reduced DNA-binding activity, indicating a role for this serine residue in transcriptional activity [32]. Further experiments indicate that the serine 311 phosphorylation promotes the interaction of p65 with CBP and the recruitment of CBP and RNA Polymerase II to the IL-6 promoter [34]. Interestingly, rPKCζ has also been shown to phosphorylate IKKβ directly in vitro at serine 177 and 181, leading to IKKβ activation and IκB degradation [46]. Finally, an in vitro interaction between PKCζ and the MKK-MAPK complex has been demonstrated and together with the NF-κB pathway, may contribute to the mechanism by which PKCζ controls cell proliferation [64].
Our studies reported here have focused on understanding the effect of the MOR activation on chemokine expression, and we believe that this is the first report showing that MOR induces the activation of PKCζ, and this signaling event is necessary for optimal induction of NF-κB activity. The requirement for PKCζ in the induction of chemokine expression is consistent with published reports showing that this kinase can play an important role in the function and activation of the inflammatory response [32, 65, 66]. It is likely that there are several signaling pathways relevant to the immune response that are initiated through the activation of MOR. It will be interesting to determine whether activation of NF-κB and/or PKCζ is involved in the induction of other proinflammatory genes, which are known to be induced following activation of MOR.
ACKNOWLEDGMENTS
This work was supported by United States Public Health Service grants DA-14230, DA-25532, P01DA-23860, P30DA-13429, T32DA-07237 (to C.H.), F31 DA-05894 (to M.K.), and DA-06650 from the National Institute on Drug Abuse.
Footnotes
- BAY 11-7082
- (E)-3-(4-methylphenylsulfonyl)-2-propenenenitrile
- CBP
- CREB-binding protein
- ChIP
- chromatin immunoprecipitation assay
- CHX
- cycloheximide
- DAMGO
- [d-Ala2,N-Me-Phe4-Gly5-ol]enkephalin
- HEK
- human embryonic kidney
- HNE
- (E)-4-hydroxynonenal
- MOR
- μ-opioid receptor
- PDK-1
- phosphoinositol-dependent kinase 1
- PSI
- pseudosubstrate inhibitor
- SEAP
- secreted alkaline phosphatase
- Sp1
- specificity protein 1
- TAD
- transactivation domain
AUTHORSHIP
All authors participated in all phases of the generation of these studies, including the planning and conduct of the experiments and the writing of the manuscript.
REFERENCES
- 1. Bidlack J. M. (2000) Detection and function of opioid receptors on cells from the immune system. Clin. Diagn. Lab. Immunol. 7, 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wick M. J., Minnerath S. R., Roy S., Ramakrishnan S., Loh H. H. (1996) Differential expression of opioid receptor genes in human lymphoid cell lines and peripheral blood lymphocytes. J. Neuroimmunol. 64, 29–36 [DOI] [PubMed] [Google Scholar]
- 3. Wybran J., Appelboom T., Famaey J. P., Govaerts A. (1979) Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lymphocytes. J. Immunol. 123, 1068–1070 [PubMed] [Google Scholar]
- 4. Rogers T. J., Peterson P. K. (2003) Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 24, 116–121 [DOI] [PubMed] [Google Scholar]
- 5. McCarthy L., Wetzel M., Sliker J. K., Eisenstein T. K., Rogers T. J. (2001) Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 62, 111–123 [DOI] [PubMed] [Google Scholar]
- 6. Alicea C., Belkowski S., Eisenstein T. K., Adler M. W., Rogers T. J. (1996) Inhibition of primary murine macrophage cytokine production in vitro following treatment with the κ-opioid agonist U50,488H. J. Neuroimmunol. 64, 83–90 [DOI] [PubMed] [Google Scholar]
- 7. Wetzel M. A., Steele A. D., Eisenstein T. K., Adler M. W., Henderson E. E., Rogers T. J. (2000) μ-Opioid induction of monocyte chemoattractant protein-1, RANTES, and IFN-γ-inducible protein-10 expression in human peripheral blood mononuclear cells. J. Immunol. 165, 6519–6524 [DOI] [PubMed] [Google Scholar]
- 8. Happel C., Steele A. D., Finley M. J., Kutzler M. A., Rogers T. J. (2008) DAMGO-induced expression of chemokines and chemokine receptors: the role of TGF-β1. J. Leukoc. Biol. 83, 956–963 [DOI] [PubMed] [Google Scholar]
- 9. Kuprash D. V., Udalova I. A., Turetskaya R. L., Rice N. R., Nedospasov S. A. (1995) Conserved κB element located downstream of the tumor necrosis factor α gene: distinct NF-κB binding pattern and enhancer activity in LPS activated murine macrophages. Oncogene 11, 97–106 [PubMed] [Google Scholar]
- 10. Mukaida N., Okamoto S., Ishikawa Y., Matsushima K. (1994) Molecular mechanism of interleukin-8 gene expression. J. Leukoc. Biol. 56, 554–558 [PubMed] [Google Scholar]
- 11. Stein B., Baldwin A. S., Jr. (1993) Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-κB. Mol. Cell. Biol. 13, 7191–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiscott J., Marois J., Garoufalis J., D'Addario M., Roulston A., Kwan I., Pepin N., Lacoste J., Nguyen H., Bensi G., et al. (1993) Characterization of a functional NF-κB site in the human interleukin 1 β promoter: evidence for a positive autoregulatory loop. Mol. Cell. Biol. 13, 6231–6240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galien R., Evans H. F., Garcia T. (1996) Involvement of CCAAT/enhancer-binding protein and nuclear factor-κ B binding sites in interleukin-6 promoter inhibition by estrogens. Mol. Endocrinol. 10, 713–722 [DOI] [PubMed] [Google Scholar]
- 14. Martin T., Cardarelli P. M., Parry G. C., Felts K. A., Cobb R. R. (1997) Cytokine induction of monocyte chemoattractant protein-1 gene expression in human endothelial cells depends on the cooperative action of NF-κB and AP-1. Eur. J. Immunol. 27, 1091–1097 [DOI] [PubMed] [Google Scholar]
- 15. Moriuchi H., Moriuchi M., Fauci A. S. (1997) Nuclear factor-κ B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J. Immunol. 158, 3483–3491 [PubMed] [Google Scholar]
- 16. Nelson P. J., Ortiz B. D., Pattison J. M., Krensky A. M. (1996) Identification of a novel regulatory region critical for expression of the RANTES chemokine in activated T lymphocytes. J. Immunol. 157, 1139–1148 [PubMed] [Google Scholar]
- 17. Shyy Y. J., Li Y. S., Kolattukudy P. E. (1990) Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem. Biophys. Res. Commun. 169, 346–351 [DOI] [PubMed] [Google Scholar]
- 18. Shyy Y. J., Li Y. S., Kolattukudy P. E. (1993) Activation of MCP-1 gene expression is mediated through multiple signaling pathways. Biochem. Biophys. Res. Commun. 192, 693–699 [DOI] [PubMed] [Google Scholar]
- 19. Li Y. S., Kolattukudy P. E. (1994) Functional role of the cis-acting elements in human monocyte chemotactic protein-1 gene in the regulation of its expression by phorbol ester in human glioblastoma cells. Mol. Cell. Biochem. 141, 121–128 [DOI] [PubMed] [Google Scholar]
- 20. Ping D., Jones P. L., Boss J. M. (1996) TNF regulates the in vivo occupancy of both distal and proximal regulatory regions of the MCP-1/JE gene. Immunity 4, 455–469 [DOI] [PubMed] [Google Scholar]
- 21. Ueda A., Okuda K., Ohno S., Shirai A., Igarashi T., Matsunaga K., Fukushima J., Kawamoto S., Ishigatsubo Y., Okubo T. (1994) NF-κB and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J. Immunol. 153, 2052–2063 [PubMed] [Google Scholar]
- 22. Ueda A., Ishigatsubo Y., Okubo T., Yoshimura T. (1997) Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-κB sites and NF-κB/Rel subunit specificity. J. Biol. Chem. 272, 31092–31099 [DOI] [PubMed] [Google Scholar]
- 23. Liu A. M., Wong Y. H. (2005) μ-Opioid receptor-mediated phosphorylation of IκB kinase in human neuroblastoma SH-SY5Y cells. Neurosignals 14, 136–142 [DOI] [PubMed] [Google Scholar]
- 24. Chen Y. L., Law P. Y., Loh H. H. (2006) Nuclear factor κB signaling in opioid functions and receptor gene expression. J. Neuroimmune Pharmacol. 1, 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Siebenlist U., Franzoso G., Brown K. (1994) Structure, regulation and function of NF-κB. Annu. Rev. Cell Biol. 10, 405–455 [DOI] [PubMed] [Google Scholar]
- 26. Baeuerle P. A., Baichwal V. R. (1997) NF-κB as a frequent target for immunosuppressive and anti-inflammatory molecules. Adv. Immunol. 65, 111–137 [PubMed] [Google Scholar]
- 27. Brown K., Gerstberger S., Carlson L., Franzoso G., Siebenlist U. (1995) Control of I κB-α proteolysis by site-specific, signal-induced phosphorylation. Science 267, 1485–1488 [DOI] [PubMed] [Google Scholar]
- 28. Chen L. F., Greene W. C. (2004) Shaping the nuclear action of NF-κB. Nat. Rev. Mol. Cell Biol. 5, 392–401 [DOI] [PubMed] [Google Scholar]
- 29. Ghosh S., May M. J., Kopp E. B. (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 [DOI] [PubMed] [Google Scholar]
- 30. Henkel T., Machleidt T., Alkalay I., Kronke M., Ben-Neriah Y., Baeuerle P. A. (1993) Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature 365, 182–185 [DOI] [PubMed] [Google Scholar]
- 31. Schmitz M. L., Mattioli I., Buss H., Kracht M. (2004) NF-κB: a multifaceted transcription factor regulated at several levels. ChemBioChem 5, 1348–1358 [DOI] [PubMed] [Google Scholar]
- 32. Leitges M., Sanz L., Martin P., Duran A., Braun U., Garcia J. F., Camacho F., Diaz-Meco M. T., Rennert P. D., Moscat J. (2001) Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol. Cell 8, 771–780 [DOI] [PubMed] [Google Scholar]
- 33. LaVallie E. R., Chockalingam P. S., Collins-Racie L. A., Freeman B. A., Keohan C. C., Leitges M., Dorner A. J., Morris E. A., Majumdar M. K., Arai M. (2006) Protein kinase Cζ is up-regulated in osteoarthritic cartilage and is required for activation of NF-κB by tumor necrosis factor and interleukin-1 in articular chondrocytes. J. Biol. Chem. 281, 24124–24137 [DOI] [PubMed] [Google Scholar]
- 34. Duran A., Diaz-Meco M. T., Moscat J. (2003) Essential role of RelA Ser311 phosphorylation by ζPKC in NF-κB transcriptional activation. EMBO J. 22, 3910–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Le Good J. A., Ziegler W. H., Parekh D. B., Alessi D. R., Cohen P., Parker P. J. (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281, 2042–2045 [DOI] [PubMed] [Google Scholar]
- 36. Chou M. M., Hou W., Johnson J., Graham L. K., Lee M. H., Chen C. S., Newton A. C., Schaffhausen B. S., Toker A. (1998) Regulation of protein kinase Cζ by PI3-kinase and PDK-1. Curr. Biol. 8, 1069–1077 [DOI] [PubMed] [Google Scholar]
- 37. Berger J., Hauber J., Hauber R., Geiger R., Cullen B. R. (1988) Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene 66, 1–10 [DOI] [PubMed] [Google Scholar]
- 38. Malim M. H., Freimuth W. W., Liu J., Boyle T. J., Lyerly H. K., Cullen B. R., Nabel G. J. (1992) Stable expression of transdominant Rev protein in human T cells inhibits human immunodeficiency virus replication. J. Exp. Med. 176, 1197–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baeuerle P. A., Baltimore D. (1996) NF-κB: ten years after. Cell 87, 13–20 [DOI] [PubMed] [Google Scholar]
- 40. Peltz G. (1997) Transcription factors in immune-mediated disease. Curr. Opin. Biotechnol. 8, 467–473 [DOI] [PubMed] [Google Scholar]
- 41. Baeuerle P. A. (1998) Pro-inflammatory signaling: last pieces in the NF-κB puzzle? Curr. Biol. 8, R19–R22 [DOI] [PubMed] [Google Scholar]
- 42. Yan C., Boyd D. D. (2006) Histone H3 acetylation and H3 K4 methylation define distinct chromatin regions permissive for transgene expression. Mol. Cell. Biol. 26, 6357–6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Teferedegne B., Green M. R., Guo Z., Boss J. M. (2006) Mechanism of action of a distal NF-κB-dependent enhancer. Mol. Cell. Biol. 26, 5759–5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Polakiewicz R. D., Schieferl S. M., Gingras A. C., Sonenberg N., Comb M. J. (1998) μ-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J. Biol. Chem. 273, 23534–23541 [DOI] [PubMed] [Google Scholar]
- 45. Toker A. (2003) PDK-1 and protein kinase C phosphorylation. Methods Mol. Biol. 233, 171–189 [DOI] [PubMed] [Google Scholar]
- 46. Lallena M. J., Diaz-Meco M. T., Bren G., Paya C. V., Moscat J. (1999) Activation of IκB kinase β by protein kinase C isoforms. Mol. Cell. Biol. 19, 2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Winston J. T., Strack P., Beer-Romero P., Chu C. Y., Elledge S. J., Harper J. W. (1999) The SCFβ-TRCP-ubiquitin ligase complex associates specifically with destruction motifs IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13, 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakayama K., Furusu A., Xu Q., Konta T., Kitamura M. (2001) Unexpected transcriptional induction of monocyte chemoattractant protein 1 by proteasome inhibition: involvement of the c-Jun N-terminal kinase-activator protein 1 pathway. J. Immunol. 167, 1145–1150 [DOI] [PubMed] [Google Scholar]
- 49. Apte R. N., Durum S. K., Oppenheim J. J. (1990) Opioids modulate interleukin 1 production and secretion by bone-marrow macrophages. Immunol. Lett. 24, 141–148 [DOI] [PubMed] [Google Scholar]
- 50. Roy S., Cain K. J., Chapin R. B., Charboneau R. G., Barke R. A. (1998) Morphine modulates NF κ B activation in macrophages. Biochem. Biophys. Res. Commun. 245, 392–396 [DOI] [PubMed] [Google Scholar]
- 51. Peng X., Mosser D. M., Adler M. W., Rogers T. J., Meissler J. J., Jr., Eisenstein T. K. (2000) Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J. Leukoc. Biol. 68, 723–728 [PubMed] [Google Scholar]
- 52. Wang J., Barke R. A., Charboneau R., Roy S., Wang J. (2005) Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J. Immunol. 174, 426–434 [DOI] [PubMed] [Google Scholar]
- 53. Hou Y. N., Vlaskovska M., Cebers G., Kasakov L., Liljequist S., Terenius L. (1996) A μ-receptor opioid agonist induces AP-1 and NF-κB transcription factor activity in primary cultures of rat cortical neurons. Neurosci. Lett. 212, 159–162 [DOI] [PubMed] [Google Scholar]
- 54. Kraus J., Borner C., Giannini E., Hollt V. (2003) The role of nuclear factor κB in tumor necrosis factor-regulated transcription of the human μ-opioid receptor gene. Mol. Pharmacol. 64, 876–884 [DOI] [PubMed] [Google Scholar]
- 55. Chen Y. L., Law P. Y., Loh H. H. (2006) Sustained activation of phosphatidylinositol 3-kinase/Akt/nuclear factor κB signaling mediates G protein-coupled δ-opioid receptor gene expression. J. Biol. Chem. 281, 3067–3074 [DOI] [PubMed] [Google Scholar]
- 56. Law P. Y., Loh H. H., Wei L. N. (2004) Insights into the receptor transcription and signaling: implications in opioid tolerance and dependence. Neuropharmacology 47 (Suppl. 1), 300–311 [DOI] [PubMed] [Google Scholar]
- 57. Boekhoudt G. H., Guo Z., Beresford G. W., Boss J. M. (2003) Communication between NF-κB and Sp1 controls histone acetylation within the proximal promoter of the monocyte chemoattractant protein 1 gene. J. Immunol. 170, 4139–4147 [DOI] [PubMed] [Google Scholar]
- 58. Sakurai H., Chiba H., Miyoshi H., Sugita T., Toriumi W. (1999) IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274, 30353–30356 [DOI] [PubMed] [Google Scholar]
- 59. Sizemore N., Lerner N., Dombrowski N., Sakurai H., Stark G. R. (2002) Distinct roles of the Iκ B kinase α and β subunits in liberating nuclear factor κ B (NF-κB) from IκB and in phosphorylating the p65 subunit of NF-κB. J. Biol. Chem. 277, 3863–3869 [DOI] [PubMed] [Google Scholar]
- 60. Buss H., Dorrie A., Schmitz M. L., Hoffmann E., Resch K., Kracht M. (2004) Constitutive and interleukin-1-inducible phosphorylation of p65 NF-κB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-α, IKKβ, IKKε, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. J. Biol. Chem. 279, 55633–55643 [DOI] [PubMed] [Google Scholar]
- 61. Mattioli I., Sebald A., Bucher C., Charles R. P., Nakano H., Doi T., Kracht M., Schmitz M. L. (2004) Transient and selective NF-κB p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I κ B kinase β and controls the kinetics of p65 nuclear import. J. Immunol. 172, 6336–6344 [DOI] [PubMed] [Google Scholar]
- 62. Sakurai H., Suzuki S., Kawasaki N., Nakano H., Okazaki T., Chino A., Doi T., Saiki I. (2003) Tumor necrosis factor-α-induced IKK phosphorylation of NF-κB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J. Biol. Chem. 278, 36916–36923 [DOI] [PubMed] [Google Scholar]
- 63. Jiang X., Takahashi N., Ando K., Otsuka T., Tetsuka T., Okamoto T. (2003) NF-κB p65 transactivation domain is involved in the NF-κB-inducing kinase pathway. Biochem. Biophys. Res. Commun. 301, 583–590 [DOI] [PubMed] [Google Scholar]
- 64. Diaz-Meco M. T., Dominguez I., Sanz L., Dent P., Lozano J., Municio M. M., Berra E., Hay R. T., Sturgill T. W., Moscat J. (1994) ζ PKC induces phosphorylation and inactivation of IκB-α in vitro. EMBO J. 13, 2842–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martin P. A., Duran S., Minguet M. L., Gaspar, Diaz-Meco M. T., Rennert P., Leitges M., Moscat J. (2002) Role of ζPKC in B-cell signaling and function. EMBO J. 21, 4049–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Savkovic S. D., Koutsouris A., Hecht G. (2003) PKCζ participates in activation of inflammatory response induced by enteropathogenic E. coli. Am. J. Physiol. Cell Physiol. 285, C512–C521 [DOI] [PubMed] [Google Scholar]