Abstract
Objectives
To examine the association between the Mediterranean diet (MeDi) and Alzheimer disease (AD) in a different AD population and to investigate possible mediation by vascular pathways.
Design, Setting, Patients, and Main Outcome Measures
A case-control study nested within a community-based cohort in New York, NY. Adherence to the MeDi (0- to 9-point scale with higher scores indicating higher adherence) was the main predictor of AD status (194 patients with AD vs 1790 nondemented subjects) in logistic regression models that were adjusted for cohort, age, sex, ethnicity, education, apolipoprotein E genotype, caloric intake, smoking, medical comorbidity index, and body mass index (calculated as weight in kilograms divided by height in meters squared). We investigated whether there was attenuation of the association between MeDi and AD when vascular variables (stroke, diabetes mellitus, hypertension, heart disease, lipid levels) were simultaneously introduced in the models (which would constitute evidence of mediation).
Results
Higher adherence to the MeDi was associated with lower risk for AD (odds ratio, 0.76; 95% confidence interval, 0.67–0.87; P<.001). Compared with subjects in the lowest MeDi tertile, subjects in the middle MeDi tertile had an odds ratio of 0.47 (95% confidence interval, 0.29–0.76) and those at the highest tertile an odds ratio of 0.32 (95% confidence interval, 0.17–0.59) for AD (P for trend <.001). Introduction of the vascular variables in the model did not change the magnitude of the association.
Conclusions
We note once more that higher adherence to the MeDi is associated with a reduced risk for AD. The association does not seem to be mediated by vascular comorbidity. This could be the result of either other biological mechanisms (oxidative or inflammatory) being implicated or measurement error of the vascular variables.
Dietary pattern analysis has recently received growing attention in relation to many diseases (ie, cirrhosis or various cancers) because individuals do not consume foods or nutrients in isolation but rather as components of their daily diet. Defining diet by dietary patterns has the ability to capture its multidimensionality while reducing its apparent complexity because patterns can integrate complex or subtle interactive effects of many dietary constituents and bypass problems generated by multiple testing and the high correlations that may exist among these constituents.1 Part of the explanation for some of the conflicting findings in the literature regarding dietary constituents and Alzheimer disease (AD) risk could be the result of individual food or nutrient approach. Nevertheless, there is paucity of data regarding the effect of composite dietary patterns on the risk for AD.
One such dietary pattern is the Mediterranean diet (MeDi), which has been associated with lower risk for several forms of cancer,2 obesity,3,4 dyslipidemia,5,6 hypertension,3,5–7 abnormal glucose metabolism,3,6 coronary heart disease,6,8–10 and overall mortality.8,10,11 In a recent study, we demonstrated that higher adherence to the MeDi at baseline evaluation was associated with lower risk of developing AD during follow-up.12 All subjects in that study were nondemented at baseline and AD incidence was prospectively assessed. Because that was the first study of its kind in the neurological literature, it is very important to observe a similar association in a different set of participants. In this article, we examine whether the association between MeDi and AD still holds when a different population of AD subjects is used: those who were found to have AD at the baseline evaluation (prevalent AD) of the Washington Heights–Inwood Columbia Aging Project (WHICAP). We hypothesized that higher adherence to the MeDi would be associated with lower risk for AD in this AD population too.
One of the possible mechanisms via which the MeDi may be exerting its protective effect for AD could be vascular: higher adherence to the MeDi could be related to lower cardiovascular-cerebrovascular disease and hence lower dementia rates. In an attempt to shed preliminary light on this possibility of vascular mediation, in this study we examined whether the association between MeDi and AD is mediated by vascular comorbidity.
METHODS
SAMPLE AND PROCEDURES
The study included participants of 2 related cohorts recruited in 1992 (WHICAP 1992) and 1999 (WHICAP 1999), which were identified (via ethnicity and age stratification processes) from a probability sample of Medicare beneficiaries residing in an area of 3 contiguous census tracts within a geographically defined area of northern Manhattan.12–16 The same assessments and study procedures were used in both cohorts. At the baseline assessment, a physician elicited each subject’s medical and neurological history and conducted a standardized physical and neurological examination. All available ancillary information (medical records, computed tomographic scans, or magnetic resonance images) was considered in the evaluation. A global summary score on the Clinical Dementia Rating (CDR)17 was also assigned. Each subject also underwent a structured in-person interview, including an assessment of health and function and a neuropsychological battery.18 The neuropsychological battery contained tests of memory (short- and long-term verbal and nonverbal); orientation; abstract reasoning (verbal and nonverbal); language (naming, verbal fluency, comprehension, and repetition); and construction (copying and matching).
A consensus diagnosis for the presence or absence of dementia was made at a diagnostic conference of neurologists and neuropsychologists where information from all these evaluations was presented. Evidence of cognitive deficit (based on the neuropsychological scores as described), evidence of impairment in social or occupational function (as assessed by the Blessed Dementia Rating Scale, the Schwab and England Activities of Daily Living Scale, and the physician’s assessment), and evidence of cognitive and social-occupational function decline as compared with the past were the criteria used for the diagnosis of dementia as required by the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R). The type of dementia was subsequently determined. For the diagnosis of probable or possible AD, the criteria of the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association19 were used. Because in these criteria stroke does not preclude the diagnosis of AD (unless cerebrovascular disease was considered the primary cause of the dementia), the diagnosis of AD with concomitant stroke was also assigned. Dietary data were not available to the consensus panel and were not considered in the diagnostic process. Only data from the baseline WHICAP evaluation were included in the analyses.
EVALUATION
Diet
Dietary data regarding average food consumption over the past year were obtained using a 61-item version of Willett’s semiquantitative food frequency questionnaire.20 Trained interviewers administered the questionnaire in English or Spanish. We have previously reported validity (using two 7-day food records) and reliability (using two 3-month frequency assessments) of various components of the questionnaire in WHICAP.13–15
Similarly to our previous work,12 we followed the method previously described by Trichopoulou et al8 for the construction of the MeDi score. Specifically, we first regressed caloric intake (in kilocalories) and calculated the derived residuals of daily gram intake (as recommended by Willet and Stampfer21) for each of the following 7 categories (which define the components of the MeDi as previously defined by Trichopoulou et al8): dairy, meat, fruits, vegetables, legumes, cereals, and fish. A value of 0 or 1 was assigned to each of the 7 groups using sex-specific medians as cutoffs. For beneficial components (fruits, vegetables, legumes, cereals, and fish), each subject whose consumption was below the median was assigned a value of 0, and each subject whose consumption was at or above the median was assigned a value of 1. For components presumed to be detrimental (meat and dairy products), each subject whose consumption was below the median was assigned a value of 1, and each subject whose consumption was at or above the median was assigned a value of 0. For fat intake (the eighth food category), we used the ratio of daily consumption (in grams) of monounsaturated lipids to saturated lipids8 (again using sex-specific median cutoffs for assignment values of 0 for low and 1 for high). For alcohol intake (the ninth food category), each subject was assigned a score of 0 for either no consumption (0 g/d) or more than moderate (≥30 g/d) consumption and a value of 1 for mild to moderate alcohol consumption (>0 to <30 g/d). This is agreement with previous reports8 that consider a moderate amount of alcohol consumption as another characteristic component of the MeDi. We classified alcohol consumption dichotomously, also because of the skewed distribution of alcohol in our population (68% reporting no alcohol intake, 31% reporting less than 30 g/d [mild to moderate intake], and 1% reporting ≥30 g/d [heavy intake]). The MeDi score was generated for each participant by adding the scores in the food categories (theoretically ranging 0–9) with a higher score indicating higher adherence to the MeDi.
Covariates
Age (years), education (years), caloric intake (kilocalories), and body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared)22 were used as continuous variables. We also considered cohort (1992 cohort as reference), sex (male sex as reference), and smoking status at baseline evaluation (nonsmoking as reference). Ethnic group was based on self-report using the format of the 1990 census.23 Participants were then assigned to 1 of 4 groups: black (non-Hispanic), Hispanic, white (non-Hispanic), or other. Ethnicity was used as a dummy variable with white (non-Hispanic) as the reference. Apolipoprotein E (APOE) genotype was used dichotomously: absence of ε4 allele vs presence of either 1 or 2 ε4 alleles. A modified version24,25 of the Charlson Index of Comorbidity26 (referred to as the comorbidity index) was included as a continuous variable.
The diagnosis of stroke was based on questioning of the participant or relatives and supplemented by a neurological examination or review of medical records. Heart disease, diabetes mellitus, and hypertension were defined by self-report or by the use of disease-specific medications. In an alternative definition of hypertension, we used continuous blood pressure measurements instead of history of hypertension. Self-report data on diabetes, hypertension, stroke, and heart disease have been shown to be reliable (test-retest reliability, κ=0.85) and to have high sensitivity and specificity (more than 90% for either) using medical records as the gold standard in this study’s cohort.27 Fasting plasma total cholesterol and triglyceride levels were determined at initial assessment using standard enzymatic techniques. Levels of high-density lipoprotein cholesterol were determined after precipitation of apolipoprotein B–containing lipoproteins with phosphotungstic acid.28 Levels of low-density lipoprotein cholesterol were recalculated using a previously published formula.29
STATISTICAL ANALYSES
Characteristics of patients by outcome of interest and by MeDi tertiles were compared using a t test or analysis of variance for continuous variables and a χ2 test for categorical variables.
MeDi Score Stability
Because dietary assessment and AD diagnosis occurred synchronously, it is possible that subjects had changed their dietary habits as a result of AD. To minimize this possibility, AD subjects with a CDR higher than 1 were not included in the analyses. To further address this issue, we used generalized estimating equations to test whether there were significant changes of MeDi score over time for a separate subset of subjects who were nondemented at baseline and developed AD during follow-up for whom we had available repeated dietary assessment. Generalized estimating equations take into account the multiple visits per subject and the fact that the characteristics of the same individual over time are likely to be correlated. The repeated measures for each subject are treated as a cluster. The generalized estimating equation model included the MeDi score as the dependent variable and time (years) as predictor. A significant time effect would indicate a significant change of MeDi score over time.
Logistic Regression Analyses
Basic Model
We calculated logistic regression models with diagnosis at baseline evaluation as the dichotomous outcome: AD (only CDR=1) vs nondementia (only CDR=0). The main predictor was MeDi score (from the baseline visit) as a continuous variable initially and in tertiles form subsequently (used for trend test calculation). In exploratory models, we repeated the analyses, excluding subjects who were diagnosed with AD with stroke (ie, using only AD without stroke as the outcome).
Adjusted Model
Because of demographic and clinical differences among patients with AD and nondemented controls, in subsequent models we simultaneously adjusted for the following variables: cohort, age at intake in the study, sex, ethnicity, education, APOE genotype, smoking, comorbidity index, and BMI. Although caloric intake–adjusted residuals were used in the MeDi score calculation, we also included caloric intake as a covariate in the models (as recommended by Willet and Stampfer21).
Vascular Comorbidity Model
We simultaneously included all vascular risk factors (history of stroke, diabetes, hypertension, or heart disease and levels of plasma total cholesterol, triglyceride, high-density lipoprotein, and low-density lipoprotein) in the adjusted logistic regression model and observed for changes in the magnitude of the coefficient pertaining to the relation between MeDi and risk for AD using the following formula: Δβ%=100 * (θ−β)/β where θ denotes the crude estimator from the model that does not contain the potential mediator (adjusted model without the vascular variables) and β denotes the adjusted estimator from the model that does include the potential mediator (adjusted model with the vascular variables).30 Changes of the β coefficient values of greater than 15% were considered evidence of mediation (ie, vascular variables are in the causal pathway between MeDi and AD).30 We finally recalculated the models excluding subjects who were diagnosed with AD with stroke (ie, using only AD without stroke as the outcome).
RESULTS
MISSING DATA ANALYSES
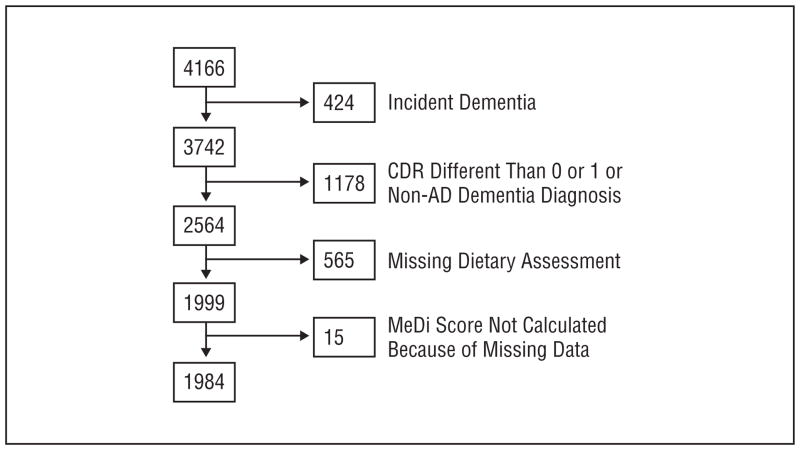
The following 3 groups of subjects were excluded from the current analyses (Figure 1): subjects who were non-demented at baseline and developed dementia at follow-up (incident dementia group, the relation of this group with MeDi has been previously examined12); subjects who were deemed nondemented but had a CDR higher than 0; and subjects who were deemed demented but had either a non-AD diagnosis or a CDR higher than 1. From the 2564 subjects remaining, there were missing dietary assessments for 565.
Figure 1.
Flowchart describing sample size. AD indicates Alzheimer disease; CDR, Clinical Dementia Rating; MeDi, Mediterranean diet.
Subjects with missing dietary information (compared with subjects with available dietary information) were older (79.1 vs 76.3 years, P<.001) and less educated (8.6 vs 10.7 years, P<.001) and had lower BMIs (26.8 vs 27.7, P=.009), lower total cholesterol levels (5.03 mmol/L [194.5 mg/dL] vs 5.23 mmol/L [202.1 mg/dL], P=.003), and lower low-density lipoprotein levels (3.02 mmol/L [116.6 mg/dL] vs 3.18 mmol/L [122.9 mg/dL], P=.005). Subjects with missing dietary information also were more likely to be Hispanic and less likely to be white (white, 22%; black, 35%; Hispanic, 43%; other, 1%; vs white, 32%; black, 33%; Hispanic, 33%; other, 2%; P<.001), were more likely to have AD (48% vs 10%, P<.001), and were less likely to be smokers (4% vs 11%, P<.001). There were no significant differences between subjects with missing data and those with available dietary information in sex (32% male vs 32% male, P=.86); medical comorbidity index (2.0 vs 1.9, P=.47); APOE genotype (31% ε4 carriers vs 27% noncarriers, P=.11); history of stroke (11% vs 8%, P=.09), diabetes (18% vs 19%, P=.71), hypertension (60% vs 64%, P=19), or heart disease (27% vs 25%, P=.49); high-density lipoprotein levels (1.23 mmol/L [47.4 mg/dL] vs 1.24 mmol/L [48.1 mg/dL], P=.45); or triglyceride levels (1.72 mmol/L [152.3 mg/dL] vs 1.76 mmol/L [155.5 mg/dL], P=.56).
STABILITY OF MeDi SCORE
There were 89 subjects with multiple dietary assessments who were not demented at baseline and who developed AD during follow-up (a different set of subjects than those used in the logistic regression analyses): for 78 participants, there were 2 assessments available; for 8 participants, 3 assessments; and for 3 participants, 4 assessments. The mean (SD) time interval between dietary assessments was 8.1 (1.9) years (range 1.8–11.9 years). The reported MeDi score did not change over time (β=−0.05, P=.09).
CLINICAL-DEMOGRAPHIC-DIETARY CHARACTERISTICS
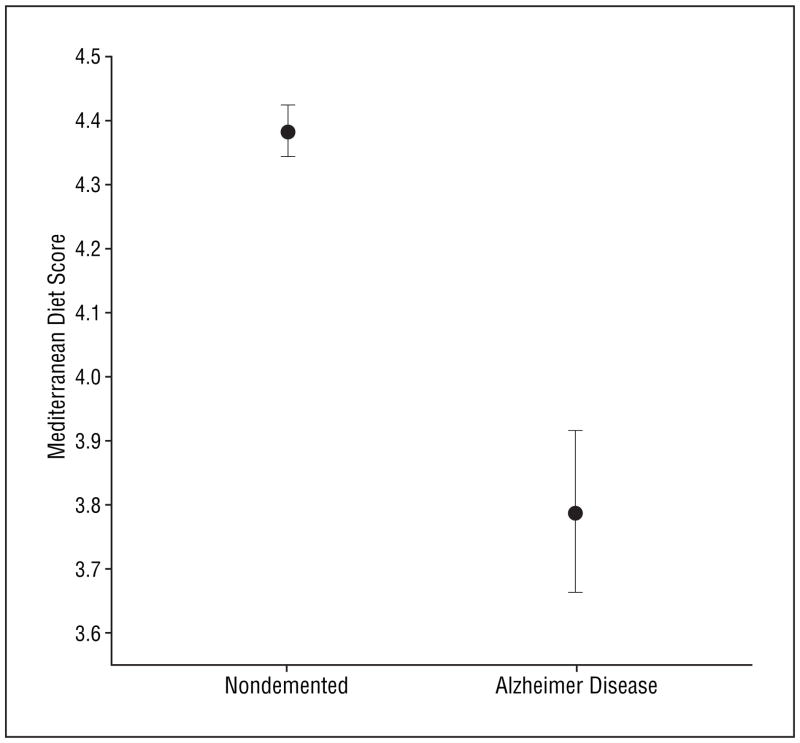
Compared with nondemented controls, subjects with AD were older and less educated and had lower BMIs (Table 1). There was a lower proportion of men, a higher proportion of Hispanic subjects, and a lower proportion of white (non-Hispanic) subjects who had AD. There was a borderline nonsignificant higher presence of ε4 allele in subjects with AD. In accordance to our previous report,31 history of stroke was more common in subjects with AD. Nondemented controls and subjects with AD did not differ in smoking status; medical comorbidity index; caloric intake; history of diabetes, hypertension, and heart disease; and levels of total cholesterol, high-density lipoprotein, triglyceride, or low-density lipoprotein. Compared with subjects who remained nondemented, subjects with AD had lower MeDi scores (Table 1 and Figure 2).
Table 1.
Demographic, Clinical, and Dietary Characteristics for Subjects With Alzheimer Disease and Nondemented Subjects
| Characteristic | Nondemented (n = 1790) | Prevalent AD (n = 194) | All (n = 1984) | P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 75.6 (6.1) | 82.3 (7.5) | 76.3 (6.6) | <.001 |
| Male sex, No. (%) | 584 (33) | 46 (24) | 630 (32) | .01 |
| Education, mean (SD), y | 11.2 (4.4) | 6.1 (4.3) | 10.7 (4.7) | <.001 |
| Ethnicity, No. (%) | ||||
| White | 626 (35) | 17 (9) | 643 (32) | <.001 |
| Black | 595 (33) | 61 (31) | 656 (33) | |
| Hispanic | 539 (30) | 115 (59) | 654 (33) | |
| Other | 30 (2) | 1 (1) | 31 (2) | |
| Presence of ε4 allele, No. (%) | 382 (26) | 50 (33) | 432 (27) | .06 |
| Smoking, No. (%) | 206 (12) | 17 (9) | 223 (11) | .27 |
| Comorbidity index, mean (SD) | 1.9 (1.4) | 2.1 (1.5) | 1.9 (1.4) | .29 |
| Energy, mean (SD), kcal | 1440 (520) | 1489 (655) | 1444 (535) | .22 |
| Body mass index, mean (SD)* | 27.8 (5.7) | 26.6 (5.9) | 27.7 (5.7) | .008 |
| MeDi score, mean (SD) | 4.4 (1.7) | 3.8 (1.8) | 4.3 (1.7) | <.001 |
| MeDi tertiles, No. (%) | ||||
| Low | 574 (32) | 93 (48) | 667 (33) | <.001 |
| Middle | 740 (41) | 70 (36) | 810 (41) | |
| High | 476 (27) | 31 (16) | 507 (26) | |
| Stroke, No. (%) | 122 (7) | 31 (16) | 153 (8) | <.001 |
| Diabetes mellitus, No. (%) | 307 (18) | 45 (23) | 352 (19) | .08 |
| Hypertension, No. (%) | 1069 (63) | 123 (65) | 1192 (64) | .65 |
| Heart disease, No. (%) | 428 (25) | 44 (23) | 472 (25) | .46 |
| TC, mean (SD), mg/dL | 201.5 (39.0) | 197.9 (42.4) | 201.0 (39.5) | .19 |
| HDL, mean (SD), mg/dL | 48.3 (15.4) | 47.4 (15.4) | 48.2 (15.4) | .52 |
| TG, mean (SD), mg/dL | 156.9 (85.0) | 142.5 (78.2) | 155.5 (84.4) | .05 |
| LDL, mean (SD), mg/dL | 122.9 (34.0) | 122.4 (37.0) | 122.8 (34.2) | .87 |
Abbreviations: AD, Alzheimer disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MeDi, Mediterranean diet; TC, total cholesterol; TG, triglycerides.
SI conversion factors: To convert total cholesterol, high-density lipoprotein, and low-density lipoprotein to mmol/L, multiply by 0.02586; triglycerides to mmol/L, multiply by 0.01129.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
Figure 2.
Means and standard errors of Mediterranean diet score for subjects with Alzheimer disease and nondemented subjects.
There was no association between MeDi score and age, sex, education, APOE genotype, medical comorbidity index, BMI, and any of the vascular variables (Table 2). Hispanic subjects adhered more and black subjects less to the MeDi pattern. Subjects adhering more to the MeDi tended to smoke less and had lower caloric intake.
Table 2.
Demographic and Clinical Characteristics by Mediterranean Diet Score Tertile
| Characteristic | Low Tertile (MeDi Score 0–3) | Middle Tertile (MeDi Score 4–5) | High Tertile (MeDi Score 6–9) | P Value |
|---|---|---|---|---|
| Cohort (WHICAP 1999), No. (%) | 402 (60) | 500 (62) | 321 (63) | .66 |
| Age, mean (SD), y | 76.5 (6.8) | 76.4 (6.6) | 75.8 (6.2) | .15 |
| Male sex, No. (%) | 210 (32) | 241 (30) | 179 (35) | .10 |
| Education, mean (SD), y | 10.5 (4.5) | 10.7 (4.8) | 10.9 (4.6) | .45 |
| Ethnicity, No. (%) | ||||
| White | 220 (33) | 265 (33) | 158 (31) | .006 |
| Black | 250 (38) | 261 (32) | 145 (29) | |
| Hispanic | 190 (29) | 271 (34) | 193 (38) | |
| Other | 7 (1) | 13 (2) | 11 (2) | |
| Presence of ε4 allele, No. (%) | 131 (25) | 180 (27) | 121 (28) | .46 |
| Smoking, No. (%) | 96 (14) | 88 (11) | 39 (8) | .001 |
| Comorbidity index, mean (SD) | 1.9 (1.4) | 2.0 (1.5) | 1.9 (1.3) | .46 |
| Energy, mean (SD), kcal | 1512 (590) | 1400 (473) | 1425 (545) | <.001* |
| Body mass index, mean (SD)† | 27.8 (6.0) | 27.6 (5.3) | 27.6 (6.0) | .68 |
| Stroke, No. (%) | 58 (9) | 64 (8) | 31 (6) | .21 |
| Diabetes mellitus, No. (%) | 112 (18) | 151 (20) | 89 (19) | .72 |
| Hypertension, No. (%) | 381 (61) | 500 (65) | 311 (64) | .33 |
| Heart disease, No. (%) | 161 (26) | 189 (25) | 122 (25) | .86 |
| TC, mean (SD), mg/dL | 201.0 (38.2) | 201.8 (39.8) | 204.0 (39.7) | .48 |
| HDL, mean (SD), mg/dL | 48.0 (15.2) | 48.4 (15.8) | 48.2 (15.1) | .89 |
| TG, mean (SD), mg/dL | 153.9 (84.3) | 158.3 (86.4) | 153.2 (81.6) | .55 |
| LDL, mean (SD), mg/dL | 122.3 (33.5) | 121.7 (34.5) | 125.2 (34.6) | .25 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MeDi, Mediterranean diet; TC, total cholesterol; TG, triglycerides; WHICAP, Washington Heights–Inwood Columbia Aging Project.
SI conversion factors: To convert total cholesterol, high-density lipoprotein, and low-density lipoprotein to mmol/L, multiply by 0.02586; triglycerides to mmol/L, multiply by 0.01129.
Post hoc Scheffe test for energy (kilocalorie): low vs middle tertile, P<.001; low vs high tertile, P = .02.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
MeDi AND AD
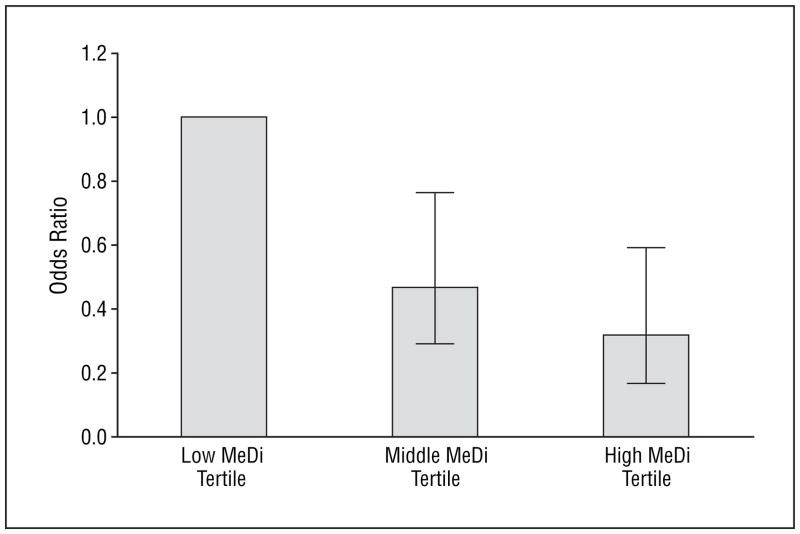
Higher adherence to the MeDi was associated with significantly lower risk for AD (Table 3 [models 1 and 2] and Figure 3). The results were similar in adjusted and unadjusted models. Each additional unit of the MeDi score was associated with 19% to 24% less risk of developing AD. In unadjusted models, compared with subjects in the lowest MeDi tertile (low adherence to the MeDi), subjects in the middle MeDi score tertile had 42% less risk of developing AD while those at the highest tertile (high adherence to the MeDi) had 60% less risk of developing AD with a significant trend for a dose-response effect. Adjustment for all potential covariates made the associations even stronger: 53% less risk for the middle and 68% less risk for the highest MeDi tertile.
Table 3.
Odds Ratios for Subjects With Alzheimer Disease vs Nondemented Subjects by Mediterranean Diet Score in Continuous and Tertile Form
| Model | Nondemented, No. | AD, No. | MeDi Continuous OR (95% CI) | P Value | MeDi Tertiles OR (95% CI) | P for Trend | |
|---|---|---|---|---|---|---|---|
| 1* | 1790 | 194 | 0.81 (0.74–0.88) | <.001 | Low | 1 (Reference) | <.001 |
| Middle | 0.58 (0.42–0.81) | ||||||
| High | 0.40 (0.26–0.61) | ||||||
| 2† | 1300 | 137 | 0.76 (0.67–0.87) | <.001 | Low | 1 (Reference) | <.001 |
| Middle | 0.47 (0.29–0.76) | ||||||
| High | 0.32 (0.17–0.59) | ||||||
| 3‡ | 1259 | 135 | 0.76 (0.66–0.86) | <.001 | Low | 1 (Reference) | <.001 |
| Middle | 0.48 (0.29–0.79) | ||||||
| High | 0.31 (0.16–0.58) | ||||||
Abbreviations: AD, Alzheimer disease; CI, confidence interval; MeDi, Mediterranean diet; OR, odds ratio.
Model 1 is unadjusted.
Model 2 is adjusted for cohort, age, sex, ethnicity, education, apolipoprotein E genotype, caloric intake, smoking, comorbidity index, and body mass index (calculated as weight in kilograms divided by height in meters squared).
Model 3 is adjusted for all variables of model 2 plus the following additional vascular variables: history of stroke, diabetes mellitus, hypertension, and heart disease and plasma levels of total cholesterol, high-density lipoprotein, triglycerides, and low-density lipoprotein.
Figure 3.
Odds ratios and 95% confidence intervals (bars) for subjects with Alzheimer disease vs nondemented subjects, for each Mediterranean diet (MeDi) adherence tertile based on logistic regression models that adjusted for cohort, age, sex, ethnicity, education, apolipoprotein E genotype, caloric intake, smoking, comorbidity index, and body mass index (calculated as weight in kilograms divided by height in meters squared).
When the same models were run with probable AD without stroke as the outcome (n=131), the associations were unchanged: odds ratio, 0.82 (95% confidence interval, 0.73–0.91); P<.001; tertile analyses P for trend, .001. Adjusted models produced similar results: odds ratio, 0.78 (95% confidence interval, 0.67–0.92); P=.003; tertile analyses P for trend, .009.
VASCULAR COMORBIDITY MEDIATION
When all vascular variables were simultaneously included in the logistic regression model (which was already adjusted for all other covariates), odds ratio values did not change in either the continuous or the tertile analyses (Table 3 [model 3]). When we used continuous blood pressure measurements instead of the dichotomous history of hypertension variable, the results did not change (data not presented). When we simultaneously included both the dichotomous history of hypertension variable and the continuous blood pressure variables (along with all the other variables) in the same model, the results were similar (data not presented).
COMMENT
Similarly to our previous findings,12 in this different AD population we observe that higher adherence to the MeDi is associated with reduced disease odds. Similarly to our previous report,12 we note a gradual reduction in AD risk for higher tertiles of MeDi adherence, suggesting a possible dose-response effect. Additionally, in accordance with our previous results,12 the associations between MeDi and AD remain unchanged and significant even when simultaneously adjusting for the most commonly considered potential confounders for AD, such as age, sex, ethnicity, education, APOE genotype, caloric intake, and BMI. Higher adherence to MeDi reduced risk for probable AD either with or without coexisting stroke.
Given the growing evidence for contribution of vascular risk factors in AD risk, vascular mechanisms are important to consider.32–34 At the same time, there is strong evidence relating the MeDi to lower risk of vascular risk factors such as dyslipidemia,5,6 hypertension,3,5–7 abnormal glucose metabolism,3,6 and coronary heart disease.6,8–10 Thus, vascular variables are likely to be in the causal pathway between MeDi and AD and should be considered as possible mediators. However, when we considered vascular risk factors in our models, the association between MeDi and AD did not change. This was the case despite our attempt to capture vascular comorbidity in the most complete possible way by simultaneously considering both a long list and alternative definitions of vascular variables. Additionally, the associations between MeDi and odds for AD were present even in the subset of subjects with AD without any history of stroke. There are at least 2 possible explanations for our findings.
First, lack of significant associations may be related to measurement error of the vascular variables biasing our results toward the null hypothesis. Many of the vascular risk factors in our final analyses were ascertained by self-report, which is likely to underestimate the real prevalence of disease, as is, for example, the case with diabetes.35 Although the prevalence of self-reported diabetes in our sample is comparable with that in previous reports for the same age groups and ethnic composition,35 the prevalence of diabetes in the general population is higher than what is diagnosed,35 and self-reported diabetes underestimates the true prevalence. Self-report of vascular risk factors has been shown to be reliable and accurate in our data,27 and we additionally attempted to include even more detailed measurements of some vascular variables (ie, blood pressure measurements and lipid measurements). However, we lacked data on the precise duration and severity of all vascular risk factors. This calls for a more accurate assessment of vascular pathways. For example, it is possible that a mediating vascular effect could be detected if measurement of vascular co-morbidity was performed with use of instruments or bio-markers more accurate and more biologically proximal to either vascular disease or to vascular-mediated neural damage (ie, left ventricular hypertrophy, direct measures of blood vessel atherosclerosis, homocysteine, glycosylated hemoglobin, brain imaging data such as strokes or white matter hyperintensities, etc).
Second, the MeDi effect may be mediated by nonvascular mechanisms, such as oxidation or inflammation. Growing evidence implicates oxidative damage in the pathogenesis of AD36,37 with neurons at risk for AD degeneration having increased lipid peroxidation, nitration, free carbonyls, and nucleic acid oxidation. Complex phenols and many other substances with important antioxidant properties such as olive oil,38,39 wine, fruits and vegetables, vitamins C and E, and carotenoids40–45 are found in high concentrations in the typical components of the MeDi.46 Typical Mediterranean meals47 or meals rich in typical Mediterranean food elements such as olive oil48 or pomegranate juice49 have been shown to increase enzymes with antioxidant properties such as paraoxonase and plasma carotenoids.47 Food intervention studies with either different tomato products (a very commonly used food in Mediterranean areas46) or a typical Mediterranean dish such as gazpacho (a cold vegetable soup that contains about 75% vegetables [tomato, cucumber, pepper], 2%–10% olive oil, and other minor components [onion, garlic, wine vinegar, and sea salt])50 have indicated significant reductions of markers of oxidative stress such as isoprostanes. Therefore, the MeDi could be capturing the composite effect of dietary antioxidants and this could, at least partially, explain the association with a lower risk of AD.
Inflammation is another potential mechanism for AD pathogenesis.34,51–54 Inflammation has been found to be associated with a higher risk for AD and cognitive decline.55 For example, C-reactive protein (CRP) (an inflammatory marker that has been detected in neuritic plaques and neurofibrillary tangles in the brains of patients with AD56,57 and is up-regulated in AD brains58–60 and serum61) has been proposed as a possible biomarker for AD.62 Higher adherence to the MeDi has been associated with lower CRP levels in both observational5,63,64 and interventional3,47 studies. In the ATTICA epidemiological study, participants on the highest tertile of the MeDi score had 20% lower CRP levels,5 and in the Nurses’ Health Study, there was a 24% reduction in CRP levels for subjects belonging to the upper quintile of a MeDi adherence index.64 As another example, IL-6, a cytokine that mediates inflammatory reactions, has been consistently detected in diffuse early amyloid plaques without neuritic pathologic abnormalities of cortical regions of patients with AD62 and has been associated with greater cognitive decline65 and an increased risk of dementia during follow-up.66 Additionally, polymorphisms of the IL-6 gene related to reduced IL-6 activity have been associated with delayed age at onset and reduced risk for AD.67 Tyrosol and caffeic acid, both found in extra virgin olive oil and in wine (which are essential components of the MeDi), have been shown to significantly reduce IL-6 production from peripheral blood mononuclear cells of healthy volunteers.68 In the ATTICA study, subjects in the highest tertile of MeDi adherence have been reported to have 17% lower IL-6 serum levels,5 and in the Nurses’ Health Study, there was a 16% reduction in IL-6 levels for subjects belonging to the upper quintile of a MeDi adherence index.64 In a clinical trial, as compared with subjects assigned to the control diet, the subjects assigned to the MeDi had significantly reduced levels of IL-6.3 Higher adherence to the MeDi is in general associated with significant reduction in a series of other inflammatory markers, including white blood cell counts, etc.5 Thus, it is possible that the MeDi may (at least partially) lower the risk for AD by affecting inflammatory processes.
This study has limitations. The use of an a priori distribution-derived MeDi score assumes underlying monotonic effects, does not address possible thresholds or the shape of the underlying curve, and weighs equally the underlying individual food categories, which in turn consist of different numbers of foods. Frequencies of food intake are based on relatively few diet constituents, which may underestimate the overall quantity of food in each food category; a common limitation of studies of diet and disease is misclassification of exposure because of limited accuracy. However, assuming that the measurement error was random, our results may actually underestimate the association between high MeDi adherence and lower AD risk.
Despite the use of standard criteria, the diagnostic expertise of our center, and the thorough workup, there is always the possibility of disease misclassification bias.69 As compared with subjects included in the present analyses, subjects with missing dietary information were more likely to have AD and to be Hispanic and nonsmokers. Given that subjects with AD have lower MeDi adherence while Hispanic individuals and nonsmokers have higher, it seems that subjects with missing dietary information should not preferentially adhere more or less to the MeDi. Additionally, subjects with missing dietary information were older and less educated and had lower BMIs, all parameters unrelated to MeDi adherence. Therefore, although we cannot completely exclude it, it does not seem likely that our results could be explained by biases related to missing dietary information.
It is possible that diet is related to socioeconomic status or to other habits or characteristics related to better health and a lower risk for AD. In our data, MeDi was not related to the overall burden of medical and vascular comorbidities or to education but was related to ethnicity. We addressed this potential bias by adjusting for years of education, ethnic group, medical comorbidity index, smoking, and vascular variables, but we cannot completely rule out residual confounding as an explanation for our findings.
Another explanation for our findings is that lower adherence to the MeDi could represent a consequence and not a cause of AD. Although we cannot completely exclude it, we addressed this possibility in several ways. First, we included in our analyses only patients with AD at the early stages of the disease (a CDR not higher than 1). Second, we found remarkable stability in MeDi scores over intervals greater than 7 years for subjects with multiple dietary assessments who were not demented at baseline but developed AD during follow-up. Additionally, we have previously reported similar stability for MeDi adherence even for nondemented subjects.12 Finally, we have previously noted an association between MeDi adherence and risk for incident AD in a longitudinal prospective design.12
Confidence in our findings is strengthened by the following factors. Dietary data were collected with a previously validated instrument that has been used widely in epidemiological studies.20 We used an a priori developed dietary pattern.1,8 Measures for multiple potential AD risk factors have been carefully recorded and adjusted for in the analyses. The diagnosis of AD took place in a university hospital with expertise in dementia and was based on comprehensive assessment and standard research criteria. The study is community-based and the population is multiethnic, increasing the external validity of the findings.
Acknowledgments
Funding/Support: This study was supported by grants AG07232, AG07702, AG15294-06, 1K08AG20856-01, and RR00645 from the National Institute on Aging; the Charles S. Robertson Memorial Gift for Research in Alzheimer’s Disease; the Blanchette Hooker Rockefeller Foundation; the New York City Council Speaker’s Fund for Public Health Research; and the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Study concept and design: Scarmeas and Stern. Acquisition of data: Scarmeas, Stern, and Mayeux. Analysis and interpretation of data: Scarmeas, Stern, and Luchsinger. Drafting of the manuscript: Scarmeas. Critical revision of the manuscript for important intellectual content: Stern, Mayeux, and Luchsinger. Statistical analysis: Scarmeas and Luchsinger. Obtained funding: Mayeux and Luchsinger. Administrative, technical, and material support: Mayeux. Study supervision: Scarmeas and Stern.
References
- 1.Jacques PF, Tucker KL. Are dietary patterns useful for understanding the role of diet in chronic disease? Am J Clin Nutr. 2001;73:1–2. doi: 10.1093/ajcn/73.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomarkers Prev. 2000;9:869–873. [PubMed] [Google Scholar]
- 3.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 4.Schroder H, Marrugat J, Vila J, Covas MI, Elosua R. Adherence to the traditional Mediterranean diet is inversely associated with body mass index and obesity in a Spanish population. J Nutr. 2004;134:3355–3361. doi: 10.1093/jn/134.12.3355. [DOI] [PubMed] [Google Scholar]
- 5.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: the ATTICA Study. J Am Coll Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360:1455–1461. doi: 10.1016/S0140-6736(02)11472-3. [DOI] [PubMed] [Google Scholar]
- 7.Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2004;80:1012–1018. doi: 10.1093/ajcn/80.4.1012. [DOI] [PubMed] [Google Scholar]
- 8.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 9.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 10.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 11.Trichopoulou A, Orfanos P, Norat T, et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005;330:991. doi: 10.1136/bmj.38415.644155.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 14.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 15.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 16.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 18.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population: development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semi-quantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Willett W, Stampfer M. Implications of total energy intake for epidemiological analyses. In: Willett W, editor. Nutritional Epidemiology. New York, NY: Oxford University Press; 1998. pp. 273–301. [Google Scholar]
- 22.Kuczmarski R, Carroll M, Flegal K, Troiano R. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994) Obes Res. 1997;5:542–548. doi: 10.1002/j.1550-8528.1997.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 23.Census of Population and Housing. Summary Tape File 1, Technical Documentation. Washington, DC: Bureau of the Census; 1991. [Google Scholar]
- 24.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64:1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 28.Lopes-Virella MF, Stone P, Ellis S, Colwell JA. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23:882–884. [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York, NY: John Wiley & Sons Inc; 1999. Interpretation of a fitted proportional hazards regression model: multiple-covariate models; pp. 129–137. [Google Scholar]
- 31.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 32.Breteler MM. Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging. 2000;21:153–160. doi: 10.1016/s0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 33.Luchsinger J, Mayeux R. Cardiovascular risk factors and Alzheimer’s disease. Curr Atheroscler Rep. 2004;6:261–266. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- 34.Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 35.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: the Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 36.Giasson BI, Ischiropoulos H, Lee VM, Trojanowski JQ. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer’s and Parkinson’s diseases. Free Radic Biol Med. 2002;32:1264–1275. doi: 10.1016/s0891-5849(02)00804-3. [DOI] [PubMed] [Google Scholar]
- 37.Pratico D. Alzheimer’s disease and oxygen radicals: new insights. Biochem Pharmacol. 2002;63:563–567. doi: 10.1016/s0006-2952(01)00919-4. [DOI] [PubMed] [Google Scholar]
- 38.Fito M, Cladellas M, de la Torre R, et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: a randomized, crossover, controlled, clinical trial. Atherosclerosis. 2005;181:149–158. doi: 10.1016/j.atherosclerosis.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 39.Alarcon de la Lastra C, Barranco MD, Motilva V, Herrerias JM. Mediterranean diet and health: biological importance of olive oil. Curr Pharm Des. 2001;7:933–950. doi: 10.2174/1381612013397654. [DOI] [PubMed] [Google Scholar]
- 40.Szeto YT, Tomlinson B, Benzie IF. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: implications for dietary planning and food preservation. Br J Nutr. 2002;87:55–59. doi: 10.1079/BJN2001483. [DOI] [PubMed] [Google Scholar]
- 41.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 42.Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- 43.Byers T, Perry G. Dietary carotenes, vitamin C, and vitamin E as protective antioxidants in human cancers. Annu Rev Nutr. 1992;12:139–159. doi: 10.1146/annurev.nu.12.070192.001035. [DOI] [PubMed] [Google Scholar]
- 44.Owen RW, Haubner R, Wurtele G, Hull E, Spiegelhalder B, Bartsch H. Olives and olive oil in cancer prevention. Eur J Cancer Prev. 2004;13:319–326. doi: 10.1097/01.cej.0000130221.19480.7e. [DOI] [PubMed] [Google Scholar]
- 45.Stupans I, Kirlich A, Tuck KL, Hayball PJ. Comparison of radical scavenging effect, inhibition of microsomal oxygen free radical generation, and serum lipoprotein oxidation of several natural antioxidants. J Agric Food Chem. 2002;50:2464–2469. doi: 10.1021/jf0112320. [DOI] [PubMed] [Google Scholar]
- 46.Mancini M, Parfitt VJ, Rubba P. Antioxidants in the Mediterranean diet. Can J Cardiol. 1995;11(suppl G):105G–109G. [PubMed] [Google Scholar]
- 47.Blum S, Aviram M, Ben-Amotz A, Levy Y. Effect of a Mediterranean meal on post-prandial carotenoids, paraoxonase activity and C-reactive protein levels. Ann Nutr Metab. 2006;50:20–24. doi: 10.1159/000089560. [DOI] [PubMed] [Google Scholar]
- 48.Wallace AJ, Sutherland WH, Mann JI, Williams SM. The effect of meals rich in thermally stressed olive and safflower oils on postprandial serum paraoxonase activity in patients with diabetes. Eur J Clin Nutr. 2001;55:951–958. doi: 10.1038/sj.ejcn.1601250. [DOI] [PubMed] [Google Scholar]
- 49.Aviram M, Dornfeld L, Rosenblat M, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000;71:1062–1076. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Moreno C, Cano MP, de Ancos B, et al. Mediterranean vegetable soup consumption increases plasma vitamin C and decreases F2-isoprostanes, pros-taglandin E2 and monocyte chemotactic protein-1 in healthy humans. J Nutr Biochem. 2006;17:183–189. doi: 10.1016/j.jnutbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Walsh S, Aisen PS. Inflammatory processes and Alzheimer’s disease. Expert Rev Neurother. 2004;4:793–798. doi: 10.1586/14737175.4.5.793. [DOI] [PubMed] [Google Scholar]
- 52.Sastre M, Klockgether T, Heneka MT. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int J Dev Neurosci. 2006;24:167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Weninger SC, Yankner BA. Inflammation and Alzheimer disease: the good, the bad, and the ugly. Nat Med. 2001;7:527–528. doi: 10.1038/87839. [DOI] [PubMed] [Google Scholar]
- 54.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 56.Iwamoto N, Nishiyama E, Ohwada J, Arai H. Demonstration of CRP immunoreactivity in brains of Alzheimer’s disease: immunohistochemical study using formic acid pretreatment of tissue sections. Neurosci Lett. 1994;177:23–26. doi: 10.1016/0304-3940(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 57.Duong T, Nikolaeva M, Acton PJ. C-reactive protein-like immunoreactivity in the neurofibrillary tangles of Alzheimer’s disease. Brain Res. 1997;749:152–156. doi: 10.1016/s0006-8993(96)01359-5. [DOI] [PubMed] [Google Scholar]
- 58.McGeer EG, Yasojima K, Schwab C, McGeer PL. The pentraxins: possible role in Alzheimer’s disease and other innate inflammatory diseases. Neurobiol Aging. 2001;22:843–848. doi: 10.1016/s0197-4580(01)00288-3. [DOI] [PubMed] [Google Scholar]
- 59.Strauss S, Bauer J, Ganter U, Jonas U, Berger M, Volk B. Detection of interleukin-6 and alpha 2-macroglobulin immunoreactivity in cortex and hippocampus of Alzheimer’s disease patients. Lab Invest. 1992;66:223–230. [PubMed] [Google Scholar]
- 60.Wood JA, Wood PL, Ryan R, et al. Cytokine indices in Alzheimer’s temporal cortex: no changes in mature IL-1 beta or IL-1RA but increases in the associated acute phase proteins IL-6, alpha 2-macroglobulin and C-reactive protein. Brain Res. 1993;629:245–252. doi: 10.1016/0006-8993(93)91327-o. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 62.Frank RA, Galasko D, Hampel H, et al. Biological markers for therapeutic trials in Alzheimer’s disease: proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer’s disease. Neurobiol Aging. 2003;24:521–536. doi: 10.1016/s0197-4580(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 63.Paschos GK, Rallidis LS, Liakos GK, et al. Background diet influences the anti-inflammatory effect of alpha-linolenic acid in dyslipidaemic subjects. Br J Nutr. 2004;92:649–655. doi: 10.1079/bjn20041230. [DOI] [PubMed] [Google Scholar]
- 64.Fung TT, McCullough ML, Newby P, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 65.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 66.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 67.Papassotiropoulos A, Bagli M, Jessen F, et al. A genetic variation of the inflammatory cytokine interleukin-6 delays the initial onset and reduces the risk for sporadic Alzheimer’s disease. Ann Neurol. 1999;45:666–668. doi: 10.1002/1531-8249(199905)45:5<666::aid-ana18>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 68.Bertelli AA, Migliori M, Panichi V, et al. Oxidative stress and inflammatory reaction modulation by white wine. Ann N Y Acad Sci. 2002;957:295–301. doi: 10.1111/j.1749-6632.2002.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 69.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]