Abstract
Purpose
Bortezomib is a small-molecule proteasome inhibitor with single-agent activity in patients with non-small cell lung carcinoma (NSCLC) and synergy with gemcitabine in preclinical studies. This phase II study of bortezomib in combination with gemcitabine/carboplatin was conducted in chemotherapy-naïve advanced NSCLC patients to assess efficacy and safety.
Patients and Methods
Patients with selected stage IIIB/IV NSCLC, performance status 0–1, and no history of brain metastasis received up to six 21-day cycles of gemcitabine 1,000 mg/m2, days 1 and 8, carboplatin AUC 5.0, day 1, and bortezomib 1.0 mg/m2, days 1, 4, 8, and 11.
Results
114 patients (52% adenocarcinoma, 85% stage IV) received a median of 3.6 treatment cycles. Median follow-up was > 3 years. Median overall survival (OS) was 11 months; 1-year and 2-year survival rates were 47% and 19%, respectively. Median PFS was 5 months; 1-year PFS rate was 7%. Response rate was 23%, and disease control rate (responses + stable disease) was 68%. The most common grade 3/4 toxicities were thrombocytopenia (63%) and neutropenia (52%). One patient experienced febrile neutropenia. Grade 3/4 neuropathy occurred in 4%.
Conclusions
Bortezomib plus gemcitabine/carboplatin resulted in a notable survival benefit in patients with advanced NSCLC, with the anticipated primary toxicity of myelosuppression. Further studies designed to investigate the role of bortezomib in advanced NSCLC are warranted.
Keywords: advanced NSCLC, bortezomib, carboplatin, gemcitabine, proteasome, stage IV
INTRODUCTION
For the past decade, doublet chemotherapy with cisplatin or carboplatin plus a third-generation drug (paclitaxel, docetaxel, vinorelbine or gemcitabine) has been considered the standard of care for first-line treatment of advanced NSCLC.1-8 Gemcitabine/carboplatin is a commonly used regimen due to its favorable toxicity profile.4,9-11 5,7,12 Despite the benefits of chemotherapy, prognosis for these patients remains poor,1,8,13 with median overall survival (OS) of typically 8–10 months and 1-year survival rates of 35–40%.1,8 Thus, integrating novel agents into front-line therapy is of tremendous interest and importance in advancing the field of lung cancer and patient outcomes.
Bortezomib (VELCADE®, Millennium Pharmaceuticals, Inc. and Johnson & Johnson Pharmaceutical Research & Development, L.L.C.) inhibits the 26S proteasome, thereby affecting the levels of numerous proteins involved in processes such as cell-cycle control, apoptosis, cell adhesion, angiogenesis, and chemoresistance.14,15 Bortezomib disrupts multiple cellular pathways shown to be important in NSCLC (Table 1).14-16 In particular, most NSCLC cells have a dysregulated apoptotic pathway involving activated nuclear factor-κB (NF-κB).17 NF-κB activates the transcription of anti-apoptotic and proliferation genes, mediating tumor cell survival in response to cytotoxic stress and resulting in chemoresistance. Bortezomib attenuates this pathway by preventing proteasomal degradation of IκB, the inhibitor of NF-κB.14,15 Bortezomib also modulates levels of the anti-apoptotic gene Bcl-2 and the tumor suppressor p53.14-16 Overexpression of Bcl-2, a key mediator of resistance to apoptosis following chemotherapy, is evident in 70–80% of NSCLC cases, while mutations leading to functional loss or decreased expression of p53 are present in up to 50% of cases.14-16
Table 1.
Effect of bortezomib on protein targets of relevance to lung cancer.16
| Protein and effect of bortezomib | Function of protein | Protein role in lung cancer |
|---|---|---|
| p27kip1 stabilization | Cell-cycle inhibition, apoptosis | Tumor suppressor |
| p53 stabilization | DNA damage repair, cell-cycle inhibition, apoptosis | Tumor suppressor, therapy resistance |
| NF-κB downregulation | Transcription factor, apoptosis suppressor | Cell survival, therapy resistance |
| Bcl-2, Bcl-xL downregulation | Anti-apoptosis | Cell survival, therapy resistance |
| Bax stabilization | Pro-apoptosis | Promote apoptosis |
| Cyclin D, E, A stabilization | Cell-cycle progression | Oncogenic |
Bortezomib has demonstrated single-agent anti-tumor activity in NSCLC, both in preclinical 18-20 and in early-phase clinical studies.21-23 In preclinical studies, bortezomib sensitized NSCLC and pancreatic cancer cells to gemcitabine-induced apoptosis in vitro and in vivo,24,25 and sequence-dependent enhanced activity was observed with bortezomib plus gemcitabine/carboplatin in NSCLC cells.26 A phase I California Cancer Consortium study demonstrated bortezomib plus gemcitabine/carboplatin to be well tolerated in patients with advanced NSCLC, with the primary toxicity being myelosuppression. The maximum tolerated dose (MTD) was determined to be bortezomib 1.0 mg/m2 days 1, 4, 8, and 11, gemcitabine 1,000 mg/m2 days 1 and 8 and carboplatin AUC 5.0 on day 1.27 The combination showed encouraging activity. Here, we report the subsequent Southwest Oncology Group (SWOG) phase II study to evaluate the efficacy and toxicity of this regimen as first-line treatment in patients with advanced NSCLC.
Methods
Patients
Chemotherapy-naïve patients with histologically or cytologically proven selected stage IIIB (T4 lesion due to malignant pleural effusion) or stage IV NSCLC were eligible. Patients were required to have measurable or assessable disease, and to have a SWOG performance status of 0–1. Patients with recurrent disease following previous surgery and/or radiation were also eligible. Recurrent disease had to be outside previous radiation fields or have a new lesion inside the field. Patients with brain metastases were not eligible. Adequate organ function was required: serum creatinine less than or equal to the institutional limit of normal, or calculated creatinine clearance of ≥ 60 cc/min (Cockcroft-Gault formula28); absolute neutrophil count (ANC) ≥ 1,500 cells/μL; platelets ≥ 100,000 cells/μL; and adequate hepatic function. Patients with grade ≥ 2 peripheral neuropathy (based on National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE] version 3.0) were excluded. No other prior malignancies were allowed except adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, or other cancers from which the patient had been disease-free for 5 years. Eligibility criteria were consistent with previous SWOG trials in advanced NSCLC.12,29-31 The Institutional Review Board at each participating institution approved the study; all patients gave written informed consent in accordance with institutional and federal guidelines before undergoing any study-related procedures.
Study design
Patients were accrued at 33 SWOG institutions between February and September 2004. Data cut-off for this report was October 11, 2007. Dosing was based on the MTD in the California Cancer Consortium phase 1 study of this combination.27 Patients received gemcitabine 1,000 mg/m2 on days 1 and 8, carboplatin AUC 5.0 on day 1, and bortezomib 1.0 mg/m2 on days 1, 4, 8, and 11, in 21-day treatment cycles. Bortezomib was administered 60 minutes after gemcitabine/carboplatin on days 1 and 8, based on preclinical data demonstrating sequence specificity.26 Patients without progression could receive up to six treatment cycles of the triplet regimen; those tolerating treatment and without disease progression could continue receiving bortezomib alone for up to 1 year.
Gemcitabine/carboplatin dose reductions were required for treatment delays of > 7 days due to neutropenia and/or thrombocytopenia, or significant hematologic or non-hematological toxicities in the preceding cycle. Bortezomib dose reductions (to 0.8 mg/m2, then 0.6 mg/m2) were required for unacceptable hematologic toxicities that persisted following gemcitabine/carboplatin dose reduction, and for any grade ≥ 2 neurological toxicities.
Supportive care, including use of anti-emetics, was at the discretion of the treating physician. Routine use of granulocyte colony stimulating factors (GCSFs) was not permitted; administration had to follow American Society of Clinical Oncology (ASCO) guidelines and be discontinued 48 hours prior to the start of the next cycle.
Evaluations
The primary objective of this phase II study was to assess OS. Progression free survival (PFS), response rate, and safety were secondary objectives. Patient archival specimens and blood samples were submitted for exploratory molecular analyses. Radiological investigations occurred at baseline and every 6 weeks during treatment, and responses were evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST).32 Safety was assessed throughout the study; toxicities were graded according to NCI CTCAE version 3.0.
Statistical analyses
Target accrual was 99 patients, to provide 85% power to rule out the null hypothesis of an 8-month median OS at the .05 alpha level versus an alternative of a 12-month median OS. Median OS of ≥ 10 months was considered as warranting phase III testing of gemcitabine/carboplatin ± bortezomib. The target accrual allowed for estimation of response and toxicity rates to within ± 10% (95% confidence interval [CI]). OS and PFS were evaluated by Kaplan–Meier methods.
Results
Of 121 patients accrued, 6 were ineligible due to the following: timing of registration following surgery, elevated SGOT and bilirubin levels, low creatinine clearance, lack of stage information, and timing of disease assessment (2). One additional patient did not receive treatment. Of the 114 patients that were treated, 2 patients completed planned treatment with 6 cycles of gemcitabine/carboplatin/bortezomib and single-agent bortezomib for a total of one year; 112 patients discontinued treatment due to progression/relapse (n=55), adverse events (AEs, n = 44), refusal unrelated to AEs (n = 6), death (n = 3; all unrelated to treatment), and other non-specified reasons (n = 4). Baseline patient characteristics are shown in Table 2. Slightly more than half of patients had adenocarcinoma, and 85% had stage IV NSCLC. Patients received a median of 3.6 treatment cycles. Median follow-up was > 3 years.
Table 2.
Baseline patient characteristics (N = 114). Percentages in categories do not necessarily total 100% due to rounding.
| Parameter | n | % |
|---|---|---|
| Median age, years (range) | 64 (28–79) | |
| Male | 69 | 61 |
| Race | ||
| White | 103 | 90 |
| Black | 4 | 4 |
| Asian | 3 | 3 |
| Native American | 1 | 1 |
| Multi-racial | 1 | 1 |
| Unknown | 2 | 2 |
| Histology | ||
| Adenocarcinoma | 59 | 52 |
| Squamous cell | 23 | 20 |
| Large cell | 7 | 6 |
| Bronchioloalveolar | 4 | 4 |
| NSCLC, NOS | 1 | 1 |
| Other | 20 | 18 |
| Performance status | ||
| 0 | 50 | 44 |
| 1 | 64 | 56 |
| Disease stage | ||
| IIIB | 14 | 12 |
| IV | 97 | 85 |
| Recurrent | 3 | 3 |
| Weight loss during past 6 months | ||
| < 5% | 79 | 69 |
| ≥ 5% | 32 | 28 |
| Missing | 3 | 3 |
NOS, not otherwise specified; NSCLC, non-small cell lung cancer
Efficacy
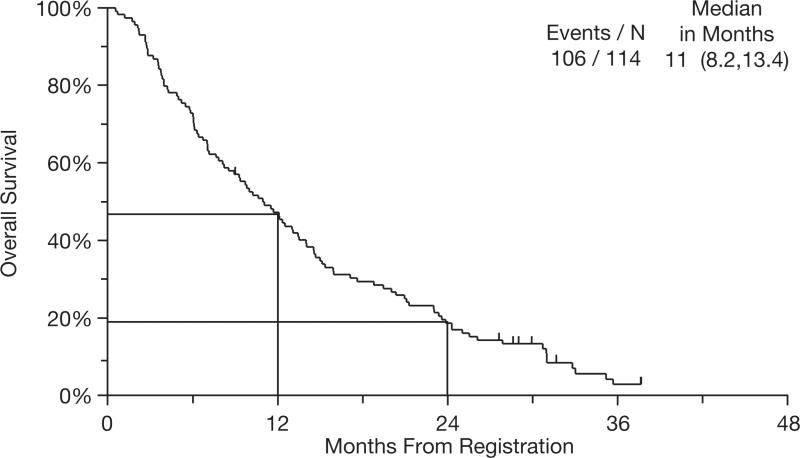
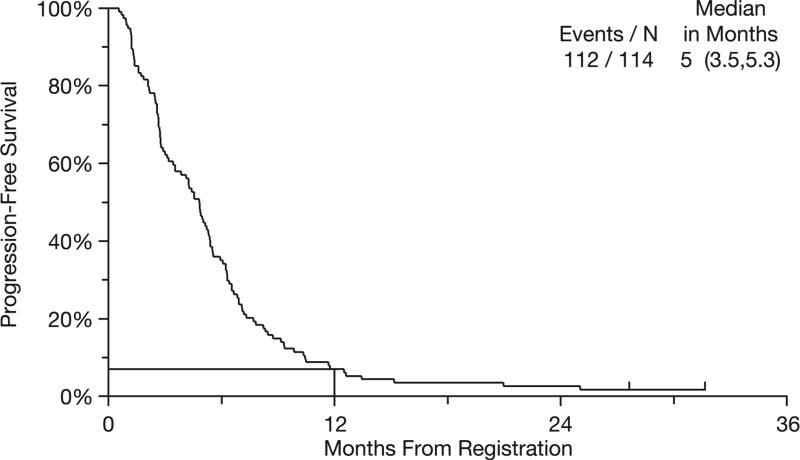
Median OS was 11 months (95% CI: 8.2–13.4 months) (Figure 1). Survival rates at 1 and 2 years were 47% and 19%, respectively. Median PFS was 5 months (95% CI: 3.5–5.3 months) (Figure 2). The 1-year PFS rate was 7%. Best response to therapy is shown in Table 3. The overall response rate (ORR) for all 114 registered patients was 23%, with a disease control rate (ORR + stable disease) of 68%.
Figure 1.
Kaplan–Meier curve for overall survival (N = 114).
Figure 2.
Kaplan–Meier curve for progression-free survival (N = 114).
Table 3.
Response to therapy by RECIST.
| Measurable disease (N = 108) | Non-measurable disease (N = 6) | All patients (N = 114) | ||||
|---|---|---|---|---|---|---|
| Response | n | % | n | % | n | % |
| CR/CRu | 2 | 2 | 0 | 0 | 2 | 2 |
| PR/PRu | 24 | 22 | 0 | 0 | 24 | 21 |
| ORR (CR + PR) | 26 | 24 | 0 | 0 | 26 | 23 |
| SD | 49 | 45 | 3 | 50 | 52 | 46 |
| Disease control rate (ORR + SD) | 75 | 69 | 3 | 50 | 78 | 68 |
| PD | 19 | 18 | 2 | 33 | 21 | 18 |
| Assessment inadequate | 14 | 13 | 1 | 17 | 15 | 13 |
CR, complete response; CRu, unconfirmed CR; ORR, overall response rate; PD, progressive disease (includes assessments of increasing disease and symptomatic deterioration); PR, partial response; PRu, unconfirmed PR; SD, stable disease.
Safety
A total of 113 patients were evaluable for safety; AEs were not reported for one patient. The most common grade 3 and 4 AEs are shown in Table 4. While the incidences of grade 3/4 thrombocytopenia and neutropenia were 63% and 52%, respectively, only 3 patients (3%) had grade 3 hemorrhage (associated with grade 4 thrombocytopenia, grade 3 thrombocytopenia, and no thrombocytopenia, respectively) and 1 patient (1%) had febrile neutropenia. Sensory neuropathy was seen in 26 patients (23%), including 3 (3%) with grade 3/4 toxicity. In total, 99 (87%) patients were reported as requiring dose reductions during the first six cycles of treatment. The most common AEs resulting in dose reduction were neutropenia and thrombocytopenia. There were four deaths possibly related to treatment, one due to multi-organ failure, one due to pneumonitis, one due to diarrhea and dehydration, and one sudden death in a patient with grade 4 thrombosis/embolism and grade 4 thrombocytopenia.
Table 4.
Most common grade ≥3 hematologic and non-hematologic toxicities (N = 113).
| Total grade ≥ 3 | Grade 3 | Grade 4 | ||||
|---|---|---|---|---|---|---|
| Adverse event | N | % | N | % | N | % |
| Hematologic toxicities | ||||||
| Thrombocytopenia | 71 | 63 | 30 | 27 | 41 | 36 |
| Neutropenia | 59 | 52 | 37 | 33 | 22 | 19 |
| Anemia | 15 | 13 | 13 | 12 | 2 | 2 |
| Non-hematologic toxicities | ||||||
| Fatigue | 15 | 13 | 12 | 11 | 3 | 3 |
| ALT/AST | 14 | 12 | 13 | 12 | 1 | 1 |
| Neuropathy | 5 | 4 | 3 | 3 | 2 | 2 |
| Dehydration | 5 | 4 | 5 | 4 | – | – |
| Hypokalemia | 4 | 4 | 3 | 3 | 1 | 1 |
| Pneumonitis | 4* | 4 | 3 | 3 | – | – |
| Anorexia | 3 | 3 | 3 | 3 | – | – |
| Diarrhea | 3 | 3 | 3 | 3 | – | – |
| Dyspnea | 3 | 3 | 3 | 3 | – | – |
| Hyponatremia | 3 | 3 | 3 | 3 | – | – |
| Hypotension | 3 | 3 | 2 | 2 | 1 | 1 |
| Lung infection | 3 | 3 | 3 | 3 | – | – |
| Nausea | 3 | 3 | 3 | 3 | – | – |
| Thrombosis/embolism | 3 | 3 | 2 | 2 | 1 | 1 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Includes one grade 5 event.
Discussion
Modern platinum-based chemotherapy is associated with benefits in survival, symptom palliation and quality of life in patients with advanced NSCLC. Nevertheless, the overall impact is modest at best, and novel approaches are needed. Bortezomib both potentiates chemotherapy in NSCLC cell lines and has demonstrated single-agent activity in clinical trials. Gemcitabine/carboplatin was selected as the chemotherapeutic backbone for S0339 because it is largely devoid of neuropathy, a potential overlapping toxicity with other agents commonly employed in NSCLC, and because of encouraging data in in vitro and in vivo models with this three drug regimen. Indeed, the median OS of 11 months and 1 year survival of 47% achieved in this phase 2 study surpass results of prior SWOG trials (S9509, S9806, S0003) in advanced NSCLC.12,29,31 Historically, recent SWOG studies in advanced NSCLC have maintained consistent eligibility criteria and therefore patient populations have been relatively comparable from study to study.12,29-31 In addition, all studies were conducted in the era of PET scanning for disease staging, minimizing the possibility that the prolonged survival seen in this study compared with historical data may be related to stage-migration effects. The survival data reported here are further validated by the multi-institutional, cooperative-group nature of our study, and by the large number of patients enrolled.
While our survival results exceeded the predefined statistical end point for proceeding with phase III investigation of gemcitabine/carboplatin plus bortezomib compared with gemcitabine/carboplatin alone, it is important to note that changes in the therapeutic landscape for advanced NSCLC have occurred since our study was initiated. Most notably, the Eastern Cooperative Oncology Group (ECOG) 4599 study of paclitaxel/carboplatin alone or in combination with bevacizumab, which demonstrated a significant survival advantage with the triplet regimen (12.3 vs 10.3 months),33 has established this combination as a new first-line standard of care in selected patients with advanced NSCLC. Results from studies in this setting must now be considered in the context of the ECOG 4599 data. However, it is worth noting that the patient populations in the current study and the ECOG 4599 trial differ somewhat due to eligibility limitations for patients receiving bevacizumab. The ECOG 4599 trial did not enroll patients with a histology of squamous cell carcinoma, hemoptysis, history of hemorrhagic diathesis, or coagulopathy due to the risk of serious hemorrhagic events, which limits the widespread applicability of this triplet regimen.33
An additional reason for improved survival in S0339 may be the more widespread use of second-line therapy in NSCLC, such as docetaxel, pemetrexed, and erlotinib. The median PFS of 5 months is similar to that reported previously, 4–5 months,12,29-31 as is the overall response rate of 23% and disease control rate of 68%.12,29-31
Therapy with bortezomib plus gemcitabine/carboplatin was generally well tolerated. As expected the most common grade 3/4 AEs were hematologic. Bortezomib-induced thrombocytopenia and neutropenia have been described as transient and cyclical, with rapid recovery of platelet count and neutrophils toward baseline during the rest period of each cycle, in studies in relapsed and/or refractory multiple myeloma.34-36 While the hematologic toxicity was not clinically significant in terms of AEs (bleeding and febrile neutropenia), it resulted in a substantial number of dose reductions (87%), which limited dose intensity. Due to the conservative criteria for dose reductions for thrombocytopenia and neutropenia specified in the protocol, they were commonly implemented and may have affected efficacy by reducing the ability to deliver full-dose gemcitabine/carboplatin.
Another common toxicity associated with bortezomib in multiple myeloma studies is peripheral sensory neuropathy.37,38 The overall incidence of grade 3/4 sensory neuropathy seen in this study was lower than in studies of bortezomib 1.3 mg/m2 in relapsed/refractory multiple myeloma;37,38 this may be attributed to the lower dose of bortezomib (1.0 mg/m2) used and the different patient population.
Lastly, a biomarker for bortezomib sensitivity has yet to be established. Considering the large number of potential molecular targets of proteasome inhibition, it may be unlikely that a single predictive biomarker will be identified. The results of our study suggest that bortezomib-based combinations could prove promising for advanced NSCLC, particularly if a biomarker for efficacy could be identified to predict which patients are most likely to benefit.
Acknowledgments
The authors would like to thank Steve Hill and Rosemary Washbrook for editorial assistance in the development of this manuscript. Steve Hill is a medical writer and Rosemary Washbrook is a medical editor with Gardiner-Caldwell London.
Acknowledgments of research support:
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA38926, CA32102, CA46282, CA35178, CA45808, CA46441, CA45560, CA45807, CA67575. CA35128, CA27057, CA35431, CA20319, CA46368, CA35281, CA86780, CA63850, CA35119, CA63844, CA42777, CA35090, CA58882.
Footnotes
Statement of originality:
The authors confirm that this manuscript contains original material.
Prior presentations of this study:
Bortezomib + gemcitabine (Gem)/carboplatin (Carbo) results in encouraging survival in advanced non-small cell lung cancer (NSCLC): Results of a phase II Southwest Oncology Group (SWOG) trial (S0339). Davies AM, McCoy J, Lara Jr PN, et al. J Clin Oncol 2006;24 (Suppl 18S Part I of II):368s (Abstract 7017). Oral Presention at the 2006 Annual Meeting of the American Society of Clinical Oncology, June 2–6, Atlanta, GA.
Disclaimers:
None
Protocol ID:
NCT00052338/NCT00075751, S0339
References
- 1.Ettinger DS. Is there a preferred combination chemotherapy regimen for metastastic non-small cell lung cancer? Oncologist. 2002;7:226–233. doi: 10.1634/theoncologist.7-3-226. [DOI] [PubMed] [Google Scholar]
- 2.Lilenbaum RC, Herndon JE, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol. 2005;23:190–196. doi: 10.1200/JCO.2005.07.172. [DOI] [PubMed] [Google Scholar]
- 3.Ohe Y, Ohashi Y, Kubota K, et al. Randomized phase III study of cisplatin plus irinotecan versus carboplatin plus paclitaxel, cisplatin plus gemcitabine, and cisplatin plus vinorelbine for advanced non-small-cell lung cancer: Four-Arm Cooperative Study in Japan. Ann Oncol. 2007;18:317–323. doi: 10.1093/annonc/mdl377. [DOI] [PubMed] [Google Scholar]
- 4.Ramalingam S, Belani CP. Carboplatin/gemcitabine combination in advanced NSCLC. Oncology (Williston Park) 2004;18:21–26. [PubMed] [Google Scholar]
- 5.Scagliotti GV, De MF, Rinaldi M, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–4291. doi: 10.1200/JCO.2002.02.068. [DOI] [PubMed] [Google Scholar]
- 6.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd FA. Current paradigms in first-line treatment of non-small-cell lung cancer. Oncology (Williston Park) 2004;18:13–20. [PubMed] [Google Scholar]
- 8.Weiss GJ, Bunn PA, Jr., Camidge DR. From radiotherapy to Targeted therapy: 20 years in the management of non-small-cell lung cancer. Oncology (Williston Park) 2006;20:1515–1524. [PubMed] [Google Scholar]
- 9.Grigorescu AC, Draghici IN, Nitipir C, et al. Gemcitabine (GEM) and carboplatin (CBDCA) versus cisplatin (CDDP) and vinblastine (VLB) in advanced non-small-cell lung cancer (NSCLC) stages III and IV: a phase III randomised trial. Lung Cancer. 2002;37:9–14. doi: 10.1016/s0169-5002(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 10.Mazzanti P, Massacesi C, Rocchi MB, et al. Randomized, multicenter, phase II study of gemcitabine plus cisplatin versus gemcitabine plus carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer. 2003;41:81–89. doi: 10.1016/s0169-5002(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 11.Zatloukal P, Petruzelka L, Zemanova M, et al. Gemcitabine plus cisplatin vs. gemcitabine plus carboplatin in stage IIIb and IV non-small cell lung cancer: a phase III randomized trial. Lung Cancer. 2003;41:321–331. doi: 10.1016/s0169-5002(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 12.Kelly K, Crowley J, Bunn PA, Jr., et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001;19:3210–3218. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 13.Mandrekar SJ, Schild SE, Hillman SL, et al. A prognostic model for advanced stage nonsmall cell lung cancer. Pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2006;107:781–792. doi: 10.1002/cncr.22049. [DOI] [PubMed] [Google Scholar]
- 14.Scagliotti G. Proteasome inhibitors in lung cancer. Crit Rev Oncol Hematol. 2006;58:177–189. doi: 10.1016/j.critrevonc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Schenkein DP. Use of proteasome inhibition in the treatment of lung cancer. Clin Lung Cancer. 2004;6(Suppl 2):S89–S96. doi: 10.3816/clc.2004.s.021. [DOI] [PubMed] [Google Scholar]
- 16.Mack PC, Davies AM, Lara PN, et al. Integration of the proteasome inhibitor PS-341 (Velcade) into the therapeutic approach to lung cancer. Lung Cancer. 2003;41:S89–S96. doi: 10.1016/s0169-5002(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 17.Denlinger CE, Rundall BK, Jones DR. Modulation of antiapoptotic cell signaling pathways in non-small cell lung cancer: the role of NF-kappaB. Semin Thorac Cardiovasc Surg. 2004;16:28–39. doi: 10.1053/j.semtcvs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Ling YH, Liebes L, Jiang JD, et al. Mechanisms of proteasome inhibitor PS-341-induced G(2)-M-phase arrest and apoptosis in human non-small cell lung cancer cell lines. Clin Cancer Res. 2003;9:1145–1154. [PubMed] [Google Scholar]
- 19.Ling YH, Liebes L, Zou Y, et al. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–33723. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Ikezoe T, Saito T, et al. Proteasome inhibitor PS-341 induces growth arrest and apoptosis of non-small cell lung cancer cells via the JNK/c-Jun/AP-1 signaling. Cancer Sci. 2004;95:176–180. doi: 10.1111/j.1349-7006.2004.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aghajanian C, Soignet S, Dizon DS, et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8:2505–2511. [PubMed] [Google Scholar]
- 22.Fanucchi MP, Fossella FV, Belt R, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2006;24:5025–5033. doi: 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson JP, Nho CW, Johnson SW, et al. Phase II/ pharmacodynamic trial of PS-341 (bortezomib, Velcade) in advanced non-small cell lung cancer. 2004.
- 24.Denlinger CE, Rundall BK, Keller MD, et al. Proteasome inhibition sensitizes non-small-cell lung cancer to gemcitabine-induced apoptosis. Ann Thorac Surg. 2004;78:1207–1214. doi: 10.1016/j.athoracsur.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–17. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 26.Mortenson MM, Schlieman MG, Virudachalam S, et al. Effects of the proteasome inhibitor bortezomib alone and in combination with chemotherapy in the A549 non-small-cell lung cancer cell line. Cancer Chemother Pharmacol. 2004;54:343–353. doi: 10.1007/s00280-004-0811-4. [DOI] [PubMed] [Google Scholar]
- 27.Davies AM, Ruel C, Lara PN, et al. The proteasome inhibitor bortezomib in combination with gemcitabine and carboplatin in advanced non-small cell lung cancer: A California Cancer Consortium phase I study. J Thorac Oncol. 2007 doi: 10.1097/JTO.0b013e31815e8b88. in press. [DOI] [PubMed] [Google Scholar]
- 28.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 29.Williamson SK, Crowley JJ, Lara PN, Jr., et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. 2005;23:9097–9104. doi: 10.1200/JCO.2005.01.3771. [DOI] [PubMed] [Google Scholar]
- 30.Kelly K, Herbst RS, Crowley JJ, et al. Concurrent chemotherapy plus cetuximab or chemotherapy followed by cetuximab in advanced non-small cell lung cancer (NSCLC): A randomized phase II selectional trial SWOG 0342. J Clin Oncol. 2006;24:367s. (abstr) [Google Scholar]
- 31.Edelman MJ, Clark JI, Chansky K, et al. Randomized phase II trial of sequential chemotherapy in advanced non-small cell lung cancer (SWOG 9806): carboplatin/gemcitabine followed by paclitaxel or cisplatin/vinorelbine followed by docetaxel. Clin Cancer Res. 2004;10:5022–5026. doi: 10.1158/1078-0432.CCR-04-0002. [DOI] [PubMed] [Google Scholar]
- 32.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 33.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 34.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 35.Lonial S, Richardson P, Sonneveld P, et al. Hematologic profiles in the phase 3 APEX trial. Blood. 2005;106:970a. (abstr 3474) [Google Scholar]
- 36.Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106:3777–3784. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richardson PG, Briemberg H, Jagannath S, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 38.San Miguel JF, Richardson P, Sonneveld P, et al. Frequency, characteristics, and reversibility of peripheral neuropathy (PN) in the APEX trial. Blood. 2005;106:111a. (abstr 366) [Google Scholar]